Abstract
Meissner's corpuscles (MCs) are tactile mechanoreceptors found in the glabrous skin of primates, including fingertips. These receptors are characterized by sensitivity to light touch, and therefore might be associated with the evolution of manipulative abilities of the hands in primates. We examined MCs in different primate species, including common marmoset (Callithrix jacchus,n = 5), baboon (Papio anubis,n = 2), rhesus macaque (Macaca mulatta,n = 3), chimpanzee (Pan troglodytes,n = 3), bonobo (Pan paniscus,n = 1) and human (Homo sapiens,n = 8). Fingertips of the first, second and fourth digits were collected from both hands of specimens, dissected and histologically stained using hematoxylin and eosin. The density (MCs per 1 mm2) and the size (cross-sectional diameter of MCs) were quantified. Overall, there were no differences in the densities of MCs or their size among the digits or between the hands for any species examined. However, MCs varied across species. We found a trend for higher densities of MCs in macaques and humans compared with chimpanzees and bonobos; moreover, apes had larger MCs than monkeys. We further examined whether the density or size of MCs varied as a function of body mass, measures of dexterity and dietary frugivory. Among these variables, only body size accounted for a significant amount of variation in the size of MCs.
Keywords: body mass, dietary frugivory, digital dexterity, Meissner's corpuscles, primates
Introduction
Meissner's corpuscles (MCs) are tactile mechanoreceptors found in the glabrous (i.e. hairless) skin of primates, including fingertips (Bolanowski & Pawson, 2003; Hoffmann et al. 2004). They are localized in the dermal papillae (Fig.1) and are characterized by sensitivity to light touch. MCs are rapidly adapting receptors that signal transient changes in pressure applied to the skin surface (Hoffmann et al. 2004; Organ et al. 2011; Zimmerman et al. 2014). As touch receptors, they provide high tactile acuity and are especially numerous in the fingertips (Johansson & Vallbo, 1979; Caruso et al. 1994). Given their characteristics and distribution patterns, MCs have been likened to retinal photoreceptors in that they make up ‘tactile fovea’ of the fingers that provides fine spatial resolution for cutaneous sensation (Hoffmann et al. 2004).
Figure 1.
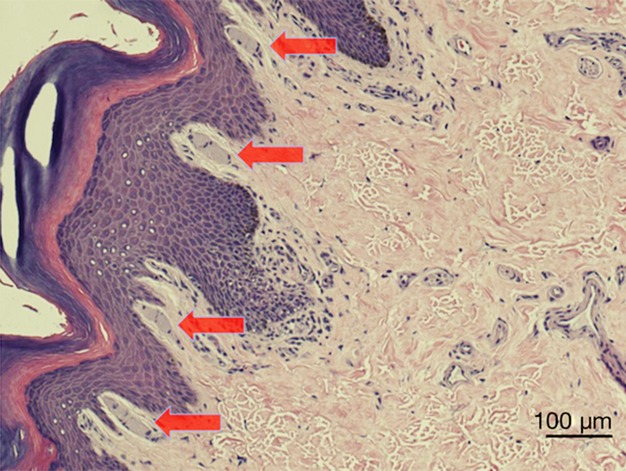
MCs of a rhesus macaque (Macaca mulatta). MCs are indicated by red arrows.
Functionally, MCs have been hypothesized to provide important sensory feedback for the effective control of grip (Purves et al. 2008). For example, Martin (1990) proposed that their association with papillary ridges enhances tactile perception and reduces slippage by allowing compensatory changes in the force of the grip (see also Preuss, 2009). This sensory control of grasping may have been especially important in the fine-branch niche of arboreal environments in which primates evolved. Others have suggested that MCs may aid in the assessment of fruit texture (Hoffmann et al. 2004). Overall, it has been suggested that MCs improve manipulative abilities in primates by providing important sensory feedback from the surface of the volar skin (Hoffmann et al. 2004).
Previous research has examined MCs in a number of primate species. For example, Winkelman (1963) published a comprehensive review describing MCs in apes, Old World monkeys, New World monkeys and strepsirrhine species. MCs were reported to be present in the glabrous skin of all primate species examined, but to be lacking in tree shrews, which are among the closest living relatives of primates. This led Martin (1990) to conclude that MCs likely evolved early in ancestral primates and were associated with adaptations to arboreal foraging. Despite their significance in primate evolution, it is notable that little comparative quantitative data exist on the distribution or size of MCs across primates.
Bolanowski & Pawson (2003) examined the spatial organization and densities of MCs in rhesus monkey fingertips. The quantitative analysis was then extended to other monkey species (Güçlü et al. 2003) and apes (Hoffmann et al. 2004) for individual fingers and different hands. From these analyses it would appear that the average densities of MCs differ dramatically among primate species. For example, the density of MCs was reported to be 5.7 per mm2 for baboon (Güçlü et al. 2003) and 45.8 per mm2 for gibbon (Hylobates lar; Hoffmann et al. 2004). However, these studies differed considerably in their methodology, such as techniques of histological staining (e.g. cholinesterase vs. Masson's trichrome), sectioning procedures (e.g. transverse vs. longitudinal), and digits and/or number of sections per digit examined. Because these methodological differences may affect the estimates of density and size, a more systematic quantitative analysis of MCs in a range of primate species is warranted.
It has been previously argued that the evolution of prehension in primates involved independent control of digits (Bishop, 1964; Heffner & Masterton, 1975; Napier, 1993), which may have evolved in the context of manipulation of small food items and other objects (Bishop, 1964; Jolly, 1970; Welles, 1976; Hoffmann et al. 2004). Primates differ in their manual ability, and several authors have attempted to rank primates in their manipulation skills according to a dexterity index developed by Heffner & Masterton (1975) (see also Iwaniuk et al. 1999). Given the sensory characteristics of MCs, their role in the control of grip and the extent to which primates use their hands, it is possible that MCs may be associated with the evolution of manipulative abilities in primates, including the use of different grips in manipulation.
In the current study, we examined these relationships by analyzing MCs in six primate species, including humans (Fig.2). We quantified the densities of MCs per mm2 and their size. Further, we examined whether MCs varied as a function of body mass, digital dexterity and dietary frugivory across the species in our sample. This allowed us to test the role of sensory feedback in the enhanced manual control of humans compared with other primates (Napier, 1961, 1993), as well as the fruit texture hypothesis of Hoffmann et al. (2004). Moreover, we examined how the density and the size of MCs were associated with the presence of the precision grip vs. power grip in the behavioral repertoire of the species. Specifically, given that the precision grip involves the use of the thumb in coordination with another digit (usually the index finger), we examined the distribution of MCs across fingers in relation to the ability to perform this grip type.
Figure 2.
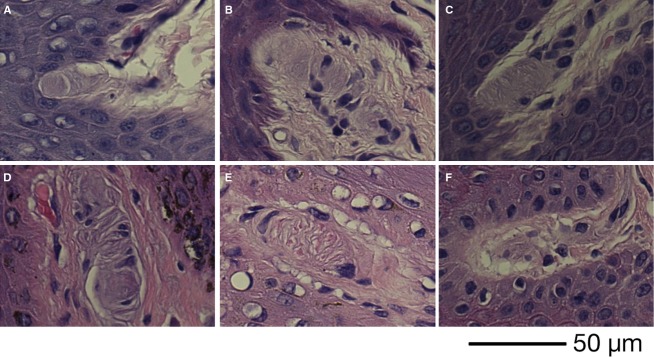
MCs of Callithrix jacchus (A), Papio anubis (B), Macaca mulatta (C), Pan troglodytes (D), Pan paniscus (E) and Homo sapiens (F).
Another purpose of the present study was to examine how MCs may be related to handedness. Although hand preference is widespread and well documented in humans (Marchant & Mcgrew, 1994; Raymond & Pontier, 2004), it may not be restricted to our species (Mcgrew & Marchant, 1997; Hopkins et al. 2007). For example, bias in hand use has been documented in chimpanzees (Hopkins et al. 2007) and other primate species (Fragaszy & Mitchell, 1990; Westergaard & Suomi, 1996; Spinozzi & Truppa, 1999). Given that handedness involves one (i.e. dominant) hand performing the task with the other providing stabilizing support, we sought to examine whether this asymmetry in hand use (especially in humans) is reflected in the distribution patterns of MCs between the hands.
Materials and methods
Subjects
The non-human primate fingertip tissue was collected opportunistically at necropsy from specimens provided by Southwest National Primate Research Center (San Antonio, TX, USA), Yerkes National Primate Research Center (Atlanta, GA, USA) and the Iowa Primate Learning Sanctuary (Des Moines, IA, USA). Fingertips from human cadavers were obtained from The George Washington University School of Medicine and Health Sciences. No animals were killed for the purpose of the present study. The specimens used are described in Table1.
Table 1.
Specimens used in the present study, including sex and age of each specimen when known
| Species | Sex | Age | Not included | Species | Sex | Age | Not included |
|---|---|---|---|---|---|---|---|
| Common marmoset (Callithrix jacchus) | M | 2 | R1, R4 | Chimpanzee (Pan troglodytes) | F | 24 | |
| M | 2 | R1 | F | 57 | |||
| M | 2 | F | 50 | ||||
| M | 2 | L1, L2, L4 | |||||
| M | 5 | L1 | |||||
| Baboon (Papio anubis) | F | 9 | Human (Homo sapiens) | F | 78 | ||
| M | 7 | F | 89 | ||||
| M | 91 | ||||||
| Rhesus macaque (Macaca mulatta) | F | 13 | L1 | M | 85 | L1, L2, L4 | |
| M | 20 | F | ? | ||||
| ? | ? | R2 | F | ? | |||
| M | ? | ||||||
| Bonobo (Pan paniscus) | F | 44 | M | ? |
Not all fingertips were available for MCs quantification (due to poor quality tissue) and fingertips ‘not’ included in the analysis are listed in the ‘Not Included’ section of the table.
Histological preparation
Fingertips (i.e. skin sample from the digital pad) of the first (thumb), second (index) and fourth (ring) digits were collected from both hands of specimens, and stored in formalin solution. Fingertips were collected from these digits specifically to test the hypothesis of differential MC distribution in relationship to the use of the thumb and index finger in precision grip compared with other digits (i.e. ring finger). The fingertips were then embedded in paraffin, and the digital pad was dissected vertically into thin (5 μm) transverse sections (3–4 per finger) in distal–proximal gradient and histologically stained using hematoxylin and eosin. The histological preparations were performed by Histo-Scientific Research Laboratories (HSRL, Jackson, VA, USA).
Microscopy and data collection
All tissue sections were coded prior to data collection to remove possible measurement bias. MCs were identified and quantified using a Zeiss Axioplan 2 photo-microscope equipped with a Ludl XY motorized stage (Ludl Electronic Products, Hawthorne, NY, USA), Heidenhain z-axis encoder, and an Optronics MicroFire color video camera (Optronics, Goleta, CA, USA) coupled to a Dell PC workstation running StereoInvestigator software (MBF Bioscience, Williston, VT, USA). The length of the epidermis in the sample was measured along the surface of stratum granulosum using a 10 × objective lens. MCs were quantified at a magnification of 20 × along this length. The size of a corpuscle was measured as its cross-sectional diameter using StereoInvestigator software (in μm) by tracing a straight line across the approximate middle portion of each corpuscle and parallel to the surface of stratum granulosum. The average counts of MCs for each finger were determined by applying the Abercrombie correction formula (Abercrombie, 1946): N = n*(T/T + H), where n is the average number of MCs across all sections for the given fingertip, T is the average section thickness, H is the average size of MCs (averaged across all sections for the given fingertip) and N is the corrected average number of MCs for each finger for the given length of epidermis. To estimate the average density of MCs (per mm2), we multiplied N by 1 mm2 and divided it by the average area of epidermis measured for each fingertip calculated across all sections, the product being the average density of MCs per mm2 for each finger.
Several fingertips were excluded from the data collection due to poor quality of tissue (e.g. large portions of epidermis missing making it impossible to measure densities of MCs per mm2 of epidermal length). The excluded samples are detailed in Table1; these were also excluded from the statistical analyses.
Statistical analyses
Because only one specimen of bonobo was available, MCs data for the bonobo and three chimpanzees were pooled and analyzed together as the genus Pan. One-way analyses of variance (anova) were used to compare the density and size of MCs among the fingers and between the hands within each species. To compare MC densities and size among species, means were calculated across fingertips for each individual and these were used in cross-species comparisons using the non-parametric Kruskal–Wallis one-way anova test. When appropriate, we used the Bonferroni correction for post hoc multiple comparisons. For comparisons between apes and monkeys, the data were pooled and analyzed together using an independent samples t-test. Ordinary least-squares regression analyses on species means were used to assess the relationship between MC densities or size and body mass, manual ability, and dietary frugivory. Digital dexterity scores found in Iwaniuk et al. (1999) were used as a proxy for manual ability. These scores were originally developed by Heffner & Masterton (1975) on the basis of the anatomy of the hand described by Napier & Napier (1967; see also Napier, 1961; e.g. opposable thumb vs. non-opposable thumb). Dietary frugivory was measured as the percentage of fruit in the diet including the feeding time, food intake or stomach content devoted to fruit, seeds or gums (Chivers, 1984). Previously published data on dietary frugivory were used (Clutton-Brock & Harvey, 1977; Lindburg, 1977; Hubrecht, 1984, 1985). Humans were excluded from analyses of percentage of fruit consumption in the diet because meaningful data are not available. All significance levels were set at α = 0.05.
Data from closely related species do not satisfy the conditions of independence due to shared common ancestry, implicit in traditional parametric and non-parametric statistical tests (Iwaniuk et al. 1999; see also Felsenstein, 1985; Harvey & Pagel, 1991). Therefore, we also performed phylogenetic generalized least-squares regressions (PGLS) using the caper package in r statistical software (Orme, 2013).
Results
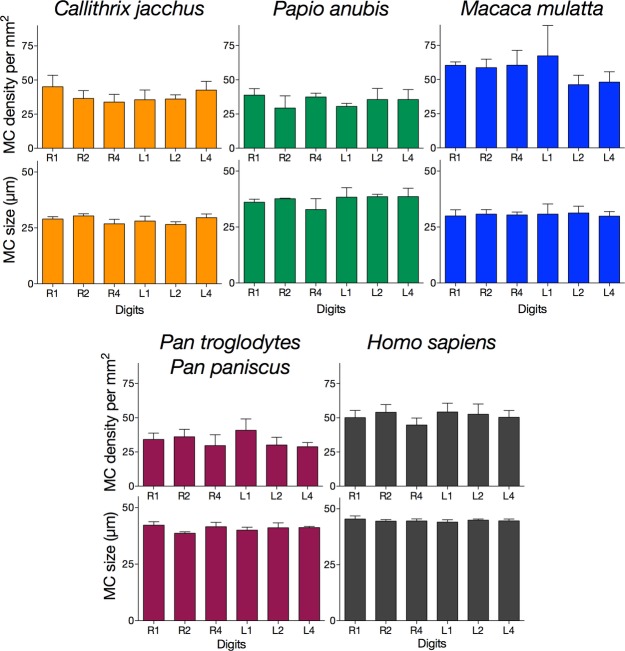
Overall, we found no differences in MC densities or size among the digits or between the hands for any species examined (Fig.3). In other words, for any given species, MCs were similar in both their density and size across the digits and between the hands. In the anova across species, omnibus Kruskall–Wallis one-way anova showed significant species differences in both the density [Χ2(4) = 13.717, P = 0.008] and the size [Χ2(4) = 18.620; P = 0.001] of MCs. Bonferroni-adjusted post hoc pairwise comparisons revealed a trend for lower MC densities in genus Pan than in Homo sapiens (P = 0.099) and Macaca mulatta (P = 0.068). In terms of MC size, Bonferroni-adjusted post hoc pairwise comparisons revealed significantly larger MCs in H. sapiens than in Callithrix jacchus (P = 0.001) and M. mulatta (P = 0.038); no other comparisons reached statistical significance (all P > 0.05; Fig.4).
Figure 3.
Meissner's corpuscles (MCs) density per mm2 and MCs size across digits for each primate species. Error bars indicate SEM. One-way anova showed no differences in MCs density or size among the digits or between the hands for any species examined (all P values non-significant). See text for more details.
Figure 4.
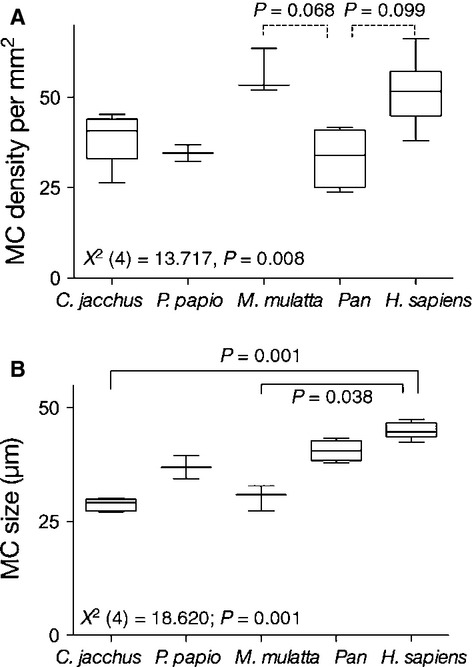
Comparison of Meissner's corpuscles (MCs) density (A) and size (B) among the primate species. Box-plots indicate the 25th percentile, the median and the 75th percentile; the whiskers indicate the range. Non-parametric Kruskall–Wallis one-way anova showed significant species differences in both the density and the size of MCs. Bonferroni-adjusted post hoc pairwise comparisons revealed significant differences between the species (solid line) or differences that approached statistical significance (dotted lines).
To further explore phylogenetic variation, we compared MC densities and size between the apes (humans and chimpanzee species) and the monkeys (macaques, baboons and marmosets). Independent samples t-test revealed no differences in MC densities (t20 = 0.424; P = 0.676) between these groups; however, apes had larger MCs than the monkeys (t20 = 8.650; P < 0.001; Fig.5).
Figure 5.
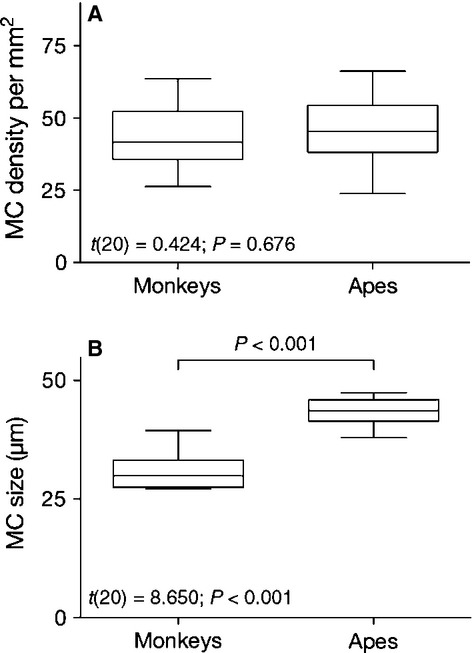
Comparison of Meissner's corpuscles (MCs) density (A) and size (B) between apes (humans and two chimpanzee species) and monkeys (marmosets, baboons and rhesus). Box-plots indicate the 25th percentile, the median and the 75th percentile; the whiskers indicate the range. Independent samples t-test revealed a significant difference between these groups in MCs size but not density.
It is possible that the observed species differences in the size of MCs reflect variation in body mass among the species in our sample. For example, the difference in body mass between chimpanzees and marmosets is over 100-fold. To assess the effects of body mass on species differences in MCs, we examined the relationship between species mean body mass (data provided in Smith & Jungers, 1997) and MC density or size using linear regression analyses. These analyses revealed a significant relationship between body mass and MC size (R2 = 0.917; F1,3 = 33.054, P = 0.01), but not MC density (R2 = 0.020; F1,3 = 0.062, P = 0.819; Fig.6).
Figure 6.
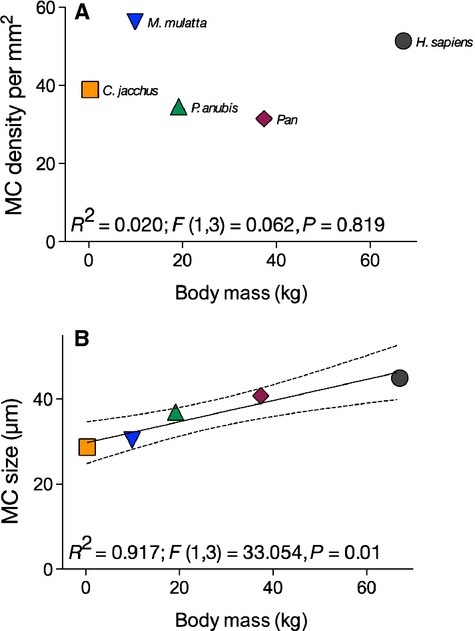
Relationships between Meissner's corpuscles (MCs) density (A), size (B) and body mass (kg). Body mass data are taken from Smith & Jungers (1997). The regression line and the confidence intervals are derived from ordinary least-squares regression analysis. See text for more details.
Next we examined MCs in relation to dexterity and dietary frugivory. Overall, regression analyses revealed no significant relationships between MC density or size and either dexterity or percentage of fruit consumption in diet (all P > 0.05; see Fig.7 for the summary of results).
Figure 7.
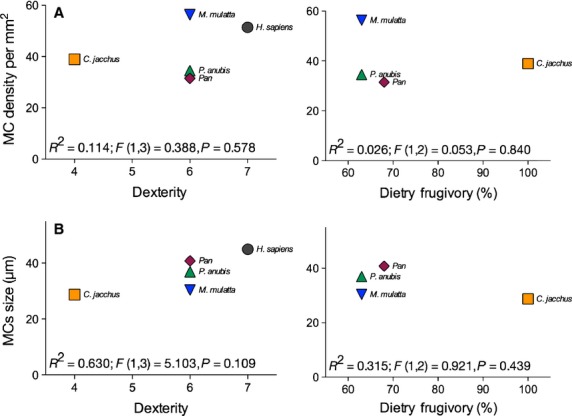
Relationship between Meissner's corpuscles (MCs) density (A) and size (B), and either dexterity or dietary frugivory. The dexterity index was originally developed by Heffner & Masterton (1975) on the basis of the anatomy of the hand described by Napier & Napier (1967; see also Napier, 1961). The dietary frugivory was compiled from available data. See text for more details.
We also performed analyses of the relationship between MCs data with body mass, dexterity score and percentage of fruit consumption in the diet using PGLS. Because there was no evidence for phylogenetic signal in our data set (all λ = 0), the results of PGLS parallel those of ordinary least-squares regression described above and are not reported here.
Discussion
Meissner's corpuscles are tactile mechanoreceptors found in the glabrous skin of primates. They are especially numerous in the fingertips (Johansson & Vallbo, 1979; Caruso et al. 1994) and are characterized by sensitivity to movement across the skin (Zimmerman et al. 2014). Their association with papillary ridges has led to the hypothesis that MCs play a role in detection and reduction of slippage by allowing compensatory changes in the force of the grip (Martin, 1990; Preuss, 2009). This sensory control of grasping may have been especially important in the fine-branch niche of arboreal environments in which primates evolved. Additionally, MCs may aid in the evaluation of fruit texture, thus allowing selection of ripe fruit (Hoffmann et al. 2004). Overall, it has been suggested that MCs improve manipulation abilities in primates by providing important sensory feedback from the surface of the hands and fingertips (Hoffmann et al. 2004).
The present study is a comparative quantitative analysis of MC density and size in different primate species. Previous quantitative analyses of MCs in primates used inconsistent histological staining techniques and counting methods, which sometimes resulted in data that are difficult to interpret. For example, Bolanowski & Pawson (2003) measured the average density of MCs in rhesus monkey fingertips and found it to be about 45 per mm2. A later study by the same group, however, measured the average density of MCs for the same species to be 26.7 per mm2 (Güçlü et al. 2003). Because these differences can likely be explained on methodological grounds, in the present study we used a systematic approach to quantify MCs in several digits and both hands of specimens. Specifically, MC densities and size across digits and between hands were measured, and their relationship to precision grip, handedness, digital dexterity and dietary frugivory were assessed.
We found no differences in MC densities or size among digits or between hands for any species examined. This suggests no relationship between MCs and either the use of precision grip or handedness. First, among the species examined in our sample, all but the common marmoset (C. jacchus) are capable of employing precision grip in either the wild or captivity (Rose, 1977; Marzke & Wullstein, 1996; Marzke, 1997; see also Napier, 1961); these species are also known for complex manipulation skills in relation to the use of feeding tools (Van Schaik et al. 1999; see also Torigoe, 1985). The precision grip usually involves the use of the thumb in association with another digit (usually the index finger; Napier, 1956; Landsmeer, 1962). No differences in MC distribution or size among digits were found, suggesting there is no relationship between variation in MCs across the digits and ability to perform the precision grip. It should be noted, however, that the precision grip is not a uniform behavior, and varies across species, maturity levels and object parameters (Marzke, 1997; Pouydebat et al. 2009). Precision grip is also largely determined by the anatomy of the hand. For example, chimpanzees and humans use different grips owing to different hand morphologies, reflecting differential proportion of thumb involvement relative to other digits. Nonetheless, assuming that the thumb is necessary for the ‘true precision grip’ (Napier, 1961), and finding no differences in MC distribution between the thumb and other digits, the present data do not support a relationship between MCs and the use of the precision grip.
Second, the comparison of MCs between hands likewise revealed no differences between hands for any species examined, suggesting that there is no relationship between handedness and MC distribution. Although we do not know the handedness for our human specimens, assuming the well-recorded ratio of 9 : 1 right-handedness (Marchant & Mcgrew, 1994; Raymond & Pontier, 2004) in our sample, there is no evidence of a relationship between hand preference and MC distribution.
Comparison of MCs across all species revealed phylogenetic differences in MC density and size. Notably, humans had significantly larger MCs than marmosets and macaques. Overall, apes (humans and Pan species) had larger MCs than monkeys, although there was no difference in MC densities between the two groups. Given the extreme diversity in body mass among the species in the present study, we analyzed the relationship between body mass and MC size. Indeed, we found that body mass accounts for 92% of the variance of MC size in our sample. This increase in the size of MCs might be related to proportional increase in the size of the finger pads in larger animals.
It is interesting to note that macaque monkeys and humans had higher densities of MCs than other species. Moreover, MC density was slightly higher (but not statistically significant) in rhesus macaques compared with humans. These results are consistent with previous reports showing higher densities per mm2 in rhesus macaques relative to humans (Bolanowski & Pawson, 2003). It should also be noted, however, that MC density steadily declines with age (Cauna, 1956, 1965; Bolton et al. 1966; Iwasaki et al. 2003). All our human specimens were of advanced age; those of documented age were 78 years old or older. It is therefore possible that the density of MCs is higher in younger adults.
Regarding the lack of a relationship between MCs and either dexterity or dietary frugivory, we should point out a couple of important caveats. First, the dexterity score proposed by Heffner & Masterton (1975) and later employed by Iwaniuk et al. (1999) was originally used for mammals at large. As a digital dexterity index, it was largely developed based on anatomical feature of thumb opposability (Napier & Napier, 1967). As such, it may not accurately represent the more subtle variation in manipulative abilities across primates (for other criticisms of this dexterity index, see Heffner & Masterton, 1975; Iwaniuk et al. 1999). Four out of six species in the present study received the score of 6 according to this dexterity index, with marmosets receiving 4 and humans a score of 7. A different dexterity index based on behavioral observations developed specifically for primates is needed to more precisely characterize the diverse manipulative abilities of primate species (Torigoe, 1985).
Second, our species sample may have been too small to properly measure the relationship between MCs and dietary frugivory. With humans excluded from the analysis, our sample was effectively divided into two groups – Old World monkeys, and apes consuming 63–68% of fruit in their diet and marmosets whose diet mostly consists of fruit. With such a binary distribution, meaningful analyses are perhaps not possible. An additional limitation of the data on dietary frugivory used here is that a single measure may not adequately account for relevant population and intra-annual variability in fruit consumption in the wild. To address this issue, a larger interspecific sample with more diverse dietary habits would be needed.
The present study is the first to systematically examine MC densities and size in a number of primate species and across different digits and between hands of individuals. We also examined MC distribution in relation to a number of different behavioral characteristics. We show that MC size, like other anatomical features, varies with body mass. Future studies should attempt to examine other tactile mechanoreceptors in relation to these variables.
Acknowledgments
The authors thank Dr Raymond Walsh for facilitating specimen collection from human cadavers. This work was supported by the National Institutes of Health (NS042867 and NS073134) and the James S. McDonnell Foundation (220020293).
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Bishop A. Use of the hand in lower primates. In: Buettner-Janusch J, editor. Evolutionary and Genetic Biology of Primates. Vol. 2. New York: Academic Press; 1964. pp. 133–225. In:, Vol. [Google Scholar]
- Bolanowski SJ, Pawson L. Organization of Meissner corpuscles in the glabrous skin of monkey and cat. Somatosens Mot Res. 2003;20:223–231. doi: 10.1080/08990220310001622915. [DOI] [PubMed] [Google Scholar]
- Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Neurology. 1966;16:1–9. doi: 10.1212/wnl.16.1.1. [DOI] [PubMed] [Google Scholar]
- Caruso G, Nolano M, Lullo F. Median nerve sensory responses evoked by tactile stimulation of the finger proximal and distal phalanx in normal subjects. Muscle Nerve. 1994;17:269–275. doi: 10.1002/mus.880170303. [DOI] [PubMed] [Google Scholar]
- Cauna N. Nerve supply and nerve endings in Meissner corpuscles. Am J Anat. 1956;99:315. doi: 10.1002/aja.1000990206. [DOI] [PubMed] [Google Scholar]
- Cauna N. The effects of aging on the receptor organs of the human dermis. In: Montagna W, editor. Advances in Biology of Skin, Vol. VI: Aging. New York: Pergamon; 1965. pp. 63–96. [Google Scholar]
- Chivers DJ. A summary of feeding and ranging in Gibbons. In: Preuschoft H, Chivers DJ, Brockelman WY, Creel N, editors. The Lesser Apes. Edinburgh: Edinburgh University Press; 1984. pp. 267–281. [Google Scholar]
- Clutton-Brock TH, Harvey PH. Species differences in feeding and ranging behaviour in primates. In: Clutton-Brock TH, editor. Primate Ecology. London: Academic Press; 1977. pp. 557–584. [Google Scholar]
- Felsenstein J. Phylogenies and comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- Fragaszy DM, Mitchell SR. Hand preference and performance on unimanual and bimanual tasks in capuchin monkeys (Cebus apella. J Comp Psychol. 1990;104:272–282. doi: 10.1037/0735-7036.104.3.275. [DOI] [PubMed] [Google Scholar]
- Güçlü B, Bolanowski SJ, Pawson L. End-to-end linkage (EEL) clustering algorithm: a study on the distribution of Meissner corpuscles in the skin. J Comput Neurosci. 2003;15:19–28. doi: 10.1023/a:1024466617694. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Heffner R, Masterton B. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain Behav Evol. 1975;12:161–200. doi: 10.1159/000124401. [DOI] [PubMed] [Google Scholar]
- Hoffmann JN, Montag AG, Dominy NJ. Meissner corpuscles and somatosensory acuity: the prehensile appendages of primates and elephants. Anat Rec. 2004;281:1138–1147. doi: 10.1002/ar.a.20119. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Lambeth S. Handedness and neuroanatomical asymmetries in captive chimpanzees: a summary of 15 years of research. In: Hopkins WD, editor. Evolution of Hemispheric Specialization in Primates. Oxford: Elsevier; 2007. pp. 146–181. [Google Scholar]
- Hubrecht RC. Field observations on group size and composition of the common marmoset (Callithrix jacchus jacchus) at Tacapura, Brazil. Primates. 1984;25:13–21. [Google Scholar]
- Hubrecht RC. Home range size and use and territorial behaviour in the common marmoset (Callithrix jacchus jacchus) at the Tacapura field station, Recife, Brazil. Int J Primatol. 1985;6:533–550. [Google Scholar]
- Iwaniuk AN, Pellis SM, Whishaw IQ. Is digital dexterity really related to corticospinal projections? A re-analysis of the Heffner and Masterton data set using modern comparative statistics. Behav Brain Res. 1999;101:173–187. doi: 10.1016/s0166-4328(98)00151-x. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Goto N, Goto J. The aging of human Meissner's corpuscles as evidenced by parallel sectioning. Okajimas Folia Anat. Jpn. 2003;79:185–190. doi: 10.2535/ofaj.79.185. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly CJ. The seed-eaters: a new model of hominid differentiation based on a baboon analogy. Man. 1970;5:5–26. [Google Scholar]
- Landsmeer J. Power grip and precision handling. Ann Rheum Dis. 1962;21:164–170. doi: 10.1136/ard.21.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindburg DG. Feeding behavior and diet of rhesus monkeys (Macaca mulatta) in a Siwalik Forest in North India. In: Clutton-Brock TH, editor. Primate Ecology: Studies of Feeding and Ranging Behaviour in Lemurs, Monkeys and Apes. London: Academic Press; 1977. pp. 223–249. [Google Scholar]
- Marchant LF, Mcgrew WC. Human handedness: an ethological perspective. Hum Evol. 1994;13:221–228. [Google Scholar]
- Martin R. Primate Origins and Evolution: a Phylogenetic Reconstruction. Princeton, NJ: Princeton University Press; 1990. [Google Scholar]
- Marzke MW. Precision grips, hand morphology, and tools. Am J Phys Anthropol. 1997;102:91–110. doi: 10.1002/(SICI)1096-8644(199701)102:1<91::AID-AJPA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Marzke MW, Wullstein KL. Chimpanzee and human grips: a new classification with a focus on evolutionary morphology. Int J Primatol. 1996;17:117–139. [Google Scholar]
- Mcgrew WC, Marchant LF. On the other hand: current issues in and metaanalysis of the behavioral laterality of hand function in non-human primates. Yearb Phys Anthropol. 1997;40:201–232. [Google Scholar]
- Napier JR. The prehensile movements of the human hand. J Bone Joint Surg. 1956;38B:902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- Napier JR. Prehensility and opposability in the hands of primates. Symp Zool Soc Lond. 1961;5:115–132. [Google Scholar]
- Napier JR. Hands. Princeton, NJ: Princeton University Press; 1993. [Google Scholar]
- Napier JR, Napier PH. A Handbook of Living Primates. New York: Academic Press; 1967. [Google Scholar]
- Organ JM, Muchlinski MN, Deane AS. Mechanoreceptivity of prehensile tail skin varies between ateline and cebine primates. Anat Rec. 2011;294:2064–2072. doi: 10.1002/ar.21505. [DOI] [PubMed] [Google Scholar]
- Orme D. 2013. R package versionThe caper package: comparative analysis of phylogenetics and evolution in R 0.5, 2.
- Pouydebat E, Gorce P, Coppens Y. Biomechanical study of grasping according to the volume of the object: human versus non-human primates. J Biomech. 2009;42:266–272. doi: 10.1016/j.jbiomech.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Primate brain evolution. In: Kaas JH, editor. Evolutionary Neuroscience. Oxford: Academic Press; 2009. pp. 793–825. [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D. Neuroscience. 4th edn. Sunderland, MA: Sinauer; 2008. [Google Scholar]
- Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- Rose MD. Positional behaviour of olive baboons (Papio anubis) and its relationship to maintenance and social activities. Primates. 1977;18:59–116. [Google Scholar]
- Smith RJ, Jungers WL. Body mass in comparative primatology. J Hum Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Truppa V. Hand preferences in different tasks by tufted capuchins (Cebus apella. Int J Primatol. 1999;20:827–849. [Google Scholar]
- Torigoe T. Comparison of object manipulation among 74 species of non-human primates. Primates. 1985;26:182–194. [Google Scholar]
- Van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: implications for the evolution of material culture. J Hum Evol. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- Welles JF. A comparative study of manual prehension in anthropoids. Saugetierlaundliche Mitteilungen. 1976;24:26–38. [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta. J Comp Psychol. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]
- Winkelman RK. Nerve endings in the skin of primates. In: Buettner-Janusch J, editor. Evolutionary and Genetic Biology of Primates. New York: Academic Press; 1963. pp. 229–259. [Google Scholar]
- Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346:950–954. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]