Abstract
In vertebrates, paired appendages (limbs and fins) are derived from the somatic mesoderm subsequent to the separation of the lateral plate mesoderm into somatic and splanchnic layers. This is less clear for teleosts, however, because the developmental processes of separation into two layers and of extension over the yolk have rarely been studied. During teleost evolution, the position of pelvic fins has generally shifted rostrally (Rosen; Nelson, 1982, 1994), although at the early embryonic stage the presumptive pelvic fin cells are initially located near the future anus region – the anterior border ofhoxc10a expression in the spinal cord – regardless of their final destination. Our previous studies in zebrafish (abdominal pelvic fins) and Nile tilapia (thoracic pelvic fins) showed that the presumptive pelvic fin cells shift their position with respect to the body trunk after its protrusion from the yolk surface. Furthermore, in Nile tilapia, presumptive pelvic fin cells migrate anteriorly on the yolk surface. Here, we examined the embryonic development of the lateral plate mesoderm at histological levels in the pufferfishTakifugu niphobles, which belongs to the highly derived teleost order Tetraodontiformes, and lacks pelvic fins. Our results show that, inT. niphobles, the lateral plate mesoderm bulges out as two separate layers of cells alongside the body trunk prior to its further extension to cover the yolk sphere. Once the lateral plate mesoderm extends laterally, it rapidly covers the surface of the yolk. Furthermore, cells located near the anterior border ofhoxc10a expression in the spinal cord reach the anterior-most region of the yolk surface. In light of our previous and current studies, we propose that anterior migration of presumptive pelvic fin cells might be required for them to reach the thoracic or more anterior positions as is seen in other highly derived teleost groups.
Keywords: evolution, lateral plate mesoderm, pelvic fin, pufferfish
Introduction
The lateral plate mesoderm forms alongside the body trunk during early embryogenesis of vertebrates, and it separates into somatic and splanchnic layers during development. The somatic layers give rise to limbs as well as the body wall, whereas splanchnic layers give rise to the heart, blood, blood vessels and epithelium of the internal organs (Funayama et al. 1999). The separation of the lateral plate mesoderm seems to be conserved among vertebrates – even in agnathan lampreys, which diversified prior to the origin of paired appendages (Onimaru et al. 2011). In chick embryos,Irx3 appears in the somatic mesoderm subsequent to subdivision (Funayama et al. 1999). Similarly, in lampreys, the lateral plate mesoderm subdivides into two layers at the cardiac level, and the somatic layer expressesLjIrx1/3, although the posterior lateral plate mesoderm does not separate into two layers and does not expressLjIrx1/3 (Onimaru et al. 2011). This observation suggests that separation of the somatic and splanchnic layers might have been a prerequisite for the acquisition of paired fins during evolution (Onimaru et al. 2011; Nuno de la Rosa et al. 2014).
In zebrafish, medaka and Nile tilapia, a thin layer of the lateral plate mesoderm is first observed on the surface of the yolk alongside the body trunk and then spreads ventrally (Murata et al. 2010; Kaneko et al. 2014). Early during embryogenesis, pectoral fin buds arise from this thin layer. However, the process of physical separation of the lateral plate mesoderm has not been studied in these three fishes, although expression ofirx3a has been observed in pectoral fin buds of zebrafish embryos (McDonald et al. 2010).
Pectoral fin buds of teleosts arise from the lateral plate mesoderm within a few days post-fertilization (dpf), whereas pelvic fin buds initiate their development at much later stages – at the time of larval-to-juvenile metamorphosis (Grandel & Schulte-Merker, 1998; Fujimura & Okada, 2007). In teleosts, the fate of presumptive pelvic fin cells seems to be determined during early embryogenesis, and pelvic fin development is initiated at their final location during metamorphosis (Tanaka, 2011). Among teleosts, pelvic fin position varies from abdominal to jugular levels (Greenwood et al. 1966; Gosline, 1971; Rosen, 1982; Tanaka, 2011). Analyses of the temporal distribution of the lateral plate mesoderm in zebrafish and Nile tilapia revealed that presumptive pelvic fin cells are originally located near the anus but, upon body trunk protrusion, shift their position from the anus to abdominal (zebrafish) or thoracic (Nile tilapia) position (Murata et al. 2010; Tanaka, 2011). Fate mapping in Nile tilapia embryos also revealed that presumptive pelvic fin cells migrate anteriorly toward their final destination (Murata et al. 2010). Thus, our understanding of the basis of evolution of pelvic fin positioning is predicated on defining the temporal distribution of lateral plate mesodermal cells.
The pufferfishTakifugu niphobles belongs to Tetraodontiformes, the teleost order that diversified most recently (Nelson, 1994; Yamanoue et al. 2009), and lacks entire structures of pelvic fins as in the pufferfishTakifugu rubripes (Tanaka et al. 2005). Here, we investigated the embryonic development of the lateral plate mesoderm inT. niphobles at both morphological and histological levels. Our results show that the lateral plate mesoderm bulges out as two separate layers of cells alongside the body trunk prior to its lateral extension to cover the yolk surface. Furthermore, the lateral plate mesoderm rapidly covers the yolk surface during embryogenesis, and cells at the anterior border ofhoxc10a expression in the spinal cord actively migrate towards the anterior end of the yolk surface, i.e. the thoracic position. Based on our previous and current findings, we propose that acquisition of the ability of presumptive pelvic fin cells to actively migrate anteriorly may be required for them to reach the thoracic or more anterior position.
Materials and methods
Biological materials
Freshly laidT. niphobles eggs were collected on Arai beach, Kanagawa prefecture, Japan, during the breeding season (May–June), and cultured in seawater at 14–16 °C. Forin situ hybridization, embryos were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline, dehydrated in a graded methanol series, and stored in 100% methanol at −20 °C.
Gene isolation andin situ hybridization
Total RNA fromT. niphobles was extracted from 6- to 7-dpf embryos using the RNeasy kit (Qiagen, Hilden, Germany). cDNA forT. niphobles hoxc10a (Tnhoxc10a) was synthesized by reverse transcription and used as a template for polymerase chain reaction with the following primers:Tnhoxc10a, 5′-GGGAGGAWCATCTYCYTTCAG-3′ and 5′-GACCTGTCTGTCKGTSAGGTTGAT-3′. The partial coding sequence forTnhoxc10a (805 bp) was submitted to GenBank under accession number KM819574. Phylogenetic analysis was used to confirm the orthology of newly identifiedT. niphobles genes. Amino acid sequences were aligned using clustalw (Thompson et al. 1997). Regions that could not be aligned were excluded from the analysis. A maximum likelihood phylogenetic tree based on amino acid sequence datasets was constructed with mega6 (Tamura et al. 2013). Bootstrapping was carried out with 1000 replicates. GenBank and Ensembl IDs for the phylogenetic reconstruction are:Homo sapiens_Hoxa10, NP_061824.3;Mus musculus_Hoxa10, NP_032289.2;Gallus gullus_Hoxa10, XP_001235693.2;Xenopus tropicalis_Hoxa10, XP_002933440.2;Danio rerio_hoxa10b, NP_571230.1;Oryzias latipes_hoxa10a, XP_004073905.1;Oreochromis niloticus_hoxa10, ENSONIP00000005789;Takifugu rubripes_hoxa10a, ENSTRUP00000040854;Homo sapiens_Hoxc10, NP_059105.2;Mus musculus_Hoxc10, NP_034592.2;Gallus gullus_Hoxc10, XP_001233806.2;Xenopus laevis_Hoxc10, NP_001083948.1;Danio rerio_hoxc10a, NP_001241873.1;Oryzias latipes_hoxc10a, ENSORLP00000009924;Oreochromis niloticus_hoxc10a, AAV97685.1;Takifugu rubripes_hoxc10a, KM819574;Ciona intestinalis_Hox10, NP_001027696.1. AmplifiedTnhoxc10a was cloned into pGEM-Teasy (Promega) and used as templates for riboprobe synthesis. Whole-mountin situ hybridization was performed as described (Westerfield, 2000).
DiO application to track cells
Application of DiO (3,3′-dioctadecyloxacarbocyanine perchlorate) was performed as described (Murata et al. 2010; Kaneko et al. 2014), with slight modifications. Briefly, eggs were treated with hatching enzyme collected fromT. niphobles embryos and dechorionated. A small amount of DiO (5 mg mL−1 in dimethylformamide; Invitrogen) was injected via a microelectrode into the mesoderm using a microinjector (Narishige). After injection, embryos were returned to filtered seawater and incubated at 20 °C. The location of the tracer in each embryo was followed over time using fluorescence microscopy.
Histology
Embryos ofT. niphobles were prepared for histology as described (Kaneko et al. 2014). Briefly, staged embryos were fixed with Serra's fixative or 4% paraformaldehyde. Embryos were then dehydrated in ethanol and acetone, and embedded in Technovit 8100 resin (Heraues-Kulzer, Wehrheim, Germany). Sections were cut at a thickness of 8 μm, and stained with hematoxylin and eosin.
Three-dimensional (3D) reconstruction
Three-dimensional images were reconstructed as described (Kaneko et al. 2014). Briefly, stained sections were digitized using Virtual Slide (CLARO, Japan). Differences in the sizes of the digitized sections were corrected by overlying each section with a virtual grid of squares that were colored similarly to establish a suitable background for 3D reconstruction. On the digitized sections, each embryonic component was colored and reconstructed using the Avizo 3D visualization framework (Maxnet, Tokyo, Japan).
Results
Distribution of the lateral plate mesoderm on the yolk surface ofT. niphobles embryos
We histologically examined the distribution of the lateral plate mesoderm ofT. niphobles (Figs3). First, we made serial sections of embryos at 3 dpf (n = 1), 4 dpf (n = 9), 5 dpf (n = 4), 6 dpf (n = 1), 7 dpf (n = 5), 8 dpf (n = 1), 9 dpf (n = 4) and 11 dpf (n = 2). Observation of sections allowed us to estimate the distribution of lateral plate mesoderm cells on the yolk surface at each stage. In medaka and Nile tilapia embryos, it is very difficult to distinguish the lateral plate mesodermal cells on the yolk surface prior to the initiation of pectoral fin buds (Kaneko et al. 2014). However, we were able to detect the lateral plate mesodermal cells in 8-somite-stageT. niphobles embryos as a single-cell layer comprised of both the somatic and splanchnic mesoderm (Fig.1). Thus, to understand the distribution of the lateral plate mesoderm, we made a 3D reconstruction from sequential sections of an embryo starting at the 8-somite stage (4 dpf). When swelling of the presumptive pectoral fin region became apparent, we regarded the cell layers extending from the pectoral fin region between the ectoderm and yolk surface as the lateral plate mesodermal cells. Thus, we reconstructed sections of an embryo at the 20-somite stage (6 dpf), when the lateral plate mesoderm thickens in the presumptive pectoral fin region; reconstruction was also carried out for the Low-pec stage (7 dpf), when the lateral plate mesoderm covered most of the yolk surface. For 3D reconstructions, one embryo at each of the 8-somite, 20-somite and Low-pec stages was sectioned at a thickness of 8 μm, and 79, 80 and 81 sections were made, respectively. Each section was digitally colorized prior to 3D reconstruction (Figs1A–D, 2A–D and 3A–D). For clarity, we did not color the blood vessels, blood cells, pharyngeal mesoderm, cardiac mesoderm or epithelium of the other internal organs, although they are indeed derived from the lateral plate mesoderm.
Figure 3.
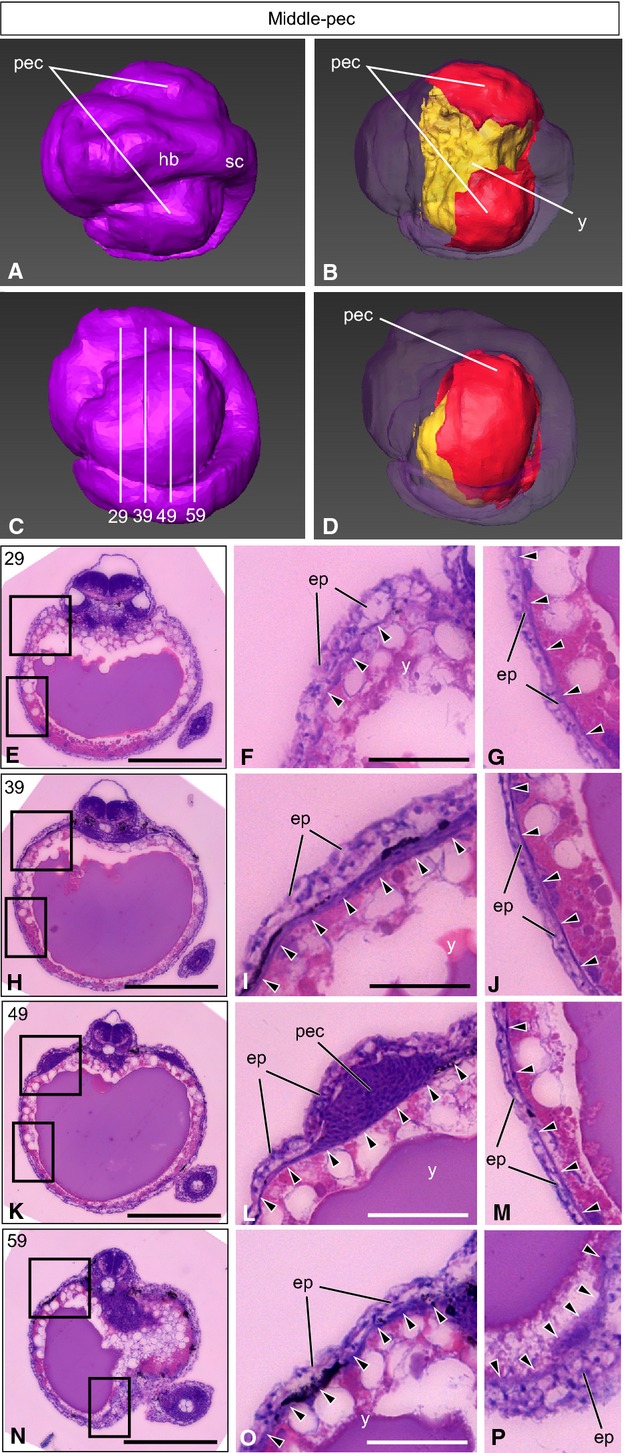
Low-pecT. niphobles embryo. (A–D) A 3D-reconstructed embryo, seen from the dorsal view (A, B) and the lateral view (C, D). Anterior is to the left. (A, C) The surface layer is colored purple. (B, D) Oblique view of the same embryo, with the surface component rendered translucent to show the yolk (yellow) and the lateral plate mesoderm and tissues derived from it (red), except for the heart, blood and blood vessels. Although the anterior ventral region of yolk surface is not colored red, the lateral plate mesoderm has already differentiated to the cardiac mesoderm. (C) Lines indicate the levels (numbers) of transverse histological sections. (E–P) Transverse histological sections of the same embryo, as indicated in (C). (F and G, I and J, L and M, O and P) Higher magnification of the photographs shown in (E), (H), (K) and (N), respectively. Arrowheads indicate the lateral plate mesoderm. ep, epidermis; hb, hindbrain; pec, pectoral fin buds; sc, spinal cord; y, yolk. Scale bars: 500 μm (E, H, K, N); 100 μm (F, G, I, J, L, M, O, P).
Figure 1.
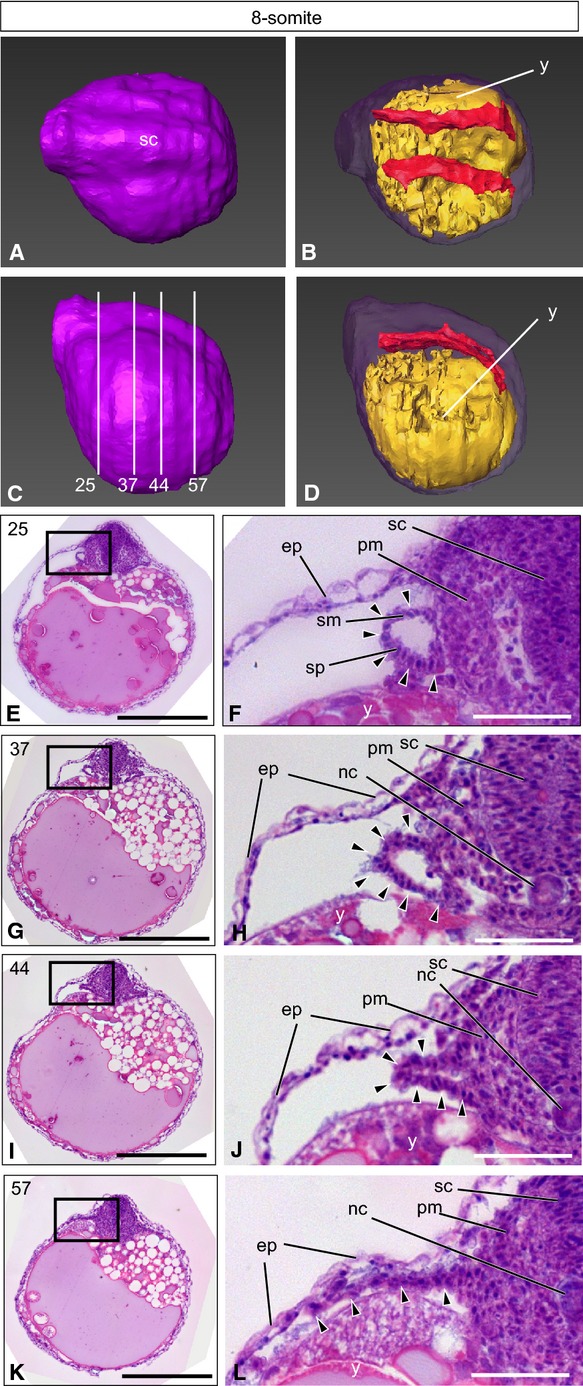
The 8-somiteT. niphobles embryo. (A–D) A 3D-reconstructed embryo, seen from the dorsal view (A, B) and the lateral view (C, D). Anterior is to the left. (A, C) The surface layer is colored purple. (B, D) Oblique view of the same embryo, with the surface component rendered translucent to show the yolk (yellow) and the lateral plate mesoderm and tissues derived from it (red), except for the heart, blood and blood vessels. (C) Lines indicate the levels (numbers) of transverse histological sections. (E–L) Transverse histological sections of the same embryo, as indicated in (C). (F, H, J, L) Higher magnification of the photographs shown in (E), (G), (I) and (K), respectively. Arrowheads indicate the lateral plate mesoderm. ep, epidermis; nc, notochord; pm, paraxial mesoderm; sc, spinal cord; sm, somatic mesoderm; sp, splanchnic mesoderm; y, yolk. Scale bars: 500 μm (E, G, I, K); 100 μm (F, H, J, L).
Figure 2.
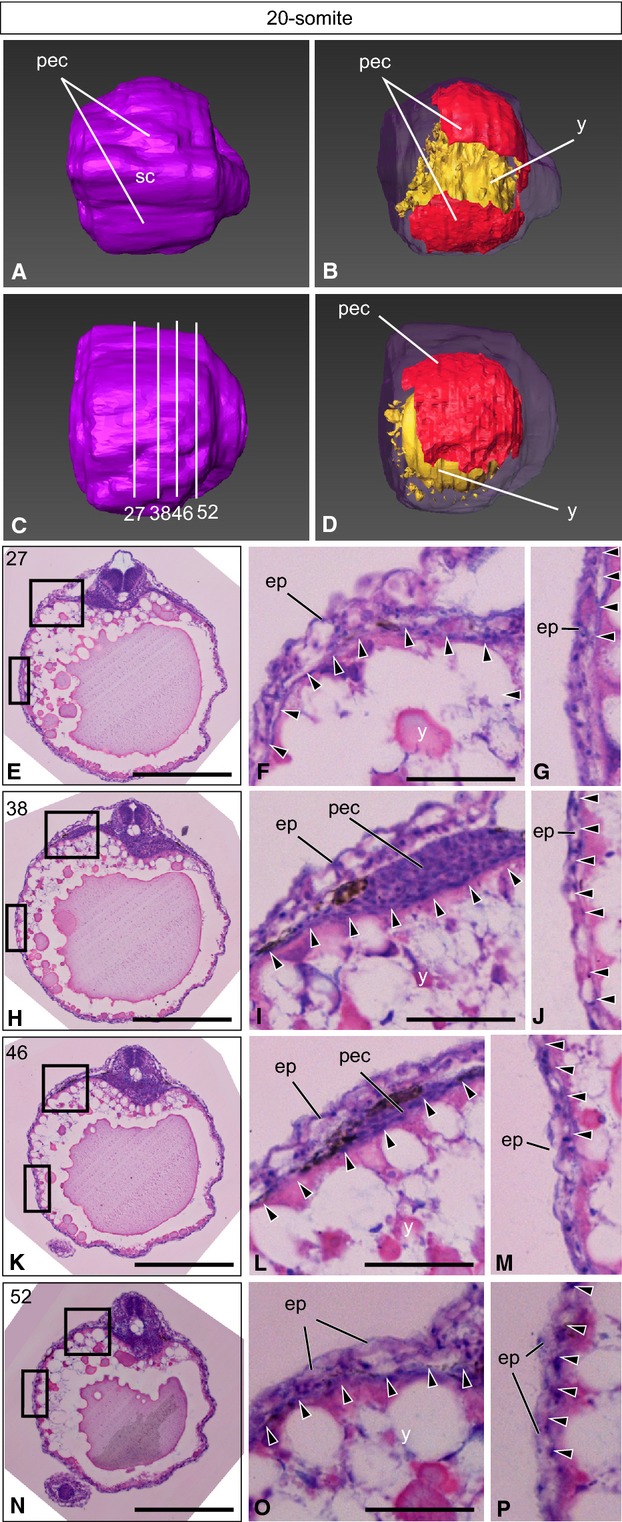
The 20-somiteT. niphobles embryo. (A–D) A 3D-reconstructed embryo, seen from the dorsal view (A, B) and the lateral view (C, D). Anterior is to the left. (A, C) The surface layer is colored purple. (B, D) Oblique view of the same embryo, with the surface component rendered translucent to show the yolk (yellow) and the lateral plate mesoderm and tissues derived from it (red), except for the heart, blood and blood vessels. (C) Lines indicate the levels (numbers) of transverse histological sections. (E–P) Transverse histological sections of the same embryo, as indicated in (C). (F and G, I and J, L and M, O and P) Higher magnification of the photographs shown in (E), (H), (K) and (N), respectively. Arrowheads indicate the lateral plate mesoderm. ep, epidermis; pec, pectoral fin buds; sc, spinal cord; y, yolk. Scale bars: 500 μm (E, H, K, N); 100 μm (F, G, I, J, L, M, O, P).
8-somite stage (4 dpf)
At the anterior yolk level (Fig.1E,F), the lateral plate mesoderm emerged as a single layer of tube-like protrusions next to the paraxial mesoderm (arrowheads in Fig.1F). At the middle level (Fig.1G,H), this tube-like structure was comprised of a few cell layers (arrowheads in Fig.1H). At the more posterior level (Fig.1I,J), the lateral plate mesod-erm formed two separate cell layers on the yolk surface (arrowheads in Fig.1J). At the posterior level (Fig.1K,L), thin layers of the lateral plate mesodermal cells extended laterally (arrowheads in Fig.1L). Thus, inT. niphobles, the lateral plate mesodermal cells form tube-like structures at ∼4 dpf, and this tube folds from the lateral side and forms two separate layers of cells corresponding to the soma-tic and splanchnic mesoderm, respectively, of tetrapods. Three-dimensional reconstruction showed that the population of the lateral plate mesodermal cells distributed alongside the body trunk (red in Fig.1B,D).
20-somite stage (6 dpf)
On the yolk surface just anterior to the pectoral fin buds (Fig.2E–G), a few layers of lateral plate mesodermal cells were observed near the body trunk, and a single layer of cells extended laterally to cover nearly 50% of the surface (arrowheads in Fig.2F,G). At the pectoral fin bud level (Fig.2H,I), the lateral plate mesodermal cells contributed to the formation of pectoral fin buds (arrowheads in Fig.2I), and one to a few layers of the cells extended laterally to cover 50–60% of the yolk surface (arrowheads in Fig.2I,J). At the posterior pectoral fin bud level (Fig.2K–M), the lateral plate mesodermal cells thickened around the pectoral fin buds (arrowheads in Fig.2L), and a thin layer of cells extended laterally to cover more than half of the yolk sphere (arrowheads in Fig.2M). At the posterior yolk (Fig.2N–P), the lateral plate mesodermal cells covered ∼50% of the yolk surface (arrowheads in Fig.2O,P). Three-dimensional construction showed that > 50% of the yolk surface was already covered by the lateral plate mesoderm at this stage (red in Fig.2D).
Low-pec stage (7 dpf)
At the anterior yolk level (Fig.3E–G), the lateral plate mesoderm seemed to cover > 60% of the yolk sphere (arrowheads in Fig.3F,G). Just anterior to the pectoral fin buds (Fig.3H–J), > 80% of the yolk surface was covered by the lateral plate mesoderm (arrowheads in Fig.3I,J). The lateral plate mesoderm thickened around the pectoral fin buds (arrowheads in Fig.3L). Unlike medaka or Nile tilapia (Kaneko et al. 2014), inT. niphobles the mesoderm of the pectoral fin buds remained undifferentiated (Fig.3L), although the lateral plate mesoderm had already covered > 90% of the yolk surface (arrowheads in Fig.3B,D,M). At the posterior yolk (Fig.3N–P), one to a few layers of the lateral plate mesodermal cells seemed to cover the entire yolk surface (arrowheads in Fig.3O,P). Three-dimensional reconstruction showed that the yolk surface was almost entirely covered by the lateral plate mesoderm (red in Fig.3D), except for the anterior–ventral region.
In conclusion, our histological analysis ofT. niphobles shows that the lateral plate mesoderm separates into two layers prior to its extension over the yolk surface. Our results also show that the lateral plate mesoderm rapidly covers the yolk surface when mesenchymal cells of the pectoral fin buds are yet undifferentiated.
The lateral plate mesodermal cells at the anterior border ofhoxc10a expression extend rostrally and reach the anterior margin of the yolk
To investigate the developmental fate of the lateral plate mesodermal cells, we labeled the cells on the yolk surface and followed their movement (Fig.4). We previously showed that, in zebrafish and Nile tilapia, presumptive pelvic fin cells originally reside near the anterior margin ofhoxc10a expression in the spinal cord (Murata et al. 2010; Tanaka, 2011), as is the case for the presumptive leg cells of tetrapods (Lance-Jones et al. 2001; Choe et al. 2006). Thus, we identified thehoxc10a cDNA fragment fromT. niphobles (Fig. S1) and investigated the fate of the lateral plate mesodermal cells originally located at the anterior margin ofhoxc10a expression in the spinal cord inT. niphobles embryos (n = 4; Fig.4). When we labeled cells near the anterior border ofhoxc10a expression (purple arrowhead in Fig.4A) at 5 dpf (15-somite stage; Fig.4B; white arrowheads in Fig.4E,H), DiO-labeled cells expanded ventrally by 6 dpf (20-somite stage; Fig.4C; white arrowheads in Fig.4F,I) and expanded rostrally by 7 dpf (Low-pec stage; Fig.4D; white arrowheads in Fig.4G). By 8 dpf, DiO-labeled cells actively migrated anteriorly and reached the anterior margin of the yolk surface, adjacent to the heart (Middle-pec stage; white arrowheads in Fig.4J). These results suggested that, inT. niphobles embryos, the lateral plate mesodermal cells at the anterior margin ofhoxc10a expression in the spinal cord reach the anterior margin of the yolk surface (Fig.4K).
Figure 4.
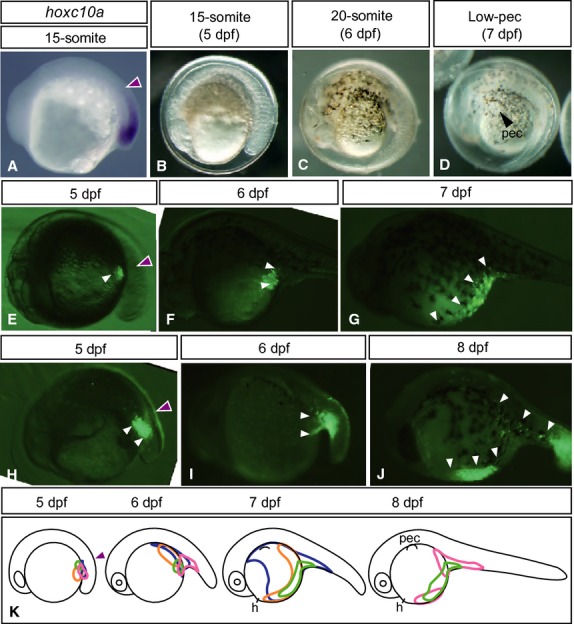
Fate mapping of lateral plate mesodermal cells duringT. niphobles development. (A) Expression ofhoxc10a in a 15-somite embryo. Each purple arrowhead in this figure indicates the anterior margin ofhoxc10a expression in the spinal cord. (B–D) Lateral views ofT. niphobles embryos at the 15-somite (5 dpf, B), 20-somite (6 dpf, C) and Low-pec (7 dpf, D) stages. (E–J) Examples of cell lineage analysis inT. niphobles embryos. White arrowheads indicate the locations of DiO-labeled cells (images in E–G were flipped). (K) Schematic diagram summarizing the lineage of labeled cells. h, heart; pec, pectoral fin buds.
Discussion
Budding of the lateral plate mesoderm
In this study, we showed that the lateral plate mesoderm separates into two layers inT. niphobles embryos. In 8-somite stageT. niphobles, the lateral plate mesoderm was observed to bud out as a single layer of tube-like protrusions adjacent to the paraxial mesoderm at the anterior level, and this tube-like mesoderm folded at the lateral margin and expanded laterally at the middle level. Unlike what we observed inT. niphobles, the lateral plate mesoderm in other vertebrate embryos, including lampreys, chick and mouse, extends laterally prior to its separation into somatic and splanchnic layers (Funayama et al. 1999; Onimaru et al. 2011). In zebrafish, medaka and Nile tilapia embryos, the lateral plate mesoderm observed on the yolk surface in our previous studies seems to be the somatic mesoderm because it gives rise to paired fins (Murata et al. 2010; Kaneko et al. 2014). Thus, in teleosts, the lateral plate mesoderm likely separates into two layers as soon as it appears, and then the splanchnic mesoderm moves toward the ventral side of the body trunk to form the gut epithelium. However, it remains unclear whether the development of the lateral plate mesoderm inT. niphobles is specific to the pufferfish lineage or conserved in teleosts. This issue could be addressed via detailed examination of the distribution of specific molecular markers for the somatic and splanchnic mesoderm of various teleosts at early stages.
Dynamics of the lateral plate mesoderm relative to pelvic fin position
InT. niphobles, the lateral plate mesodermal cells adjacent to the anterior border ofhoxc10a expression in the spinal cord reached the anterior end of the yolk sphere. It is a conserved feature that the lateral plate mesoderm adjacent to the anterior border ofhoxc10a expression in the spinal cord (i.e. adjacent to the future anus region of the trunk) is determined to be the posterior appendages (hindlimbs/pelvic fins) among tetrapods and teleosts (Murata et al. 2010). Our current and previous studies suggest that the rostral shift of pelvic fins from the anus region could be caused by trunk protrusion and active anterior migration of the presumptive pelvic cells. The zebrafish has abdominal pelvic fins, and our previous work showed that the presumptive pelvic fin cells on the yolk surface shift their position with respect to the body trunk after trunk protrusion and reach the future pelvic fin position (Fig.5A; Murata et al. 2010). In zebrafish, anterior migration of the presumptive pelvic fin cells is barely observed at the time the lateral plate mesodermal cells cover the yolk surface (Murata et al. 2010). In Nile tilapia (Murata et al. 2010; Kaneko et al. 2014) and pufferfish, however, cells adjacent to the anterior border ofhoxc10a expression in the spinal cord were found to actively migrate anteriorly to cover the yolk surface (Fig.5C,D). Especially, in pufferfish the lateral plate mesoderm rapidly covered the yolk surface, and caudal cells actively migrated toward the anterior end of the yolk – immediately adjacent to the heart. Pufferfish belong to the highly derived teleost order Tetraodontiformes (Nelson, 1994), and some Tetraodontiformes fishes have pelvic fins or spines at the thoracic or jugular position. Thus, we expect that, in teleosts that diversified more recently – such as Nile tilapia and some Tetraodontiformes – presumptive pelvic fin cells reach the thoracic position not just because of trunk protrusion but also via their active anterior migration. In zebrafish, the yolk tube forms as a consequence of trunk protrusion, and thus such a difference in yolk shape might be the reason why presumptive pelvic fin cells hardly migrate anteriorly. Although medaka belongs to Acanthopterygii, as do Nile tilapia and pufferfish, pelvic fins arise at the abdominal position. In medaka, DiO-labeled lateral plate mesodermal cells extended anteriorly from the caudal-most region (Fig.5B), although they did not show drastic anterior migration as seen in Nile tilapia or pufferfish (Fig.5C,D). Taken together, it is likely that the rostral shift of pelvic fins from the anus to the abdominal position is caused only by trunk protrusion, whereas active anterior migration of presumptive pelvic fin cells would also be required for them to reach the thoracic or jugular position (Fig.5). It is possible that anterior migration of the presumptive pelvic fin cells is aborted if the yolk surface area decreases, for example, via formation of a narrow yolk tube as seen in zebrafish or by absorbing yolk more rapidly as seen in medaka. Alternatively, an unknown factor(s) that promotes the migration of lateral plate mesodermal cells might be produced from neighboring cells in highly derived teleosts. Investigation of the distribution of the lateral plate mesoderm in other teleosts, or identification of the factor(s) that promotes extension of the lateral plate mesoderm, is required to address these possibilities.
Figure 5.
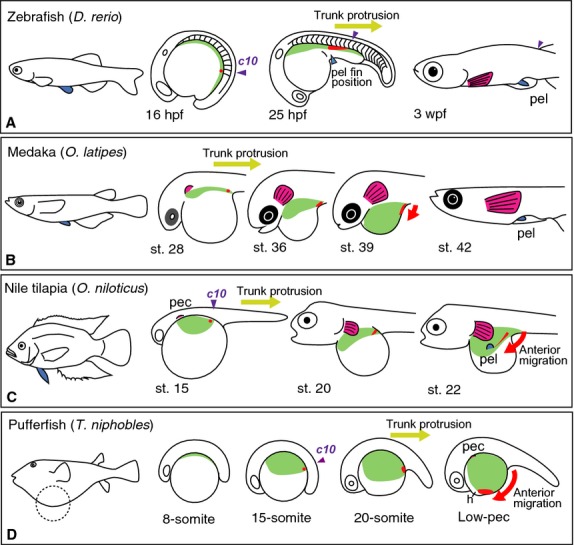
Schematic depiction of the development of the lateral plate mesoderm in embryos of zebrafish, medaka, Nile tilapia andT. niphobles. (A) In zebrafish, the distribution of the lateral plate mesoderm was observed by Murata et al. (2010) by examining the expression ofprrx1a (light green) and tracing DiI-labeled cells (red), which denote the lateral plate mesodermal cells. The population ofprrx1a-positive cells is observed alongside the body trunk at 16 hpf. When the body trunk protrudes, theprrx1a-positive cells narrow toward the caudal region. DiI-labeling experiments showed that cells adjacent to the anterior border ofhoxc10a in the spinal cord (c10, purple arrowhead) reach the prospective pelvic fin position, such as the abdominal position (light-blue arrowhead), after trunk protrusion. Thus, in zebrafish, the allometric growth of the body trunk leads to the rostral shift of the presumptive pelvic fin cells. (B) In medaka, distribution of the lateral plate mesoderm was observed by Kaneko et al. (2014) using histological analysis (light green) and DiI-labeling (red). The population of lateral plate mesodermal cells (light green) is observed alongside the body at stage 28. The lateral plate mesodermal cells extend anterior–laterally to cover the yolk sphere, and they entirely cover the yolk by stage 39. The DiI-labeled caudal-most cells extend anteriorly after trunk protrusion (red). (C) In Nile tilapia, distribution of the lateral plate mesoderm was observed previously by histological analysis (light green; Kaneko et al. 2014) and DiI-labeling (red; Murata et al. 2010). The lateral plate mesodermal cells (light green) expand laterally around the pectoral fin buds at stage 15. The cells expand laterally alongside the body by stage 20. By stage 22, the cells expand laterally and cover of the more-anterior than posterior surface of the yolk sphere, and pelvic fin buds appear at the prospective pelvic fin position (Kaneko et al. 2014). Cells at the anterior border ofhoxc10a expression (c10, purple arrowhead) shift their position slightly anterior with respect to the body trunk after trunk protrusion. These cells also seem to actively migrate anteriorly (red arrow) on the yolk surface until they reach the prospective pelvic fin position (Murata et al. 2010). (D) In the pufferfishT. niphobles, the lateral plate mesodermal cells bulge out alongside the body trunk at the 8-somite stage (light green). From the 15- to 20-somite stage, the cells cover > 50% of the yolk surface. The yolk surface is rapidly covered by the lateral plate mesodermal cells by the Low-pec stage. DiO-labeled cells at the anterior border ofhoxc10a expression (red) actively migrate toward the anterior end of the yolk surface (red arrow) adjacent to the heart. h, heart; pec, pectoral fin buds; pel, pelvic fin buds. Schematic diagrams in (A, C) and (B, C) are modified after Murata et al. (2010) and Kaneko et al. (2014), respectively.
Acknowledgments
The authors thank Yumie Murata for critical suggestions. This work was supported in part by a Grant-in-Aid for Scientific Research (B) (25291086) to MT and DK, the Toray Science Foundation, the Nakajima Foundation, and the Narishige Zoological Science Award to MT. The authors also thank reviewers whose comments greatly improved the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Molecular phylogenetic tree for Hox10.
References
- Choe A, Phun HQ, Tieu DD. Expression patterns of Hox10 paralogous genes during lumbar spinal cord development. Gene Expr Patterns. 2006;6:730–737. doi: 10.1016/j.modgep.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Fujimura K, Okada N. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae). Developmental staging system. Dev Growth Differ. 2007;49:301–324. doi: 10.1111/j.1440-169X.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Funayama N, Sato Y, Matsumoto K. Coelom formation: binary decision of the lateral plate mesoderm is controlled by the ectoderm. Development. 1999;126:4129–4138. doi: 10.1242/dev.126.18.4129. [DOI] [PubMed] [Google Scholar]
- Gosline WA. Functional Morphology and Classification of Teleostean Fishes. Honolulu: The University Press of Hawaii; 1971. [Google Scholar]
- Grandel H, Schulte-Merker S. The development of the paired fins in the zebrafish (Danio rerio. Mech Dev. 1998;79:99–120. doi: 10.1016/s0925-4773(98)00176-2. [DOI] [PubMed] [Google Scholar]
- Greenwood PH, Rosen DE, Weitzman SH. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bull Am Mus Nat Hist. 1966;131:339–456. [Google Scholar]
- Kaneko H, Nakatani Y, Fujimura K. Development of the lateral plate mesoderm in medakaOryzias latipes and Nile tilapiaOreochromis niloticus: insight into the diversification of pelvic fin position. J Anat. 2014;225:659–674. doi: 10.1111/joa.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance-Jones C, Omelchenko N, Bailis A. Hoxd10 induction and regionalization in the developing lumbosacral spinal cord. Development. 2001;128:2255–2268. doi: 10.1242/dev.128.12.2255. [DOI] [PubMed] [Google Scholar]
- McDonald LA, Gerrelli D, Fok Y. Comparison of Iroquois gene expression in limbs/fins of vertebrate embryos. J Anat. 2010;216:683–691. doi: 10.1111/j.1469-7580.2010.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Tamura M, Aita Y. Allometric growth of the trunk leads to the rostral shift of the pelvic fin in teleost fishes. Dev Biol. 2010;347:236–245. doi: 10.1016/j.ydbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Nelson JS. Fishes of the World. New York, NY: Wiley-Interscience; 1994. [Google Scholar]
- Nuno de la Rosa L, Muller GB, Metscher BD. The lateral mesodermal divide: an epigenetic model of the origin of paired fins. Evol Dev. 2014;16:38–48. doi: 10.1111/ede.12061. [DOI] [PubMed] [Google Scholar]
- Onimaru K, Shoguchi E, Kuratani S. Development and evolution of the lateral plate mesoderm: comparative analysis of amphioxus and lamprey with implications for the acquisition of paired fins. Dev Biol. 2011;359:124–136. doi: 10.1016/j.ydbio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Rosen DE. Teleostean interrelationships, morphological function and evolutionary inference. Am Zool. 1982;22:261–273. [Google Scholar]
- Tamura K, Stecher G, Peterson D. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. Revealing the mechanisms of the rostral shift of pelvic fins among teleost fishes. Evol Dev. 2011;13:382–390. doi: 10.1111/j.1525-142X.2011.00493.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hale LA, Amores A. Developmental genetic basis for the evolution of pelvic fin loss in the pufferfish Takifugu rubripes. Dev Biol. 2005;281:227–239. doi: 10.1016/j.ydbio.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon Press; 2000. A guide for the laboratory use for zebrafish (Danio rerio), 4th ed. [Google Scholar]
- Yamanoue Y, Miya M, Matsuura K. Explosive speciation of Takifugu: another use of fugu as a model system for evolutionary biology. Mol Biol Evol. 2009;26:623–629. doi: 10.1093/molbev/msn283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Molecular phylogenetic tree for Hox10.


