Abstract
The role of livestock grazing in regulating woody cover and biomass in grass-dominant systems is well recognized. However, the way in which woody plant populations in respond when livestock are removed from grazing in the absence of other disturbances, such as fire, remains unclear.
We conducted a 10-year, replicated fencing experiment in a sandy semiarid rangeland in northern China (which has a mean annual rainfall of 365 mm), where fires have been actively suppressed for decades.
Fencing dramatically influenced the growth and age structure of the native tree species, Ulmus pumila, which is the sole dominant tree in the area. After a decade, the density of the U. pumila tree population in the fencing plots increased doubly and canopy cover increased triply. The proportion of both saplings (U2) and young trees (U3) increased in fencing plots but decreased in grazing plots after the 10-year treatment period. The effects of fencing on U. pumila trees varied by age class, with potential implications for the future structure of the U. pumila tree community. Decadal fencing led to approximately 80-fold increase in recruitment and a nearly 2.5-fold decrease in the mortality of both U2 and U3. Further, livestock grazing generated a “browsing trap” to the recruitment of both U2 and U3, and had a small impact on the mortality of old trees. A long-term, fencing-driven shift in woody species composition was mediated via its effects on both recruitment and mortality rates.
Synthesis and applications. Our results demonstrate that in the long-term absence of both fire and livestock, native woody plant encroachment tends to occur in sandy rangelands, transforming the woody plant demography in the process. The feasibility of full livestock exclusion in sandy rangelands requires further discussion. A balanced amount of livestock grazing may provide critical ecosystem services by regulating woody cover and mediating woody plant encroachment.
Keywords: Fencing, Hunshandake, livestock exclusion, livestock grazing, semiarid rangeland, Ulmus pumila, woody plant encroachment
Introduction
Increased woody plant abundance (encroachment) is occurring in rangelands worldwide (Roques et al. 2001; Rundel et al. 2014). Rangelands constitute approximately 50% of the earth's land surface and contain more than 30% of the world's human population (Anadón et al. 2014). These rangelands carry profound consequences for community structure, biodiversity and the functioning of rangeland ecosystems (Huxman et al. 2005; Hu et al. 2008; Richardson and Rejmánek 2011; Huang et al. 2012; Ratajczak et al. 2012). For example, the encroachment of woody plants into rangelands can alter the soil moisture (Pressland 1973) and nutrient and microclimate conditions (Belsky 1992), suppress grass productivity associated with animal production and wildlife conservation (Stuart-Hill and Tainton 1989; Hudak 1999; Dalle et al. 2006; Belay et al. 2013), and reduce stream flow and groundwater recharge (Archer 2010). It is necessary to study the factors that can cause woody plant encroachment to understand rangeland ecology and management (Holdo et al. 2009; Gordijn et al. 2012).
Woody plant encroachment appears to be caused by a combination of climate change, livestock grazing, fire suppression, and an increase in atmospheric CO2 (Archer et al. 1995; Morgan et al. 2007; Wigley et al. 2010; Eldridge et al. 2011; Buitenwerf et al. 2012; Kulmatiski and Beard 2013). Water availability plays a critical role in regulating tree cover and biomass in rangelands by effectively providing a climatic limit to the tree biomass (Park et al. 2012; Ratajczak et al. 2012; Su et al. 2014). However, “top-down” forces, such as herbivores and fire, also exert significant effects on tree cover and biomass and can profoundly alter the structure and composition of many rangeland ecosystems (Sankaran et al. 2013). Fires often limit tree cover and biomass by preventing seedling recruitment and sapling maturation to adults rather than directly killing adult trees (Hoffmann 1999; Higgins et al. 2000; Staver et al. 2009; Hean and Ward 2012). Fires are incapable of suppressing woodland regeneration (Holdo 2007). Fire and browsing, whose effects on vegetation are somewhat analogous (Bond 2005, 2008), must act synergistically to prevent increases in tree cover (Cabral et al. 2003; Holdo et al. 2013). However, in contrast to fire, the effects of herbivores on woody encroachment are difficult to generalize across rangeland ecosystems (Augustine and McNaughton 1998; Staver et al. 2009; Sankaran et al. 2013).
Livestock grazing, which is the most common form of land use in the world (Foley et al. 2005), has grown by 600% over the last three decades (Asner et al. 2004). Livestock grazing leads to woody encroachment once the stocking rate exceeds a threshold determined by the long-term mean annual rainfall (Jeltsch et al. 1997). It is widely accepted that overgrazing has facilitated woody plant invasion through a combination of factors (Jeltsch et al. 1997; Roques et al. 2001; Kambatuku et al. 2011), such as reducing competition from grasses (Auken 2000), dispersal of seeds of woody plants (Auken 2000; Tews et al. 2006), and changing rodent and insect populations (Jeltsch et al. 1997; Auken 2000; Roques et al. 2001; Kambatuku et al. 2011). However, browsing pressure can reduce the establishment of woody seedlings (Prins and van der Jeugd 1993) and retard the growth of woody plants (Pellew 1983). In semiarid rangelands, native browsing ungulates influence the recruitment, growth, and mortality of woody vegetation, and they consequently regulate woody cover. The removal of native browsing ungulates can lead to rapid woody encroachment (Sankaran et al. 2013). For example, Maher et al. (2010) found that an absence of kangaroos in fenced plots in kwongan of southwestern Australia would result in the much more extensive and rapid encroachment of Allocasuarina huegeliana than it would in unfenced plots. Livestock removal is often considered an essential tool for vegetation restoration and conservation in arid and semiarid rangelands (Frank et al. 2014). However, little is known about how livestock removal affects woody encroachment because isolating the effects of browsing on rangeland woody plant dynamics requires long-term, controlled livestock exclusion experiments, which are rare in Eurasian rangelands (Wesche and Treiber 2012).
We conducted a decade-long, replicated livestock exclusion experiment (fencing) with normal livestock grazing (1.4 sheep unit per ha) as a control in semiarid Hunshandake Sandy Land in northern China, where fires have been actively suppressed for decades. The site is savanna-like (Liu et al. 2013; Su et al. 2014) and is characterized as a continuous grass layer with scattered trees solely dominated by Ulmus pumila trees (Wallis de Vries et al. 1996; Solla et al. 2005; Jiang et al. 2006; Tang et al. 2014). Our specific objectives were to (1) quantify the long-term effects of fencing on U. pumila tree population dynamics in the absence of external perturbations, such as fire; and (2) determine whether fencing affects the recruitment and mortality of U. pumila trees.
Materials and Methods
Study site
The study was conducted at the Hunshandake Sandy Land Ecological Research Station (41°54′N, 116°0′E) of the Institute of Botany, Chinese Academy of Science in Inner Mongolia, northern China. The elevation is approximately 1300 m. The soil is composed primarily of eolian sands, which are often distributed in a mosaic pattern with sandy dark chestnut soil (Xu and Zou 1998). Meteorological data obtained from a weather station located in Zhenglanqi County (approximately 100 km from the site) indicate that the mean annual rainfall is 250–450 mm, with an average 367.1 mm for the period 2002–2012. Approximately 70% of the yearly rainfall events occur between June and August. The annual mean temperature is −1.7°C, with the lowest monthly mean value of −23.4°C in January and the highest monthly mean value of 14.7°C in July. The average frost-free period lasts approximately 110 days. Vegetation in the area is characterized as a savanna-like woodland community (Liu et al. 2013; Su et al. 2014) and a discontinuous layer of perennial grasses (Jiang et al. 2006). Ulmus pumila, the sole dominant tree species, is distributed sparsely on the fixed dunes, typically with an open canopy structure (Su et al. 2014). The shrub layer is dominated by Salix gordejevii,S. microstachya, and Caragana microphylla (Su et al. 2009). The herbaceous layers are dominated by grassland plants that are common to Eurasian steppe zone, including Leymus chinensis,Agropyron cristatum, and the forb Artemisia frigida.
The most common livestock at the study zone are cattle, sheep, and camel (Ding et al. 2005). By the 1980s, the national government had encouraged nomadic herders to move to villages and increase their herds to boost incomes. The average stocking rate reached approximately 2.6 sheep unit ha−1, a rate that is much greater than the theoretical stocking rate (1.2–1.8 sheep unit ha−1) (Ding et al. 2005). Year-long continuous livestock grazing-induced directional shift degraded most of the available rangelands by 2000 (Ding et al. 2005; Zheng et al. 2006). A demonstration project was launched in 2001 that excluded livestock from 2670 hectares of communal rangeland.
Field investigations
A large enclosure (2670 ha) protected with 1.5-m-tall wire mesh fences was established at the study site in 2001. To ensure the exclusion of all domestic animals, the local government hired people to conduct daily checks. Meanwhile, the remaining rangeland (approximately 5000 ha) was used for livestock grazing at a stocking rate of 1.4 ± 0.4 sheep unit per ha. Five plots (100 × 100 m) with similar landscapes and soil conditions were selected in the aforementioned fencing and grazing sites. We mapped the individual U. pumila trees in each fencing and grazing plot and measured their density, basal area (at 15 cm aboveground level), diameter, height, aboveground biomass, and canopy cover (the proportion of the floor covered by the vertical projection of the tree crowns).
In 2002, we measured the base diameter (d) and height of all trees in plots using a diameter tape. The diameter at breast height (dbh) of trees higher than 1.5 m was also measured. dbh is a strong indicator of tree biomass (Zianis 2008). The aboveground biomass (AGB) of the U. pumila trees in the plots was estimated based on the allometric equation, where AGB = 0.1196 dbh2.2201 (R2 = 0.9968, bias error = 5.61%). To develop the above allometric equation, fifty individual trees with different sizes were randomly selected and destructively sampled during their most vigorous growth period (August). The aboveground portion of each tree was air-dried to a constant weight and weighed on a large platform scale (Yaohua Co. Ltd., Shanghai, China) in the field (±10 g) for AGB. The AGB data were then analyzed using an allometric equation following that of Kuyah et al. (2012). All of the AGB data for the other U. pumila trees (height ≤1.5 m) were obtained through actual measurements. Canopy cover was ocular and estimated by three experienced forestry professionals (Korhonen et al. 2006). The total canopy cover of large trees (height >1.5 m) was recorded.
In 2012, all of the measurements except the canopy cover measurements were retaken. We then estimated the canopy cover using digital photographs that were obtained by the methods described by Korhonen et al. (2006). The original images were converted into binary images using ImageJ image analysis software (1.42q, National Institutes of Health, Bethesda, MD). Crowns in binary images were painted black such that the result would be closer to the traditional canopy cover estimate.
To better understand fencing effects on U. pumila tree population dynamics in this system, changes in the biomass, basal area, height, recruitment, and mortality were analyzed separately for each age class: seedlings (U1, d < 0.5 cm), saplings (U2, 0.5 ≤ d < 5 cm), young trees (U3, 5 ≤ d < 20 cm), mature trees (U4, 20 ≤ d < 40 cm), and old trees (U5, d > 40 cm). The performance of each age class is presented in Table1. To compare the population structure, the proportion of each age class relative to the U. pumila tree populations in grazing and fencing plots was calculated over the 10-year treatment period. We calculated the decadal changes in AGB, basal area, height, recruitment, and mortality at each age class level over the 10-year treatment period. Recruitment here refers to all new individuals of each age class recorded in the 2012 census but not in the 2002 census. Recruitment is thus an integrated measure of effective recruitment over a 10-year period and does not include data on individuals that were recruited and died within that period. Mortality similarly refers to the fraction of all living individuals in each age class that were present in the 2002 census but died by the 2012 census.
Table 1.
Performance of seedlings (U1), saplings (U2), young (U3), mature (U4), and old (U5) Ulmus pumila trees in Hunshandake Sandy Land
| Class | Age (yr) | Diameter (cm) | Height (m) |
|---|---|---|---|
| U1 | <2 | <0.5 | <0.5 |
| U2 | 2–10 | 0.5–5 | 0.5–2 |
| U3 | 10–30 | 5–20 | 2–5 |
| U4 | 31–50 | 20–40 | 5–8 |
| U5 | >50 | >40 | >8 |
We evaluated the effects of fencing on the survival rates of U1 (divided into the current year and a 2-year period) and U2. We counted the number of U1 and U2 individuals in the grazing and fencing plots (n0) in May 2002. After 3 months, the living individuals in the plots (na) were counted again, and the survival rate was calculated as follows: survival rate (%) = 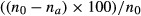 . We repeated the measurements in 2004, 2006, and 2008.
. We repeated the measurements in 2004, 2006, and 2008.
Statistical analysis
A two-way repeated-measures analysis of variance (ANOVA) was employed to detect the significance of canopy cover, AGB, tree density, and basal area of the U. pumila tree population at the whole-plot level and in each age class, using treatment (fencing and grazing) and year, and their interactions as fixed factors. We used paired t-tests to evaluate the effects of fencing on the decadal recruitment and decadal mortality of the U. pumila tree population between 2002 and 2012. The statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL). A post hoc least significant difference (LSD) test was implemented in P = 0.05 (significant at P < 0.05).
Results
Responses of total Ulmus pumila tree population
Ulmus pumila is nearly the sole dominant native tree species growing on the study site (Liu et al. 2013; Su et al. 2014). The decade-long fencing dramatically influenced the U. pumila tree population attributes in plots (Figs.1, 2). Woody plant encroachment substantially increased in the fencing plots during the 10-year period (Fig.1B). Long-term fencing significantly changed the canopy cover (P < 0.05) and tree density (P < 0.05) (Table2). In fencing plots, the total canopy cover of the U. pumila tree population rose significantly (P < 0.05), from 9.2% in 2002 to 24.7% in 2012, a nearly threefold increase over the 10-year treatment period (Fig.2A). The total density of U. pumila trees doubled (P < 0.05), from 20.03 individuals per ha in 2002 to 37.93 individuals per ha in 2012 (Fig.2C). The effects of fencing on the aboveground biomass and basal area of the U. pumila trees were slight and insignificant (P > 0.05). Time has significant effect on the canopy cover, tree density, aboveground biomass, and basal area of the U. pumila tree population (P < 0.05). Both aboveground biomass and basal area increased more than 2 times from 2002 to 2012 (Fig.2B and D).
Figure 1.
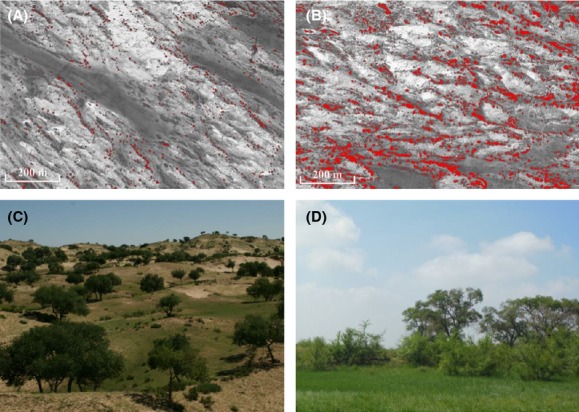
Satellite images show the distribution of Ulmus pumila trees (trees whose canopy area is larger than 2 m2 were painted red) in grazing (A) and fencing (B) plots. Field photos show the generational status of U. pumila trees in grazing (C) and fencing (D) plots. The background images were created by Google Earth.
Figure 2.
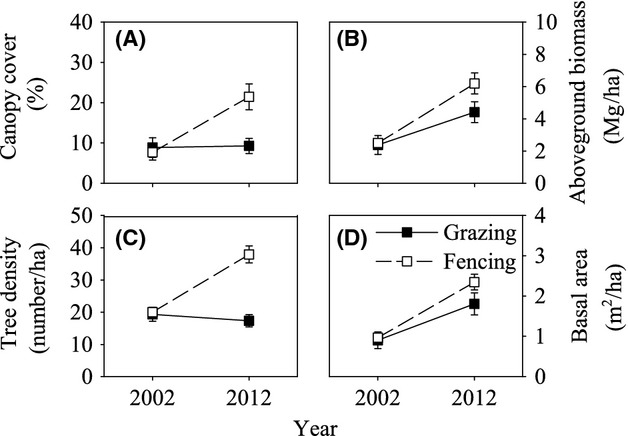
Decadal changes in canopy cover (A), total aboveground biomass (B), tree density (C), and total basal area (D) of Ulmus pumila trees in grazing and fencing plot. Basal area and biomass values have been scaled up from the plot level (100 × 100 m) and are reported on a per hectare basis. Error bars represent ±SE.
Table 2.
Results of two-way repeated-measures ANOVA testing the effects of treatment (fencing and grazing) and time (before and after) on canopy cover, aboveground biomass, tree density, and basal area of the studied Ulmus pumila trees
| Variables | Canopy cover |
Aboveground biomass |
Tree density |
Basal area |
||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Treatment | 23.75 | 0.02* | 1.46 | 0.31ns | 35.25 | 0.01* | 1.08 | 0.38ns |
| Time | 73.95 | 0.00* | 17.11 | 0.03* | 80.73 | 0.00* | 60.95 | 0.00* |
| Treatment × Time | 15.49 | 0.03* | 1.20 | 0.35ns | 50.69 | 0.01* | 0.52 | 0.52ns |
F-test values and P-values are given.
represents P < 0.05 and nsrepresents P > 0.05.
Decadal fencing altered the age structure of the U. pumila trees, the proportion of each age class was significantly changed (P < 0.05) (Table3). The proportion of saplings (U2) and young trees (U3) in fencing plots significantly increased (P < 0.05), accounting for approximately 31.2% and 13.5%, respectively, in 2012, higher than that in 2002 by 13.3% and 74.5%, respectively (Fig.3A and B). By contrast, the proportion of U2 and U3 in grazing plots declined over the 10-year treatment period, from 25.8% and 11.0%, respectively, in 2002 to 10.5% and 6.5%, respectively, in 2012 (P < 0.05). The reduction achieved was 59.3% and 41.0%, respectively (Fig.3A and B). Fencing had no significant effects on mature U. pumila trees (U4) and old U. pumila trees (U5) (P > 0.05) (Table3). The proportion of U4 decreased in both the grazing and fencing plots (Fig.3C), and the proportion of U5 climbed in both grazing and fencing plots (Fig.3D).
Table 3.
Results of two-way repeated-measures ANOVA testing the effects of treatment (fencing and grazing) and time (before and after) on the proportion of saplings (U2), young (U3), mature (U4), and old (U5) Ulmus pumila trees
| Variables |
U2 |
U3 |
U4 |
U5 |
||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Treatment | 27.42 | 0.01* | 12.05 | 0.04* | 0.02 | 0.88ns | 2.28 | 0.23ns |
| Time | 9.91 | 0.05* | 2.91 | 0.19ns | 1.06 | 0.38ns | 18.72 | 0.02* |
| Treatment × Time | 15.74 | 0.03* | 35.91 | 0.01* | 12.23 | 0.04* | 11.64 | 0.04* |
F-test values and P-values are given.
represents P < 0.05 and nsrepresents P > 0.05.
Figure 3.
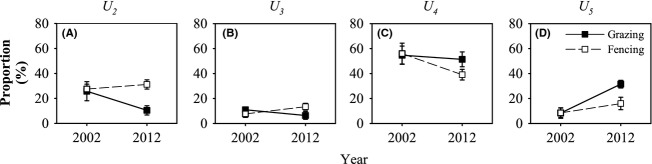
Decadal changes in the proportion of each age class relative to total Ulmus pumila trees in grazing and fencing plots. U2: U. pumila saplings, U3: young U. pumila tree, U4: mature U. pumila tree, U5: old U. pumila tree. Error bars represent ±SE.
Responses of Ulmus pumila trees in different age classes
Fencing was beneficial for the height increment of U. pumila trees except for U5 (Fig.4A–D). U2 and U3 changed significantly in response to grazing and fencing, and U4 and U5 changed insignificantly (P < 0.05) (Table4). In the absence of livestock, the height of U2 and U3 increased from 15.84 ± 6.44 cm and 35.11 ± 30.14 cm, respectively. By contrast, livestock grazing significantly reduced the height of U2 and U3, from 16.16 ± 9.08 cm and 20.46 ± 9.32 cm, respectively. In the absence of livestock, U. pumila trees in each age class showed substantial increases in aboveground biomass over the 10-year period (Fig.4E–F). However, fencing had significant effects on both U2 and U3 (P < 0.05), and insignificant effects on both U4 and U5 (P > 0.05) (Table4). The aboveground biomass of different age classes showed significant different response magnitudes to fencing. U3 increased the most (Fig.4F), with a value of 229.84 ± 46.47 kg ha−1, followed by U4, U5, and U2. We found no net changes in the aboveground biomass of U2 and U3 in grazing plots over the 10-year period, unlike U4 and U5, whose aboveground biomass increased even in the presence of livestock grazing (Fig.4G and H). Similar to the patterns observed for aboveground biomass, fencing had significant effects on the basal area of both U2 and U3 (P < 0.05) and insignificant effects on both U4 and U5 (P > 0.05) (Table4). In the fencing plots, decadal changes were the largest in the basal area of U5, with a value of 9918.14 ± 188.32 cm2 ha−1, followed by U4 (3242.35 ± 70.25 cm2 ha−1) and U3 (550.56 ± 193.80 cm2 ha−1). Decadal changes in the basal area of U2 were undetectable. There were no detectable changes in the basal area of all four age classes in grazing plots over the 10-year period.
Figure 4.
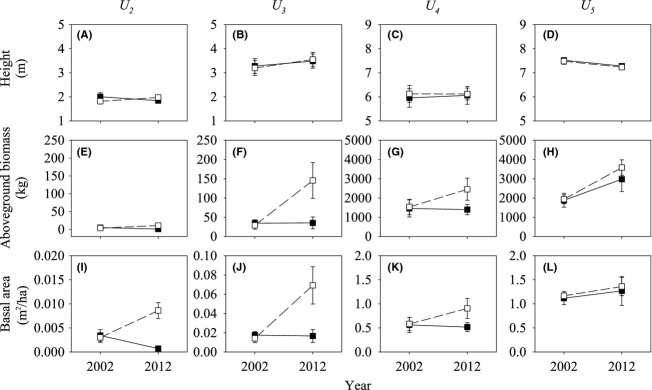
Decadal changes in aboveground biomass (A), height (B), and basal area (C) of saplings (U2), young (U3), mature (U4), and old (U5) Ulmus pumila trees in grazing and fencing plots. Basal area and biomass values have been scaled up from the plot level (100 × 100 m) and are reported on a per hectare basis. Error bars represent ±SE.
Table 4.
Results of two-way repeated-measures ANOVA testing the effects of treatment (fencing and grazing) and time (before and after) on the height, aboveground biomass, and basal area of saplings (U2), young (U3), mature (U4), and old (U5) Ulmus pumila trees
| Variables |
U2 |
U3 |
U4 |
U5 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | ||
| Height | Treatment | 5.07 | 0.01* | 5.63 | 0.09* | 0.07 | 0.80ns | 0.04 | 0.85ns |
| Time | 0.00 | 0.99ns | 0.24 | 0.66ns | 0.01 | 0.93ns | 3.83 | 0.15ns | |
| Treatment × Time | 0.33 | 0.6ns | 0.04 | 0.86ns | 0.02 | 0.89ns | 0.00 | 0.98ns | |
| Aboveground biomass | Treatment | 7.65 | 0.07* | 8.51 | 0.06* | 1.08 | 0.38ns | 0.41 | 0.57ns |
| Time | 1.05 | 0.38ns | 1.44 | 0.32ns | 0.76 | 0.45ns | 27.47 | 0.01* | |
| Treatment × Time | 2.26 | 0.23ns | 2.75 | 0.20ns | 0.95 | 0.40ns | 0.57 | 0.50ns | |
| Basal area | Treatment | 11.23 | 0.04* | 12.70 | 0.04* | 1.18 | 0.36ns | 0.07 | 0.81ns |
| Time | 1.71 | 0.28ns | 1.77 | 0.28ns | 0.77 | 0.44ns | 37.66 | 0.01* | |
| Treatment × Time | 2.52 | 0.21ns | 3.43 | 0.16ns | 1.27 | 0.34ns | 0.01 | 0.94ns | |
F-test values and P-values are given.
represents P < 0.05 and nsrepresents P > 0.05.
All age classes except U5 showed significantly higher recruitment in fencing plots than in grazing plots (Fig.5A). Compared with grazing, the recruitment of both U2 and U3 increased approximately 80-fold in the fencing plots (P < 0.05). Unlike the pattern observed for recruitment, significant changes in decadal mortality were found in both grazing plots and fencing plots (P < 0.05) (Fig.5B). Protection from livestock dramatically reduced mortality rates for U2, U3, and U4, but not for U5 (Fig.5B). U2 had the highest mortality in grazing plots, at approximately 79.2%, followed by U3, with a value of approximately 67.8%. In the absence of livestock, the mortality rates did not differ significantly among U2, U3, and U4, which averaged approximately 27.2% per decade (Fig.5B). U5 had the highest mortality rate (∼36.7%) in fencing plots, but nearly the lowest (∼46.4%) in grazing plots.
Figure 5.
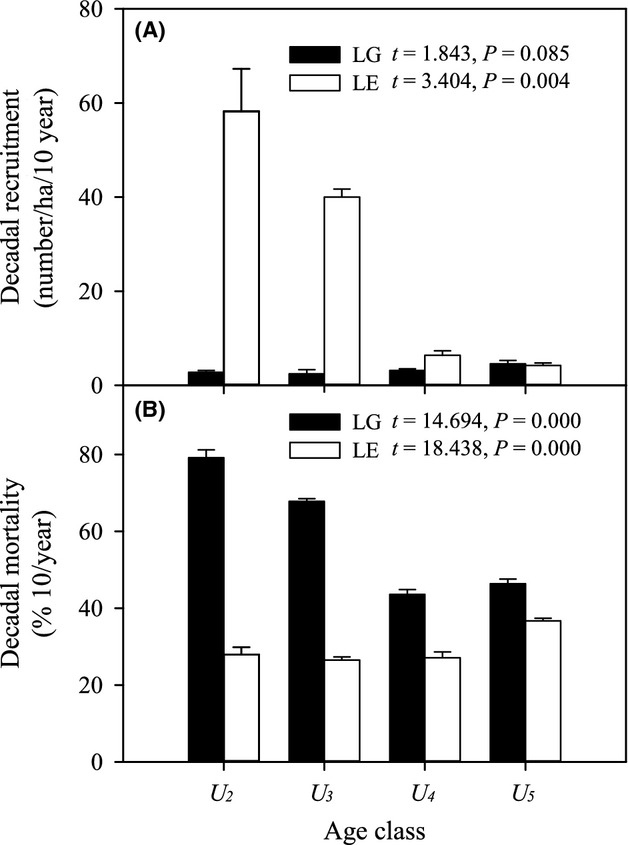
Decadal recruitment (A) and decadal mortality (B) of saplings (U2), young (U3), mature (U4), and old (U5) Ulmus pumila trees in grazing and fencing plots. Estimate of recruitment has been scaled up from the plot level (100 × 100 m) and reported on a per hectare basis. Error bars represent ±SE.
Responses of generation recruitment of Ulmus pumila tree
Fencing significantly increased the survival rates of U. pumila seedlings and saplings (Fig.6). For example, after fencing for 2 years, the survival rate of current-year, 2-year, and older U. pumila were 10.7%, 60.6%, and 85.5%, respectively, which were much higher than the survival rates prior to fencing (similar to grazing plots) (Fig.6). However, there were no meaningful differences among the survival rates after excluding livestock for a longer period of time, regardless of whether the trees were seedlings or saplings. Livestock grazing controlled generation recruitment effectively, with almost no seedlings surviving prior to fencing (Fig.6). However, the survival rate of saplings was approximately 20.3%, even in grazing plots (Fig.6).
Figure 6.
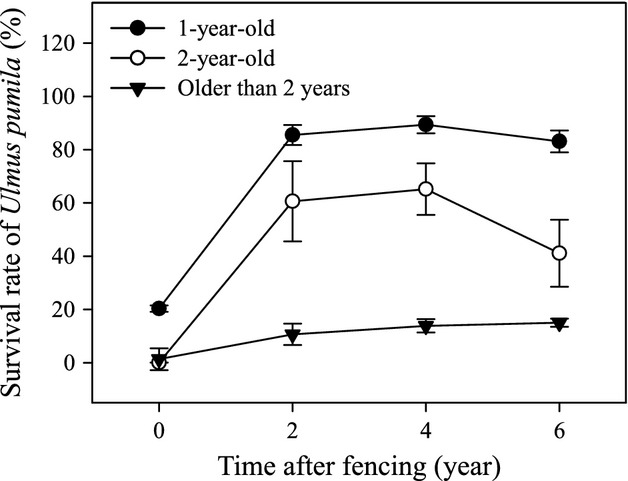
The effect of grazing and fencing on the survival rates of current-year and 2-year-old seedlings (U1) and U. pumila saplings (U2). Error bars represent ±SE.
Discussion
Ulmus pumila is widely distributed in the north temperate zone (Solla et al. 2005) and contributes to a special savanna-like woodland in the rangelands of northern China (Liu et al. 2013; Su et al. 2014) and Mongolia (Dulamsuren et al. 2009a,b). Dulamsuren et al. (2009a) presumed that U. pumila cannot encroach onto the south-facing slopes covered with steppe and savanna-like U. pumila woodland, even without livestock grazing. Nonetheless, the results from our decade-long fencing experiment indicate that livestock exclusion is capable of inducing U. pumila encroachment in the Hunshandake sandy rangeland in northern China, thereby dramatically altering the structure and function of native dominant trees. The decade-long livestock exclusion not only resulted in a double to triple increase in the canopy cover and density of the U. pumila tree population but also led to approximately an 80-fold increase in recruitment and a nearly 2.5-fold decrease in mortality in both saplings (U2) and young trees (U3) in this ecosystem.
The large increment of total U. pumila tree biomass that was observed in fencing plots (Fig.2B) is in accordance with the results of Riedel et al. (2013), who found that woody plant biomass in Mediterranean woodland doubled in undisturbed, nongrazed areas. However, the results are attributable to significant tree growth over time and not to fencing (Table2). The abundance of native tree species increased significantly following the exclusion of browsing herbivores (Fig.2C and Table2). Coincidentally, the same phenomenon has been observed in temperate savanna (Weltzin et al. 1997), temperate grassy woodland (Kirkpatrick 2004), grassland (Noble et al. 2007), and other areas. However, the fact that total tree biomass also increased in grazing plots, although to a lesser extent than in fencing plots (Fig.2B), indicates that the current grazing regimes presented here (1.4 sheep unit ha−1) were able to modulate but not stop the succession of tree vegetation toward the climax vegetation types, which would consist of U. pumila forest or Pinus sylvestris var. mongolica forest (Liu et al. 2009a). The results obtained from Mediterranean areas (Casasús et al. 2007; Riedel et al. 2013), whose landscapes are similar to our study site, show that livestock grazing at moderate stocking rates can halt woody plant encroachment.
Our results indicate that the effects of fencing on U. pumila trees varied by age classes, with potential implications for the future structure of the U. pumila tree community. In particular, fencing had exerted a significant influence on the dynamics of U2 and U3 compared with other age classes (Table3 and 4, Figs.3, 4). The proportion of U2 and U3 climbed by 13.3% and 74.5%, respectively, after the 10-year fencing (Fig.3). Livestock grazing significantly influenced the recruitment of U2 and U3 (Fig.5A) and had virtually no impact on the mortality of U4 and U5 (Fig.5B). Consequently, only U5 showed a net increase in basal area and biomass in grazing plots over the decade (Fig.4), which suggests a long-term livestock grazing-induced directional shift in the U. pumila age structure toward aging. Our results are consistent with similar long-term experiments that were conducted in the semiarid savanna (Sankaran et al. 2013). Year-round chronic browsing by domestic livestock constrains woody biomass by generating a “browsing trap,” particularly for U2 and U3. Significant differences were found in the structure of U. pumila trees between fencing and grazing plots after decadal treatment (Fig.3 and Table3). At the beginning of our study, U4 occupied the largest proportion (approximately 55%) of the site. However, the proportion of U2, U3, and U5 increased after a decade of fencing, and only U5 increased in the grazing plots. The recruitment of both U2 and U3 (0.8 ± 0.04 and 0.5 ± 0.09 individual ha−1 year−1) was substantially controlled by livestock grazing. The results may indicate that livestock grazing can easily control young trees but not adult trees because of the latter's large size (Archibald and Bond 2003; Ward and Esler 2011; Hean and Ward 2012). However, herbivores did not cause lethal damage in U. pumila trees beyond the age of seedlings (Dulamsuren et al. 2009a), but cause stunting and twisting in mature U. pumila trees (Liu et al. 2013). Domestic livestock (e.g., cattle and sheep) prefer young leaves, which have higher nitrogen and water levels, to mature leaves (Coley and Barone 1996). Between 5% and 10% of the leaf area of U. pumila trees was consumed by herbivores (Dulamsuren et al. 2009a), which is sufficient to reduce plant fitness and substantially impact the growth and survival of plants (Coley and Barone 1996), consequently depressing the survival of seedlings and small trees. We did not quantify the indirect effects of livestock, such as those arising from changes in grass biomass and the strength of tree–grass competition (Riginos and Young 2007; Riginos 2009), as well as the abundance of other cryptic consumers, such as rodents, which can be important to tree survival in some ecosystems (Goheen et al. 2004; Maclean et al. 2011). Although future studies would benefit from a quantification of both the direct and indirect effects of different age classes of trees, our results suggest that the direct effects of livestock, particularly on sapling and young trees, are substantial.
Our results support the opinion that U. pumila seedlings older than 2 years of age have increased survival rates (Dulamsuren et al. 2009b). Dulamsuren et al. (2009b) analyzed whether xeric microclimate and high herbivore densities limit the success of seedling establishment in U. pumila tree and found that most of the seedlings older than 2 years survived after suffering from feeding damage by insects and small mammals, from nitrogen deficiency, and, to a lesser degree, from drought. The survival rates of seedlings older than 2 years could exceed 20% even with livestock grazing (Fig.6). The germination of U. pumila seeds is not a problem in U. pumila open woodland (Guo and Liu 2004), but the high susceptibility of newly emerged seedlings to environmental stresses and disturbances is a serious bottle neck for U. pumila that prevents them from encroachment (Dulamsuren et al. 2009b). Livestock grazing considerably suppressed the survival of seedlings younger than 2 years of age and kept their survival rate below 5% (Fig.6). Livestock exclusion stimulates the outbreak of seedlings and protects more seedlings across their receptive stage successfully (Fig.6). The density of U. pumila seedlings can reach 288 per m2 in fenced areas (Liu et al. 2005), and their density is only 4 per m2 in heavily grazed sites (Li 2003). The survival rates can exceed 10% and 60% for the current-year and 2-year-old seedlings, respectively (Fig.6). This finding may be explained by the fact that U. pumila, as the dominant native species, produces a number of seeds that easily geminate in the local soil (Liu et al. 2009b). However, the survival of the seedlings can be affected by the presence of livestock through consumption and physical damage, such as trampling, which in turn can impact their growth and reproduction patterns (Cairns and Moen 2004).
Trees are typically long-lived, and tree dynamics in arid and semiarid areas often tend to be “event-driven,” where the timing and magnitude of episodic rainfall events drive plant growth, mortality, and other ecosystem processes (Sankaran et al. 2013). Consequently, general trends are difficult to show in short-term studies that are likely to miss significant recruitment or dieback events and thus incorrectly estimate transitions. Our findings show that livestock grazing maintains the woody layer in a low-density state dominated by U4 individuals and that fencing generates a high-density state dominated by younger U2 and U3 (Fig.3). These shifts occur not just because livestock exclusion substantially increases rates of recruitment and plant growth but also because over longer timescales, fencing reduces rates of mortality for older ones (Fig.5). Our results highlight the importance of long-term experimental studies for a detailed understanding of fencing or grazing effects on woody vegetation dynamics. A short-term effect may be misleading (Bakker 1998). For example, our prediction, which was made 3 years after fencing, was that natural restoration would be practicable without any artificial management (Normile 2007). However, after 10 years, our results indicate that artificial management is necessary to prevent woody plant encroachment. Modest livestock grazing appears to stabilize the functioning of these rangeland ecosystems (Bai et al. 2004).
Reduced grazing pressure or even the disappearance of grazing livestock in some areas has been consistently reported, for example, Li and Huntsinger (2011) and Zhou et al. (2013) in China, which may have detrimental effects on sandy rangelands. However, woody plant encroachment does not necessarily occur after the cessation of grazing (Bakker 1998). The results may depend on the vegetation type, grazing regime, and the socioeconomic environment that is associated with livestock grazing systems in the study area. Grazing can control the dominance of certain plant species and enhance structural heterogeneity by selective defoliation, trampling, nutrient cycling, and propagule dispersal (Fig.7, Rook and Tallowin 2003). Therefore, the feasibility of full livestock exclusion requires further discussion. Before an expanding exclosure, the ecological consequences of additional exclosures should be investigated because long-term exclosures can increase woody plant encroachment.
Figure 7.
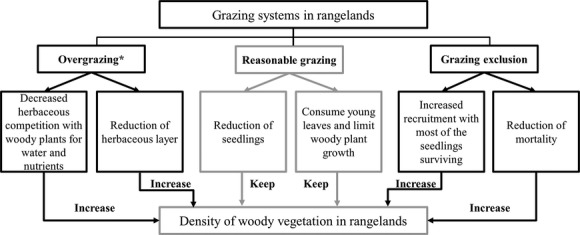
Processes mediating woody vegetation encroachment in different grazing systems. * refers to Asner and Martin (2004).
Acknowledgments
This research was jointly supported by the National Natural Science Foundation of China (Grant No. 31400338) and the Strategic Priority Program of Chinese Academy of Sciences (Grant No. XDA05070102). We thank Nasongwuritu, Huhetuga, and Peiguo Deng for their significant assistances while conducting field works. We thank Wiley English Language Services for the careful editing services.
Conflict of Interest
None declared.
References
- Anadón JD, Sala OE, Turner BL. Bennett EM. Effect of woody-plant encroachment on livestock production in North and South America. Proc. Natl Acad. Sci. 2014;111:12948–12953. doi: 10.1073/pnas.1320585111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SR. Rangeland conservation and shrub encroachment: new perspectives on an old problem. In: Toit JTD, Kock R, Deutsch J, editors. Wild rangelands: conserving wildlife while maintaining livestock in semi-arid ecosystems. Oxford: Wiley-Blackwell; 2010. pp. 53–97. in, eds. [Google Scholar]
- Archer S, Schimel D. Holland E. Mechanisms of shrubland expansion: land use, climate or CO2. Clim. Change. 1995;29:91–99. [Google Scholar]
- Archibald S. Bond WJ. Growing tall vs. growing wide: tree architecture and allometry of Acacia karroo in forest, savanna, and arid environments. Oikos. 2003;102:3–14. [Google Scholar]
- Asner GP. Martin RE. Ecosystems and Land Use Change. In: Defries RS, Asner GP, Houghton RA, editors; Biogeochemistry of desertification and woody encroachment in grazing systems, in ecosystems and land use change. Washington, DC: American Geophysical Union; 2004. pp. 99–116. eds., and. [Google Scholar]
- Asner GP, Elmore AJ, Olander LP, Martin RE. Harris AT. Grazing systems, ecosystem responses, and global change. Annu. Rev. Environ. Resour. 2004;29:261–299. [Google Scholar]
- Augustine DJ. McNaughton SJ. Ungulate effects on the functional species composition of plant communities: herbivore selectivity and plant tolerance. J. Range Manag. 1998;62:1165–1183. [Google Scholar]
- Auken OWV. Shrub invasions of North American semiarid grasslands. Annu. Rev. Ecol. Syst. 2000;31:197–215. [Google Scholar]
- Bai Y, Han X, Wu J, Chen Z. Li L. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- Bakker JP. The impact of grazing on plant communities. In: WallisDeVries MF, Van Wieren SE, Bakker JP, editors. Grazing and conservation management. The Netherlands: Springer; 1998. pp. 137–184. [Google Scholar]
- Belay T, Totland Ø. Moe S. Ecosystem responses to woody plant encroachment in a semiarid savanna rangeland. Plant Ecol. 2013;214:1211–1222. [Google Scholar]
- Belsky AJ. Effects of grazing, competition, disturbance and fire on species composition and diversity in grassland communities. J. Veg. Sci. 1992;3:187–200. [Google Scholar]
- Bond WJ. Large parts of the world are brown or black: a different view on the ‘Green World’ hypothesis. J. Veg. Sci. 2005;16:261–266. [Google Scholar]
- Bond WJ. What limits trees in C4 grasslands and savannas? Annu. Rev. Ecol. Evol. Syst. 2008;39:641–659. [Google Scholar]
- Buitenwerf R, Bond WJ, Stevens N. Trollope WSW. Increased tree densities in South African savannas: > 50 years of data suggests CO2 as a driver. Glob. Change Biol. 2012;18:675–684. [Google Scholar]
- Cabral AC, De Miguel JM, Rescia AJ, Schmitz MF. Pineda FD. Shrub encroachment in Argentinean savannas. J. Veg. Sci. 2003;14:145–152. [Google Scholar]
- Cairns DM. Moen J. Herbivory influences tree lines. J. Ecol. 2004;92:1019–1024. [Google Scholar]
- Casasús I, Bernués A, Sanz A, Villalba D, Riedel J. Revilla R. Vegetation dynamics in Mediterranean forest pastures as affected by beef cattle grazing. Agric. Ecosyst. Environ. 2007;121:365–370. [Google Scholar]
- Coley PD. Barone JA. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 1996;27:305–335. [Google Scholar]
- Dalle G, Maass BL. Isselstein J. Encroachment of woody plants and its impact on pastoral livestock production in the Borana lowlands, southern Oromia, Ethiopia. Afr. J. Ecol. 2006;44:237–246. [Google Scholar]
- Ding G, Li S, Cai J, Zhao T, Wang X. Ling X. Pasture resources evaluation and stocking density in Hunshandake Sandy Land: case study of Zhenglan Banner, Inner Mongolia (in Chinese with English abstract) Chin. J. Ecol. 2005;24:1038–1042. [Google Scholar]
- Dulamsuren C, Hauck M, Nyambayar S, Bader M, Osokhjargal D, Oyungerel S, et al. Performance of Siberian elm (Ulmus pumila) on steppe slopes of the northern Mongolian mountain taiga: drought stress and herbivory in mature trees. Environ. Exp. Bot. 2009a;66:18–24. [Google Scholar]
- Dulamsuren C, Hauck M, Nyambayar S, Osokhjargal D. Leuschner C. Establishment of Ulmus pumila seedlings on steppe slopes of the northern Mongolian mountain taiga. Acta Oecol. 2009b;35:563–572. [Google Scholar]
- Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF. Whitford WG. Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol. Lett. 2011;14:709–722. doi: 10.1111/j.1461-0248.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Frank ASK, Wardle GM, Dickman CR. Greenville AC. Habitat- and rainfall-dependent biodiversity responses to cattle removal in an arid woodland-grassland environment. Ecol. Appl. 2014;24:2013–2028. [PubMed] [Google Scholar]
- Goheen JR, Keesing F, Allan BF, Ogada D. Ostfeld RS. Net effects of large mammals on Acacia seedling survival in an African savanna. Ecology. 2004;85:1555–1561. [Google Scholar]
- Gordijn PJ, Rice E. Ward D. The effects of fire on woody plant encroachment are exacerbated by succession of trees of decreased palatability. Perspect. Plant Ecol. Evol. Syst. 2012;14:411–422. [Google Scholar]
- Guo K. Liu H. A comparative researches on the development of elm seedlings in four habitats in the Hunshandake Sandland, Inner Mongolia, China (in Chinese with English abstract) Acta Ecol. Sin. 2004;24:2024–2028. [Google Scholar]
- Hean JW. Ward D. Fire and herbivory are not substitutable: evidence from regrowth patterns and changes in physical and chemical defences in Acacia seedlings. J. Veg. Sci. 2012;23:13–23. [Google Scholar]
- Higgins SI, Bond WJ. Trollope WSW. Fire, resprouting and variability: a recipe for grass-tree coexistence in savanna. J. Ecol. 2000;88:213–229. [Google Scholar]
- Hoffmann WA. Fire and population dynamics of woody plants in a neotropical savanna: matrix model projections. Ecology. 1999;80:1354–1369. [Google Scholar]
- Holdo RM. Elephants, fire, and frost can determine community structure and composition in Kalahari woodlands. Ecol. Appl. 2007;17:558–568. doi: 10.1890/05-1990. [DOI] [PubMed] [Google Scholar]
- Holdo RM, Holt RD. Fryxell JM. Grazers, browsers, and fire influence the extent and spatial pattern of tree cover in the Serengeti. Ecol. Appl. 2009;19:95–109. doi: 10.1890/07-1954.1. [DOI] [PubMed] [Google Scholar]
- Holdo RM, Holt RD. Fryxell JM. Herbivore-vegetation feedbacks can expand the range of savanna persistence: insights from a simple theoretical model. Oikos. 2013;122:441–453. [Google Scholar]
- Hu YL, Zeng DH, Fan ZP, Chen GS, Zhao Q. Pepper D. Changes in ecosystem carbon stocks following grassland afforestation of semiarid sandy soil in the southeastern Keerqin Sandy Lands, China. J. Arid Environ. 2008;72:2193–2200. [Google Scholar]
- Huang G, Zhao XY, Li YQ. Cui JY. Restoration of shrub communities elevates organic carbon in arid soils of northwestern China. Soil Biol. Biochem. 2012;47:123–132. [Google Scholar]
- Hudak AT. Rangeland mismanagement in South Africa: failure to apply ecological knowledge. Hum. Ecol. 1999;27:55–78. [Google Scholar]
- Huxman TE, Wilcox BP, Breshears DD, Scott RL, Snyder KA, Small EE, et al. Ecohydrological implications of woody plant encroachment. Ecology. 2005;86:308–319. [Google Scholar]
- Jeltsch F, Milton SJ, Dean WRJ. Rooyen NV. Analysing shrub encroachment in the Southern Kalahari: a grid-based modelling approach. J. Appl. Ecol. 1997;34:1497–1508. [Google Scholar]
- Jiang G, Han X. Wu J. Restoration and management of the Inner Mongolia grassland require a sustainable strategy. Ambio. 2006;35:269–270. doi: 10.1579/06-s-158.1. [DOI] [PubMed] [Google Scholar]
- Kambatuku JR, Cramer MD. Ward D. Savanna tree–grass competition is modified by substrate type and herbivory. J. Veg. Sci. 2011;22:225–237. [Google Scholar]
- Kirkpatrick JB. Vegetation change in an urban grassy woodland 1974–2000. Aust. J. Bot. 2004;52:597–608. [Google Scholar]
- Korhonen L, Korhonen KT, Rautiainen M. Stenberg P. Estimation of forest canopy cover: a comparison of field measurement techniques. Silva Fenn. 2006;40:577. [Google Scholar]
- Kulmatiski A. Beard KH. Woody plant encroachment facilitated by increased precipitation intensity. Nat. Clim. Chang. 2013;3:833–837. [Google Scholar]
- Kuyah S, Dietz J, Muthuri C, Jamnadass R, Mwangi P, Coe R, et al. Allometric equations for estimating biomass in agricultural landscapes: I Aboveground biomass. Agric. Ecosyst. Environ. 2012;158:216–224. [Google Scholar]
- Li Y. Ecophysiological adaptive strategies of elm (Ulmus pumila L.) in Hunshandake Sandland. Beijing: Chinese Academy of Sciences; 2003. [Google Scholar]
- Li W. Huntsinger L. Chinas grassland contract policy and its impacts on herder ability to benefit in Inner Mongolia: tragic feedbacks. Ecol. Soc. 2011;16:1. [Google Scholar]
- Liu J, Liu F, Dong M. Wang R. Size structure and neighbor pattern of Ulmus pumila L. population at southern edge of the Hunshandake sandy land (in Chinese with English abstract) J. Desert Res. 2005;24:29–34. [Google Scholar]
- Liu H, Wang L, Yang J, Nakagoshi N, Liang C, Wang W, et al. Predictive modeling of the potential natural vegetation pattern in northeast China. Ecol. Res. 2009a;24:1313–1321. [Google Scholar]
- Liu M, Jiang G, Yu S, Li Y. Li G. The role of soil seed banks in natural restoration of the degraded Hunshandak Sandlands, northern China. Restor. Ecol. 2009b;17:127–136. [Google Scholar]
- Liu L, Wang H, Lin C. Wang D. Vegetation and community changes of elm (Ulmus pumila) woodlands in Northeastern China in 1983–2011 (in Chinese with English abstract) Chin. Geogr. Sci. 2013;23:321–330. [Google Scholar]
- Maclean JE, Goheen JR, Doak DF, Palmer TM. Young TP. Cryptic herbivores mediate the strength and form of ungulate impacts on a long-lived savanna tree. Ecology. 2011;92:1626–1636. doi: 10.1890/10-2097.1. [DOI] [PubMed] [Google Scholar]
- Maher KA, Hobbs RJ. Yates CJ. Woody shrubs and herbivory influence tree encroachment in the sandplain heathlands of southwestern Australia. J. Appl. Ecol. 2010;47:441–450. [Google Scholar]
- Morgan JA, Milchunas DG, LeCain DR, West M. Mosier AR. Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe. Proc. Natl Acad. Sci. 2007;104:14724–14729. doi: 10.1073/pnas.0703427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JC, Müller WJ, Detling JK. Pfitzner GH. Landscape ecology of the burrowing bettong: Warren distribution and patch dynamics in semiarid eastern Australia. Austral Ecol. 2007;32:326–337. [Google Scholar]
- Normile D. Getting at the roots of killer dust storms. Science. 2007;317:314–316. doi: 10.1126/science.317.5836.314. [DOI] [PubMed] [Google Scholar]
- Park YD, Lee DK, Batkhuu NO, Tsogtbaatar J, Combalicer MS, Park GE, et al. Woody species variations in biomass allocation, photosynthetic WUE and carbon isotope composition under natural drought condition in Mongolia. J. Environ. Sci. Manage. 2012:29–37. [Google Scholar]
- Pellew R. The impacts of elephant, giraffe and fire upon the Acacia tortilis woodlands of the Serengeti. Afr. J. Ecol. 1983;21:41–74. [Google Scholar]
- Pressland A. Rainfall partitioning by an arid woodland (Acacia aneura F. Muell.) in south-western Queensland. Aust. J. Bot. 1973;21:235–245. [Google Scholar]
- Prins HH. van der Jeugd HP. Herbivore population crashes and woodland structure in East Africa. J. Ecol. 1993;81:305–314. [Google Scholar]
- Ratajczak Z, Nippert JB. Collins SL. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology. 2012;93:697–703. doi: 10.1890/11-1199.1. [DOI] [PubMed] [Google Scholar]
- Richardson DM. Rejmánek M. Trees and shrubs as invasive alien species – a global review. Divers. Distrib. 2011;17:788–809. [Google Scholar]
- Riedel JL, Bernues A. Casasus I. Livestock grazing impacts on herbage and shrub dynamics in a Mediterranean Natural Park. Rangeland Ecol. Manag. 2013;66:224–233. [Google Scholar]
- Riginos C. Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology. 2009;90:335–340. doi: 10.1890/08-0462.1. [DOI] [PubMed] [Google Scholar]
- Riginos C. Young TP. Positive and negative effects of grass, cattle, and wild herbivores on Acacia saplings in an East African savanna. Oecologia. 2007;153:985–995. doi: 10.1007/s00442-007-0799-7. [DOI] [PubMed] [Google Scholar]
- Rook AJ. Tallowin JR. Grazing and pasture management for biodiversity benefit. Anim. Res. 2003;52:181–189. [Google Scholar]
- Roques KG, O'Connor TG. Watkinson AR. Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 2001;38:268–280. [Google Scholar]
- Rundel PW, Dickie IA. Richardson DM. Tree invasions into treeless areas: mechanisms and ecosystem processes. Biol. Invasions. 2014;16:663–675. [Google Scholar]
- Sankaran M, Augustine DJ. Ratnam J. Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna. J. Ecol. 2013;101:1389–1399. [Google Scholar]
- Solla A, Martín JA, Corral P. Gil L. Seasonal changes in wood formation of Ulmus pumila and U. minor and its relation with Dutch elm disease. New Phytol. 2005;166:1025–1034. doi: 10.1111/j.1469-8137.2005.01384.x. [DOI] [PubMed] [Google Scholar]
- Staver AC, Bond WJ, Stock WD, van Rensburg SJ. Waldram MS. Browsing and fire interact to suppress tree density in an African savanna. Ecol. Appl. 2009;19:1909–1919. doi: 10.1890/08-1907.1. [DOI] [PubMed] [Google Scholar]
- Stuart-Hill G. Tainton N. The competitive interaction between Acacia karroo and the herbaceous layer and how this is influenced by defoliation. J. Appl. Ecol. 1989;26:285–298. [Google Scholar]
- Su H, Li Y, Lan Z, Xu H, Liu W, Wang B, et al. Leaf-level plasticity of Salix gordejevii in fixed dunes compared with lowlands in Hunshandake Sandland, North China. J. Plant. Res. 2009;122:611–622. doi: 10.1007/s10265-009-0249-1. [DOI] [PubMed] [Google Scholar]
- Su H, Li Y, Liu W, Xu H. Sun O. Changes in water use with growth in Ulmus pumila in semiarid sandy land of northern China. Trees. 2014;28:41–52. [Google Scholar]
- Tang Y, Jiang DM. Lu XT. Effects of exclosure management on elm (Ulmus Pumila) recruitment in Horqin Sandy Land, Northeastern China (in Chinese with English abstract) Arid Land Res. Manage. 2014;28:109–117. [Google Scholar]
- Tews J, Esther A, Milton SJ. Jeltsch F. Linking a population model with an ecosystem model: assessing the impact of land use and climate change on savanna shrub cover dynamics. Ecol. Model. 2006;195:219–228. [Google Scholar]
- Wallis de Vries MF, Manibazar N. Dügerlham S. The vegetation of the forest-steppe region of Hustain Nuruu, Mongolia. Plant Ecol. 1996;122:111–127. [Google Scholar]
- Ward D. Esler KJ. What are the effects of substrate and grass removal on recruitment of Acacia mellifera seedlings in a semi-arid environment? Plant Ecol. 2011;212:245–250. [Google Scholar]
- Weltzin JF, Archer S. Heitschmidt RK. Small-mammal regulation of vegetation structure in a temperate savanna. Ecology. 1997;78:751–763. [Google Scholar]
- Wesche K. Treiber J. Abiotic and biotic determinants of steppe productivity and performance–a view from Central Asia. In: Werger MJA, van Staalduinen MA, editors; Eurasian steppes. Ecological problems and livelihoods in a changing world. The Netherlands: Springer; 2012. pp. 3–43. in, ed., and. [Google Scholar]
- Wigley BJ, Bond WJ. Hoffman MT. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob. Change Biol. 2010;16:964–976. [Google Scholar]
- Xu WD. Zou CJ. Sandy forest ecosystem of China. Beijing: China Forestry Publishing House; 1998. [Google Scholar]
- Zheng YR, Xie ZX, Robert C, Jiang LH. Shimizu H. Did climate drive ecosystem change and induce desertification in Otindag sandy land, China over the past 40 years? J. Arid Environ. 2006;64:523–541. [Google Scholar]
- Zhou L, Zhu Y, Yang G. Luo Y. Quantitative evaluation of the effect of prohibiting grazing policy on grassland desertification reversal in northern China. Environ. Earth Sci. 2013;68:2181–2188. [Google Scholar]
- Zianis D. Predicting mean aboveground forest biomass and its associated variance. For. Ecol. Manage. 2008;256:1400–1407. [Google Scholar]


