Abstract
A novel strain of Lactobacillus johnsonii No. 1088 was isolated from the gastric juice of a healthy Japanese male volunteer, and characterized for its effectiveness in the stomach environment. Lactobacillus johnsonii No. 1088 was found to have the strongest acid resistance among several lactobacilli examined (>10% of cells survived at pH 1.0 after 2 h), and such a high acid resistance property was a specific characteristic of this strain of L. johnsonii. When cultured with various virulent bacteria, L. johnsonii No. 1088 inhibited the growth of Helicobacter pylori,Escherichia coli O-157, Salmonella Typhimurium, and Clostridium difficile, in which case its effectiveness was more potent than that of a type strain of L. johnsonii,JCM2012. In addition to its effect in vitro, L. johnsonii No. 1088 inhibited the growth of H. pylori in human intestinal microbiota-associated mice in both its live and lyophilized forms. Moreover, L. johnsonii No. 1088 suppressed gastric acid secretion in mice via decreasing the number of gastrin-positive cells in the stomach. These results taken together suggest that L. johnsonii No. 1088 is a unique lactobacillus having properties beneficial for supporting H. pylori eradication by triple therapy including the use of a proton pump inhibitor (PPI) and also for prophylaxis of gastroesophageal reflux disease possibly caused after H. pylori eradication as a side effect of PPI.
Keywords: Gastroesophageal reflux disease, Helicobacter pylori, lactic acid bacteria, Lactobacillus johnsonii, probiotics
Introduction
Recently, probiotics have become widely recognized as the way to regulate the intestinal bacterial environment and to confer heath benefit on the host. Although the World Health Organization defined probiotics as “live micro-organisms which when administered in adequate amounts confer a health benefit on the host” (Gilliland et al. 2001), the main benefit of probiotics is now recognized as its effect in the intestines as originally suggested by Metchnikoff (1908). Although lactobacillus and bifidobacterium are typical genera used as probiotics (Prasad et al. 1998), other bacteria belonging to enterococcus or bacillus are also used for this purpose (Franz et al. 2003; Bader et al. 2012). It is said that probiotics are also good to prevent or alleviate cancer, constipation, inflammatory diseases, etc. through improving the intestinal microbiota (Mercenier et al. 2003).
In addition to the benefits of probiotic bacteria in the intestines, some beneficial effects on the stomach have been reported recently. Among them is the anti-Helicobacter pylori activity of selected probiotic bacterial strains. Midolo et al. (1995) reported that some strains of lactic acid-producing bacteria inhibit the growth of H. pylori in vitro, where not only lactic acid but also other extracellular compounds produced by these bacteria were suggested to be anti-H. pylori agents. Kabir et al. (1997), using a gnotobiotic murine system, found that gnotobiotic mice bearing with Lactobacillus salivarius or lactobacilli that originated from murine stomach were largely protected from colonization by H. pylori, whereas Enterococcus faecalis and Staphylococcus aureus did not have such a preventive effect. Using a mouse model we confirmed the therapeutic effect of L. salivarius but not that of Lactobacillus casei or Lactobacillus acidophilus; that is, L. salivarius could eliminate or suppress H. pylori colonization when administered after a H. pylori infection (Aiba et al. 1998).
In clinical situations, H. pylori is eliminated by treatment with multiple antibiotics in combination with a proton pump inhibitor (PPI) (Malfertheiner et al. 2007). However, even after successful elimination of H. pylori, lowering of the pH in the stomach after H. pylori eradication is possibly problematic, resulting in gastroesophageal reflux disease (GERD), especially in Asian populations (Xie et al. 2013). Although some studies have not supported the negative relationship between eradication H. pylori and risk of GERD (McColl et al. 2000; Malfertheiner et al. 2002), prevention of excess gastric acid secretion might be beneficial to prevent GERD. Since we demonstrated that some species of lactobacilli even as heat-killed forms decreased the number of gastrin-positive cells in stomach and elevated the pH of gastric juice (Takahashi et al. 2011), appropriate species of lactobacilli are thought to be beneficial for both eradication of H. pylori and lowering the risk of GERD after H. pylori eradication.
In the present study we report the characteristics of a new strain of Lactobacillus johnsonii No. 1088, which was highly acid resistant and showed strong anti-H. pylori activity in mice. This strain also decreased the number of gastrin-positive cells and improved the acidic state of the stomach after H. pylori eradication in mice.
Materials and Methods
Bacterial strains
Lactobacillus johnsonii No. 1088 was isolated from gastric juice of a healthy Japanese male, and identified as L. johnsonii according to the method described in Bergey's Manual of Systematic Bacteriology vol. 2 and by comparing its genomic DNA sequence encoding 16S ribosomal RNA with that of type and reference strains (L. johnsonii ATCC33200 and L. johnsonii NCC533, respectively). This strain was deposited at the National Institute of Technology and Evolution (Chiba, Japan) as Accession No. NITE P-278. Lactobacillus johnsonii La1, L. gasseri OLL2716, L. casei shirota, L. rhamnosus GG, L. acidophilus BF, and L. brevis KB290 were isolated from commercially available fermented products. Lactobacillus johnsonii JCM2012, L. gasseri JCM1131, and Clostridium difficile JCM1296 were obtained from the Japan Collection of Microorganisms (RIKEN, Ibaraki, Japan). Helicobacter pylori No. 130, Escherichia coli O-157, Salmonella Typhimurium LT2, Candida albicans TI3001 were strains isolated at Tokai University Hospital.
Resistance of lactobacilli to low pH
Lactobacilli were cultured in de Man, Rogosa and Sharpe (MRS) broth (BD, NJ, USA) for 18 h at 37°C to prepare growth-phase bacteria to be tested. The grown bacterial cultures were diluted with 0.1 mol/L HCl-citrate buffer (pH 2.0, 1.5 or 1.0) to about 107 CFU/mL, and incubated at 37°C up to 120 min. The residual numbers of viable bacteria were determined at various time points.
Mixed-culture study of L. johnsonii with other bacteria
Lactobacillus johnsonii No. 1088 or JCM2012 (106 CFU/mL) was cocultured with H. pylori No. 130 (107 CFU/mL) in Brain-Heart Infusion (BHI) broth (BD) supplemented with 5% horse serum at 37°C under microaerobic culture conditions up to 48 h, with the number of viable H. pylori being determined at various time points. As a control, H. pylori was cultured without lactobacilli. To examine the effect of L. johnsonii cocultured with other pathogenic bacteria instead of H. pylori, we cocultured E. coli O-157 (107 CFU/mL), S. Typhimurium LT2 (107 CFU/mL), C. difficile JCM1296 (107 CFU/mL) or C. albicans TI3001 (106 CFU/mL) with L. johnsonii No. 1088 or JCM2011 (106 CFU/mL) in Gifu Anaerobic Medium (GAM) broth (Nissui, Tokyo, Japan) supplemented with 0.5% glucose, BHI broth, BHI broth (anaerobic condition), BHI broth, respectively, and then determined the numbers of viable bacteria at various time points.
Anti-H. pylori activity of L. johnsonii No. 1088 in human intestinal microbiota-associated mice
All animal experiments reported in this study were carried out in accordance with the institutional guidelines of Tokai University. Male germ-free mice were purchased from Clea Japan, Inc. (Tokyo, Japan) and maintained in Trexler-type flexible-film plastic isolators with sterile food and water. At the age of 4 weeks, human feces obtained from a healthy Japanese male volunteer were orally administered to each animal (0.5 mL of about 10 mg/mL of feces diluted in phosphate buffer). After 4 weeks, H. pylori No. 130 (109 CFU/mice) was orally administered to these animals four times (once a day for 4 days). After 11 days after the last administration of H. pylori (day 0), oral administration of L. johnsonii No. 1088 was started. For examination of the effect of culture broth of L. johnsonii No. 1088, six animals were sacrificed at day 0 to know the number of H. pylori before L. johnsonii treatment. Twenty animals were administered 0.5 mL of culture broth of L. johnsonii No. 1088 (about 109 CFU) once a day. In another experiment, lyophilized powder of L. johnsonii No. 1088 containing the same number of bacteria was used instead of the culture broth. After 2 weeks (day 14), 10 animals were sacrificed; and then 2 weeks later (day 28), the remaining 10 animals were sacrificed to examine the number of H. pylori in the stomach. As controls, 10 animals without L. johnsonii No. 1088 administration were maintained under the same conditions and sacrificed at day 14, with another 10 killed at day 28.
Gastric acid production in germ-free mice
Male germ-free Balb/c mice (8-weeks old) were administered 109 CFU of L. johnsonii No. 1088 (n = 5) suspended in phosphate-buffered saline (PBS). The control group received PBS without bacteria (n = 5). After 10 days from the administration, mice were anesthetized with Nembutal, their stomach was exposed, and the pylorus and duodenum were clamped with forceps. After 2 h, gastric juice was collected and centrifuged at 3000g for 5 min, after which its volume, total acidity, and pH were determined. Total acidity was determined by titration with 0.1 N NaOH, and pH was measured with a pH meter (Horiba, Tokyo, Japan).
Immunohistochemical analysis of gastrin-positive cells
The number of gastrin-positive cells in the stomach of mice was examined by an immunohistochemical method described previously (Takahashi et al. 2011) to examine the effect of PPI and L. johnsonii No. 1088 on the number of gastrin-positive cells. As shown schematically in Figure1, at 8 weeks of age, s.c. administration of 200 μg of the PPI omeprozaol every 2 days was started in 4–5 mice (8-week PPI treatment group). At 12 weeks of age, 3–5 other mice in each group were started on the PPI as above (4-week PPI treatment group). All mice were then sacrificed when 16 weeks old, and their stomach weight and number of gastrin-positive cells were determined. After being sacrificed, mice were weighed and their stomachs were resected. The resected stomachs were rinsed with PBS, weighed, and then fixed in 10% formalin buffered with PBS. The fixed tissues were embedded in paraffin and cut into 2-μm-thick sections. The tissue sections were deparaffinized, microwaved for 10 min in Target Retrieval Solution (Dako, Glostrup, Denmark), and stained with rabbit polyclonal antigastrin antibody (Dako) as the primary antibody followed by goat anti-rabbit IgG labeled with Alexa488 (Molecular Probes, OR, USA) as the secondary antibody. Finally, the sections were stained with DAPI (4,6-diamidino-2-phenylinodole). The stained slides were observed with a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan). AxioVision release 4.8 (Zeiss, Jena, Germany) was used to count the number of gastrin-positive cells in sections in random fields 1 mm in length along the corpus-antrum axis.
Figure 1.

Schematic drawing of time schedule of the experiment to examine the effect of proton pump inhibitor (PPI) and Lactobacillus johnsonii No. 1088 on the number of gastrin-positive cells and stomach weight.
Immunological quantification of serum gastrin concentration
The serum concentration of gastrin was determined by use of a radio immunoassay (Gastirn RIA kit II; TFB, Tokyo, Japan) according to the manufacturer's instruction at SRL (Tokyo, Japan).
Statistics
Statistical significance between two groups was examined with Student's t-test. Comparison between multiple groups (>2) was performed either with Tukey's honestly significant difference test (for analyzing between all groups) or Dunnett's test (for analyzing between control and other groups).
Results
Isolation and identification of L. johnsonii No. 1088
To obtain extremely acid-resistant lactobacilli, we isolated lactobacilli from human gastric juice. Gastric juices (1 mL each) were sampled from 10 healthy human volunteers and used as the sources for bacterial isolation. The samples were variously diluted with sterile saline, cultured on BL (glucose-Blood-Liver; Nissui) or MRS (BD) agar under anaerobic conditions for 48 h at 37°C. The colonies that appeared were isolated and cultured in MRS broth (BD Biosciences). Colony picking and cultivation were repeated three times to obtain purified bacterial strains. Totally 15 strains were selected as potential lactobacilli (gram positive, rod shape, catalase negative, motility negative, sporogenesis negative, and lactic acid production positive). The strain used in this study was selected as the most acid resistant one among them, that is, giving the maximum number of viable bacteria after incubation for 120 min at 37°C at pH 2.0 (0.1 mol/L sodium citrate-hydrochloric acid buffer).
This strain was identified as L. johnsonii according to Bergeys' Manual of Systematic Bacteriology vol. 2, and further confirmed by comparing its genomic DNA sequence encoding 16S ribosomal RNA to that of type and reference strains (L. johnsonii ATCC33200 and L. johnsonii NCC533, respectively). The sequence was 99.7% and 100% identical to that of L. johnsonii ATCC33200 and L. johnsonii NCC533, respectively. To deny the possibility of contamination from a commercially available strain in yogurt (L. johnsonii La1), PCR products amplified by using several short primers (atgagagacg, gcacgcgaat, agcgagatgt, ctgccgattg, cgaggtcagt, or gtgagttgca) were compared (Williams et al. 1990) and found to be different from those of La1 (data not shown). We named this new strain as L. johnsonii No. 1088.
Acid resistance of L. johnsonii No. 1088 and other lactobacilli
Next we compared the viability of L. johnsonii No. 1088 in an acidic environment with that of other lactobacilli. Figure2 shows the viabilities of commercially available lactobacilli when incubated at pH 1.0 (Fig.2A), 1.5 (Fig.2B) or 2.0 (Fig.2C) at 37°C up to 120 min. Among the lactobacilli examined, L. johnsonii No. 1088, L. johnsonii La1, and L. gasseri OLL2716 were highly acid resistant (>1–10% were viable even after 120 min at pH 1.0), with L. johnsonii No. 1088 being the most. Lactobacillus casei shirota, L. rhamnosus GG, and L. brevis KB290 were dead within 30 min at pH 1.0, whereas L. acidophilus BF showed intermediate resistance to acid. Figure2A also shows the viabilities of type strains of L. johnsonii JCM2012 and L. gasseri JCM1131. As shown in this figure, the strong acid resistance property was strain specific rather than a common property of the respective species.
Figure 2.

Viability of Lactobacillus johnsonii No. 1088 and other lactobacilli at pH 1.0 (A), 1.5 (B), and 2.0 (C). Growing bacteria (L. johnsonii No. 1088, L. johnsonii La1, L. gasseri OLL2716, L. casei shirora, L. rhamnosus GG, L. acidophilus BF, and L. brevis KB290 for pH 1.0, 1.5, and 2.0; and type strains L. johnsonii JCM2012 and L. gasseri JCM1131 added for pH 1.0) were diluted at a density around 107 CFU/mL in acidic buffer and then incubated at 37°C up to 120 min. Counts of living bacterial counts were made at various time points.
Antibacterial effect of L. johnsonii No. 1088 in mixed cultures
Since lactobacilli have been reported to have antimicrobial activity (Kailasapathy and Chin 2000), next we examined L. johnsonii for such an effect on various virulent bacteria. Figure3 shows the viabilities of various virulent bacteria when these bacteria were cocultured with L. johnsonii JCM2012 or No. 1088 up to 48 h. Although H. pylori No. 130 alone grew about threefold in 48 h, when co-cultured with L. johnsonii JCM2012 or No. 1088 the number of H. pylori decreased to about 1/300 or 1/3000, respectively (Fig.3A). The growth of E. coli O-157 (Fig.3B), S. Typhimurium LT2 (Fig.3C), and C. difficile JCM1296 (Fig.3D) was also strongly inhibited when these bacteria were co-cultured with L. johnsonii JCM2012 or No. 1088, in which case L. johnsonii No. 1088 was slightly more effective than JCM2012. The number of viable C. albicans TI3001 cells was not decreased when these cells were cocultured with L. johnsonii (Fig.3E), but their growth in cocultures was inhibited. These results suggest that L. johnsonii No. 1088 and JCM2012 were effective in suppressing the growth of various virulent bacteria and that such an antibacterial effect was stronger for No. 1088 than for JCM2012, especially against H. pylori.
Figure 3.
Antibacterial effect of Lactobacillus johnsonii No. 1088 and its type strain L. johnsonii JCM2012 under mixed-culture conditions. Lactobacillus johnsonii No. 1088 or JCM2012 (106 CFU/mL) was co-cultured with various virulent bacteria (A, Helicobacter pylori No. 130 [107 CFU/mL]; B, Escherichia coli O-157 [107 CFU/mL]; C, Salmonella. Typhimurium LT2 [107 CFU/mL]; D, Clostridium difficile JCM1296 [107 CFU/mL] or E, Candida albicans TI3001 [106 CFU/mL]), and numbers of viable bacteria were determined at the various time points indicated. Symbols are defined in the figure itself.
Anti-H. pylori activity of L. johnsonii No. 1088 in human intestinal microbiota-associated mice
To confirm the anti-H. pylori activity of L. johnsonii No. 1088 found in vitro (Fig.3A), we examined its activity in vivo. Figure4 shows the effect of L. johnsonii No. 1088 on the number of H. pylori No. 130 in human intestinal microbiota-associated mice. As shown in Figure4A and B, L. johnsonii No. 1088 reduced the number of H. pylori in the stomach to about 1/100 after 4 weeks' oral administration; and L. johnsonii No. 1088 was effective when administered not only as culture broth (Fig.4A) but also as its lyophilized powder form (Fig.4B). Administration of either form of L. johnsonii No. 1088 resulted in living L. johnsonii No. 1088 in the stomach (Fig.4C and 4D).
Figure 4.
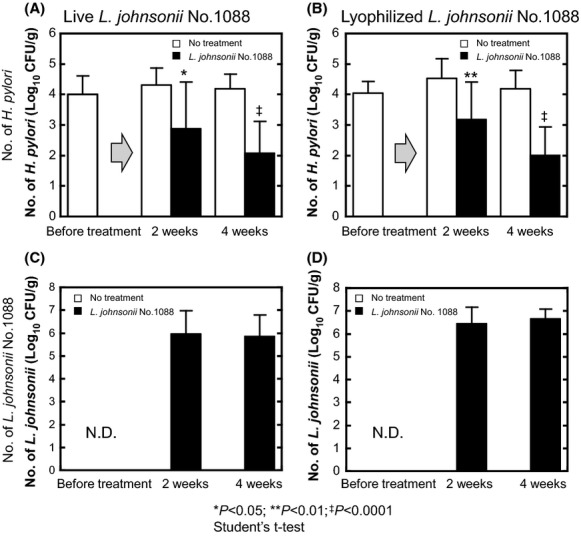
Anti-Helicobacter pylori effect of Lactobacillus johnsonii No. 1088 in human intestinal microbiota-associated mice. Mice associated with human intestinal microbiota were prepared by using germ-free mice and then infected with Helicobacter pylori No. 130 (109 CFU/mice) as described in Materials and Methods. Helicobacter pylori-bearing mice were orally and daily administered growing live L. johnsonii No. 1088 (A and C) or a comparable number of lyophilized cells (B and D) for 2 or 4 weeks. In both mice treated with live or the lyophilized form of L. johnsonii No. 1088, the number of H. pylori in the stomach significantly decreased (A and B). Along with the decrease in the number of H. pylori, a steady number of L. johnsonii No. 1088 was detected in the stomach (C and D). Statistical significance was determined by use of Student's t-test (*P < 0.05, **P < 0.01, ‡P < 0.0001 versus no treatment for comparable time periods).
Effect of L. johnsonii No. 1088 on gastric acid production
Triple therapy including PPI is now widely used to eradicate H. pylori in clinical situations (Malfertheiner et al. 2007). But lowering of the gastric pH after successful eradication of H. pylori has been recognized as a potential risk factor of GERD (Xie et al. 2013). As demonstrated above, L. johnsonii No. 1088 was found useful for eradication of H. pylori. Next we examined whether this lactobacillus strain would also be beneficial for lowering the risk of GERD. Table1 shows volume, total acidity, and pH of gastric juice of germ-free Balb/c mice 10 days after having been administered 109 CFU of L. johnsonii No. 1088. Although the volumes of gastric juice were the same irrespective of the administration of L. johnsonii No. 1088, the pH of the gastric juice was significantly (P < 0.005) higher when L. johnsonii No. 1088 was administered (pH 6.1) than when the control PBS was given (pH 3.0). Moreover, the total acidity in the L. johnsonii No. 1088-treated group (5.2 mEq/mL) tended to be lower than that in the control one (19.0 mEq/mL), although there was no statistically significant difference (P = 0.051).
Table 1.
Volume, acid concentration, and pH of gastric juice after administration of Lactobacillus johnsonii No. 1088
| Treatment | Volume of gastric juice (mL) | Acid concentration (mEq/mL) | pH | n |
|---|---|---|---|---|
| PBS | 0.6 ± 0.2 | 19.0 ± 4.4 | 3.0 ± 0.5 | 5 |
| L. johnsonii No. 1088 | 0.6 ± 0.3 | 5.2 ± 5.1* | 6.1 ± 0.7** | 5 |
PBS, phosphate-buffered saline.
Significance of difference versus PBS:
P = 0.051;
P < 0.005.
Effect of L. johnsonii No. 1088 on PPI-induced increase in gastrin-positive cells and weight of stomach
To know the underlying mechanism, we next examined the effect of L. johnsonii No. 1088 on the number of gastrin-positive cells. Since gastrin is a hormone produced by G-cells in the pyloric antrum of the stomach and stimulates the secretion of gastric acid, it is interesting to know whether PPI treatment and administration of L. johnsonii No. 1088 would affect the number of these cells. Germ-free Balb/c mice (4 weeks old) were orally administered 109 CFU of live L. johnsonii No. 1088 or PBS. The effects of treatment with PPI with and without L. johnsonii No. 1088 on the number of gastrin-positive cells and stomach weight are shown in Figure5A and B, respectively; and the statistical analysis of these data (Tukey's honestly significant difference test) are summarized in Table2. As shown in Figure5A, the administration of L. johnsonii No. 1088 decreased the number of gastrin-positive cells (compared with PPI for 0W; P = 0.0003), whereas the stomach weight was not different (Fig.5B). PPI administration increased both the number of gastrin-positive cells (Fig.5A, open bars) and stomach weight (Fig.5B, open bars). Since gastrin stimulates the growth of the fundic mucosa (Kinoshita and Ishihara 2000), this result can be explained by a probable increase in gastrin secretion. On the other hand, preadministration with L. johnsonii No. 1088 significantly prevented the increase in the number of gastrin-positive cells (Fig.5A, closed bars) and also that in stomach weight (Fig.5B, closed bars).
Figure 5.

Effect of proton pump inhibitor (PPI) and Lactobacillus johnsonii No. 1088 on the number of gastrin-positive cells and stomach weight. Germ-free Balb/c mice (4 weeks old) were orally administered 109 CFU of live L. johnsonii No. 1088 (closed bars) or PBS (open bars). At 8 weeks of age, 4–5 mice in each group were administered s.c. with 200 μg of the PPI omeprozaol every 2 days (8-week PPI treatment group). At 12 weeks of age, the remaining 3–5 mice in each group were administered PPI as above (4-week PPI treatment group). At 16 weeks of age, all mice were sacrificed; and the number of gastrin-positive cells (A) and stomach weight (B) were analyzed as described in Materials and Methods. Statistical significance of difference between groups was determined by Tukey's honestly significant difference test and summarized in Table2, whereas selected results are indicated in the graph (**P < 0.01; ***P < 0.001 as indicated by the brackets).
Table 2.
P-values after determination of gastrin-positive cells/mm and stomach weight for Figure5
| Test condition | PBS |
Lactobacillus johnsonii No. 1088 |
||||
|---|---|---|---|---|---|---|
| 0 Week | 4 Weeks | 8 Weeks | 0 Week | 4 Weeks | 8 Weeks | |
| (A) Gastrin-positive cells/mm | ||||||
| PBS | ||||||
| 0 Week | – | – | – | – | – | – |
| 4 Weeks | 0.0004 | – | – | – | – | – |
| 8 Weeks | 0.0001 | 0.9691 | – | – | – | – |
| L. johnsonii No. 1088 | ||||||
| 0 Week | 0.0003 | <0.0001 | <0.0001 | – | – | – |
| 4 Weeks | 0.0012 | <0.0001 | <0.0001 | 0.9501 | – | – |
| 8 Weeks | 0.0014 | <0.0001 | <0.0001 | 0.9886 | 0.9999 | – |
| (B) Relative stomach weight | ||||||
| PBS | ||||||
| 0 Week | – | – | – | – | – | – |
| 4 Weeks | 0.102 | – | – | – | – | – |
| 8 Weeks | <0.0001 | 0.0604 | – | – | – | – |
| L. johnsonii No. 1088 | ||||||
| 0 Week | 0.6342 | 0.7772 | 0.0005 | – | – | – |
| 4 Weeks | 0.0063 | 0.9949 | 0.0809 | 0.3125 | – | – |
| 8 Weeks | 0.0426 | 1 | 0.0165 | 0.725 | 0.9792 | – |
Statistical significances were determined by Tukey's honestly significant difference test. P < 0.05 is indicated by bold letters. PBS, phosphate-buffered saline.
Decrease in serum gastrin concentration by ingested heat-killed L. johnsonii No. 1088
Finally, we examined the effect of oral administration of L. johnsonii No. 1088 on the serum concentration of gastrin. As shown in Figure6, oral administration of heat-killed L. johnsonii No. 1088 for 10 days to germ-free Balb/c mice dose dependently decreased their serum concentration of gastrin; the gastrin concentration was significantly lower at a dose of 109 or 1010 CFU/mouse than that before treatment (P < 0.05).
Figure 6.

Decrease in serum gastrin concentration after oral treatment of germ-free mice with heat-killed Lactobacillus johnsonii No. 1088 for 10 days. Various numbers of heat-killed L. johnsonii No. 1088 were orally administered for 10 days, and serum concentrations of gastrin were determined with ELISA. Administration with 109 or 1010 CFU of heat-killed L. johnsonii No. 1088 significantly decreased the serum gastrin concentration. *P < 0.05 versus PBS (Dunnett's test).
Discussion
Acid resistance has been recognized as one of the important factors for selecting probiotic strains of lactic acid bacteria, since survival during passage through the stomach is a key factor for the bacteria to reach the intestines alive. Although L. acidophilus and bifidobacteria have been reported to be resistant to acid, such a resistance is not intrinsic to the species but is highly dependent on the strain (Charteris et al. 1998; Kailasapathy and Chin 2000). In the present study, L. johnsonii No. 1088 was found to be in the group of the most acid-resistant lactobacilli. This group also contained L. gasseri OLL2716, which had earlier been selected from 203 strains of lactobacilli (Sakamoto et al. 2001). Since other strains of L. johnsonii and L. gasseri were not so resistant to acid (Fig.1A), such high acid resistance of L. johnsonii No. 1088 and L. gasseri OLL2716 may be considered to be specific to these strains. Although several mechanisms have been proposed to explain the acid resistance of gram-positive bacteria (Cotter and Hill 2003), the underlying mechanism of the high acid resistance of L. johnsonii No. 1088 remains to be elucidated.
Lactobacillus acidophilus and bifidobacteria have been reported to have wide-spectrum antipathogenic bacterial activity, including that against S. aureus,S. Typhimurium, Yersinia enterocolitica, and Clostridium perfringens (Kailasapathy and Chin 2000). Furthermore, anti-H. pylori activity of lactobacilli has been reported for several probiotic strains, including L. acidophilus,L. casei,L. salivarius,L. reuteri,L. johnsonii, and L. gasseri (Midolo et al. 1995; Kabir et al. 1997; Michetti et al. 1999; Felley et al. 2001; Francavilla et al. 2008; De Vuyst et al. 2010). Although competitive colonization and production of various compounds having microbial activities (organic acids, bacteriocins, hydrogen peroxide, carbon dioxide, etc.) have been proposed as the probable mechanisms underlying the antibacterial activity of lactobacilli (Kailasapathy and Chin 2000), the main mechanism of the anti-H. pylori activity of L. johnsonii No. 1088 has not yet been addressed. One of the possible anti-H. pylori agents might be peptides derived from L. johnsonii No. 1088 as suggested for anti-H. pylori activity of L. johnsonii La1 (De Vuyst et al. 2010). The clinical effectiveness of lactobacilli is controversial depending on the strains used for studies, but some promising results have been reported regarding L. gasseri OLL2716 (Sakamoto et al. 2001), L. reuteri (Francavilla et al. 2014), and L. johnsonii La1 (Felley et al. 2001). However, complete eradication of H. pylori solely by ingestion of lactobacilli cannot be attained. Instead, it is a promising strategy to use lactobacilli as a support for eradication with other therapies including multiple antibiotics. Since L. johnsonii No. 1088 was extremely acid resistant, a strong supportive effect of it for H. pylori eradication in clinical situations might be expected as observed in L. gasseri OLL2716 (Fujimura et al. 2006).
Another interesting characteristic of L. johnsonii No. 1088 was its ability to inhibit gastric acid production by decreasing the number of gastrin-positive cells in the stomach. In a previous study, we demonstrated that such an effect of L. johnsonii No. 1088 was slightly stronger than that of L. gasseri OLL2716 (Takahashi et al. 2011). In the present study, we demonstrated that L. johnsonii No. 1088 suppressed the gastrin production increased by PPI administration (Fig.5). These results clearly suggest that L. johnsonii No. 1088 should be useful for not only supporting H. pylori eradication therapy but also prevention of the risk of GERD after H. pylori eradication therapy including PPI. Although the mechanism by which L. johnsonii No. 1088 decreases the number of gastrin-positive cells is not clear to date, activation of Toll-like receptor 2 (TLR2) by cell surface components of L. johnsonii No. 1088 might be one candidate, since a known TLR2 ligand, pam3 (Takeda et al. 2002), but not TLR4 ligand, Lipopolysaccharide (LPS), reduced the number of gastrin-producing cells as well (Y. Nakano, unpubl. result). Further study will be necessary to confirm this hypothesis.
In conclusion, L. johnsonii No. 1088 was presently shown to be a unique lactobacillus, having an extremely acid resistance property, strong antibacterial activity including that against H. pylori and also inhibiting gastrin-induced acid production in germ-free mice. We propose the combination of these properties to be beneficial for supporting H. pylori eradication by antibiotics triple therapy including PPI, and also for prophylaxis of GERD possibly caused after H. pylori eradication as a side effect of PPI treatment.
Conflict of Interest
Y. Aiba, Y. Nakano, K. Takahashi, and Y. Komatsu are employees of Snowden Co., Ltd.
References
- Aiba Y, Suzuki N, Kabir AM, Takagi A. Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am. J. Gastroenterol. 1998;93:2097–2101. doi: 10.1111/j.1572-0241.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- Bader J, Albin A. Stahl U. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes. 2012;3:67–75. doi: 10.3920/BM2011.0039. [DOI] [PubMed] [Google Scholar]
- Charteris WP, Kelly PM, Morelli L. Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998;84:759–768. doi: 10.1046/j.1365-2672.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- Cotter PD. Hill C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003;67:429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst L, Vincent P, Makras E, Leroy F. Pot B. Peptide extracts from cultures of certain lactobacilli inhibit Helicobacter pylori. Probiotics Antimicrob. Proteins. 2010;2:26–36. doi: 10.1007/s12602-009-9029-4. [DOI] [PubMed] [Google Scholar]
- Felley CP, Corthesy-Theulaz I, Rivero JL, Sipponen P, Kaufmann M, Bauerfeind P, et al. Favourable effect of an acidified milk (LC-1) on Helicobacter pylori gastritis in man. Eur. J. Gastroenterol. Hepatol. 2001;13:25–29. doi: 10.1097/00042737-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Francavilla R, Lionetti E, Castellaneta SP, Magista AM, Maurogiovanni G, Bucci N, et al. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 2008;13:127–134. doi: 10.1111/j.1523-5378.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J. Clin. Gastroenterol. 2014;48:407–413. doi: 10.1097/MCG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- Franz CM, Stiles ME, Schleifer KH. Holzapfel WH. Enterococci in foods–a conundrum for food safety. Int. J. Food Microbiol. 2003;88:105–122. doi: 10.1016/s0168-1605(03)00174-0. [DOI] [PubMed] [Google Scholar]
- Fujimura S, Kato S, Oda M, Miyahara M, Ito Y, Kimura K, et al. Detection of Lactobacillus gasseri OLL2716 strain administered with yogurt drink in gastric mucus layer in humans. Lett. Appl. Microbiol. 2006;43:578–581. doi: 10.1111/j.1472-765X.2006.02017.x. [DOI] [PubMed] [Google Scholar]
- Gilliland SE, Morelli L. Reid G. 2001. and Health and nutrition properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutrirional properties of probiotics in food including powder milk with live lactic acid bacteria.
- Kabir AM, Aiba Y, Takagi A, Kamiya S, Miwa T. Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41:49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailasapathy K. Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000;78:80–88. doi: 10.1046/j.1440-1711.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y. Ishihara S. Mechanism of gastric mucosal proliferation induced by gastrin. J. Gastroenterol. Hepatol. 2000;15(Suppl):D7–D11. doi: 10.1046/j.1440-1746.2000.02145.x. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Dent J, Zeijlon L, Sipponen P, Veldhuyzen Van Zanten SJ, Burman CF, et al. Impact of Helicobacter pylori eradication on heartburn in patients with gastric or duodenal ulcer disease – results from a randomized trial programme. Aliment. Pharmacol. Ther. 2002;16:1431–1442. doi: 10.1046/j.1365-2036.2002.01285.x. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O'morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl KE, Dickson A, El-Nujumi A, El-Omar E. Kelman A. Symptomatic benefit 1-3 years after H. pylori eradication in ulcer patients: impact of gastroesophageal reflux disease. Am. J. Gastroenterol. 2000;95:101–105. doi: 10.1111/j.1572-0241.2000.01706.x. [DOI] [PubMed] [Google Scholar]
- Mercenier A, Pavan S. Pot B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr. Pharm. Des. 2003;9:175–191. doi: 10.2174/1381612033392224. [DOI] [PubMed] [Google Scholar]
- Metchnikoff E. Lactic acid as inhibiting intestinal putrefaction. In: Mitchell PC, editor. The prolongation of life: optimistic studies. London: G. P. Putnam's Sons; 1908. pp. 160–183. [Google Scholar]
- Michetti P, Dorta G, Wiesel PH, Brassart D, Verdu E, Herranz M, et al. Effect of whey-based culture supernatant of Lactobacillus acidophilusjohnsonii) La1 on Helicobacter pylori infection in humans. Digestion. 1999;60:203–209. doi: 10.1159/000007660. [DOI] [PubMed] [Google Scholar]
- Midolo PD, Lambert JR, Hull R, Luo F. Grayson ML. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 1995;79:475–479. doi: 10.1111/j.1365-2672.1995.tb03164.x. [DOI] [PubMed] [Google Scholar]
- Prasad J, Gill H, Smart J. Gopal PK. Selection and characterisatino of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1998;8:993–1002. [Google Scholar]
- Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T. Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J. Antimicrob. Chemother. 2001;47:709–710. doi: 10.1093/jac/47.5.709. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakano Y, Matsuoka T, Kumaki N, Asami Y. Koga Y. Role of indigenous lactobacilli in gastrin-mediated acid production in the mouse stomach. Appl. Environ. Microbiol. 2011;77:6964–6971. doi: 10.1128/AEM.05230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O. Akira S. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin Res. 2002;8:459–463. doi: 10.1179/096805102125001073. [DOI] [PubMed] [Google Scholar]
- Williams JG, Kubelik AR, Livak KJ, Rafalski JA. Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Cui X, Zheng H, Chen D, He L. Jiang B. Meta-analysis: eradication of Helicobacter pylori infection is associated with the development of endoscopic gastroesophageal reflux disease. Eur. J. Gastroenterol. Hepatol. 2013;25:1195–1205. doi: 10.1097/MEG.0b013e328363e2c7. [DOI] [PubMed] [Google Scholar]



