Abstract
Heart failure (HF) has reached epidemic proportions in the United States and is one of the most important challenges to public health. Severe congestive HF is associated with substantial morbidity and mortality. HF afflicts approximately 5 million patients and contributes to 3 million hospitalizations and 300,000 deaths yearly.1 Late-stage HF has a poor prognosis, and therapeutic options are limited. Defective excitation–contraction (EC) coupling in HF may result from altered density or function of proteins relevant for Ca2+ homeostasis.
EC COUPLING
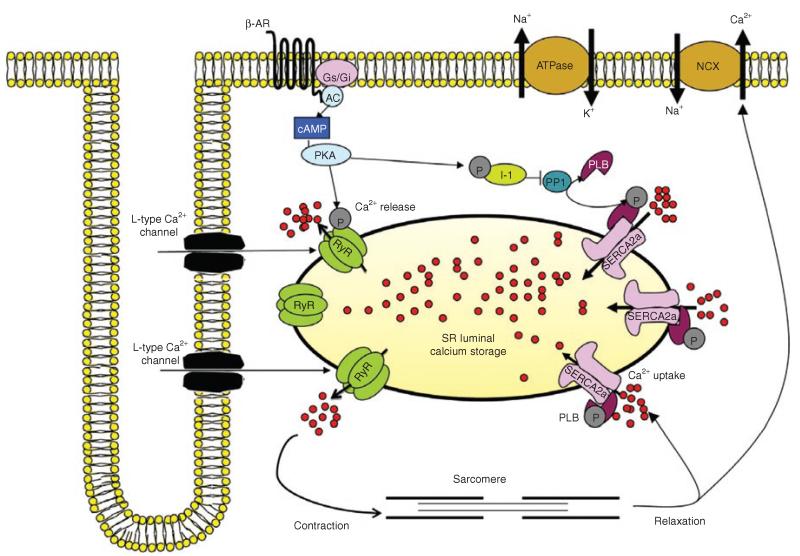
EC coupling is the process of electrical excitation of myocytes leading to contraction of the heart. EC coupling consists of processes involved in Ca2+ activation of contractile proteins and the subsequent removal of calcium, thereby facilitating relaxation. During the cardiac action potential, Ca2+ enters through L-type Ca2+ channels that trigger Ca2+ release from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyRs). The resulting rise in intracellular calcium concentration causes sarcomeric shortening and muscle contraction.2 Ca2+ reuptake into the SR through SR Ca2+-ATPase (SERCA2a) and, via sarcolemmal Na+/Ca2+ exchanger, lowers intracellular Ca2+ concentration, thereby causing dissociation of Ca2+ from troponin C and resulting in cardiac relaxation (Figure 1).2 Alterations in EC coupling play a critical role in the pathophysiology and electrical remodeling in human HF. This review focuses on the contributions of abnormal calcium cycling protein handling to the deterioration of ventricular myocyte contractility in the failing heart.
Figure 1.
Excitation-contraction signaling in cardiomyocytes. Ca2+ influx through L-type Ca2+ channels. AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; Gi, inhibitory G protein; Gs; stimulatory G protein; I-1, protein phosphatase inhibitor-1; NCX, sodium–calcium exchanger; PKA, protein kinase A; PLB, phospholamban; PP1, protein phosphatase 1; RyR, ryanodine receptor; SERCA2a, sarco/endoplasmic reticulum Ca2+-ATPase 2a; β-AR, β-adrenergic receptor.
Alteration of protein calcium cycling in HF
The pathophysiologic effects of altered calcium homeostasis result from either an upregulation or a downregulation of calcium cycling proteins; these effects are categorized as ventricular remodeling or electrical remodeling, a series of arrhythmogenic effects that involves prolongation of both the duration of the action potential and the Ca2+ signal.2 The most notable EC coupling abnormalities are (i) a decrease in the Ca2+ re-entry mechanism into SR, predominantly regulated by the activity of SERCA2a, and (ii) dysregulated SR Ca2+ release from RyRs (Figure 1).
β-Adrenergic receptors
Cardiac sympathetic function plays an important role in the regulation of heart function and has been studied extensively in recent decades. The human heart expresses two subtypes of adrenergic receptors (ARs): β1 and β2.3 Both subtypes can couple to stimulatory G proteins to activate adenylyl cyclase, increase cyclic adenosine monophosphate (cAMP) production, and activate protein kinase A (PKA). PKA phosphorylates at least three key Ca2+ regulators: (i) L-type Ca2+ channels, (ii) RyR, which enhances Ca2+ influx and increases the velocity and the amplitude of SR Ca2+ release and therefore the contractility, thereby mediating the inotropic and chronotropic effects of βARs,4 and (iii) phospholamban (PLB; in serine 16), which relieves SERCA2a inhibition and contributes to SR Ca2+ reuptake, thereby mediating the lusitropic effect of βARs.5 The β1AR subtype is implicated in dysfunction of the adrenergic system in HF.6 Studies in mice have shown that overexpression of β1ARs causes early hypertrophy and interstitial fibrosis, followed by marked cardiac dysfunction with aging.7,8 By contrast, transgenic mice overexpressing β2ARs at higher absolute levels did not develop cardiomyopathy with age.9 Moreover, adenovirus-mediated overexpression of β2ARs resulted in improved ventricular function and functional recovery of unloaded HF in a rabbit model.10 β2 agonists have also been shown to have a beneficial effect on left ventricular (LV) remodeling after myocardial infarction in a rat model.4 Furthermore, stimulation of cardiac myocytes with β2-agonists appeared to provide protection against apoptosis.11 The reported differences between the effects of β1- and β2-receptor overexpression are remarkable. Given that both subtypes activate cAMP signaling, the observed differences must be due to nonclassical, receptor-specific pathways such as β2-receptor coupling to protein G (Gαi) and mitogen-activated protein kinases. In addition, compartmentation of cAMP signaling may be responsible for differences between β1- and β2-receptor-generated cAMP.6 Moreover, the remaining βARs are desensitized in the failing heart because of increased levels of G-protein-coupled receptor kinase activity. Inhibition of upregulated G-protein-coupled receptor kinase activity has been proposed as a therapeutic strategy for HF.6
RyR
RyR2 is a macromolecule complex; its phosphorylation level is most likely caused by changes in the local complex of kinases (PKA, Ca2+-calmodulin-dependent protein kinase (CaMKII)), phosphatases (PP1 and PP2A), and phosphodiesterase 4D3.6 Alteration in RyR2 and associated molecules can cause functional and/or structural changes in the heart, leading to HF and sudden cardiac death. Marks and colleagues reported that chronic hyperphosphorylation of RyR2 on serine 2809 by PKA is characterized by dissociation of calstabin 2 from RyR, followed by instability of RyR2.12 This results in a diastolic SR Ca2+ leak that depletes the SR of Ca2+ and contributes to impaired contractility. Indeed, mice engineered with RyR2 lacking the PKA phosphorylation site are protected from HF progression after myocardial infarction.13 Recently, a mouse model mimicking chronic PKA hyperphosphorylation of RyR2 demonstrated the mechanism through which β-blockers improve cardiac function in HF.14 It has been suggested that in failing hearts chronic PKA hyperphosphorylation can be sustained by reducing levels of phosphatase and phosphodiesterase 4D3 within the RyR2 complex.6 Phosphodiesterase 4D deficiency in the RyR complex promotes HF and arrhythmias.15 Finally, several groups have shown that CaMKII is also critical for RyR phosphorylation on serine 2814/2815, which leads to the SR Ca2+ leak.6 CaMKII expression and activity are upregulated in human HF and in animal models.6 The absence of CaMKIIδ significantly attenuated the development of pressure overload-induced HF and improved survival, cardiomyocyte apoptosis, and fibrosis. These findings suggest that CaMKIIδ-mediated changes in Ca2+ handling, including phosphorylation of RyR2 at the CaMKII site and increased Ca2+ leak in the diastolic SR, underlie the decompensation process from cardiac hypertrophy to HF.16 There is ongoing debate over the relative importance of PKA as compared with CaMKII in the regulation of RyR2 in the heart.
SERCA2a
In healthy human hearts, 75% of Ca2+ is removed by SERCA2a and ~25% by the Na+/Ca2+ exchanger. The small size and slow decay of Ca2+ transient in the failing heart has been shown to be related to reduced Ca2+ transport by SERCA2a.17 Abnormal Ca2+ handling in failing hearts is caused, in part, by decreases in SERCA2a mRNA, protein, and/or activity in animal models and also in HF in humans regardless of the etiology of the HF.17 Restoration of SERCA2a expression and increasing the activity level of SERCA2a in experimental models of HF have received a great deal of attention in the past few years, by our group and others. Improved contraction and accelerated relaxation of the heart have been shown in mice overexpressing cardiac SERCA2a.18 In neonatal rat cardiomyocytes with normal or depressed SERCA2a expression, adenovirus-mediated transfer of SERCA2a resulted in enhanced SR Ca2+ uptake and accelerated decay of Ca2+ transients.19,20 Furthermore, catheter-based transfection with adenovirus encoding SERCA2a restored LV cardiac function in rats that were in transition to HF.21,22 In addition, SERCA2a gene transfer restored energetic functions and decreased ventricular arrhythmia in a rat model of ischemia and improved LV mechanical and energetic functions in diabetes-induced HF in rats.22,23 Long-term overexpression of SERCA2a achieved by slow, selective intracoronary infusion of adeno-associated virus serotype 1 (AAV1) and AAV6 carrying SERCA2a resulted in successful transduction (increased SERCA2a protein and mRNA levels), improved ventricular remodeling, preserved systolic function, and increased coronary blood flow in an overload model of HF24,25 and also in an ischemic model of HF26 in swine. A major advantage of SERCA2a overexpression is its ability to exert beneficial effects on heart function with both long- and short-term expression. Two clinical trials using AAV encoding SERCA2 are under way: CUPID (Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease) and a phase II, randomized, double-blinded, placebo-controlled study, started in 2007, that is using AAV1–SERCA2a (Mydicar; Celladon, La Jolla, CA) in patients with congestive HF. The results of the phase I study showed an acceptable safety profile with an improvement of LV function and remodeling.27,28 In the phase II trial, 39 patients with advanced HF were randomized to receive an intracoronary low dose (6 × 1011 DNase-resistant particles), middle dose (3 × 1012 DNase-resistant particles), or higher dose (1 × 1013 DNase-resistant particles) of AAV1–SERCA2a or a placebo. At 6 months, the patients who received AAV1–SERCA2a demonstrated improvement or stabilization in NYHA class, MLWHFQ, 6MWT, VO2 max, NT-proBNP levels, and LV end-systolic volumes. Significant increases in time to adjudicated cardiovascular events and a decreased frequency of cardiovascular events per patient were observed to be associated with AAV1–SERCA2a. No increases in adverse events, disease-related events, laboratory abnormalities, or arrhythmias were observed in patients treated with AAV1–SERCA2a relative to those who received placebo.17 Other clinical trials to study the effects of overexpression of SERCA2a include one in the United Kingdom in patients with ischemia who have been put on an LV assist device using AAV6–SERCA2a or saline and a phase II single-center, double-blind, randomized placebo-controlled, parallel study that will be carried out at the Institute of Cardiology Pitié-Salpêtrière, Paris, France, with the primary objective of investigating the impact of AAV6.CMV–SERCA2a on cardiac remodeling parameters in patients with severe HF.29
PLB
PLB is an SR protein containing 52 amino acids that associates with SERCA2a and inhibits the Ca2+ transport rate of SERCA2a in its unphosphorylated state, whereas PLB phosphorylation by either PKA or CaMKII reverses this inhibition.6 An increase in PLB/SERCA2a stoichiometry, the basal level of PLB phosphorylation, or the ability of βARs to mediate PLB phosphorylation could contribute to a decrease in SR Ca2+ concentration and therefore Ca2+ homeostasis in HF. In failing hearts, the phosphorylation levels of PLB at serine 16 and threonine 17 are decreased.6 Using transgenic and gene transfer approaches, increasing the levels of PLB relative to SERCA2a in isolated cardiomyocytes has shown a significant alteration in intracellular Ca2+ mobilization and prolongation of the relaxation phase of the Ca2+ transient, decrease in Ca2+ release, and increase in resting Ca2+ concentration.6,17 On the other hand, inhibition of the effects of PLB is a promising approach to target the Ca2+ handling pathway in HF. Indeed, decreasing PLB expression by gene transfer of a dominant negative PLB mutant in a large animal HF model or by PLB knockout mice, PLB antisense RNAs, and intracellular inhibitory PLB antibodies has been shown to prevent the development of HF and to restore cardiac function5; these effects can be attributed to increased SERCA2a activity and a higher SR Ca2+ load. Successful treatment of HF was recently demonstrated in a rat model of aortic banding by RNA interference targeting PLB. RNA interference against PLB for long-term treatment in an HF animal model using rAAV9 showed that PLB protein concentration was decreased significantly, resulting in restoration of cardiac function and reduction in pathological hypertrophy, dilation, and fibrosis.30 These findings support the notion that targeting PLB can enhance cardiac contractility. PLB ablation also provided evidence that targeting PLB may rescue HF by increasing SR Ca2+ uptake and enhancing contractile performance.31 Recently, it has been reported that CaMKII-TG–PLB-KO crossbred mice demonstrated enhanced SR Ca2+ uptake through PLB ablation; improvements were also reported in myocyte Ca2+ transients in transgenic mice with CaMKII-mediated HF. However, the KO–TG mice showed exaggerated HF, with a more rapid onset of lethality and a further decrease in contractile function. The enhanced SR Ca2+ content exacerbates the already high level of diastolic Ca2+ spark activity, potentially increasing arrhythmias and further worsening overall heart function.32 The data are consistent with the hypothesis that, in the face of phosphorylation-activated RyR2 channels, depletion of Ca2+ stores through PLB ablation or during sympathetic activation can exacerbate the SR Ca2+ leak, thereby increasing mitochondrial Ca2+-mediated cell death or activating other Ca2+-dependent processes that contribute to cardiac dysfunction.32 Moreover, PLB mutations that do not directly influence the PLB–SERCA interaction may indirectly contribute to the development of HF.6 Also, human mutations encoding a premature stop codon (with no detectable PLB protein) were reported to cause hypertrophy in heterozygous individuals and severe dilated cardiomyopathy in homozygous individuals.6 Another PLB mutation (deletion of arginine in position 14) was identified in a family suffering from severe HF and was subsequently shown to encode a super-inhibitory PLB.33 These findings demonstrate that, in contrast to mice (in which PLB deficiency enhances myocardial function without adverse effects), PLB is essential for cardiac health in humans, and its absence results in lethal HF.
Protein phosphatase Inhibitor 1 (I-1)
I-1, a ubiquitously expressed 28-kDa protein, is a potent and highly specific inhibitor of phosphatase 1 (PP1) activity when it is phosphorylated by cAMP-dependent PKA. In the heart, I-1 has been associated with Ca2+ homeostasis and contractile function. In particular, upon stimulation of the β-adrenergic axis, PKA phosphorylates threonine 35 in I-1, resulting in PP1 inhibition and amplification of the contractile response.34 I-1 has been shown to be markedly downregulated in the failing human heart; this finding is consistent with increased PP1 activity and decreased PLB phosphorylation.35,36 Overexpression of the PP1 catalytic subunit in mice demonstrated significantly decreased phosphorylation of PLB and depressed cardiac function.36 Ablation of I-1 was associated with depressed basal cardiac function, and, in work-performing hearts, it was associated with a modest decrease in basal contractile parameters.36 Cardiac-specific overexpression of a truncated, mutated, and constitutively active form of I-1 (I-1c; T35D) restored contractile properties in failing rat hearts37 and in failing human cardiac myocytes under isoproterenol treatment.36 Furthermore, infection of adult and neonatal rat cardiac myocytes with an adenovirus encoding the full-length I-1 was associated with a marked increase in PLB phosphorylation and cardiac contractility.38 Recently, it has been shown that inducible expression of constitutively active I-1 enhances basal cardiac function and protects against ischemia/reperfusion injury in a mouse model.39 These data suggest that increased I-1 activity enhances Ca2+ cycling and improves mechanical recovery as well as cell survival after an ischemic insult. However, a recent study reported that, although I-1c overexpression improved cardiac contractility in young mice at rest, it was deleterious and arrhythmogenic after adrenergic stress and with aging.40
In summary, dysregulation of Ca2+ cycling proteins plays an important role in the pathophysiology of HF. Therefore, the evolution of our understanding of HF and correction of calcium cycling proteins represents a major step toward new therapies. Reversal of HF by gene transfer was made possible through the interface between basic science and clinical investigations. Pursuing this collaborative process promises future progress in our ability to manage HF.
ACKNOWLEDGMENTS
This work was supported by NIH KO1HL 103176 (L.H.) and NIH R01 HL093183, HL088434, HL071763, HL080498, HL083156, and P20HL100396 (R.J.H.). The authors gratefully acknowledge Irene C. Turnbull for her editing assistance.
Footnotes
CONFLICT OF INTEREST
R.J.H. is the scientific cofounder of Celladon, with plans to commercialize AAVI–SERCA2a for the treatment of heart failure. L.H. declared no conflict of interest.
References
- 1.Lloyd-Jones D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 3.Brodde OE, Bruck H, Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. J. Pharmacol. Sci. 2006;100:323–337. doi: 10.1254/jphs.crj06001x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 5.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin. Biol. Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lompré AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation. 2010;121:822–830. doi: 10.1161/CIRCULATIONAHA.109.890954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisognano JD, et al. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J. Mol. Cell. Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 9.Liggett SB, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 10.Tevaearai HT, Eckhart AD, Walton GB, Keys JR, Wilson K, Koch WJ. Myocardial gene transfer and overexpression of beta2-adrenergic receptors potentiates the functional recovery of unloaded failing hearts. Circulation. 2002;106:124–129. doi: 10.1161/01.cir.0000020220.79105.fd. [DOI] [PubMed] [Google Scholar]
- 11.Communal C, Colucci WS. The control of cardiomyocyte apoptosis via the beta-adrenergic signaling pathways. Arch. Mal. Coeur Vaiss. 2005;98:236–241. [PubMed] [Google Scholar]
- 12.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 13.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc. Natl. Acad. Sci. USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan J, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and ß-adrenergic receptor blockade in mice. J. Clin. Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehnart SE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling H, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J. Clin. Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawase Y, Ladage D, Hajjar RJ. Rescuing the failing heart by targeted gene transfer. J. Am. Coll. Cardiol. 2011;57:1169–1180. doi: 10.1016/j.jacc.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker DL, et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ. Res. 1998;83:1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- 19.Giordano FJ, He H, McDonough P, Meyer M, Sayen MR, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation. 1997;96:400–403. doi: 10.1161/01.cir.96.2.400. [DOI] [PubMed] [Google Scholar]
- 20.Hajjar RJ, Kang JX, Gwathmey JK, Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto MI, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl. Acad. Sci. USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Monte F, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakata S, et al. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1204–H1207. doi: 10.1152/ajpheart.00892.2006. [DOI] [PubMed] [Google Scholar]
- 24.Kawase Y, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Hadri L, et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol. Ther. 2010;18:1284–1292. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeri R, et al. Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circ. Heart Fail. 2010;3:627–634. doi: 10.1161/CIRCHEARTFAILURE.109.891184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajjar RJ, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J. Card. Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Jaski BE, et al. Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J. Card. Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaanine AH, Kalman J, Hajjar RJ. Cardiac gene therapy. Semin. Thorac. Cardiovasc. Surg. 2010;22:127–139. doi: 10.1053/j.semtcvs.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suckau L, et al. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minamisawa S, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, et al. Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ. Res. 2010;106:354–362. doi: 10.1161/CIRCRESAHA.109.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haghighi K, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J. Mol. Cell. Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc. Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Carr AN, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol. Cell. Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pathak A, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ. Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 38.El-Armouche A, et al. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003;17:437–439. doi: 10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- 39.Nicolaou P, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ. Res. 2009;104:1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wittköpper K, et al. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J. Clin. Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]