Abstract
Background Context
Primary care clinicians need to identify candidates for early interventions to prevent patients with acute pain from developing chronic pain.
Purpose
We conducted a 2-year prospective cohort study of risk factors for the progression to chronic pain and developed and internally validated a clinical decision rule (CDR) that stratifies patients into low, medium and high-risk groups for chronic pain.
Study Design/Setting
Prospective cohort study in primary care.
Patient Sample
Patients with acute low back pain (LBP; ≤30 days duration)
Outcome measures
Self-reported perceived non-recovery and chronic pain.
Methods
Patients were surveyed at baseline, 6 months and 2 years. We conducted bivariate and multivariate regression analyses of demographic, clinical and psychosocial variables for chronic pain outcomes, developed a CDR and assessed its performance by calculating the bootstrapped areas under the receiver operating characteristic curve (AUC) and likelihood ratios. This study was supported by NIH/NCCAM grants K23 AT002298, R21 AT004467, NIH/NCCAM K24 AT007827, the Research Evaluation and Allocation Committee (REAC) of the University of California San Francisco, and the Mount Zion Health Fund, San Francisco. The funding agencies played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors report no conflict of interests.
Results
605 patients enrolled. 13% had chronic pain at 6 months, 19% at 2 years. An eight-item CDR was most parsimonious for classifying patients into three risk levels. Bootstrapped AUC was 0.76 (0.70–0.82) for the 6-month CDR. Each 10-point score increase (60-point range) was associated with an odds ratio of 11.1 (10.8–11.4) for developing chronic pain. Using a <5% probability of chronic pain as the cutoff for low risk and a >40% probability for high risk, likelihood ratios were 0.26 (0.14–0.48) and 4.4 (3.0–6.3) for these groups, respectively.
Conclusions
A CDR was developed that may help primary care clinicians classify patients with strictly defined acute LBP into low, moderate and high-risk groups for developing chronic pain and performed acceptably in 1,000 bootstrapped replications. Validation in a separate sample is needed.
Keywords: Low back pain, chronic pain, acute pain, clinical decision rule, prediction, primary care
INTRODUCTION
Although most patients presenting with an episode of acute low back pain (LBP) in primary care will recover in six to eight weeks with or without medical intervention,1,2 those who subsequently develop chronic pain suffer considerably,3 often are difficult to treat, and account for most LBP-related health expenses.4 Primary care clinicians need decision support to identify candidates for early interventions for secondary prevention of chronic pain. Previous studies have identified risk factors for chronic pain, and have attempted to develop clinical decision rules for the primary care setting.5,6 The most important are the STarT-Back developed in the UK7,8 and the Chronic Pain Risk Screener (CPRS) developed in the US.9 The STarT-BACK and several instruments developed in Europe (Örebro Musculoskeletal Pain Screening Questionnaire (ÖMPSQ)10,11, Kiel Pain Inventory and Avoidance-Endurance Questionnaire, 12,13 and Heidelberger Kurz-Fragebogen (HKF).14) have not been evaluated in the US. Other limitations of the latter instruments are that they were not developed or validated in primary care patients and used delayed return-to-work as chronic pain outcomes, which only captures a subset of patients taking sick leave.
Both the STarT-BACK and CPRS have been well validated in patients shortly following an index visit at a primary care office.15 However, these index visit patients included patients with a wide range of LBP duration; less than half suffered from acute LBP. Because patients who suffer LBP for more than 3 months already have a much worse prognosis, instruments that work for this population may not perform as well in patients with acute LBP. Hence, clinicians need a tool that only addresses the prognosis of patients with truly acute LBP.1
We therefore conducted a prospective cohort study to investigate the prognosis of patients with strictly defined acute LBP16, and whether we can identify early risk factors that can help primary care clinicians determine a more accurate prognosis. If available such risk stratification would be feasible for primary care clinics and could potentially support physicians in treatment allocation decisions. We included questionnaire items representative of all risk factors known at the time of the cohort’s inception and set out to develop a novel clinical decision rule (CDR).
METHODS
Patient Selection
The Prognosis of Pain (POP) study was a 2-year longitudinal telephone survey of 18–70 year old members of Kaiser Permanente, Northern California, the largest integrated health plan in its region with 2.4 million adult members at the time. Acute LBP was defined as back pain between the rib cage and buttocks of less than one month that was severe enough to seek medical care and was not preceded by any other episodes of LBP in the past year. The 1-month criterion for acuteness of pain was chosen in part for pragmatic reasons, as we found that the time from scheduling a doctor’s visit to being seen might be more than two weeks from the date of first pain onset. Patients were included if they spoke English and had no fever, history of cancer, chronic inflammatory disease, previous spine surgery, fibromyalgia, chronic pain conditions, disabling psychiatric diseases, or ongoing prescriptions for narcotics prior to the LBP episode. Patients with sciatica (i.e. LBP radiating below the knee), were not excluded.
A computer program screened electronic medical records to identify patients seen the day before for LBP, and a written invitation was sent by mail to join the study. This invitation offered a $20 gift certificate and did not reveal the inclusion criterion of pain duration; it therefore prioritized minimization of false reporting over larger numbers of ineligible respondents. Respondents were interviewed over the phone at baseline and 6 months. For the 2-year follow-up, participants, when reached (maximum of 3 attempts), were given a choice between a phone interview and an internet-based survey using SurveyGizmo (http://www.surveygizmo.com).17 The study was approved by the Institutional Review Boards of the University of California, San Francisco and Kaiser Permanente. Two follow-up survey time points at 6 months and 2 years allowed us to determine consistency of predictors over time. The surveys were conducted between February 2008 and November 2010.
Baseline Measures
In addition to the typical demographic items (age, sex, ethnicity, foreign born, education and income) we asked about marital status, employment status, heavy or monotonous work, job satisfaction, and smoking. The following clinical parameters were assessed at baseline: duration of current episode; history of prior episodes; pain-free interval before current episode; pain location(s); sciatica; pain intensity by 11-point numeric rating scale (NRS) as average, worst, and most tolerable pain or average bothersomeness; McGill Pain Questionnaire;18 Roland-Morris Disability Questionnaire (RMDQ);19 and days on sick leave and of reduced daily activities. The complete 24-item ÖMPSQ,11 10-item HKF14 and the 4-item Perceived Stress Scale (PSS-4)20 were included. Additional psychological predictor variables were selected from validated instruments according to strong factor loadings and face validity (Table 1).
Table 1.
Prediction Items
| Numbers in parenthesis refer to item numbers in instruments. L: Linton’s ÖMPSQ11; H: Heidelberger Kurzfragebogen14,51 | |
|---|---|
| Clinical questions related to LBP | |
| When did your pain start? (L7) | Days |
| Did it ever go below the knee? | y/n |
| Does it today go below the knee most of the time? | y/n |
| How would you rate the average (H5) pain you have had during the past week? (L8) | 0–10 |
| How would you rate the pain you have had during the past week when it was most tolerable? (H6) (“…the pain you had since it began?” if answer to L7 is <1 week) | 0–10 |
| How much pain would you be willing to tolerate and still consider the therapy successful? (H7) | 0–10 |
| Are you on sick leave because of pain? | y/n |
| If yes: How many days? (L5) | Days |
| Have you been on sick leave before for back pain? | y/n |
| On how many days during the past week did back or leg pain (sciatica) cause you to cut down for more than half of the day on things you usually do? | Days |
| On how many days during the past week did back or leg pain (sciatica) cause you to stay in bed for more than half the day? | Days |
| On how many days during the past week did back or leg pain (sciatica) cause you to loose days from work or school for more than half the day? | Days |
| Do you have pain in other parts of your body in addition to your back pain? (H4) | y/n |
| Do you have pain in the □ neck □ shoulders □ upper back | |
| McGill Pain Questionnaire18 | |
| Roland Morris Disability Questionnaire (RMDQ) 52 for function or disability | 0–24 |
| Do you smoke? | y/n |
| If yes: More than 10 cigarettes per day? | y/n |
| ÖMPSQ and HKF-R10 items11,51,53 | |
| Is your work heavy or monotonous? (L4) | 0–10 |
| Based on all the things you do to cope or deal with your pain, on an average day, how much are you able to decrease it? (L11) | 0–10 |
| I can do light work for an hour. (L12) | 0–10 |
| I can walk for an hour (L13) | 0–10 |
| I can do ordinary household chores (L14) | 0–10 |
| I can do the weekly shopping (L15) | 0–10 |
| I can sleep at night (L16) | 0–10 |
| How tense or anxious have you felt in the past week? (L17) | 0–10 |
| How much have you been bothered by feeling down or depressed in the past week? (L18/H10a) | 0–10 |
| Did you cry a lot or feel like crying in the past week? (H10b) | 0–10 |
| I still enjoy doing things I liked before (H10e) | 0–10 |
| In your view, how large is the risk that your current pain may become persistent (may not go away)? (L19) | 0–10 |
| In your estimation, what are the chances that you will be working in 6 months? (L20) | 0–10 |
| If you take into consideration your work routines, management, salary, promotion possibilities, and work mates, how satisfied are you with your job? (L21) | 0–10 |
| Physical activities make my pain worse (L22) | 0–10 |
| In your view from past experience, does massage bring pain relief? (H8) | y/n/dk |
| An increase in pain is an indication that I should stop what I am doing until the pain is decreasing (L23) | 0–10 |
| I should not do my normal work with my present pain (L24) | 0–10 |
| If you were aware of pain during the last week, how often did you have the following thoughts and feelings?(H9) | |
| g I cannot stand it any longer! | 0–10 |
| h I wonder whether I have the same bad disease as… | 0–10 |
| m How much longer do I have to endure this pain? | 0–10 |
| n I wonder whether there is a bad disease behind all of this pain? | 0–10 |
| 2-item Version of Coping Strategies Questionnaire (CSQ-2i)21 | |
| Parameters: diverting attention (1 mental positive thinking); reinterpreting pain sensations + detachment (2, 9); catastrophizing (3 magnification); ignoring sensations (4, 11); praying (5); coping self-statements (6, 13 challenge appraisal, endurance); increased behavioral activity (7, 14 active/passive distraction). “People who experience pain have developed a number of ways to deal with their pain. When you feel pain, how much do you do the following: | |
| 1. I think of things I enjoy doing | 0–10 |
| 2. I just think of it as some other sensation, such as numbness | 0–10 |
| 3. It is terrible and I feel it is never going to get any better | 0–10 |
| 4. I don’t pay any attention to it | 0–10 |
| 5. I pray for the pain to stop | 0–10 |
| 6. I tell myself I can’t let the pain stand in the way of what I have to do | 0–10 |
| 7. I do something active, like household chores or projects | 0–10 |
| 9. I pretend it is not a part of me | 0–10 |
| 11. I ignore it | 0–10 |
| 13. I see it as a challenge and don’t let it bother me | 0–10 |
| 14. I do something I enjoy, such as watching TV or listening to music | 0–10 |
| More coping items from CPCI25 on “asking for assistance” (42); “seeking social support” (6); CRSS/KPI28,54 (68) (introduction as in CSQ) | |
| CPCI 42. I ask for help in carrying, lifting or pushing something | 0–10 |
| CPCI 6. I make arrangements to see a friend or family member | 0–10 |
| KPI 68. I have somebody console me | 0–10 |
| KPI 26+66. I talk with my partner or family | 0–10 |
| Selected PCS-items23 for catastrophizing: magnification (13), rumination (9), and helplessness (2) | |
| “If you were aware of pain during the last week, how often did you have the following thoughts and feelings?” | |
| 13) I wonder whether something serious may happen | 0–10 |
| 9) I can’t seem to keep the pain out of my mind | 0–10 |
| 2) I feel I can’t go on | 0–10 |
| Two work-related fear avoidance items from FABQ22: | |
| 3) Physical activity such as bending, lifting, walking or driving might harm my back | 0–10 |
| 11) My normal work might harm my back | 0–10 |
| 2-item version of self-efficacy for pain subscale21 from Arthritis Self-Efficacy Scale (ASES) | |
| As of now, how certain are you that you can decrease your pain quite a bit? | 0–10 |
| As of now, how certain are you that you can continue most of your daily activities? | 0–10 |
Perceived Stress Scale (4-item version PSS-4)20
| |
To avoid overextensive participant burden from lengthy questionnaires we limited the survey to selected items expected to perform reasonably well when a reduced item set was needed.21 In addition to the psychological items in ÖMPSQ and HKF, we included another fear-avoidance beliefs item from the Fear-Avoidance Beliefs Questionnaire (FABQ),22 another catastrophizing item from the Coping Strategies Questionnaire (CSQ)21 and two from the Pain Catastrophizing Scale (PCS)23. As additional coping style items, we included ignoring and positive distracting using single items from the CSQ, seeking instrumental and emotional support using four items from CPCI24–26 and KPI 27–29 and denial of stress using two items from Brief-COPE.30 The 2-item version of the self-efficacy for pain subscale21 from Arthritis Self-Efficacy Scale was also included. Anxiety and depression were assessed by multiple ÖMPSQ and HKF items, and positive affect by one item from the CES-D31 (Table 1).
Follow-Up Outcome Measures
No gold standard or international consensus exists regarding the outcome definition for chronic LBP in cohort studies. Following recommendations from expert LBP epidemiologists in the Netherlands,32 we applied a previously published primary outcome measure that combines a lack of perceived recovery (less than “much improved” on a 6-point Likert Perceived Recovery Scale)33 with current pain intensity of 3 or more on 0–10 Numeric Rating Scale.32,34 Its accuracy was assessed for this population sample in a prior study.34 In an exploratory fashion we also used a Grade 2 or higher chronic pain level according to the validated Graded Chronic Pain Scale (GCPS) by von Korff (this instrument yields a 4-grade-level chronic pain score as a function of pain intensity and pain-related disability for the past 6 months), but only for 2-year follow-up analyses, as it includes recall of the acute phase LBP at onset.9
Statistical Analyses
All analyses were conducted using Stata.35 We proceeded in a series of analytic steps from bivariate to multivariate analysis of predictor variables, data and consensus-driven decisions for scoring and cut-off, and the calculation of areas under the receiver operating characteristic curve (AUC) and likelihood ratios for the resulting CDR.
Step 1 was raw bivariate logistic regression analysis of predictor variables for our primary outcome at 6 months and 2 years. We used individual items assessed at baseline as predictor variables. In addition, we compared the odds ratios for predictors of the primary 2-year outcome with those of the 2-year GCPS. We selected variables that were associated with our outcome at p <0.1 consistently at both time points for inclusion in Step 2.
Step 2 used the variables selected in Step 1 in multivariate logistic regression models and proceeded to stepwise backward elimination of the least supported variables. In addition, we analyzed the correlations between predictor variables and eliminated collinear redundant variables (r >.35) to arrive at the most parsimonious sets, separately for the 6-month and the 2-year predictions.
Step 3 used the beta-coefficients of each of the final multivariable regression models (one predicting 6-month outcomes, one predicting 2-year outcomes), multiplied by 10 and rounded to the closest half integer, to create two scoring rules. The performance of each scoring rule was assessed by bootstrapped analysis with 1,000 replications and calculation of the AUROCC and associated 95% confidence intervals (CIs).
Step 4 determined the number of patients who were positive or negative on our outcome measures for each point score. We then empirically identified reasonable cutoffs for low, moderate and high-risk groups based on discussion with content experts. A minimal risk group was defined as patients that have a less than 5% chance of developing chronic pain at 6 months and less than 10% at 2 years. Conversely, if a patient has a risk of 40% or more of developing chronic pain at either 6 months or 2 years, a clinician likely would consider closer follow-up office appointments and potentially more intensive early interventions to prevent chronic pain, and these were defined as a high-risk group. In the middle range, less intensive interventions may suffice until further follow-up assessments. By simple inspection of the table with the two patient groups being either positive or negative on the outcome for each score point, we determined the cut-off for the summary score and assessed the proportion of patients in each risk group.
Step 5: We calculated likelihood ratios with 95% confidence intervals (CI’s) for the classification into these three risk groups by our CDR, according to the formula by Simel and colleagues.36
RESULTS
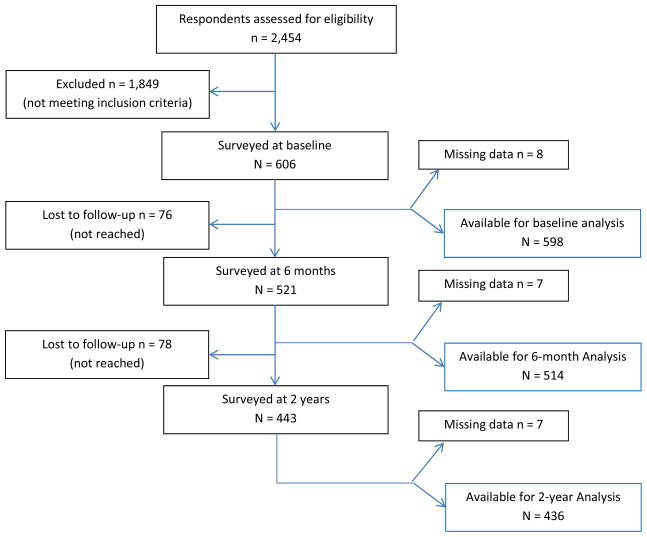
The Prognosis of Pain (POP) study enrolled 605 eligible members of Kaiser Permanente, Northern California (KPNC) from February 2008 to March 2009 (Figure 1). This represents 25% of the 2,454 respondents to invitations mailed to 42,650 patients who were seen for any kind of LBP in clinics of the health plan during the twelve months of recruitment. Overall, 521 participants (86%) responded at 6 months and 443 (73%) at the 2-year follow-up. The average age was 50.5 (±12.6) years, 56% were female, 65% Caucasian-American, 18% foreign born, 61% had a college degree, and 59% were employed full-time (for further details, see 16) The sample represented the socioeconomic and ethnic diversity of primary care patients in the San Francisco Bay Area.37 These patients sought medical care for pain of considerable intensity (average in past week 5.6 ±1.8; 2.6 ±1.8 when most tolerable; 8.6 ±1.4 when worst; 11-point NRS), bothersomeness (6.5 ±2.3) and disability (mean Roland-Morris score 15.8 ±4.7). The median duration of pain at baseline interview was 14 days; 8% had been on sick leave; and 27% had some sciatic pain to below the knee during this episode, 10% at the time of the interview. The final sample included 510 patients with complete 6-month follow-up data and 443 patients with complete 2-year data. Using our primary combined outcome criterion,34 13% of the patients (95% confidence interval [CI], 10%–16%) experienced persistent or recurrent pain at 6 months and 19% (CI, 15%–22%) at 2 years after pain onset.16 Numerous patients who self-reported as much improved at 6 months felt worse at 2 years (details in 16). Participants lost to follow-up were slightly younger and included slightly more females but did not differ in those variables that were included in the CDR.
Figure 1.
Flow Diagram
Bivariate analyses (analysis Step 1)
All variable had <2% missing responses and were used without substitution. The following 12 variables had odds ratios at significance levels of p<0.1 for our primary outcome at both time points, five were protective and seven were predictive of chronic pain. Protective were: completed college, ability to walk for 1 hour, ability to sleep tonight, coping by TV or music, and self-efficacy in ability to decrease pain; predictive were additional pain in upper back, higher level of least pain since onset, smoking, catastrophizing (2 items), expectancy of chronicity and the need to holding onto something when getting off the sofa. Additional variables that satisfied at least one of our outcome criteria and the GCPS were perceived stress, coping by ignoring, coping by prayer, belief that activity worsens pain, anxiety or tension, RMDQ items 2, 5, 18, 22, yoga at baseline, McGill overall pain intensity, worst pain since onset, sciatica since onset, African-American ethnicity, and being separated or widowed. No significant bivariate associations consistent across at least 2 outcome measures were found for age, sex, income level, born outside the US, duration of pain, sciatica at time of interview, average pain intensity since onset, pain level willing to tolerate, cut-down activity days, days in bed, days lost from work, retirement, job satisfaction, other RMDQ items, positive affect, enjoyment, positive thinking, depression, ability to do light work or household chores for 1 hour, heavy or monotonous work, multiple other pain-avoidance and catastrophizing items, coping by seeking a friend or talking with family member, staying active, detachment, reinterpretation, challenge appraisal, asking for instrumental or emotional support, other perceived stress or stress denial items.
Multivariate Analyses (analysis Step 2)
After backward elimination, eight variables remained for the 6-month prediction model and eight slightly different variables for the 2-year model. They are listed in Table 2 and discussed below. When using only these eight variables for each model, the regression models explained 16% (6-month) and 10% (2-year) of the respective outcome variance. Using Grade 2 or higher of the GCPS as outcome (instead of our primary outcome combining perceived recovery with pain intensity) at 2 years provided similar results (not shown) for included parameters, AUC, and explained variance.
Table 2.
Items used in the 6 month and 2 year risk scores
| Item | Response | 6-month model | 2-year model |
|---|---|---|---|
| Did your pain ever go below the knee during this episode of back pain? | Y/N | X | |
| Do you have additional pain in the upper back? | Y/N | X | X |
| How would you rate the pain you have had during the past week when it was most tolerable? 1 | 0–10 | X | |
| Can you sleep at night?*2 | 0–10 | X | |
| Can you walk for an hour*2 | 0–10 | X | |
| In your view, how large is the risk that your current pain may become persistent (may not go away)?2 | 0–10 | X | |
| You think it is terrible and you feel it is never going to get any better**3 | 0–10 | X | X |
| When you feel pain you ignore it**3 | 0–10 | X | X |
| You do something you enjoy, such as watching TV or listening to music**3 | 0–10 | X | X |
| In the last month, how often have you felt confident about your ability to handle your personal problems?***4 | 0–10 | X | |
| Did you complete college education (BS, BA)? | Y/N | X | X |
Item Stems:
“Could you please answer with a number on a scale from 0 - 10? The 0 means “I can NEVER do this because of pain” and the 10 means “I can ALWAYS do this without pain being a problem.”
“When you feel back pain, how much do you do the following, where a 0 indicates you never do that and a 10 indicates you always do it when you feel back pain:”
“The next question asks about your life in general, and about stress you have, not only from your back pain, but also stress from other aspects of your life including family, relationships, work, health etc. We would like for you to tell us about your feelings and thoughts during the last month. Again, use the scale from 0 to 10, where 0 is never, and 10 is always, and tell us how often you felt or thought a certain way.”
Sources:
Neubauer, E., et al., HKF-R 10 - Screening for predicting chronicity in acute low back pain (LBP): A prospective clinical trial. Eur J Pain, 2005.
Linton, S.J. and K. Hallden, Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain, 1998. 14(3): p. 209–15.
Jensen, M.P., et al., One- and two-item measures of pain beliefs and coping strategies. Pain, 2003. 104(3): p. 453–69.
Cohen, S., Perceived Stress Scale (PSS). http://www.psy.cmu.edu/~scohen/.
Point Score Creation (analysis Step 3)
Table 3 shows the beta-coefficients and odds ratios for each model. Multiplying the beta coefficients by 10 and rounding to the closest half integer created the 6-month and 2-year scoring rules. This method gives differential weights to individual predictors according to their beta-coefficients in the multivariate model. Note that multipliers for dichotomous items are based on values of 0 or 1, whereas continuous variables are multiplied by values between 0 and 10. The 6-month scoring rule ranged from −25 to 34 points. Applied to our sample, the AUC was 0.78 (95% CI 0.72–0.84; Figure 1). Applying the bootstrap procedure for 1,000 replications, a 10-point increase in the 60-point score was associated with a 11.1 odds ratio (95% CI 10.8–11.4; p <0.001) for having chronic pain 6 months after baseline. After bootstrapping, the AUC was slightly lower: 0.76 (95% CI 0.70–0.82).
Table 3.
Odds Ratios and β–Coefficients for Multivariate Regression Model for Predicting Chronic Pain at 6 Months and 2 Years and Corresponding Point Score.
| 6-month prediction model | 2-year prediction model | |||||
|---|---|---|---|---|---|---|
| Parameter | β–Coefficient | OR 6-mo (95% CI) | Point Multiplier | β–Coefficient | OR 2-y (95% CI) | Point Multiplier |
| College Education [y/n] | −.47 (−1.05–.10) | .62 (.35–1.10) | −5 | −.29 (−.83–.24) | .75 (.44–1.27) | −3 |
| Coping with TV and Music [0–10] | −.11 (−.20--.02) | .90 (.82–.98) | −1 | −.11 (−.20--.03) | .89 (.82–.97) | −1 |
| Ability to Sleep [0–10] | −.09 (−.18–.01) | .92 (.84–1.01) | −1 | |||
| Ability to Walk 1 hour [y/n] | −.05 (−.11–.02) | .95 (.89–1.02) | −.5 | |||
| Pain in Upper Back [y/n] | 1.80 (1.09–2.51) | 6.06 (2.98–12.31) | 18 | .65 (−.12–1.42) | 1.92 (.89–4.13) | 6.5 |
| Pain Below Knee [y/n] | .59 (−.01–1.19) | 1.80 (.99–3.27) | 6 | |||
| Pain Willing to Tolerate [0–10] | .16 (.00–.31) | 1.17 (1.00–1.36) | 1.5 | |||
| Expectancy of Chronic Pain [0–10] | .12 (.01–.24) | 1.13 (1.01–1.27) | 1 | |||
| Catastrophizing [0–10] | .11 (−01–.22) | 1.12 (1.01–1.24) | 1 | .08 (−.01–.17) | 1.08 (.99–1.19) | 1 |
| Coping by Ignoring Pain [0–10] | .10 (.01–.19) | 1.11 (1.01–1.21) | 1 | .15 (.06–.23) | 1.16 (1.06–1.26) | 1.5 |
| Perceived Stress 4 [0–10] | .12 (.02–.21) | 1.12 (1.02–1.24) | 1 | |||
Using the same process for creating a scoring rule for the eight strongest predictor variables at the 2-year outcome, we obtained summary scores between −18 and 28.5. Applied to our sample at the 2-year follow-up, the AUC was 0.70 (95% CI 0.64–0.76; bootstrapped 0.69; 0.62–0.75). Applying the bootstrap procedure for 1,000 replications, a 10-point increase in the score was associated with a 11.1 odds ratio (95% CI 10.7–11.5; p <0.001) for having chronic pain 2 years after baseline.
Selection of Cutpoints (analysis Step 4)
After inspection of the outcomes table for each rule, we identified optimal score cut-offs for creating the three clinically-useful risk groups at 6 months and 2 years and assessed the proportion of patients in each risk group. The results are shown in Table 4.
Table 4.
Proportion of Patients with Chronic Pain in Each Risk Group and Likelihood Ratios for Correct Risk Classification.
| Risk group (total N) | Number of Patients | Percentage with chronic back pain (95% CI) | Likelihood ratio (95% CI) | |
|---|---|---|---|---|
| with chronic back pain | without chronic back pain | |||
| 6 month model (509) | 67 | 442 | ||
|
| ||||
| Low risk (score < - 4) | 9 | 230 | 3.8 (1.7 – 7.0) | 0.26 (0.14 – 0.48) |
| Moderate risk (score -4 to 7) | 27 | 165 | 14.1 (9.5 – 19.8) | 1.1 (0.79 – 1.5) |
| High risk (score ≥8) | 31 | 47 | 39.7 (28.8 – 51.5) | 4.4 (3.0 – 6.3) |
|
| ||||
| 2 year model (440) | 82 | 358 | ||
|
| ||||
| Low risk (score < - 4) | 22 | 194 | 10.1% (6.49–15.0) | 0.50 (0.34–0.72) |
| Moderate risk (score -4 to 7) | 32 | 125 | 20.4% (14.4–27.5) | 1.12 (0.82–1.52) |
| High risk (score ≥8) | 28 | 39 | 41.8% (29.8–54.5) | 3.14 (2.06–4.78) |
Following a discussion among clinical colleagues, we assumed that a score with a predictive value of or near 5% would be a good cut-off for the lowest risk group, and that a 40% predictive value would be an appropriate cut-off for recommending further assessment and therapeutic measures. Applying these criteria to the 6-month prediction, score cutoffs were less than -4 for low-risk and above +7 for high-risk groups. The low risk group included 47% of all patients, the mid-range risk group 38%, and the high-risk group 15%. The resulting proportions of chronic pain patients in the three risk groups were 3.8%, 14.1% and 39.7%, respectively.
Applying the 2-year decision rule in the 2-year follow-up dataset, we obtained scores between −18 and 28.5 and found that relatively low scores had a higher than 5% risk of developing chronic pain. We, therefore, chose a 10% cutoff for the low-risk group, maintained the 40% cutoff for the high-risk classification, and thus classified 49% as low risk at a score of ≤1, 36% as mid-level risk at scores of >1 and <9, and 15% as high risk with scores of ≥9.
Likelihood ratios (Analysis Step 5)
Likelihood ratios for correctly classifying patient into low, medium, and high-risk categories were 0.26 (95% CI 0.14–0.48), 1.08 (0.79–1.5), and 4.35 (3.0–6.3) at six months and 0.50 (0.34–0.72), 1.12 (0.82–1.52), and 3.14 (2.06–4.78), respectively, at 2 years (Table 4).
DISCUSSION
To the best of our knowledge, this is the first attempt to develop a clinical decision rule (CDR) for the prediction of chronic LBP among patients with strictly defined acute LBP of less than four weeks duration in the US. A variety of methods exists for developing such rules.38,39 This CDR was developed using multivariable logistic regression to help primary care clinicians decide whether a patient who presents with a new episode of non-specific LBP with or without sciatica is at risk of developing chronic pain and may warrant closer follow-up and potentially a more intensive therapeutic intervention. The CDR is limited to patients who had no LBP in the previous year and never had spine surgery. Prior CDRs were developed and validated in patients with LBP of any duration; a majority of these had pain for more than 3 months and already had a higher pre-test probability for persistent pain. When a CDR constructed of items that were identical or highly similar to the 9-item STarT-Back from the UK was applied in our sample, its performance was found unsatisfactory in patients with truly acute LBP.40
The variables included in the new CDRs reflect risk factors that have been found in prior studies: College education was protective and the only significant demographic predictor.41 Pain spreading to the upper back was a consistent clinical risk factor. Sciatica42 and difficulty sleeping10 predicted poor outcomes at 6 months, the inability to walk for 1 hour10 poor outcomes at 2 years. At both follow-ups, a coping style of watching TV or listening to music43 was protective, whereas catastrophizing44 and coping with pain by ignoring13 were psychological risk factors. Five of eight predictor variables were identical for outcomes at our 2 follow-up time points, three were different. The expectancy of pain to persist was maladaptive at 6 months,45,46 while a low willingness to tolerate pain14 and perceived stress41,42 increased risk at 2 years. We do not have an explanation for the difference.
The observed likelihood ratios for the 6-month CDR of 0.26 (95% CI 0.14–0.48) for the low-risk and 4.4 (95% CI 3.0–6.3) for the high-risk classifications are moderately accurate.47 The rule is likely to be clinically useful, as almost half of all patients fell into a low-risk group that was unlikely to develop chronic pain at 6 months. Approximately 15% of patients were classified as high-risk and may warrant more intensive interventions. The remaining 38% of mid-level risk patients, assessed at an average of 2 weeks (range 2–30 days) after pain onset, had a mean risk of 14% for developing chronic pain. They may warrant closer oversight by their primary care clinician than the low-risk group, but it may be justified to suggest waiting a bit longer before prescribing more intense and costly interventions.
We previously reported for this cohort that due to the recurring course of cLBP, individuals with persistent pain at 6 months were not identical to those at 2 years, and that the proportion of persistent pain patients had increased between the two follow-up time points.16 Creating a 2-year decision rule with the 2-year follow-up made it impossible to use the 5% criterion as cutoff for low risk classification. We therefore used a 10% criterion but maintained the 40% risk for the high-risk classification cutoff. The results show that the prediction of the longer-term outcome is challenging in patients with strictly defined acute LBP. Longer-term predictions over years appear to be less precise than the prediction for 6 months, which is not surprising as the outcome is much further into the future than for the 6-month CDR.
A scoring method that assigns weights to individual predictor variables may best be used by a programmed risk classification calculator rather than by hand but it increases the precision of the prediction.38 Whereas the 6-month CDR maybe most useful for the primary care clinical practice, the 2-year CDR maybe useful for long-term clinical research. Five of the predictive items for the 6-month rule are identical with those for the 2-year rule. For validation of the rules in a separate sample and in particular for clinical application we would recommend assessing all eleven items, and then applying the relevant 8 items for 6-month predictions and the slightly different set of 8 items for a 2-year prediction.
The main limitation of our study is that we have not assessed the decision rules’ performance in a separate validation sample. The observed variance in these predictor item scores among study participants with acute LBP early into their episode is rather large and reduces their predictive power. Moreover, as shown for this cohort, a high recurrence rate leads to different individuals having persistent pain at different time points.16 This variance creates a challenge for creating a rule that performs strongly with patients where it is most needed, early in the course of a new episode of LBP.
A second limitation is that we included only questionnaire items that were known to be potentially predictive at the time of the study’s implementation. Somatization,48 reduced levels of body awareness,49 and potentially many others may be further parameters of predictive value and were not included in our questionnaire. However, we included a wide range of demographic, clinical and psychological predictor variables carefully chosen according to the best knowledge of the time.
A third limitation is that we relied on diagnostic codes from electronic medical records created by primary care providers and patient self-report. It is possible that clinical findings that indicate a more severe baseline condition, such as positive signs for spinal nerve compression or spinal claudication from spinal stenosis, can be identified as important risk factors for chronic pain at the very first onset of LBP by clinical exam and imaging studies. However, current clinical guidelines do not recommend imaging in the first weeks after new-onset LBP in patients who most likely would not need an immediate referral to a spine surgeon. Future studies would benefit from clinical exams at study entrance.
A fourth potential limitation is that the study population did not include uninsured patients; only 3% reported annual household incomes below $25,000. However, income level was not predictive of the outcome.
CONCLUSION
Despite these limitations, we conclude that our study provides a clinical decision rule that is urgently needed for one of the most frequent and most costly conditions in primary care.50 It contains 8 items for the 6-month and 8 items for the 2-year risk classification (5 are common to both) into 3 levels of risk for developing chronic pain in patients presenting in primary care with a new-onset episode of strictly defined acute low back pain. The next step is to prospectively validate this tool in an independent population.
Acknowledgments
This study was supported by NIH/NCCAM grants K23 AT002298 (Mehling), R21 AT004467 (Mehling), NIH/NCCAM K24 AT007827 (Hecht), the Research Evaluation and Allocation Committee (REAC) of the University of California San Francisco, and the Mount Zion Health Fund, San Francisco. The funding agencies played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
We would like to acknowledge the cooperation of the many patients who took time to answer our surveys in spite of being in pain and thank them. We would like to thank Timothy S. Carey, MD, MPH, for his contributions to study design, item selection and manuscript review, Alice Pressman for creating the computer program for the electronic medical records, Viranjini Gopisetty and Elizabeth Bartmess for project management, Pete Bogdanos for research assistance and the many volunteers who helped with the interviews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wolf E. Mehling, Email: mehlingw@ocim.ucsf.edu.
Mark H. Ebell, Email: ebell@uga.edu.
Andrew L. Avins, Email: andrew.avins@ucsf.edu, andy.l.avins@kp.org.
Frederick M. Hecht, Email: rhecht@php.ucsf.edu.
References
- 1.Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. Bmj. 2003;327(7410):323. doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Korff M, Saunders K. The course of back pain in primary care. Spine. 1996;21(24):2833–2837. doi: 10.1097/00007632-199612150-00004. discussion 2838–2839. [DOI] [PubMed] [Google Scholar]
- 3.Wolter T, Szabo E, Becker R, Mohadjer M, Knoeller SM. Chronic low back pain: course of disease from the patient’s perspective. Int Orthop. 2011;35(5):717–724. doi: 10.1007/s00264-010-1081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Borkan J, Van Tulder M, Reis S, Schoene ML, Croft P, Hermoni D. Advances in the field of low back pain in primary care: a report from the fourth international forum. Spine. 2002;27(5):E128–132. doi: 10.1097/00007632-200203010-00019. [DOI] [PubMed] [Google Scholar]
- 6.Borkan JM, Koes B, Reis S, Cherkin DC. A report from the Second International Forum for Primary Care Research on Low Back Pain. Reexamining priorities. Spine. 1998;23(18):1992–1996. doi: 10.1097/00007632-199809150-00016. [DOI] [PubMed] [Google Scholar]
- 7.Hill JC, Dunn KM, Lewis M, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632–641. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 8.Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560–1571. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 10.Linton SJ, Boersma K. Early identification of patients at risk of developing a persistent back problem: the predictive validity of the Orebro Musculoskeletal Pain Questionnaire. Clin J Pain. 2003;19(2):80–86. doi: 10.1097/00002508-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Linton SJ, Hallden K. Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain. 1998;14(3):209–215. doi: 10.1097/00002508-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hasenbring M. Risikofaktoren und gesundheitsfoerderndes Verhalten {Risk factors and health promoting behaviour in the transition of acute to chronic disc related low back pain} 1992. Chronifizierung bandscheibenbedingter Schmerzen. [Google Scholar]
- 13.Hasenbring MI, Hallner D, Klasen B, Streitlein-Bohme I, Willburger R, Rusche H. Pain-related avoidance versus endurance in primary care patients with subacute back pain: Psychological characteristics and outcome at a 6-month follow-up. Pain. 2012;153(1):211–217. doi: 10.1016/j.pain.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Neubauer E, Junge A, Pirron P, Seemann H, Schiltenwolf M. HKF-R 10 - Screening for predicting chronicity in acute low back pain (LBP): A prospective clinical trial. Eur J Pain. 2006;10(6):559–566. doi: 10.1016/j.ejpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Turner JA, Shortreed SM, Saunders KW, Leresche L, Berlin JA, Korff MV. Optimizing prediction of back pain outcomes. Pain. 2013;154(8):1391–1401. doi: 10.1016/j.pain.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Mehling WE, Gopisetty V, Bartmess E, et al. The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine (Phila Pa 1976) 2012;37(8):678–684. doi: 10.1097/BRS.0b013e318230ab20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.surveygizmo. 4888 Pearl East Cir. Suite 399W, Boulder, CO 80301, USA: 2010. www.surveygizmo.com. [Google Scholar]
- 18.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 19.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8(2):141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S. Perceived Stress Scale (PSS) http://www.psy.cmu.edu/~scohen/
- 21.Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One- and two-item measures of pain beliefs and coping strategies. Pain. 2003;104(3):453–469. doi: 10.1016/S0304-3959(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 22.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7(4):524–532. [Google Scholar]
- 24.Truchon M, Cote D. Predictive validity of the Chronic Pain Coping Inventory in subacute low back pain. Pain. 2005;116(3):205–212. doi: 10.1016/j.pain.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MP, Turner JA, Romano JM, Strom SE. The Chronic Pain Coping Inventory: development and preliminary validation. Pain. 1995;60(2):203–216. doi: 10.1016/0304-3959(94)00118-X. [DOI] [PubMed] [Google Scholar]
- 26.Hadjistavropoulos HD, MacLeod FK, Asmundson GJ. Validation of the Chronic Pain Coping Inventory. Pain. 1999;80(3):471–481. doi: 10.1016/S0304-3959(98)00224-3. [DOI] [PubMed] [Google Scholar]
- 27.Hallner D, Hasenbring M. Classification of psychosocial risk factors (yellow flags) for the development of chronic low back and leg pain using artificial neural network. Neurosci Lett. 2004;361(1–3):151–154. doi: 10.1016/j.neulet.2003.12.107. [DOI] [PubMed] [Google Scholar]
- 28.Hasenbring M. Kieler Schmerz Inventar (KSI) Handlungsanweisung {KPI manual} American version available. 1994. [Google Scholar]
- 29.Hasenbring M, Hallner D, Klasen B. Psychological mechanisms in the transition from acute to chronic pain: over- or underrated? Schmerz. 2001;15(6):442–447. doi: 10.1007/s004820100030. [DOI] [PubMed] [Google Scholar]
- 30.Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4(1):92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 31.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.de Vet HC, Terluin B, Knol DL, et al. Three ways to quantify uncertainty in individually applied “minimally important change” values. J Clin Epidemiol. 2009 doi: 10.1016/j.jclinepi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Beurskens AJ, de Vet HC, Koke AJ. Responsiveness of functional status in low back pain: a comparison of different instruments. Pain. 1996;65(1):71–76. doi: 10.1016/0304-3959(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 34.Mehling WE, Gopisetty V, Acree M, et al. Acute low back pain and primary care: how to define recovery and chronification? Spine (Phila Pa 1976) 2011;36(26):2316–2323. doi: 10.1097/BRS.0b013e31820c01a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stata12. StataCorp; College Station, Texas 77845 USA: 2013. http://www.stata.com. [Google Scholar]
- 36.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 37.Gordon NP. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? Unpublished Work. 2006 summary at http://www.dor.kaiser.org/external/uploadedFiles/content/research/mhs/Other_Reports/mhs_project_trends-1993-2005_notes.pdf.
- 38.Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194(3):888–894. doi: 10.1016/j.ajog.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. Jama. 1997;277(6):488–494. [PubMed] [Google Scholar]
- 40.Brage S, Sandanger I, Nygard JF. Emotional distress as a predictor for low back disability: a prospective 12-year population-based study. Spine. 2007;32(2):269–274. doi: 10.1097/01.brs.0000251883.20205.26. [DOI] [PubMed] [Google Scholar]
- 41.Hayden JA, Chou R, Hogg-Johnson S, Bombardier C. Systematic reviews of low back pain prognosis had variable methods and results-guidance for future prognosis reviews. J Clin Epidemiol. 2009 doi: 10.1016/j.jclinepi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Villarreal EA, Brattico E, Vase L, Ostergaard L, Vuust P. Superior analgesic effect of an active distraction versus pleasant unfamiliar sounds and music: the influence of emotion and cognitive style. PLoS One. 2012;7(1):e29397. doi: 10.1371/journal.pone.0029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. American journal of epidemiology. 2002;156(11):1028–1034. doi: 10.1093/aje/kwf136. [DOI] [PubMed] [Google Scholar]
- 44.Boersma K, Linton SJ. Expectancy, fear and pain in the prediction of chronic pain and disability: a prospective analysis. Eur J Pain. 2006;10(6):551–557. doi: 10.1016/j.ejpain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Linton SJ. A review of psychological risk factors in back and neck pain. Spine. 2000;25(9):1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- 46.Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet. 2005;365(9469):1500–1505. doi: 10.1016/S0140-6736(05)66422-7. [DOI] [PubMed] [Google Scholar]
- 47.Melloh M, Elfering A, Stanton TR, et al. Who is likely to develop persistent low back pain? A longitudinal analysis of prognostic occupational factors. Work. 2013;46(3):297–311. doi: 10.3233/WOR-131672. [DOI] [PubMed] [Google Scholar]
- 48.Mehling WE, Daubenmier J, Price CJ, Acree M, Bartmess E, Stewart AL. Self-reported interoceptive awareness in primary care patients with past or current low back pain. Journal of pain research. 2013;6:403–418. doi: 10.2147/JPR.S42418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 50.Roland M, Morris R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Part II: Development of guidelines for trials of treatment in primary care. Spine. 1983;8(2):141–150. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Neubauer E, Pirron P, Junge A, Seemann H, Schiltenwolf M. What questions are appropriate for predicting the risk of chronic disease in patients suffering from acute low back pain? Zeitschrift fur Orthopadie und ihre Grenzgebiete. 2005;143(3):299–301. doi: 10.1055/s-2005-836632. [DOI] [PubMed] [Google Scholar]
- 52.Neubauer E, Junge A, Pirron P, Seemann H, Schiltenwolf M. HKF-R 10 - Screening for predicting chronicity in acute low back pain (LBP): A prospective clinical trial. Eur J Pain. 2005 doi: 10.1016/j.ejpain.2005.08.002. [DOI] [PubMed] [Google Scholar]