Abstract
INTRODUCTION
Erythrocytosis is the most common dose-limiting adverse effect of testosterone therapy (TTh), but the mechanisms of T-mediated erythropoiesis remain unclear. In this study, we examine risk factors for erythrocytosis associated with TTh.
METHODS
Retrospective review of 179 hypogonadal men on TTh in a single andrology clinic was performed. Demographic data, TTh formulation and duration of treatment, and 5α reductase inhibitor (5ARI) use were assessed. Serum dihydrotestosterone (DHT), total T (TT), free T (FT), follicle stimulating hormone (FSH), luteinizing hormone (LH), Hematocrit (Hct), and lipid levels were extracted and changes during treatment determined. Spearman’s rank correlation was used to identify relationships between change in Hct (ΔHct) and study variables.
RESULTS
Of 179 patients, 49 (27%) developed a ≥10% ΔHct and 36 (20.1%) developed erythrocytosis (Hct ≥50%) at a median follow-up of 7 months. Topical gels were used by 41.3% of patients, injectable T by 52.5%, and subcutaneous pellets by 6.1%. More men who developed ΔHct ≥10% used injectable T than men with ΔHct <10% (65% vs. 48%, p=0.035), and were less likely to be on 5ARI (2% vs. 15%, p=0.017). Men with ΔHct ≥10% had higher post-treatment DHT levels (605.0 vs. 436.0 ng/dL, p=0.017) and lower LH and FSH levels than men with ΔHct <10%. Spearman’s rank correlations yielded relationships between ΔHct and post-treatment DHT (ρ=0.258, p=0.001) and TT (ρ=0.171, p=0.023).
CONCLUSION
DHT may play a role in TTh-related erythrocytosis, and monitoring of DHT levels during TTh should be considered. In men who develop erythrocytosis, 5ARIs may be therapeutic.
Keywords: Testosterone replacement, Dihydrotestosterone, Erythrocytosis
INTRODUCTION
Hypogonadism affects a growing number of men in the United States1, and as its prevalence has increased, so has the use of testosterone therapy (TTh). The beneficial effects of TTh include amelioration of hypogonadal symptoms (fatigue, erectile dysfunction and poor libido) as well as improvements in muscle mass, mood and cognitive function, bone mineral density, and reversal of the metabolic syndrome1–4. In contrast, the adverse effects of TTh include negative effects on lipids, a possible increased risk of cardiovascular disease, elevated estrogen levels, gynecomastia, local reactions, and erythrocytosis1, 3, 5–9. Of these, erythrocytosis is the most common dose-limiting adverse effect, occurring in 4–40% of men on TTh, and may worsen pre-existing vascular disease secondary to increased blood viscosity1, 2. As such, erythrocytosis secondary to TTh has been addressed with cessation or modification of treatment and periodic phlebotomy.
Despite the high incidence of TTh-related erythrocytosis, the mechanisms underlying significant hematocrit (Hct) elevations in the setting of exogenous T are poorly understood. Certainly, specific formulations, dosing, and serum T levels correlate with the development of erythrocytosis5, 10–12, and biological factors including age5, 10, 13, smoking and alcohol use10, 14, obesity15, and cardiac/lung disease16 may also play some role. Proposed mechanisms center on the actions of T on hematopoiesis in the bone marrow with previously identified links to erythropoietin stimulation17, 18, suppression of the iron regulatory peptide hepcidin13, and possible relationship with androgen receptor expression15.
The majority of these hypotheses derive from studies investigating the effects of exogenous T on hematopoiesis. However, when examining studies employing exogenous dihydrotestosterone (DHT) for androgen supplementation, elevations in Hct persist, but in the setting of suppressed plasma T levels19, 20. Limited data regarding the possible role of DHT in the development of TTh-induced erythrocytosis exist. In this retrospective study, we examine clinical factors associated with elevations in Hct, focusing on a potential role for DHT in this process in men on TTh.
METHODS
Patient Selection/Study Variables
After approval by the Institutional Review Board of Baylor College of Medicine, retrospective chart review of 245 hypogonadal men treated with TTh between 2009–2012 was performed. Of these, 66 men were excluded due to a lack of pre- or post-TTh Hct levels, being lost to follow up, or known hematologic disorders, leaving 179 men in our study. The diagnosis of hypogonadism was based on biochemical evidence of low serum T (<300 ng/dL) as well as clinical symptoms including fatigue, low energy, and worsening libido and/or erectile function. Men with pre-treatment serum T levels above 300 ng/dL, but with hypogonadal symptoms and no alternate diagnoses to explaining the symptoms, were also treated with TTh21.
After inclusion, demographic data including age, body mass index (BMI), and medical comorbidities were recorded. Testosterone formulation used for TTh, duration of TTh, change in Hct (ΔHct) levels during TTh, and concurrent use of 5α-reductase inhibitors (5ARIs) were recorded. To evaluate factors related to erythrocytosis, DHT, total T (TT), free T (FT), follicle stimulating hormone (FSH), luteinizing hormone (LH), Hct, total cholesterol (TChol), HDL and LDL cholesterol, and triglycerides (TG) drawn prior to TTh initiation and during follow up were recorded.
Data Analysis
All samples were analyzed in the Laboratory for Male Reproductive Research and Testing at Baylor College of Medicine. As the definition for erythrocytosis in the literature ranges from 50–54%, we defined erythrocytosis as Hct ≥50%, which we use in clinical practice. In this work, a Hct ≥50% was used for comparison purposes, whereas the primary analysis grouped patients based on ΔHct during TTh: 1) ΔHct ≥10% above pre-treatment values or 2) ΔHct <10%. The cutoff of 10% ΔHct was based on the finding that men with a ≥10% ΔHct were more likely to develop erythrocytosis than those with <10% ΔHct.
In a secondary analysis, patients were grouped based on whether they were taking a 5ARI during TTh. Pre- and post-treatment variables were compared between groups using Mann-Whitney U analysis for continuous variables and Chi-square or Fisher’s exact test for categorical variables. Changes in Hct, TT, FT, DHT pre- and post-treatment were also compared between groups. Spearman’s rank correlations were used to identify significant correlations between ΔHct and cohort variables during treatment. All statistical analyses were performed using SPSS for Mac Version 22 (IBM Corporation, Armonk, NY) with p<0.05 considered statistically significant.
RESULTS
Cohort Demographics
Patient characteristics of the 179 men included in the analysis are found in Table 1. The median (IQR) age within the cohort was 49 (40–60) years and BMI 28.1 (25.8–31.4) kg/m2. Within the cohort, 172 men initially presented with secondary hypogonadism, and 7 with primary hypogonadism. Seventy four (41.3%) men in our cohort used topical, 94 (52.5%) injectable, and 11 (6.1%) subcutaneous pellet T formulations. During treatment, 20 (11.2%) patients were concomitantly on a 5ARI, with 15 on finasteride and 5 on dutasteride. Men on 5ARI used 5mg daily dosing, with the exception of 2 men on 1mg of finasteride for alopecia. All men on 5ARI had been treated for at least 3 months prior to TTh initiation. The overall incidence of erythrocytosis (Hct ≥50%) within the cohort was 20.1% (36 men) after a median of 7 (3–19) months of TTh, with 27% (49 men) developing a ≥10% ΔHct during this time period.
Table 1.
Cohort Demographics
| All Patients | ↑Hct <10% | ↑Hct >10% | p-value | No 5ARI | 5ARI | p-value | |
|---|---|---|---|---|---|---|---|
| N | 179 | 130 | 49 | 159 | 20 | ||
| Age (years) | 49.0 (40.0–60.0) | 48.0(38.5–59.3) | 52.0(43.0–60.0) | 0.14 | 48.0 (39.0–59.0) | 56.0(42.8–64.7) | 0.07 |
| BMI (kg/m2) | 28.1 (25.8–31.4) | 28.3(25.8–32.1) | 28.0 (25.8–30.0) | 0.29 | 27.8(25.6–31.0) | 31.2(28.1–32.8) | 0.02 |
| Duration of TST (months) | 7.0(3.0–19.0) | 5.0(2.0–18.0) | 12.0(6.0–35.5) | 0.002 | 6.0 (2.0–19.0) | 15.0(4.8–25.5) | 0.15 |
| TST Formulation: | |||||||
| Topical | 74(41.3) | 59 (45.4) | 15(30.6) | 0.07 | 66(41.5) | 8 (40.0) | 0.90 |
| Injectable | 94 (52.5) | 62 (47.7) | 32(65.3) | 0.04 | 83 (52.2) | 11 (55.0) | 0.81 |
| Pellet | 11(6.1) | 9 (6.9) | 2(4.1) | 0.73 | 10 (6.3) | 1 (5.0) | 0.82 |
| Post-treatment Hct >50% | 36(20.1) | 16(12.3) | 20 (40.8) | O.001 | 35 (22.0) | 1 (5.0) | 0.07 |
| Prior Anabolic Steroid Use | 11(6.1) | 10 (7.7) | 1 (2.0) | 0.294 | 11(6.9) | 0(0) | 0.615 |
| 5ARI Use | 20(11.2%) | 19 (14.6%) | 1 (2.0%) | 0.02 | - | - | - |
| Comorbidities- | |||||||
| Any | 59(33.0) | 37 (28.5) | 22 (44.9) | 0.04 | 54 (34) | 5(25) | 0.42 |
| DM | 14 | 12 (9.2) | 2(4.1) | 0.36 | 10 (6.3) | 4 (20.0) | 0.06 |
| HTN | 19 | 8 (6.2) | 11 (22.4) | 0.002 | 16(10.1) | 3 (15.0) | 0.45 |
| CAD/CHF | 17 | 11(8.5) | 6(12.2) | 0.243 | 15 (9.4) | 2(10.0) | 0.935 |
| COPD | 4 | 2(1.5) | 2(4.1) | 0.03 | 4 (2.5) | 0(0) | 0.473 |
| HLD | 35 | 20 (5.4) | 15(30.6) | 0.022 | 32(20.1) | 3 (15.0) | 0.422 |
| Hypothyroid | 9 | 7 (5.4) | 2(4.1) | 0.068 | 9 (5.6) | 0(0) | 0.600 |
| OSA | 9 | 6 (4.6) | 3(6.1) | 0.763 | 8 (5.0) | 1 (5.0) | 0.995 |
| Alcohol abuse | 1 | 1 (0.8) | 0(0) | 0.538 | 1 (0.6) | 0(0) | 1.000 |
| Smoker | 26 | 18(13.8) | 8(16.3) | 0.715 | 22(13.8) | 4 (20.0) | 0.499 |
All values reported as median(IQR), or count(%) unless otherwise specified
Comparison of Men As a Function of Hct Elevation
Men with ΔHct ≥10% were of comparable age (52.0 vs. 48.0 years old, p=0.137) and BMI (28.0 vs. 28.3 kg/m2, p=0.289), but had a longer duration of TTh (12.0 vs. 5.0 months, p=0.002) than men with ΔHct <10%. Men with ΔHct ≥10% more frequently used injectable T formulations (65.3% vs. 47.7%, p=0.035), were less frequently on concomitant 5ARIs (2.0 vs. 14.6%, p=0.017) and had higher incidence of any comorbidity (44.9 vs. 28.5%), hypertension (22.4% vs. 6.2%), COPD (4.1 vs. 1.5%), and hyperlipidemia (30.6 vs. 5.4%) (p<0.05 for each).
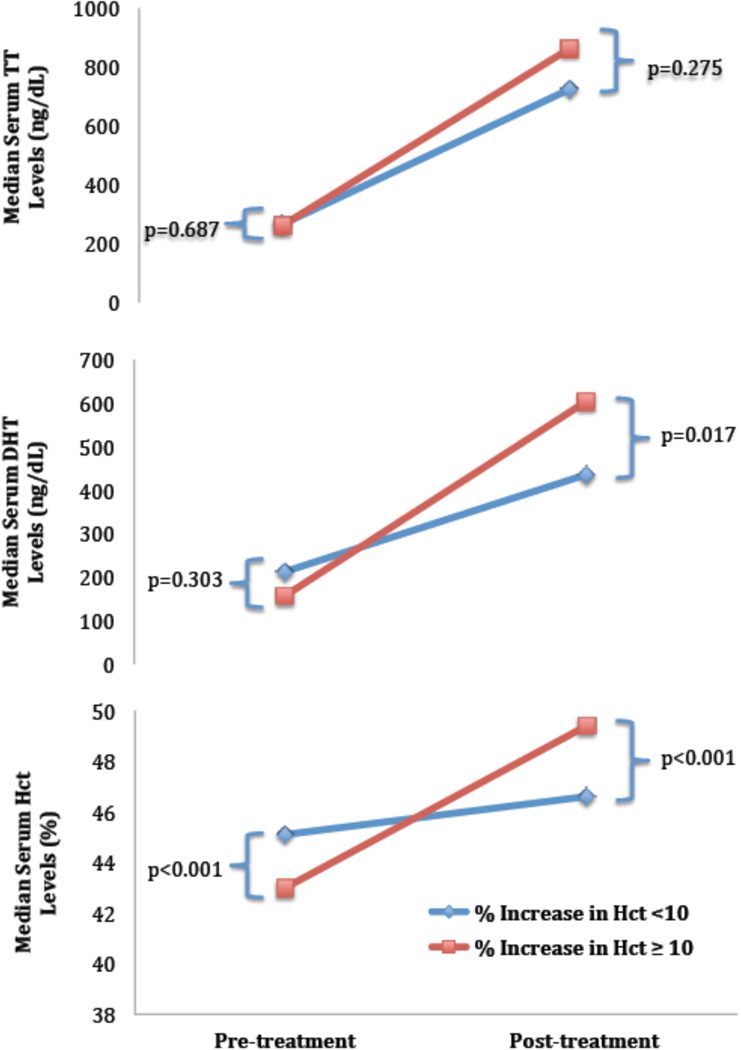
Comparing pre- and post-treatment labs in men who developed ≥10% ΔHct during TTh with those that did not yielded no significant differences between groups in pre-treatment labs, with the exception of pre-treatment Hct, which was lower in men who developed ≥10% ΔHct (43.0 vs. 45.1 %, p<0.001) (Table 2). Post-treatment, men with ≥10% ΔHct had significantly higher DHT levels (605.0 vs. 436.0 ng/dL, p=0.017), without significant differences in TT (863.0 vs. 725.0 ng/dL, p=0.275) and FT (18.4 vs. 15.2 pg/mL, p=0.268) levels (Figure 1). DHT values were higher at follow-up in men with ≥10% ΔHct, and when comparing men with ≥ and < 10% ΔHct, a relationship approaching significance was observed (354.7 vs. 215.0 ng/dL p=0.085). Changes in TT and FT were not different between the groups. Men with ≥10% ΔHct had lower post-treatment FSH (0.18 vs. 0.45 mIU/mL, p=0.012) and LH (0.19 vs. 0.30 mIU/mL, p=0.012). No significant differences in LDL, HDL, TChol, and TG levels were observed between groups.
Table 2.
Pre- and post-TTh laboratory values and magnitude change during treatment.
| Pre-Treatment | All Patients | ↑Hct <10% | ↑Hct ≥10% | p-value | No 5ARI | 5ARI | p-value |
|---|---|---|---|---|---|---|---|
| TT (ng/dL) | 264.0 (198.0–321.0) | 266.0 (203.0–322.0) | 262.5 (184.3–316.5) | 0.69 | 261.0 (191.3–320.3) | 278.0 (237.0–322.0) | 0.47 |
| DHT (ng/dL) | 210.0 (107.2–260.5) | 212.5 (98.6–267.8) | 156.5 (113.3–225.3) | 0.30 | 213.0 (128.0–273.3) | 116.0 (43.2–201.0) | 0.02 |
| Hct (%) | 44.8 (43.0–47.0) | 45.1 (43.6–47.0) | 43.0 (41.0–45.2) | <0.001 | 44.9 (43.0–47.0) | 44.3 (42.9–45.6) | 0.34 |
| Post-Treatment | |||||||
| TT (ng/dL) | 750.0 (427.5–1258.3) | 725.0 (415.0–1210.0) | 863.0 (519.0–1292.0) | 0.28 | 743.0 (427.5–1266.0) | 785.0 (441.3–1206.5) | 0.93 |
| DHT (ng/dL) | 4850 (280.0–766.0) | 436.0 (260.0–739.0) | 605.0 (354.5–872.0) | 0.02 | 536.0 (339.0–809.0) | 126.0 (69.5–253.8) | <0.001 |
| Hct (%) | 46.8 (45.1–49.4) | 46.6 (44.8–48.3) | 49.4 (46.4–51.6) | <0.001 | 47.2 (45.4–49.8) | 45.4 (43.6–46.6) | 0.77 |
| Magnitude Change | |||||||
| ΔHct (%) | 2.7 (0.6–4.6) | 1.2 (0.1–3.0) | 5.8 (5.1–7.0) | 0.001 | 3.0 (0.8–4.8) | 1.1 (0.1–2.9) | 0.013 |
| ΔDHT (ng/dL) | 224.0 (116.5–406.5) | 215.0 (152.5–570.5) | 354.7 (116.8–364.0) | 0.09 | 280.0 (145.0–502.0) | 20.8 (−25.4–103.3) | 0.007 |
| ΔT (ng/dL) | 457.5 (181.8–992.8) | 434.0 (161.8–958.0)] | 506.0 (287.8–1001.0) | 0.43 | 451.5 (202.5–962.5) | 467.0 (143.0–1128.0) | 0.89 |
| ΔFT (pg/dL) | 11.6 (4.2–22.0) | 11.2 (3.0–21.5) | 13.5 (9.0–22.9) | 0.12 | 11.6 (4.1–21.8) | 12.6 (4.6–34.6) | 0.60 |
All values reported as median(IQR)
Figure 1.
Comparison of median pre- and post-treatment TT, DHT, Hct in men as a function of ΔHct.
Comparison of Men as a Function of Concurrent 5ARI Therapy
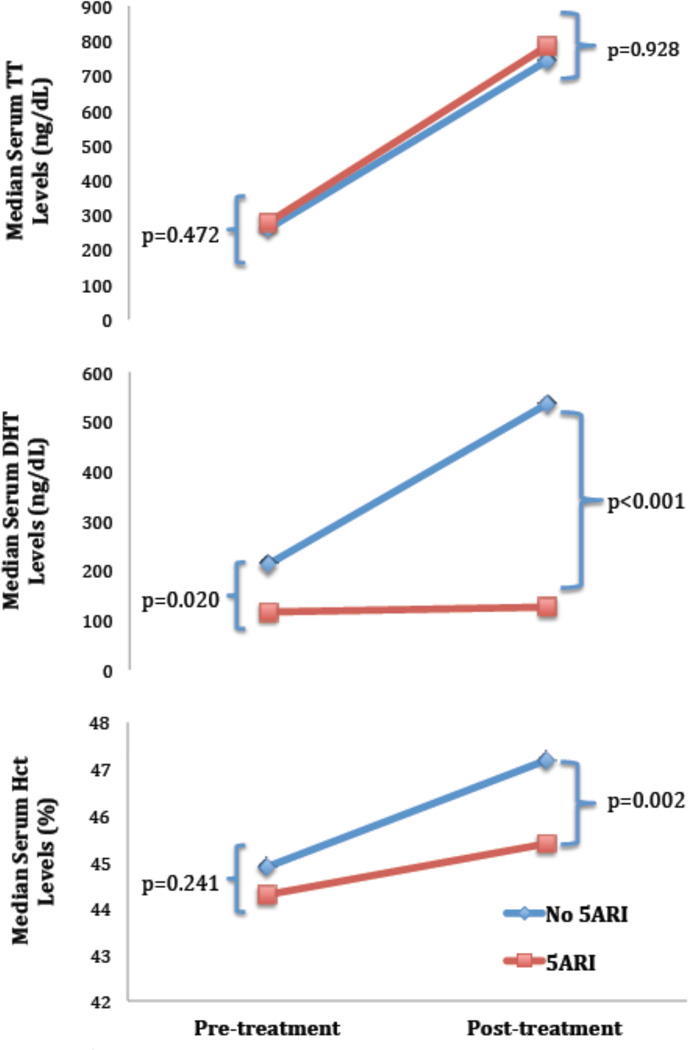
Given that 5ARIs lower serum DHT levels, a secondary analysis comparing men on 5ARIs to those who were not was performed to further assess whether DHT was involved in ΔHct. No differences in patient demographics, duration of TTh use, T formulation, or comorbidity incidence between groups were observed (Table 1). When comparing pre-and post treatment labs (Table 2, Supplementary Table 1), no differences in median pre-treatment Hct were observed between groups (44.3 vs. 44.9%, p=0.241), but post-treatment Hct was significantly higher in men not taking 5ARI (47.2 vs. 45.4%, p=0.002) (Table 2, Figure 2), with a median ΔHct of +3.0 % (0.8–4.8) in men not taking 5ARI vs. +1.1% (0.08–2.9) in men on 5ARI (p=0.013).
Figure 2.
Comparison of median pre- and post-treatment TT, DHT, and Hct in men as a function of concomitant 5ARI therapy.
As expected, a similar trend in post-treatment DHT levels was observed, with a median DHT of 536.0 ng/dL in men not taking 5ARIs vs. 126.0 ng/dL in the 5ARI group (p<0.001). Despite the discordant rise in Hct and DHT between men these groups, TT levels pre-treatment (261.0 vs. 278.0 ng/dL, p=0.472) and post-treatment (743.0 vs. 785.0 ng/dL, p=0.928) were not significantly different between these groups. Of the 36 men in the cohort that developed erythrocytosis, only 1 (2.7%) of these patients was concurrently taking a 5ARI. This patient presented with a baseline Hct of 48.0%, which then rose to 50.4% at follow-up, a 5% increase. Only 1 (5%) other patient who was concurrently taking a 5ARI had a >10% ΔHct during the course of treatment, although the patient did not, by definition, develop erythrocytosis.
Correlation Analyses
To identify variables most strongly impacting ΔHct, Spearman’s rank correlation analysis between ΔHct and cohort variables was performed, yielding significant correlations between ΔHct and follow-up TT and DHT. A larger positive correlation was observed between the ΔHct and follow-up DHT (ρ=0.258, p=0.001) than follow-up TT (ρ=0.171, p=0.023), suggesting that DHT may influence ΔHct more than TT. No significant correlation between ΔHct and FT or lipids was observed. A negative correlation was observed between ΔHct and follow-up LH (ρ=−0.212, p=0.005) and FSH (ρ=-0.254, p=0.001), as would be expected with an intact hypothalamic-pituitary-gonadal axis.
The above data suggest a previously unrecognized role for DHT in the development of erythrocytosis in men on TTh, which may be tempered using 5ARI without an impact on serum TT levels.
DISCUSSION
Erythrocytosis is the most common side effect of TTh and occurs at variable rates as a function of T formulation, although its specific etiology is unclear. Several potential mechanisms underlying the development of erythrocytosis have been proposed, including erythropoietin stimulation17, 18, suppression of hepcidin13, alone and in concert with erythropoietin18, and relation to androgen receptor CAG repeat length15. Here we provide evidence supporting a role for DHT in the development of erythrocytosis in men on TTh.
Within our cohort, 20.1% of men on TTh developed erythrocytosis, consistent with prior studies observing a 5–30% incidence of erythrocytosis as a function of T formulation and upper-limit-of-normal Hct levels ranging from 49–54%10, 12, 16, 17, 22–24. In our study, erythrocytosis was defined as Hct ≥50%; we use this value in clinical practice as a cutoff for treatment modification or therapeutic phlebotomy in men on TTh. Given the variability in the definition of erythrocytosis, we used the magnitude of change in Hct levels during TTh to identify factors associated with ΔHct.
When comparing men with ΔHct of ≥10% to those with <10% change, we observed significant increases in DHT in the absence of changes in TT and FT. Furthermore, the observed ΔHct over the course of treatment correlated more strongly with follow-up DHT than follow-up TT values. Published data regarding a possible DHT-mediated role in erythrocytosis are limited, though the current work may be compared to studies examining cohorts of hypogonadal men treated with DHT as an androgen. Several small clinical trials investigating the use of transdermal DHT in hypogonadal men have demonstrated significant increases in Hct during treatment. Although these findings were not the primary goal of these studies, the observed increases in Hct were 5.3% at 6 months25 and 6.7% at 24 months19. Moreover, the observed increases in Hct appeared to occur in a T-independent fashion, as suppression of serum TT and FT were observed during each study19, 20, 25.
To more specifically evaluate whether DHT plays a role in TTh-induced erythrocytosis, we evaluated the subgroup of men in our cohort taking 5ARIs during TTh and found that fewer men on 5ARI developed erythrocytosis than men not taking 5ARIs. Furthermore, men on 5ARIs had a lower ΔHct than men not on 5ARIs, suggesting that inhibition of 5AR may suppress erythropoiesis in this setting. Two randomized, placebo controlled studies examine the effects of TTh during 5AR inhibition. One study evaluated the effects of exogenous T in comparison to T + finasteride on bone mineral density in 70 men randomized to receive biweekly placebo, testosterone enanthate (TE), or TE + finasteride24, 26. Although not the primary goal of the study, at 36 months follow-up, men receiving TE alone experienced a 14.4% increase in Hct, whereas those receiving concomitant TE + finasteride experienced only a 9.7% increase in Hct levels, with a 30% rate of erythrocytosis (Hct >52%). However, no distinction in erythrocytosis rates was made between the TE and TE + finasteride groups. A more recent trial compared TE + high-dose dutasteride vs. TE + placebo at various TE dosages over 20 weeks27. No difference in post-treatment Hct levels between groups was observed, although the study was not powered to detect this difference28. When considering the relationship between DHT and T in the context of our findings, a previous study found that TTh administration using both topical and injectable formulations yielded transient and concomitant rises in both DHT and T, with higher Hct elevations in men with higher DHT and T levels12. A more recent study evaluating parenteral testosterone undecanoate observed a low rate of erythrocytosis in the setting of T and DHT levels within physiologic limits, ascribing these findings to a lack of supraphysiologic T and DHT levels30. A more recent study evaluating oral testosterone also found that DHT and T levels parallel each other, although erythrocytosis rates were not discussed29. Thus, while the existing literature supports our findings, whether DHT elevations are causally related to the development of erythrocytosis remains unclear.
Consistent with prior studies, our study observed a higher incidence of ΔHct ≥10% in patients on injectable T formulations11, 12. We also observed a trend towards an increased risk of erythrocytosis in older patients, which has also been previously described5, 13 and those with a higher incidence of medical comorbidities that may alone predispose to erythrocytosis1, 10, 16. However, the retrospective nature of this study and the relatively low overall incidence of specific comorbidities limits definitive conclusions about individual comorbidities.
Our study is limited by its retrospective nature, which precluded more precise control for confounding factors. Furthermore, while we present evidence suggesting a relationship between DHT and a rise in Hct during TTh, we cannot prove causality with our current dataset. In addition, we lack erythropoietin and hepcidin levels, and CAG repeat lengths, making it difficult to integrate our findings with other proposed mechanisms of increased erythropoiesis in the setting of TTh. Additional limitations include the overall small cohort size and relatively short follow-up, a longer duration of TTh, which may have further informed the rate of erythrocytosis and allowed further dissection of the role of DHT in this condition, and the small number of men with primary hypogonadism, limiting the ability to differentiate the rates of erythrocytosis in men with primary or secondary hypogonadism. Further investigation is indicated to evaluate the role of DHT in TTh-induced erythrocytosis as well as the effects of concurrent 5ARI use during TTh, particularly in the setting of erythrocytosis. Nevertheless, our findings suggest that patients with elevated Hct on TTh should be screened for elevated DHT and that 5ARIs may be an alternative therapy to cessation of TTh or phlebotomy, as is current practice.
CONCLUSIONS
Serum DHT levels in hypogonadal men on TTh correlate with T-induced erythrocytosis, suggesting a role for DHT in the development of TTh-induced erythrocytosis. As such, DHT levels should be monitored during TTh, and in men who develop erythrocytosis, 5ARIs may be considered as adjunct or alternate therapy to modification of TTh or therapeutic phlebotomy.
Supplementary Material
KEY ABBREVIATIONS
- TTh
testosterone therapy
- 5ARI
5α-reductase inhibitor
- DHT
dihydrotestosterone
- Hct
hematocrit
- TT
total testosterone
- FT
free testosterone
- FSH
follicle stimulating hormone
- LH
luteinizing hormone
- Hct
hematocrit
- ΔHct
change in hematocrit
- TChol
total cholesterol
- TG
triglycerides
Footnotes
Publisher's Disclaimer: DISCLAIMER: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
REFERENCES
- 1.Rhoden EL, Morgentaler A. Risks of Testosterone-Replacement Therapy and Recommendations for Monitoring. New England Journal of Medicine. 2004;350:482. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 2.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Therapeutics and Clinical Risk Management. 2009;5:427. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calof OM, Singh AB, Lee ML, et al. Adverse Events Associated With Testosterone Replacement in Middle-Aged and Older Men: A Meta-Analysis of Randomized, Placebo-Controlled Trials. The Journals of Gerontology: Medcial Sciences. 2005;60:1451. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Nieschlag E, Swerdloff R, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: Investigation, treatment and monitoring of late-onset hypogonadism in males. International Journal of Impotence Research. 2009;21:1. doi: 10.1038/ijir.2008.41. [DOI] [PubMed] [Google Scholar]
- 5.Coviello AD, Kaplan B, Lakshman KM, et al. Effects of Graded Doses of Testosterone on Erythropoiesis in Healthy Young and Older Men. The Journal of Clinical Endocrinology and Metabolism. 2008;93:914. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Balsells MM, Murad MH, Lane M, et al. Adverse Effects of Testosterone Therapy in Adult Men: A Systematic Review and Meta-Analysis. The Journal of Clinical Endocrinology and Metabolism. 2010;95:2560. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 7.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clinic Proceedings. 2007;82:29. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Viallard JF, Marit G, Mercié P, et al. Polycythaemia as a complication of transdermal testosterone therapy. British Journal of Haematology. 2001;110:237. doi: 10.1046/j.1365-2141.2000.02072-3.x. [DOI] [PubMed] [Google Scholar]
- 9.Surampudi PN, Wang C, Swedloff R. Hypogonadism in the Aging Male: Diagnosis, Potential BEnefits, and Risks of Testosterone Replacement Therapy. International Journal of Endocrinology. 2011;2012:1. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip FF, di Pierro I, Brown R, et al. Trough serum testosterone predicts the development of polycythemia in hypogonadal men treated for up to 21 years with subcutaneous testosterone pellets. European Journal of Endocrinology. 2010;162:385. doi: 10.1530/EJE-09-0717. [DOI] [PubMed] [Google Scholar]
- 11.Sih R, Morley JE, Kaiser FE, et al. Testosterone Replacement in Older Hypogonadal Men: A 12-Month Randomized Controlled Trial. The Journal of Clinical Endocrinology & Metabolism. 1997;82:1661. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 12.Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, Efficacy, and Safety of a Permeation-Enhanced Testosterone Transdermal System in Comparison with Bi-Weekly Injections of Testosterone Enanthate for the Treatment of Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism. 1999;84:3469. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 13.Bachman E, Feng R, Travison T, et al. Testosterone Suppresses Hepcidin in Men: A Potential Mechanism for Testosterone-Induced Erythrocytosis. The Journal of Clinical Endocrinology and Metabolism. 2010;95:4743. doi: 10.1210/jc.2010-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiels M, Rohrmann S, Menke A, et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20:877. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zitzmann M, Nieschlag E. Androgen Receptor Gene CAG Repeat Length and Body Mass Index Modulate the Safety of Long-Term Intramuscular Testosterone Undecanoate Therapy in Hypogonadal Men. Journal of Clinical Endocrinology and Metabolism. 2007;92:3844. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 16.Keohane C, Mcmullin MF, Harrison C. The diagnosis and management of erythrocytosis. British Medical Journal. 2013;347:1. doi: 10.1136/bmj.f6667. [DOI] [PubMed] [Google Scholar]
- 17.Bachman ETT, Basaria S, Davda MN, Guo W, Li M, Connor Westfall J, Bae H, Gordeuk V, Bhasin S. Testosterone Induces Erythrocytosis via Increased Erythropoietin and Suppressed Hepcidin: Evidence for a New Erythropoietin/Hemoglobin Set Point. Journal of Gerontology and Biological Sciences: Medical Sciences. 2013:1. doi: 10.1093/gerona/glt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014;69:725. doi: 10.1093/gerona/glt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idan A, Griffiths KA, Harwood DT, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: a randomized, placebo-controlled trial. Annals of Internal Medicine. 2010;153:621. doi: 10.7326/0003-4819-153-10-201011160-00004. [DOI] [PubMed] [Google Scholar]
- 20.Ly LP, Jimenez M, Zhuang TN, et al. A Double-Blind, Placebo-Controlled, Randomized Clinical Trial of Transdermal Dihydrotestosterone Gel on Muscular Strength, Mobility, and Quality of Life in Older Men with Partial Androgen Deficiency. Journal of Clinical Endocrinology and Metabolism. 2001;86:4078. doi: 10.1210/jcem.86.9.7821. [DOI] [PubMed] [Google Scholar]
- 21.Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone Replacement Therapy in Patients with Prostate Cancer after Radical Prostatectomy. Journal of Urology. 2013;190:639. doi: 10.1016/j.juro.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palumbo V, Esposito D, Visconti D, et al. Effects of Testosterone Replacement Therapy on Hepcidin Levels in young Hypogonadal Men. Endocrine Abstracts. 2013;32:659. [Google Scholar]
- 23.SIh R, Morley JE, Kaiser FE, et al. Testosterone Replacement in Older Hypogonadal Men: A 12-Month Randomized Controlled Trial. Journal of Clinical Endocrinology and Metabolism. 1997;82:1661. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 24.Amory JK, Watts N, B, Easley KA, et al. Exogenous Testosterone or Testosterone with Finasteride Increases Bone Mineral Density in Older Men with Low Serum Testosterone. Journal of Clinical Endocrinology and Metabolism. 2004;89:503. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 25.Kunelius P, ILukkarinen O, Hannuksela ML, et al. The Effects of Transdermal Dihyrotestosterone in the Aging Male: A Prospective, Randomized, Double Blind Study. Journal of Clinical Endocrinology and Metabolism. 2002;87:1467. doi: 10.1210/jcem.87.4.8138. [DOI] [PubMed] [Google Scholar]
- 26.Page Stephanie T., J. K. A., Bowman F. DuBois, Anawalt Bradley D., Matsumoto Alvin M., Bremner William J., Lisa Tenover J. Exogenous Testosterone (T) Alone or with Finasteride Increases Physical Performance, Grip Strength, and Lean Body Mass in Older Men with Low Serum T. The Journal of Clinical Endocrinology and Metabolism. 2005;90:1502. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 27.Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5α-reductase inhibitor on fat-free mass in men with suppressed testosterone production: A randomized controlled trial. JAMA. 2012;307:931. doi: 10.1001/jama.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruntmanis U. THe role of 5α-reductase inhibition in men receiving testosterone replacement therapy. JAMA. 2012;307:968. doi: 10.1001/jama.2012.259. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Rubinow K, Clark RV, et al. Pharmacokinetics of modified slow-release oral testosterone over 9 days in normal men with experimental hypogonadism. J Androl. 2012;33:420. doi: 10.2164/jandrol.111.014514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad F, Kamischke A, Yassin A, et al. More than eight years' hands-on experience with the novel long-acting parenteral testosterone undecanoate. Asian J Androl. 2007;9:291. doi: 10.1111/j.1745-7262.2007.00275.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.