Abstract
Besides secretion of antigen-specific antibodies, B cells may play an important role in the generation of immune responses by efficiently presenting antigen to T cells. We and others recently described a subpopulation of CD11c+ B cells (Age/autoimmune associated B cells, ABCs) which appear with age, during virus infections and at the onset of some autoimmune diseases and which participate in autoimmune responses by secreting autoantibodies. Here we assessed the ability of these cells to present antigen and activate antigen-specific T cells. We demonstrated that ABCs present antigen to T cells, in vitro and in vivo, better than follicular B cells (FO cells) do. Our data indicate that ABCs express higher levels of the chemokine receptor, CCR7, and have higher responsiveness to CCL21 and CCL19 than FO cells and are localized at T/B cell border in spleen. Using multiphoton microscopy we show that, in vivo, CD11c+ B cells form significantly more stable interactions with T cells than Follicular B cells do. Together these data identify a previously undescribed role for ABCs as potent antigen-presenting cells and suggest another potential mechanism by which these cells can influence immune responses and/or the development of autoimmunity.
Introduction
It is well known that B cells contribute to immune responses via their descendants, antibody secreting plasma cells. In addition, B cells are also professional antigen-presenting cells and therefore can participate in immune responses by activating antigen-specific CD4+ T cells. Interactions between B and T cells occur mainly in secondary lymphoid organs (spleen and lymph nodes) whose architecture is favorable for such events. B cells migrate to the B cell zones in secondary lymphoid organs where they may encounter antigens and become activated (1). Migration to B cell follicles is driven by a chemokine gradient of CXCL13 which is produced by follicular stromal cells and is recognized by the CXCR5 receptor on B cells (2). In the follicles, B cells can encounter antigens from the surface of neighboring cells, such as follicular dendritic cells (FDCs) (3) or macrophages (4). Alternatively, small soluble antigens can be acquired by B cells directly from the lymph (5). While B and T cells are organized into separate zones in resting lymphoid organs, upon activation, they move and interact with each other at the border between these two zones (6, 7). Such directed movement is tightly regulated by interactions of multiple chemokines produced by stromal cells and chemokine receptors expressed on the surface of lymphocytes. In particular, it has been demonstrated that B cells upregulate the expression of CCR7 upon encounter with antigen. CCR7 expression drives the cells to move towards the chemokines CCL19 and CCL21 that are produced by stromal cells in the T cell zone (8). In addition, the formation of stable and motile antigen-specific B cell/CD4 T cell conjugates has also been detected using multiphoton microscopy, indicating that B cells may indeed act as APCs during the initiation of an immune response (8). In support of this statement, it has been demonstrated that B cells are able to prime naïve CD4 T cells both in vitro (9) and in vivo (10, 11).
Several well characterized subsets of B cells are found in the spleen and/or lymph nodes. These are distinguished by the expression of distinct surface markers. In addition to phenotypic differences, each B cell subset has a unique function. The ability to present antigen also differs between the different subsets of B cells. For example, it has been demonstrated that marginal zone (MZ) B cells are more potent activators of naïve CD4 T cells than FO B cells (12). Enhanced antigen presenting capabilities have also been demonstrated for germinal center (GC) B cells (13).
We and others have recently described a novel subset of B cells in the spleens of elderly female mice (ABCs) that is characterized by expression of CD11c and the transcription factor, T-bet (14–16). B cells with a similar phenotype appear in autoimmune-prone mice, at about the time the symptoms of their disease appear and also in animals suffering from acute virus infections (14, 15, 17). Gene expression analysis as well as surface staining of these cells indicated that the cells express high levels of the co-stimulatory molecules CD80 and CD86 and of MHCII (14). These characteristics led us to hypothesize that ABCs can serve as efficient APCs to prime CD4 T cells.
Here we demonstrate that CD11c+T-bet+ B cells acquired from aged or autoimmune female mice present antigen more efficiently than follicular B cells do, both in vitro and in vivo. Moreover, these cells localize in spleens at the T cell /B cell border where antigen presentation takes place. In addition, upon antigen encounter, ABCs form more stable conjugates with T cells than FO B cells do. Taken together, these data indicate a previously undescribed function for ABCs as potent antigen presenting cells and suggest an additional way in which they may contribute to the development of autoimmunity.
Materials and Methods
Isolation of distinct B cell populations
Splenic B cells were purified by negative enrichment using biotinylated TER-119, NK1.1 and anti-TCR antibodies followed by anti-biotin microbeads (Miltenyi, Germany). All the donor mice for ABCs were 18 – 20 months old. ABCs were purified with a MoFlo sorter (Dako-Cytomation) as B220+CD19+CD11c+ to greater than 95% purity. FO B cells were identified as B220+CD19+CD11c−CD21intCD1dint, and Marginal Zone B cells were isolated as B220+CD19+CD11c−CD21highCD1dhigh. After sorting, cells were stained for CD21 and CD1d surface expression to confirm the distinction between three B cell populations: ABCs, FO and MZ B cells (Supplemental Figure 1). For analysis, events were collected on a CyAn ADP (Beckman Coulter) and data were analyzed using FlowJo version 8.8 (Tree Star).
In vitro antigen presentation assay
B cell populations were isolated as described above and 105 cells were mixed with either 105 OVA-specific H-2b restricted T cell hybridomas (BO 80.10) or CD4+ T cells from OT-II transgenic mice. Cells were incubated in the presence of whole OVA protein or OVA peptide (amino acids 323–339) for 24 hours at 37C. The presence of IL-2 in the supernatants was determined by MTT assay using IL-2-dependent HT-2 cells as described previously (18). IL-2 titers are expressed in units/ml.
In vitro proliferation assay
OVA-specific CD4+ T cells were isolated from OT-II transgenic mice, labeled with CFSE, and incubated with either FO B cells or ABCs (isolated from 18–20 months old mice) in the presence of whole OVA protein (10ug/ml) or OVA peptide (amino acids 323–339; 10ug/ml)) for 4 hours at 37C. CFSE dilution of CD4+ T cells was determined by flow cytometry.
In vivo proliferation assay
3K-specific CD4+ T cells were isolated using Miltenyi Biotec CD4 T cell isolation kit (Myltenyi) from 508 TCR transgenic mice crossed to Rag-negative background (bred at National Jewish Health (19). Cells were labeled with CFSE according to manufacturer’s protocol and injected i.v. in C57BL/6 mice (2×106 cells per mouse). 24 hours later ABCs or FO B cells were isolated from 18–20 months old mice by flow cytometric sorting as described above, incubated in the presence of 3K peptide or 3K-OVA protein for 4 hours at 37C, and injected into mice that received labeled CD4+ T cells. Dilution of CFSE by T cells was determined 4 days later.
Immunofluorescence histology
ABCs or FO B cells from 18–20 months old GFP-expressing C57BL/6 mice were isolated as described above and injected i.v. into C57BL/6 mice. Three days after injection, spleens were harvested and incubated in 4% paraformaldehyde and 10% sucrose for 2 hours at room temperature followed by incubation in 30% sucrose overnight at +4°C. Spleens were frozen at −80°C in OCT compound (EM Sciences). Tissues were cut into 5- to 7-µm sections and dried at room temperature overnight. Sections were rehydrated with PBS for 20 min and blocked for 30 min with PBS, 2% BSA, 0.05% Tween 20. Ab mixtures were added and incubated for 45 min followed by three 5-min washes with PBS. Sections were allowed to dry, mounted, and analyzed with Zeiss Axivert 200M microscope (3i Marianas System) using Slidebook 4.0 software (Intelligent Imaging Innovations).
Flow Cytometry
Lymph nodes or spleens were prepared at the indicated times and red blood cells were lysed. Single cell suspensions were stained with MHC tetramers at 37°C for 2 hours. APC-Db/NP366–74 and PE-Db/PA224–38 were produced as described (20), PE-IAb/NP311–25 tetramer was provided by the NIH Tetramer Core Facility. Antibodies to surface proteins were added and the cells incubated for a further 20 minutes at 4°C.
Cells were stained under saturating conditions with antibodies to mouse CD4 (clone GK1.5), CD8 (clone 53–6.7), B220 (clone RA3-6B2), CD11b (clone M1/70), CD11c (clone N418), CD19 (clone 1D3) purchased from Ebiosciences or BD Pharmingen, or generated in house.
Cells were analyzed by flow cytometry on CyAn (Beckman-Coulter) instrument and data were analyzed using FlowJo software (Treestar).
Chemotaxis Assay
Recombinant murine CXCL13, CCL19 or CCL21 (PeproTech) was added to the bottom well of a 24-well plate in a total of 500 µl of migration medium (Iscove's medium supplemented with Glutamax, 25 mM Hepes buffer, and 1% fatty-acid-free BSA). Total splenic cells from aged (18–20 months old) or young C57BL/6 mice were depleted of red blood cells and added to the upper insert of a transwell (5 µm pore; Costar) at a concentration of 106 cells/100 µl. After 3 hr incubation at 37°C, cells were removed from the upper and lower wells, pooled (2–6 wells were used for each condition in each experiment), stained on ice, and analyzed by flow cytometry. For relative cell counts, a CYAN was used to acquire cells for 60 sec, at which time at least 250,000–500,000 cells were collected from upper wells and 20,000–90,000 cells from lower wells. The percent of cells migrating into the bottom chamber was calculated: (number of cells in lower well) / (number of cells in lower + upper well) × 100.
Mice
C57BL/6 (B6) mice were obtained from The Jackson Laboratory. In antigen presentation assays T cells were isolated from transgenic mice expressing the F508αβTCR (T cells are specific for 3K peptide presented by I-Ab)(21) or OT-II (T cells are specific for OVA peptide presented by I-Ab)(22). B6/UBI-GFP(23) mice were generated and maintained at National Jewish Health animal facility. B6.Nba2(24) mice were purchased from Jackson Laboratories (B6.NZB-(D1Mit47-D1Mit209)/BkotJ). Female mice were used for all experiments. All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the National Jewish Health Animal Care and Use Committee.
Multiphoton microscopy
3K-specific CD4+ T cells were isolated from 508 TCR transgenic mice that were crossed to Rag-negative C57BL/6 mice (bred at National Jewish Health (19). Cells were labeled with either 20µM CMTMR, 2µM CFSE or 2µM VPD, washed 3 times, and injected i.v. into wild-type C57BL/6 recipients. 24 hours later ABCs and FO B cells were isolated from 18–20 months old mice by flow cytometric sorting as described above, incubated in the presence of 3K peptide for 4 hours at 37C, labeled with 20µM CMTMR or 2µM CFSE or 2µM VPD and injected in recipient mice that received labeled CD4+ T cells. Twelve hours following injection, mice were sacrificed and their spleens were surgically removed for imaging and immobilized on coverslips. During imaging, spleens were maintained at 35–37°C in a flow chamber perfused with RPMI medium without phenol red (Gibco) saturated with 95% O2/5% CO2. Multiphoton imaging was done using an Olympus FV1000MPE microscope with a XLPLN25XWMP Super 25x 1.05NA water immersion objective, and a Spectra Physics 10W Mai-Tai HP DeepSee-OL laser. The 450–490nm, 500–550nm, 575–640nm and 645–685nm filters were used for Blue, Green, Red, and Far Red emission channel acquisition, respectively. To exclude potential effects of the fluorescent dyes on viability and motility of ABCs and FO B cells, we swapped the dyes used to label the two transferred B cell populations between experimental repeats.
For time-lapse image acquisition, each xy plane spanned 509µm × 509µm at a resolution of 0.994µm/pixel. Images of up to 22 xy planes with 3µm Z-spacing were acquired every 30 sec for 30 min. Data was visualized and analyzed using Imaris (Bitplane) and MATLAB (Mathworks). To isolate each fluorophore to a single channel, linear unmixing was performed. The fluorescence intensity of a given fluorophore in its optimal channel was determined. The bleed-through fluorescence of the same fluorophore in each of the other channels was then assessed. The percentage “bleed” into each channel was calculated by dividing the fluorescence in the non-optimal channel by the fluorescence in the optimal channel. The fluorescence in all non-optimal channels was then subtracted out on a pixel-by-pixel basis using MATLAB and the ImarisXT ‘Image Arithmetic’ function using the percentage bleed determined. Surfaces were made in Imaris (using the ‘Surface’ function) to identify the T cells, FO B cells and ABCs. Based on these surface objects, individual B cells were identified and tracked by Imaris, and cellular speed and displacement were calculated from the tracks. Only cells that were tracked for at least 5 min were included in analyses of motility and displacement and only cells that were tracked for at least 10 min were included in analyses of interactions. Using a custom automated Matlab script T cell/B cell interactions were quantified, with T cells and B cells were scored as interacting if their cellular surfaces were within 0.994µm (1 pixel) of each other.
Statistical Analysis
All statistics were performed using Prism software using student’s t-test.
Results
ABCs are localized to the T cell/B cell border in spleen
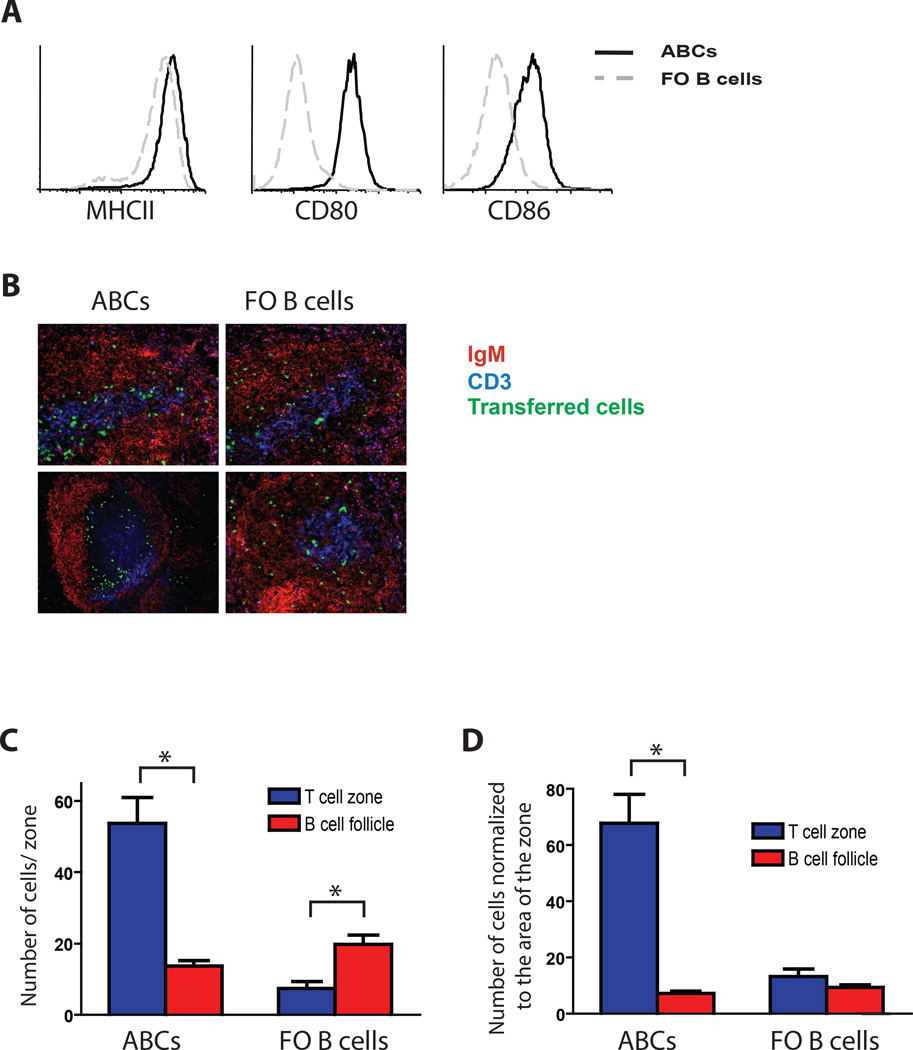
We have previously demonstrated that ABCs obtained from aged mice express high levels of MHCII, CD80 and CD86 on their surface compared to FO B cells (Fig. 1A and (14)), suggesting that these cells might be efficient at stimulating T cells. Since activated B cells are known to upregulate costimulation molecules and migrate towards the T cell B cell border in order to present antigen to T cells (25), we decided to determine the localization of ABCs in the spleen. Unfortunately, direct immunofluorescent staining of aged spleen sections was not able to distinguish definitively between ABCs and other B cell and dendritic cell populations. Therefore, we isolated ABCs and FO B cells from old C57BL/6 female mice in which all cells express GFP (UBI-GFP mice) and injected them intravenously into naïve C57BL/6 mice. After 72 hours, we determined the localization of GFP+ B cells by immunofluorescent histology. Spleen sections were examined because ABCs are found preferentially in this organ rather than in lymph nodes. While FO B cells were mostly homogenously distributed in B cell follicles, ABCs were localized either in T cell zones or at the T cell/B cell border (Fig. 1B–D). These data were quantified based on the absolute number of ABCs or FO B cells localized to the T cell zone or B cell follicle (Fig. 1C) or normalized to the area of each zone (Fig. 1D). The data clearly demonstrate that, unlike conventional B cells, ABCs are mostly localized in the T cell zone.
Figure 1. ABCs localize at the T cell/B cell border.
(A) ABCs (solid line) and FO B cells (gray dashed line) were analyzed for the expression of MHCII, CD80 and CD86. (B) Localization of GFP+ ABCs or FO B cells (green cells) in spleen 3 days after i.v. transfer. Data represent one of three independent experiments. (C and D) Quantitation of histology either by absolute number of cells in T cell or B cell zones (C) or by normalizing to the area of each zone (D). Bars represent the means +/− SEM. * p< 0.01 (t test).
ABCs express T-cell specific chemokine receptors and migrate towards both T cell- and B cell-attracting chemokines
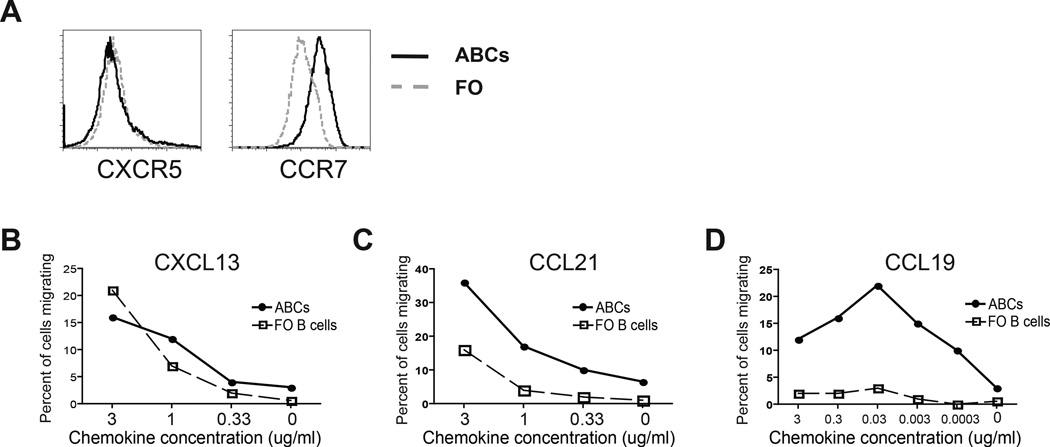
Next we tried to find out which factors are responsible for ABCs’ localization at the T cell/B cell border. It is commonly accepted that chemokines and chemokine receptors determine the localization and migration of lymphocytes within secondary lymphoid organs (26). FO B cells express the chemokine receptor, CXCR5, and thus respond to the chemokine, CXCL13, produced in the B cell follicle by follicular stromal cells. T cells, on the other hand, generally express the CCR7 receptor and migrate towards CCL19 and CCL21 chemokines (27). To understand how ABCs are localized to the T cell zone, we first examined the expression of CXCR5 and CCR7 by these cells and compared the results to those of FO B cells. Flow cytometric analysis revealed that ABCs express levels of CXCR5 that are similar to those observed on FO B cells (Fig. 2A). However, they express significantly higher levels of the T cell specific chemokine receptor CCR7 (Fig. 2A). Next we tested whether this difference in expression levels actually reflects a functional difference in the ability of these cells to migrate towards specific chemokines. Using migration transwells, we assessed the responsiveness of ABCs and FO B cells to three different chemokines: the B cell-specific chemokine, CXCL13, and two nominally T cell-specific chemokines, CCL19 and CCL21. While migration towards CXCL13 was comparable between ABCs and FO B cells (Fig. 2B), ABCs migrated significantly better toward CCL19 and CCL21 than FO B cells did (Fig. 2C and D). These transwell migration data are consistent with the expression patterns of chemokine receptors by ABCs and explain their localization to the T cell/B cell border in the spleen. During their conversion from FO B cells (16), ABCs retain responsiveness to CXCL13, and upregulate expression of CCR7, thereby gaining responsiveness to the T cell zone chemokines CCL19 and CCL21 resulting in their localization to the T cell/B cell border in the spleen.
Figure 2. ABCs express CCR7 and migrate towards both B cell- and T cell-attracting chemokines.
(A) ABCs (solid line) and FO B cells (gray dashed line) were analyzed for expression of CXCR5 and CCR7. (B–C) ABCs and FO B cells chemotactic abilities toward B cell specific chemokine CXCL13 (B) and T cell specific chemokines CCL21 (C) and CCL19 (D). Data represent one of three independent experiments.
ABCs present antigen more efficiently than FO B cells in vitro
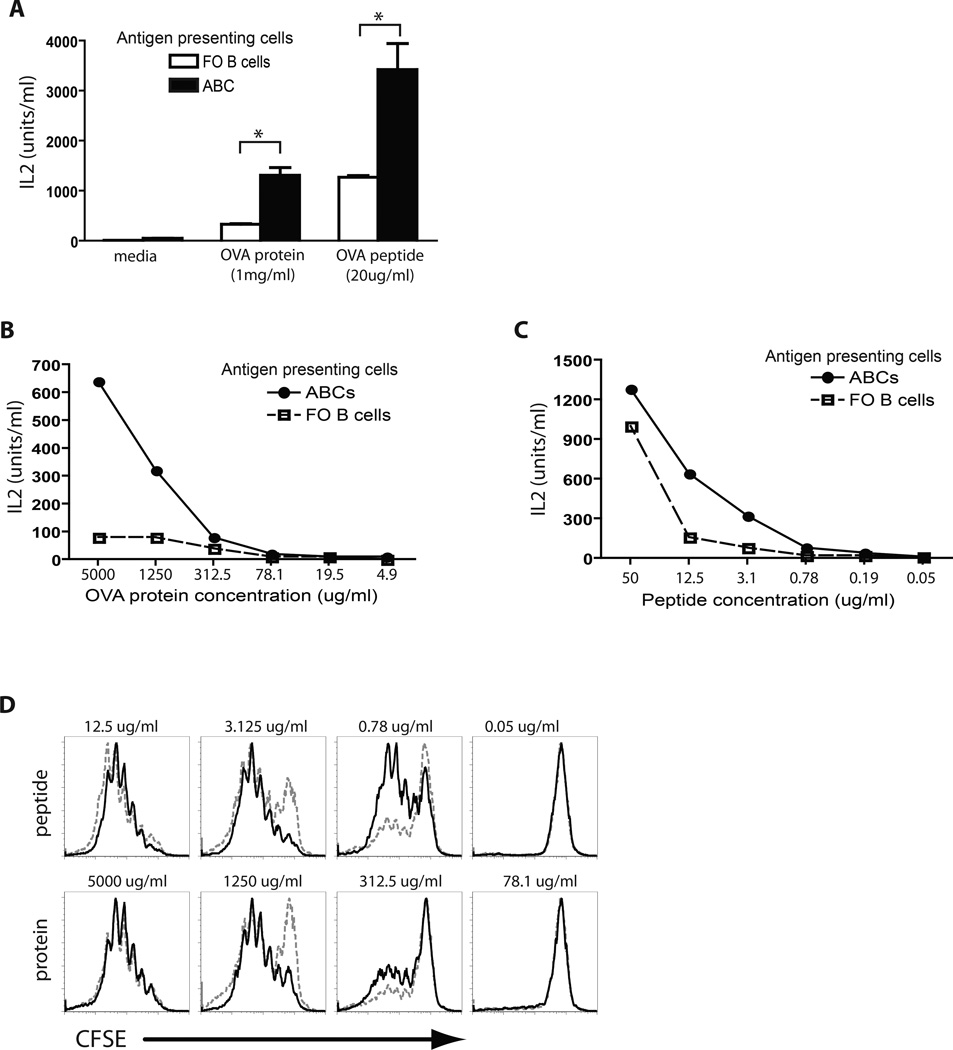
To compare the antigen-presenting abilities of ABCs and FO B cells in vitro, we isolated these cell subsets from the spleens of aged female C57BL/6 mice, and incubated them with antigen-specific T cells in the presence of either whole protein antigen (ovalbumin, OVA) or peptide antigen from the same protein. ABCs and FO B cells were compared in their ability to induce IL-2 production by OVA-specific T cell hybridomas (Fig. 3A) as well as primary OVA-specific CD4+ T cells isolated from OT-II transgenic mice (Fig. 3B and C). As shown in Figure 3, in the presence of OVA peptide, ABCs were more efficient than FO B cells at inducing secretion of IL-2 by both antigen-specific T cell hybridoma cells and primary OT-II T cells. This difference was probably due to the higher levels of MHCII and costimulatory proteins on the ABCs versus FO B cells. Similar results were obtained when intact OVA was used as the source of antigen and detection was by OVA-specific T cell hybridomas. The results were even more dramatic when naïve OTII cells were used as detectors and intact OVA as antigen. In this case ABCs were still effective antigen presenting cells, whereas FO B cells failed completely to stimulate the T cells. This result may have been due not only to differences in levels of MHCII and costimulatory proteins but also to the idea that ABCs may be able to more efficiently take up and process whole OVA protein due to their high levels of expression of genes involved in vesicular transport and cytoskeletal rearrangement (14). We also assessed T cell proliferation in vitro in response to antigen presentation by monitoring CFSE dilution by OT-II T cells 3 days after incubation with antigen-pulsed FO B cells or ABCs (Fig. 3D). Interestingly, the highest doses of antigen (either protein or peptide) led to equal T cell stimulation by FO B cells and ABCs. However, ABCs were better T cell stimulators at lower concentrations of antigen. This result contrasts with our observations of IL-2 production where the maximum differences were observed in the presence of the highest amount of antigen (Fig. 3B and C). The discrepancy is probably due to consumption of IL-2 by the proliferating T cells, causing the IL-2 assays shown in Figs 3B, C to underestimate the amounts of IL-2 produced by the T cells in each assay.
Figure 3. Antigen presentation by ABCs and FO B cells in vitro.
(A) ABCs and FO B cells were analyzed for ability to present peptide and protein antigens to antigen-specific hybridoma T cells. Bars represent the means +/− SEM of n=3 mice per group. * p<0.01 (t test). (B, C) ABCs and FO B cells were tested to present different concentrations of protein (B) or peptide (C) to primary OT-II T cells. (D) ABCs and FO B cells were tested for ability to stimulate proliferation of CFSE labeled antigen specific T cells after 3 days of coculture in the presence of peptide or protein antigen. Data represent one of three independent experiments.
These results demonstrate that in vitro ABCs are more efficient antigen presenting cells than FO B cells.
ABCs present antigen more efficiently than FO B cells in vivo
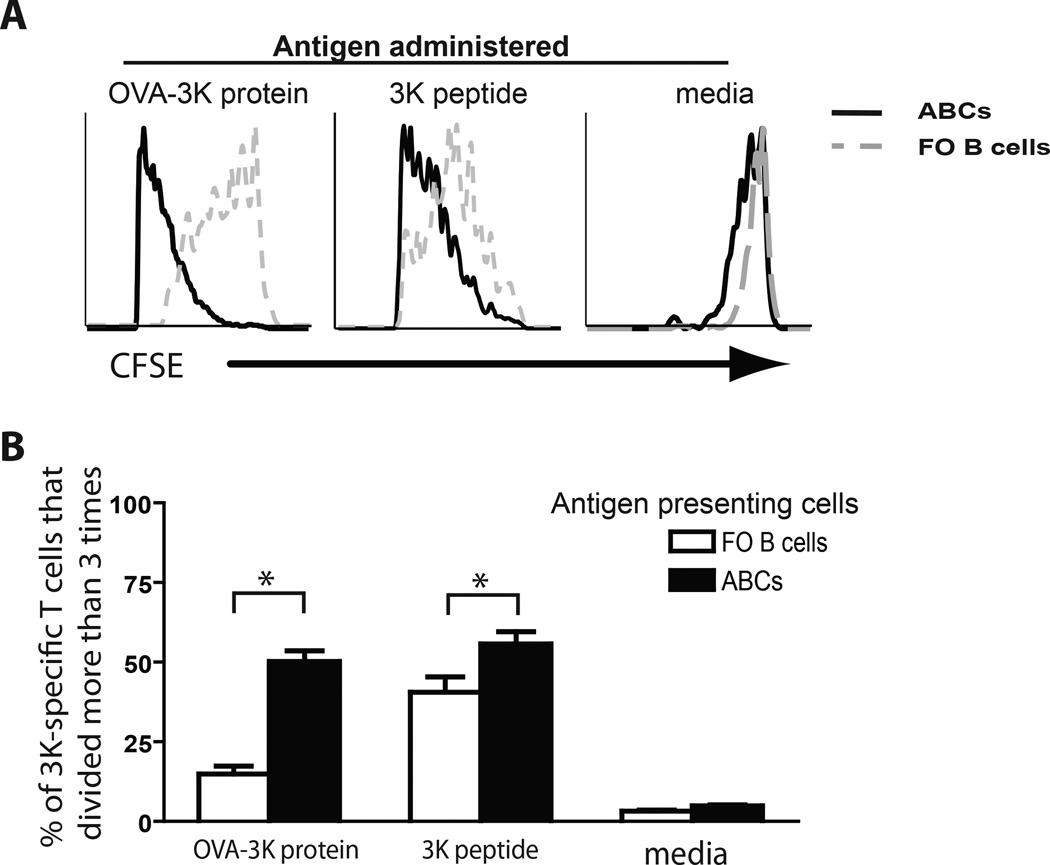
Next we explored whether the efficient antigen presenting activity by ABCs in vitro is also evident in vivo. To this end, we transferred CFSE-labeled OT-II T cells into C57BL/6 mice one day before they received antigen pre-loaded FO B cells or ABCs. CFSE dilution by T cells was determined four days after the injection of B cells and, as shown in Figure 4, ABCs were significantly more potent in stimulating T cell proliferation. ABCs were equally good T cell stimulators when given either whole protein or peptide (Figs. 4A, B). By contrast, FO B cells presented OVA peptide quite well (Fig. 4B), but intact OVA protein poorly (Fig. 4A).
Figure 4. Antigen presentation by ABCs and FO B cells in vivo.
(A) CFSE dilution by antigen specific T cells was determined in mice that received either ABCs (solid line) or FO B cells (dashed gray line) pulsed with antigen (protein, peptide or media). (B) Bar graph represents mean (±SD) of percentage of T cells divided more than 3 times after mice received ABCs or FO B cells pulsed with antigen (protein, peptide or media). Bars represent the means +/− SEM of n=5 mice per group. * p<0.05 (t test).
These data demonstrate that ABCs have an increased ability to take up, process and present antigen to T cells and induce T cell proliferation both in vivo and in vitro.
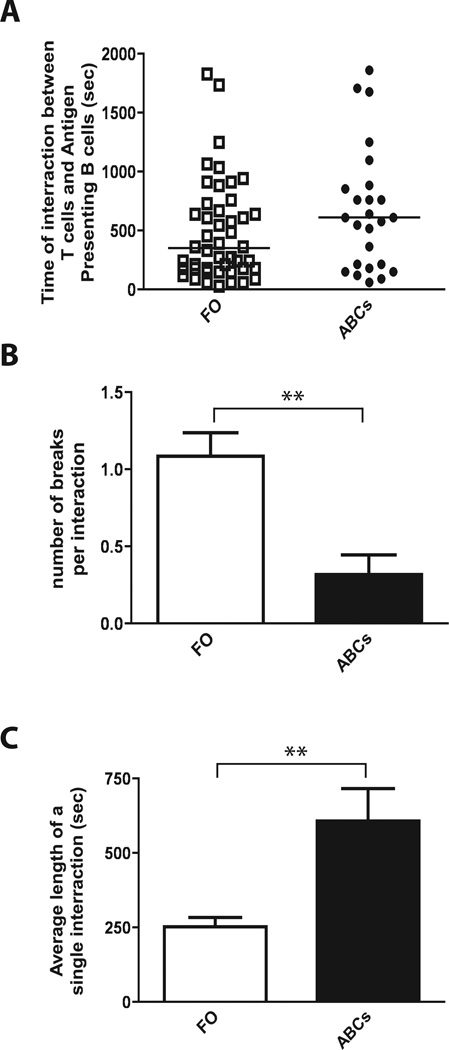
ABCs form long and stable interactions with antigen-specific T cells
There are several possible explanations for the robust antigen presenting abilities of ABCs. These include their localization to the T cell/B cell border, their ability to process antigen, and their high expression of co-stimulatory and MHCII molecules. All of these properties suggest that T cell contacts with ABCs may differ in quantity or quality from their contacts with other B cells, for example FO cells. To test this idea we used multiphoton microscopy to compare the interactions between antigen-pulsed B cells (FO or ABC) and antigen-specific T cells in spleen explants.
Fluorescent dye-labeled T cells were transferred into C57BL/6 mice and, 24 hours later, antigen pulsed B cells (FO B cells and ABCs, labeled with different fluorescent dyes) were injected. B/T cell interactions in the spleen were tracked by time-lapse multiphoton microscopy twelve hours later. We observed that ABC/T cell interactions were significantly more stable than FO B cell/T cell interactions (Fig. 5A–C and Supplemental Movie 1), resulting in longer contact times between ABCs and T cells. Previous studies on interactions between antigen presenting cells and T cells demonstrated that the duration of interactions is critical for the fate of the T cells (28). Long APC/T cell interactions, often lasting more than 12 hours, led to sustained T cell activation, while aborted or transient interactions led to tolerogenic signaling in T cells (29–31). Therefore, our results suggest that ABC/T cell interactions are more likely to lead to T cell activation than FO B cell/T cell interactions. As a control, we also injected T cells that were specific for an unrelated antigen. Neither ABCs nor FO B cells interacted with these T cells, confirming the antigen-specific nature of the presenting cell/T cell interactions we observed (Supplemental movie 2).
Figure 5. ABCs form more stable interactions with antigen specific T cells than FO B cells in vivo.
(A) Total time for which each analyzed B cell was in contact with antigen specific T cell(s). (B) Bar graph represents mean (±SD) number of breaks each interacting T cell analyzed made during time of observation. (C) Bar graph represents average (±SD) duration of each interaction between B cell (ABC or FO B cells) and antigen-specific T cell. Bars represent the means +/− SEM of at least 3 independent time-lapse multiphoton experiments. * p<0.001 (t test).
Overall, our multiphoton experiments demonstrated that ABCs not only localize to the area most suitable for interactions with antigen specific T cells but also possess some cell-intrinsic features that allow them to form more productive interactions with antigen-specific T cells, ultimately leading to more efficient T cell activation.
ABCs obtained from autoimmune mice also possess more efficient antigen presenting capabilities than FO B cells
All of the data described above indicate that ABCs obtained from aged C57BL/6 female mice are more efficient antigen presenting cells than FO B cells. This is explained by their expression of several surface markers including chemokine receptors and co-stimulatory molecules, their localization in T cell zones in the spleen and their ability to process and present protein antigens effectively.
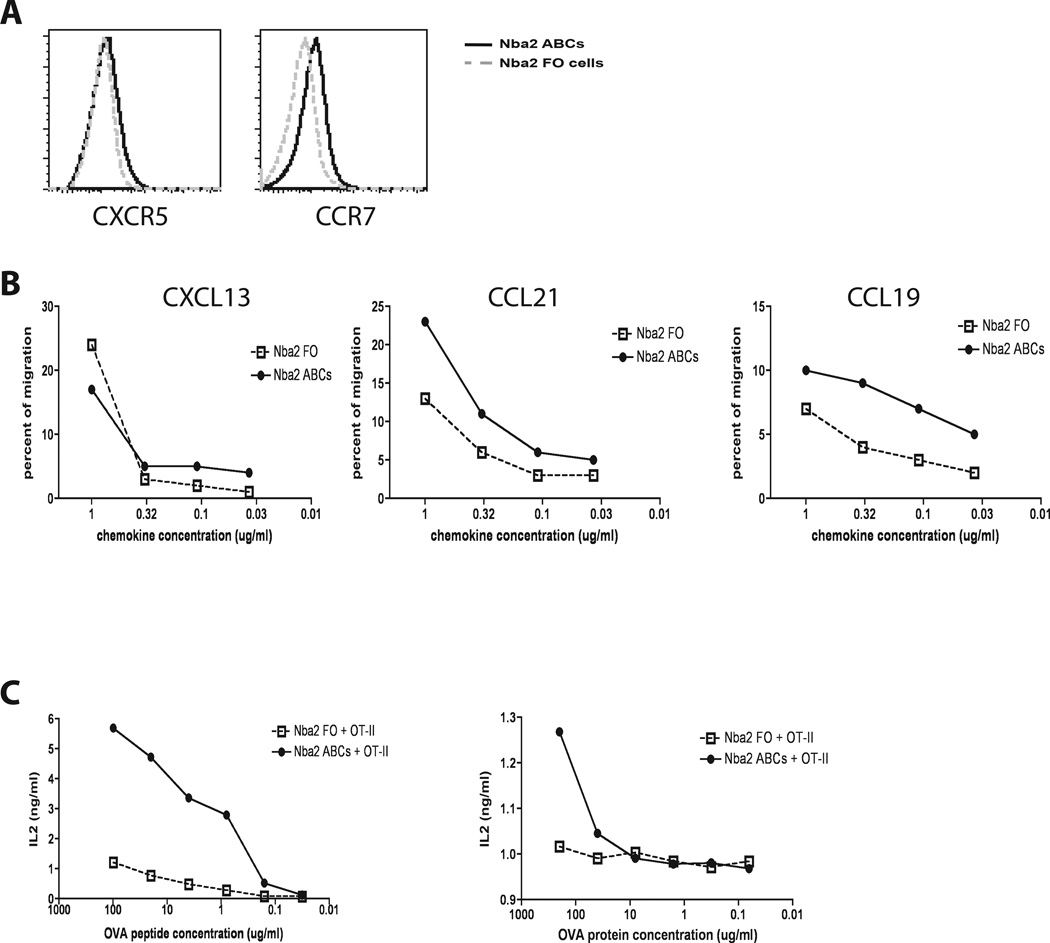
B cells expressing T-bet, CD11b and CD11c are also found in the spleens of autoimmune mice, appearing at about the same time as the first symptoms of autoimmunity (14, 17). These cells have also been termed ABCs, however, they may not share all the properties of the related population in aged female animals. To find out whether ABCs from autoimmune mice have antigen presenting capabilities similar to those of ABCs from elderly animals, ABCs were obtained from the spleens of the autoimmune-prone strain, Nba.2. The donor animals were 8–12 months old and already had autoantibodies in their sera. Expression of chemokine receptors on the surface of ABCs and FO B cells was compared using flow cytometry. As shown in Fig. 6A, ABCs obtained from B6.Nba2 mice expressed higher levels of CCR7 and similar levels of CXCR5 compared to FO B cells obtained from the same mice. Comparison of the ability of the cells to migrate in CXCL13, CCL19, and CCL21 chemokine gradients showed that ABCs and FO B cells from autoimmune mice migrated equally well toward CXCL13 (a chemokine expressed in B cell follicles). However, autoimmune ABCs migrated better towards CCL19 and CCL21 (T cell zone chemokines) than FO B cells did. Together these data suggest that, like ABCs from elderly animals, the ABCs in autoimmune animals may also be localized to the T cell/B cell border (Fig. 6B).
Figure 6. ABCs from autoimmune mice exhibit increased antigen presenting properties compared to FO B cells.
(A) ABCs (solid line) and FO B cells (gray dashed line) from Nba2 autoimmune mice were analyzed for expression of CXCR5 and CCR7. (B) ABCs and FO B cells from Nba2 mice were tested for chemotactic migration toward CXCL13, CCL21 and CCL19. (C) ABCs and FO B cells were tested for antigen presentation at different concentrations of protein (left graph) or peptide (right graph) to primary OT-II T cells. All data represent one of three independent experiments.
Finally, the antigen presenting capabilities of ABCs and FO B cells from B6.Nba2 autoimmune mice were tested in vitro. Similar to the data obtained with cells from aged female mice (Fig. 3), ABCs from autoimmune mice presented antigen (peptide and whole protein) and activated primary antigen-specific T cells significantly better than FO B cells did (Fig. 6C).
Taken together, these data demonstrate that ABCs from autoimmune mice possess characteristics that are similar to those of ABCs from aged wild type mice. Thus ABCs may influence the development and progress of autoimmunity, not only through secretion of autoantibodies (14, 17), but also through presentation of self antigens and activation of autoreactive T cells.
Discussion
We recently identified ABCs as cells that are able to produce self-reactive antibodies and accumulate both with age and during the onset of autoimmunity (14, 17). We have also described a similar subset of B cells in autoimmune patients. However, the function of these cells in both aged and autoimmune mice remained largely unknown.
B cells can contribute to the development of autoimmune disease in various ways. The most obvious and direct way is by the secretion of autoantibodies which target and destroy the animal’s own organs. Our previously published data have shown that ABCs, after activation, are capable of secreting self-reactive antibodies (14, 17). In addition, we have also shown that ABCs are responsible for the appearance of autoantibodies and that the depletion of these cells leads to a reduction in auto-reactive antibodies in the serum (17).
However, there are other ways B cells can contribute to autoimmunity including secretion of pro- or anti- inflammatory cytokines and presentation of self-antigens to autoreactive T cells (32). B cells express MHCII and costimulatory proteins and therefore may present antigen to CD4 T cells. The consequences of such presentation for the T cells depend on the state of the B cells. In some cases B cells, particularly naïve resting B cells, present antigen and tolerize the responding T cells (28). In other cases the target T cells are productively activated. Despite the fact that B cells have been demonstrated to be potent antigen presenting cells, several reports suggest that the presence of B cells in mice is not required for the generation of T cell responses to antigens such as keyhole limpet hemocyanin (KLH), alloantigens, influenza virus and human gamma globulin (33–35). Thus, it has been suggested that DCs, and not B cells, are responsible for antigen presentation and activation of CD4 T cells (36). However, further studies have demonstrated that this is not always the case and B cells, as well as other APCs, can play various roles in antigen presentation depending on the nature of antigen (11, 37). Overall, it has been suggested that, especially at low concentrations of an antigen, antigen specific B cells uptake and present antigen more efficiently than other cells (38). This might be explained by the fact that antigen-specific B cells can capture antigen via their B cell receptor (BCR), while DCs and macrophages use pinocytosis which is 1,000–10,000 less efficient (39).
The fact that B cells can efficiently present antigens makes them good candidates to function as APCs during autoimmunity, since self-antigens are usually present at low concentrations. In support of this, it has been demonstrated that B cells are the major APCs during the induction of various autoimmune diseases (40, 41). Several reports have indicated that the antigen presenting function of B cells is crucial for the development of T1D (42–44). Moreover, NOD mice that contain B cells that can react only with hen egg lysozyme fail to develop diabetes. Conversely, disease is accelerated in NOD mice that express an immunoglobulin heavy chain that is prone to react with insulin (45). These results suggest indicating that autoreactivity of the BCRs in NOD mice is critical for presentation of antigen in this model (46). The role of B cells as APCs in autoimmunity has also been suggested by an MRLlpr model of lupus like disease by generating MRLlpr mice that contain no B cells or that have B cells unable to secrete antibodies (47). In the absence of B cells the mice do not develop lupus. On the other hand, if B cells are present the animals develop disease even if the B cells cannot give rise to soluble antibodies (47).
However, the particular subset of B cells that is responsible for the presentation of the self-antigen in autoimmune settings has never been established. Here we investigated the role of ABCs in antigen presentation in aged wild type or autoimmune B6.Nba2 mice. We explored the localization of ABCs in the spleen and their ability to present antigen and activate antigen-specific T cells. Our data indicate that, due to the expression of both T and B cell specific chemokine receptors, ABCs are localized at the T cell/B cell border in the spleen, as opposed to FO B cells which localize mostly to B cell follicles. Thus, ABCs localization provides greater opportunity to form interactions with antigen-specific T cells.
Coincidental with their localization, ABCs also have better capabilities to present soluble protein antigen to antigen-specific T cells both in vitro and in vivo. The fact that this is true in in vivo assays could be due to the fact that ABCs, but not FO B cells localize to the T cell/B cell border. However, ABCs are also more potent than FO B cells in activating antigen specific T cells in vitro, indicating that ABCs possess cell intrinsic features which allow them to be more efficient at antigen presentation. These intrinsic features could include enhanced antigen uptake and/or processing by ABCs. To test this idea we cultured ABCs and FO B cells with DQ-OVA and compared their ability to generate fluorescent antigen. We did not see any difference in the rate of antigen processing between ABCs and FO B cells (data not shown). Therefore ABCs probably present antigen to T cells in vitro more efficiently than FO B cells do because ABCs express higher levels of MHC class II and higher levels of the costimulatory proteins CD80 and CD86 than FO B cells do.
We have previously showed that ABCs from autoimmune-prone mice can give rise to cells that secrete autoantibodies (14, 17), indicating the specificity of their BCRs for self-antigens. As such, ABCs can take up autoantigens through their antigen receptors and are perfect candidates for activating autoreactive T cells leading to the onset of autoimmunity.
Moreover, multiphoton data demonstrate that ABCs form significantly more stable interactions with T cells, when compared with FO B cells. The stability of APC/T cell contacts has been shown to be critical for the fate of the T cells as more stable interactions usually lead to T cell activation, while less stable ones often lead to tolerance (28). Thus, interactions between ABCs and T cells have a better chance for leading to the activation of the T cell than FO/T cell interactions.
Taken together, the data presented in this report strongly suggest that antigen presentation is one of the major functions of ABCs in both aged and autoimmune mice. This conclusion leads to several questions which have to be explored in the future. For example, how does the depletion of ABCs affect T cell activation during autoimmunity? We have already demonstrated that depletion of ABCs leads to a reduction in the titer of autoantibodies and we proposed that ABCs themselves were the main source of autoantibodies (17). However, in light of this report, it is possible that, in the absence of ABCs, CD4+ T cells do not become activated and therefore do not provide help to other autoreactive B cells.
We have recently reported the appearance of ABCs at the peak of anti-viral humoral responses (15). Our data indicated that, in the absence of ABCs, there is a reduction in anti-viral IgG2a titers and inefficient viral clearance. It will be interesting to determine whether antigen presenting capabilities of ABCs acquired from infected animals are similar to those of aged and autoimmune ABCs. If this is the case, ABCs might play several important roles during viral infection.
In summary, the data presented in this report identify a previously unappreciated function for ABCs as potent antigen presenting cells. The appearance of ABCs in autoimmune mice and autoimmune patients leads to the suggestion that their superior antigen presenting capabilities might be critical for the development of autoimmunity.
Supplementary Material
Acknowledgements
The authors thank Dr. M. Phillips and L. Noges for critical review of the manuscript. We thank Dr. P. Beemiller and B. Leavitt for programming of image analysis Matlab scripts.
This work was supported in part by U.S. Public Health Service grants AI-18785, AI-22295 and AI-046374
References
- 1.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 2.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 5.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 7.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 8.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. Journal of immunology. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 10.Morris SC, Lees A, Finkelman FD. In vivo activation of naive T cells by antigen-presenting B cells. Journal of immunology. 1994;152:3777–3785. [PubMed] [Google Scholar]
- 11.Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. Journal of immunology. 1999;162:5695–5703. [PubMed] [Google Scholar]
- 12.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. Journal of immunology. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 13.Glazier KS, Hake SB, Tobin HM, Chadburn A, Schattner EJ, Denzin LK. Germinal center B cells regulate their capability to present antigen by modulation of HLA-DO. J Exp Med. 2002;195:1063–1069. doi: 10.1084/jem.20012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110:E3216–E3224. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol Res. 2012 doi: 10.1007/s12026-012-8365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. The Journal of experimental medicine. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J Immunol. 2011;186:2889–2896. doi: 10.4049/jimmunol.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 21.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 24.Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 25.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 26.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 27.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 28.Jacobelli J, Lindsay RS, Friedman RS. Peripheral tolerance and autoimmunity: lessons from in vivo imaging. Immunol Res. 2013;55:146–154. doi: 10.1007/s12026-012-8358-7. [DOI] [PubMed] [Google Scholar]
- 29.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 30.Katzman SD, O'Gorman WE, Villarino AV, Gallo E, Friedman RS, Krummel MF, Nolan GP, Abbas AK. Duration of antigen receptor signaling determines T-cell tolerance or activation. Proc Natl Acad Sci U S A. 2010;107:18085–18090. doi: 10.1073/pnas.1010560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampe CS. B Cell in Autoimmune Diseases. Scientifica (Cairo) 2012 doi: 10.6064/2012/215308. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topham DJ, Tripp RA, Hamilton-Easton AM, Sarawar SR, Doherty PC. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. Journal of immunology. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 35.Phillips JA, Romball CG, Hobbs MV, Ernst DN, Shultz L, Weigle WO. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guery JC, Ria F, Adorini L. Dendritic cells but not B cells present antigenic complexes to class II-restricted T cells after administration of protein in adjuvant. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constant S, Sant'Angelo D, Pasqualini T, Taylor T, Levin D, Flavell R, Bottomly K. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. Journal of immunology. 1995;154:4915–4923. [PubMed] [Google Scholar]
- 38.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13:1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- 39.Watts C, Davidson HW. Endocytosis and recycling of specific antigen by human B cell lines. EMBO J. 1988;7:1937–1945. doi: 10.1002/j.1460-2075.1988.tb03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. Journal of immunology. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 41.Wong FS, Wen L, Tang M, Ramanathan M, Visintin I, Daugherty J, Hannum LG, Janeway CA, Jr, Shlomchik MJ. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53:2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 42.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. Journal of immunology. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 43.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. Journal of immunology. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 44.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 45.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. Journal of immunology. 2001;167:5535–5538. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 46.Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur J Immunol. 2002;32:3657–3666. doi: 10.1002/1521-4141(200212)32:12<3657::AID-IMMU3657>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.