Abstract
Background
Net fluid and weight loss are ubiquitously employed to monitor diuretic response in acute decompensated heart failure research and patient care. However, the performance of these metrics has never been critically evaluated. The weight and volume of aqueous fluids such as urine should be nearly perfectly correlated and with very good agreement. As a result significant discrepancy between fluid and weight loss during the treatment of acute decompensated heart failure would indicate measurement error in one or both of the parameters.
Methods
The correlation and agreement (Bland-Altman method) between diuretic-induced fluid/weight loss were examined in three acute decompensated heart failure trials and cohorts: 1) DOSE (n=254) 2) ESCAPE (n=348) the 3) Penn (n=486).
Results
The correlation between fluid and weight loss was modest (DOSE r=0.55; ESCAPE r=0.48; Penn r=0.51; p<0.001 for all) and the 95% limits of agreement were wide (DOSE −7.9 to 6.4 Kg-L; ESCAPE −11.6 to 7.5 Kg-L; Penn −14.5 to 11.3 Kg-L). The median relative disagreement ranged from ± 47.0% to 63.5%. A bias toward greater fluid than weight loss was found across populations (−0.74 to −2.1 Kg-L p≤0.002). A consistent pattern of baseline characteristics or in-hospital treatment parameters that could identify patients at risk of discordant fluid and weight loss was not found.
Conclusions
Considerable discrepancy between fluid balance and weight loss is common in patients treated for acute decompensated heart failure. Awareness of the limitations inherent to these commonly used metrics and efforts to develop more reliable measures of diuresis are critical for both patient care and research in acute decompensated heart failure.
Keywords: Decompensated heart failure, diuretics, weight loss, net fluid output
Introduction
One of the primary objectives in the treatment of acute decompensated heart failure is relief of congestion. Although limited data are available to inform the optimal method for monitoring decongestion in acute decompensated heart failure, serial weight and fluid loss are measures extensively employed in clinical care and research, and use of these metrics is endorsed by cardiovascular society guidelines.1–3 However, in practice it is widely acknowledged that net fluid output and serial changes in weight are difficult to obtain accurately.2,4,5
Given that decongestion of acute decompensated heart failure patients is one of the most common reasons for hospitalization and fluid/weight loss are ubiquitously used in both research and clinical care to monitor diuretic response, a better understanding of the performance of these parameters is critical. The objectives of this manuscript were the following; 1) Further explore the relationship between net fluid output and weight loss including assessment of agreement, bias, and patient/treatment related factors predicting lack of agreement. 2) Evaluate if discrepancy between fluid and weight loss influences discharge markers of decongestion and carries prognostic importance. Given that local practice patterns and fidelity of data collection can vary between different clinical and research populations, our objective was to explore these associations across multiple different settings to evaluate the consistency and generalizability of these findings.
Methods
The relationship between fluid and weight loss was explored in the 1) Diuretic Optimization Strategies Evaluation (DOSE) trial dataset, 2) Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial dataset and 3) the Penn acute decompensated heart failure clinical cohort. Given that DOSE was a contemporary trial of decongestive strategies; the primary analyses were undertaken in DOSE with ESCAPE and Penn acute decompensated heart failure primarily used for validation. Inclusion in this analysis required administration of intravenous loop diuretics to ensure active diuresis was a goal of the treatment team and availability of data on fluid and weight loss. Additional detail on each cohort can be found below.
DOSE Trial
The DOSE trial was a multicenter, randomized, double-blind, placebo controlled trial of diuretic strategies in patients with acute decompensated heart failure. The study design and results of have been previously published.6,7 The study used a 2×2 factorial design randomizing patients to a strategy of high- vs. low-dose furosemide treatment and continuous infusion vs. bolus furosemide administration. Eligibility criteria included an oral loop diuretic dose 80–240 mg of furosemide equivalents. The randomized intervention was continued for 72 hours and the primary ascertainment of fluid and weight loss occurred over this interval. In cases where the length of stay was less than 72 hours, the 48 hour fluid or weight loss was used to calculate the change.
ESCAPE Trial
The ESCAPE Trial was a randomized, multicenter trial of therapy guided by pulmonary artery catheter vs. clinical assessment in hospitalized patients with acute decompensated heart failure. Methods and results have been published previously.8,9 Inclusion criteria included treatment with more than 160 mg of furosemide equivalents daily and at least 1 sign and 1 symptom of congestion. Net fluid output and change in body weight were ascertained from randomization to discharge. Patients in the ESCAPE population that did not have data available to calculate net urine output (n=19) and patients that did not receive IV loop diuretics (n=24) were excluded from the analysis. Additional details of the assembly and characteristics of this subgroup of the ESCAPE trial have been previously published.10
Penn Cohort
Consecutive charts of patients with a primary discharge diagnosis of congestive heart failure who were admitted to non-interventional cardiology and internal medicine services at the Hospital of the University of Pennsylvania from 2004 to 2009 were reviewed. Briefly, inclusion required a B-type natriuretic peptide (BNP) level of > 100 pg/mL within 24 hours of admission, receipt of intravenous loop diuretics, and availability of data on fluid intake and output during the hospitalization. Additional details on the assembly of this cohort, including a consort diagram, have been previously published.10 Net fluid output and change in body weight were ascertained between baseline and discharge.
Given that the correlation between fluid and weight loss appear to be limited (when it should approach unity) and available data does not support either fluid or weight loss as the primary source of this error; the average of fluid and weight loss were taken when a reference was required (i.e., Bland-Altman plots) and expressed as Kg-L. In all cohorts estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology collaboration equation.11 When the data was available, change in markers of hemoconcentration (hemoglobin, hematocrit, albumin, and total protein) were evaluated as the relative change in each marker from baseline to discharge. The study was approved by the Yale University Institutional Review Board.
Statistical methods
The primary analytic goals were to determine the correlation, agreement, and bias between fluid and weight loss across several heart failure populations and determine patient or treatment parameters that were associated with disagreement. As a result, the primary outcomes of this analysis were the correlation coefficients and the bias and 95% limits of agreement using the methodology described by Bland and Altman.12,13 Values reported are mean ± SD, median (quartile 1 – quartile 4) and percentile. Independent Student’s t test, the Wilcoxon Rank Sum test, or the Mann Whitney U tests were used to compare continuous variables between groups of patients. The chi-square test was used to evaluate associations between categorical variables. Although previous investigation from the DOSE trial reported Pearson correlations, Spearman’s rho was utilized in this analysis to minimize the effect of outliers which are common with fluid and weight loss. Bland-Altman Plots were constructed by plotting the difference between fluid and weight loss on the X axis and the average of fluid and weight loss on the Y axis. To allow easy visual comparison of the plots between cohorts, the range of the X axis was set at 7.5 times the mean of the average of fluid and weight loss and the Y axis was set at the 1st and 99th percentile of the average of fluid and weight loss. Bias was calculated as the mean of the difference between fluid and weight loss and the 95% limits of agreement were plotted at 1.96 times the standard deviation of the bias. The hypothesis that the bias was different than zero was tested using a one sample t test. Proportionality of the bias across the spectrum of different average fluid/weight loss was evaluated using Spearman’s rho. Proportional hazards modeling was used to evaluate time-to-event associations with 1) death, rehospitalization, or emergency room visits (DOSE) 2) death or rehospitalization (ESCAPE) or 3) death (Penn). Candidate covariates entered in the model were all baseline, or in-hospital, or discharge characteristics that differed between groups of patients with discordant fluid and weight loss with a p≤0.2 (i.e., Table 1 and 3 in DOSE). Statistical analysis was performed with IBM SPSS Statistics version 19.0 (IBM Corp., Armonk, NY) and statistical significance was defined as a 2-tailed p<0.05.
Table 1.
Baseline characteristics of the DOSE trial population stratified by relative agreement between fluid and weight loss
| Characteristic | Overall cohort (n=254) | Fluid and weight loss within ± 50% (N=122) | Fluid ≥50% more than weight loss (N=84) | Weight ≥50% more than fluid loss (N=48) | p-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 67 ± 13.6 | 67 ± 14 | 66 ± 13 | 68 ± 14 | 0.594 |
| Black race | 20.1% | 15.6% | 27.4% | 18.8% | 0.338 |
| Male | 72.0% | 74.6% | 66.7% | 75.0% | 0.405 |
| Medical History | |||||
| Hypertension | 82.3% | 92.8% | 81.0% | 83.3% | 0.923 |
| Diabetes | 53.1% | 50.8% | 56.0% | 54.2% | 0.759 |
| Ischemic heart disease | 58.3% | 57.4% | 57.1% | 62.5% | 0.804 |
| Ejection fraction ≥40% | 34.1% | 32.5% | 33.7% | 39.1% | 0.719 |
| Gout | 24.8% | 23.8% | 23.8% | 29.2% | 0.739 |
| Non-ischemic cardiomyopathy | 50.0% | 53.3% | 48.8% | 43.8% | 0.516 |
| Admission Physical Exam | |||||
| Weight (lbs) | 217 ± 65 | 217 ± 61 | 218 ± 69 | 216 ± 68 | 0.855 |
| Heart rate (beats/min) | 78 ± 15 | 77 ± 17 | 76 ± 14 | 80 ± 14 | 0.424 |
| Systolic blood pressure (mm Hg) | 118 ± 19 | 118 ± 18 | 118 ± 20 | 117 ± 19 | 0.886 |
| Jugular venous distention | 63.0% | 70.6% | 57.0% | 53.3% | 0.050 |
| Severe edema | 32.7% | 36.1% | 32.1% | 25.0% | 0.380 |
| Rales | 59.5% | 56.2% | 60.7% | 66.0% | 0.494 |
| S3 | 19.8% | 18.6% | 18.3% | 25.5% | 0.552 |
| Randomized intervention | |||||
| Continuous infusion | 47.6% | 50.8% | 40.5% | 52.1% | 0.272 |
| High dose | 51.6% | 57.4% | 45.2% | 47.9% | 0.197 |
| Cardiac Function and Symptoms | |||||
| Ejection fraction (%) | 35.1 ± 17.5 | 34.6 ± 17.9 | 35.4 ± 16.6 | 35.8 ± 18.2 | 0.851 |
| NYHA Class | 3.2 ± 0.6 | 3.3 ± 0.6 | 3.3 ± 0.5 | 3.2 ± 0.6 | 0.544 |
| Laboratory Values | |||||
| Serum sodium (mEq/L) | 138.2 ± 3.9 | 138.3 ± 3.8 | 138.0 ± 4.1 | 138.3 ± 3.6 | 0.797 |
| NT-Pro BNP (pg/mL) | 4490 (2443–11327) | 4070 (2136–11472) | 5178 (3073–11799) | 5092 (2577–9882) | 0.301 |
| eGFR (mL/min/1.73m2) | 52.8 ± 24.1 | 54.6 ± 24.8 | 49.0 ± 22.0 | 55.0 ± 25.4 | 0.251 |
| Blood urea nitrogen (mg/dL) | 38.8 ± 22.6 | 36.6 ± 21.8 | 42.3 ± 22.2 | 38.3 ± 25.2 | 0.078 |
| Cystatin C (mg/L) | 1.6 ± 0.6 | 1.6 ± 0.5 | 1.7 ± 0.6 | 1.6 ± 0.6 | 0.148 |
| Hemoglobin (g/dL) | 11.7 ± 2.0 | 11.9 ± 1.9 | 11.2 ± 2.0 | 11.9 ± 2.1 | 0.075 |
| Uric Acid (mg/dl) | 9.9 ± 2.6 | 9.8 ± 2.5 | 10.3 ± 2.7 | 9.3 ± 2.7 | |
| Troponin (pg/ml) | 43.2 ± 75.9 | 48.5 ± 96.3 | 33.1 ± 37.1 | 46.9 ± 65.5 | |
| Renin (ng/ml/hr) | 4.32 (0.8–16.0) | 4.9 (0.9–15.7) | 5.4 (0.8–20.9) | 3.3 (0.7–14.2) | |
| Aldosterone (pg/ml) | 215 (116–366) | 186 (97–345) | 245 (142–451) | 225 (108–353) | |
| Medications (Admission) | |||||
| β-Blocker | 83.5% | 82.0% | 86.9% | 81.3% | |
| ACE inhibitor or ARB | 60.6% | 65.6% | 50.0% | 66.7% | |
| Digoxin | 29.1% | 24.6% | 34.5% | 31.3% | |
| Loop diuretic dose (mg) | 120 (80–160) | 120 (80–160) | 120 (80–160) | 120 (80–160) | |
| Aldosterone antagonist | 29.9% | 27.9% | 27.4% | 39.6% | |
NTpro-BNP: N terminal pro B-type natriuretic peptide, eGFR: Estimated glomerular filtration rate. ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker.
Significant p value.
Table 3.
Correlation between fluid and weight loss on the individual days of hospitalization in the DOSE trial.
| n (%) | r | p | |
|---|---|---|---|
| Baseline to 24 hours | 283 (92) | 0.47 | <0.001 |
| 24 to 48 hours | 267 (87) | 0.47 | <0.001 |
| 48 to 72 hours | 233 (76) | 0.30 | <0.001 |
| 72 to 96 hours | 194 (63) | 0.23 | 0.001 |
N (%) represents the patients in the DOSE trial with data available during this interval.
Results
DOSE Trial
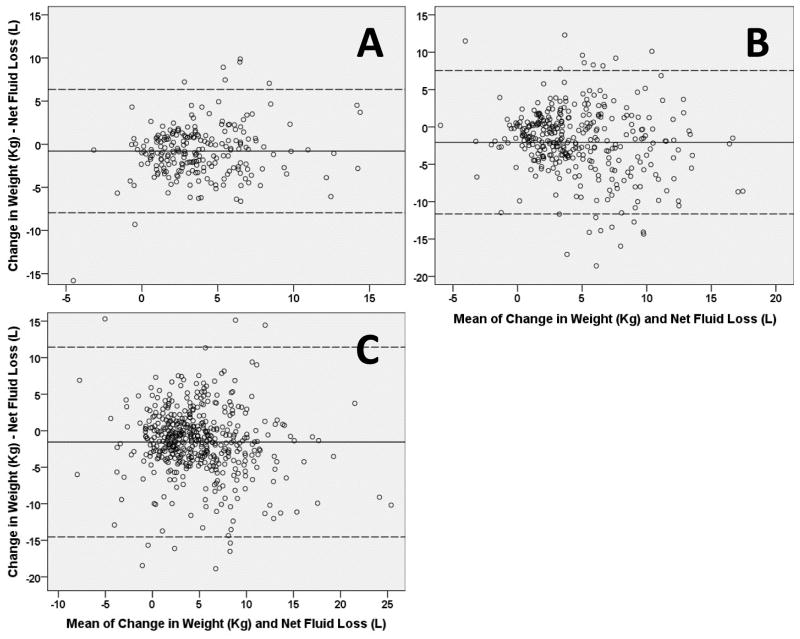
Baseline characteristics of the analyzed cohort are presented in Table 1. Overall 17.5% of the DOSE population was missing either fluid or weight loss over the 72 hour intervention period (Table 2). Amongst these patients, the correlation between net fluid and weight loss was modest (Table 2). The correlation tended to be worse on individual treatment days and decline further as the hospitalization progressed (Table 3). Agreement between the two metrics was poor with the 95% limits of agreement spanning 3.8 times the average fluid/weight loss of the population (Table 2, Figure 1A). There was a bias toward greater fluid than weight loss and this bias was largely constant across different degrees of fluid and weight loss (Table 2, Figure 1A).
Table 2.
Description of the correlation and agreement between fluid and weight loss across the different cohorts
| Trial/Cohort | |||
|---|---|---|---|
| DOSE | ESCAPE | PENN | |
| Setting | Inpatient randomized trial of loop diuretic strategies in acute decompensated heart failure | Inpatient randomized trial of pulmonary artery catheters in acute decompensated heart failure | Inpatient retrospective observational acute decompensated heart failure cohort |
| Length of stay (days) | 5 (3–9) | 7 (5–10) | 6 (4–9) |
| Diuretic observation period (days)* | 3 (3–3) | 7 (5–10) | 6 (4–9) |
| Missing weight loss | 12% | 11% | 26% |
| Missing net fluid loss | 12% | 4% | 24% |
| IV loop diuretics not administered | 0 | 6% | 7% |
| N available for primary comparisons | 254 (82.5%) | 348 (80%) | 486 (47%) |
| Mean weight loss (Kg) | 3.4 ± 4.1 | 3.6 ± 4.7 | 4.1 ± 6.2 |
| Mean net fluid loss (L) | 4.2 ± 3.1 | 5.8 ± 5.2 | 5.2 ± 6.3 |
| Correlation between net fluid and weight loss (r, p value) | r=0.55, p<0.001 | r=0.48, p<0.001 | r=0.51, p<0.001 |
| 95% limits of agreement (Kg-L) | −6.4 to 7.9 | −7.5 to 11.6 | −11.4 to 14.5 |
| Ratio of 95% limits of agreement to average fluid-weight loss‡ | 3.8 | 4.1 | 5.4 |
| Median relative disagreement (%)§ | ±47 (22.6–97.7) | ±63.5 (23.4–120.6) | ±51.7 (16.3–108.6) |
| Mean Bias (Kg-L) | −0.74 ± 3.8 | −2.1 ± 4.9 | −1.6 ± 6.6 |
| p value different than zero | 0.002 | <0.001 | <0.001 |
| Proportionality of bias across values of fluid and weight loss (r, p value) | r=0.13, p=0.06 | r=0.16, p=0.004 | r=0.13, p=0.005 |
Diuretic observation period indicates the period of time during which the fluid and weight loss were determined.
Calculated as the ratio of the span of the 95% limits of agreement divided by the mean of fluid and weight loss in the population.
Calculated as the absolute value of the percentage difference between fluid and weight loss
Figure 1. Bland-Altman plots of the agreement between fluid and weight loss in the studied HF populations.
Panel A: DOSE trial; Panel B ESCAPE trial; Panel C Penn cohort. Solid lines represent the mean bias and dashed lines the 95 percent limits of agreement.
Baseline characteristics, in-hospital treatment and outcome parameters, and discharge parameters were largely similar between categories of patients’ fluid and weight loss within ± 50%, 50% greater fluid than weight loss, and 50% greater weight than fluid loss (Tables 1 & 4). There was a small but statistically significant difference in the change in blood urea nitrogen with a greater worsening in patients with significantly greater weight than fluid loss (Table 4). In patients with >50% higher weight than fluid loss, the net fluid intake was similar, however the ratio of fluid intake to output was significantly higher (Table 4). On univariate analysis the rate of death, rehospitalization, or emergency room visits did not differ significantly between the groups (p=0.062) but outcomes were significantly worse in patients with fluid>weight loss compared to patients with similar fluid and weight loss (HR=1.5, 95% CI=1.0–2.2, p=0.041). However, this relationship was no longer significant after adjustment for baseline and in-hospital characteristics (HR=1.3, 95% CI 0.8–2.1, p=0.25). Serial measures of hemoconcentration were not available in the DOSE trial dataset.
Table 4.
In-hospital and discharge characteristics of the DOSE trial population stratified by relative agreement between fluid and weight loss
| Characteristic | Fluid and weight loss within ± 50% (N=122) | Fluid ≥50% more than weight loss (N=84) | Weight ≥50% more than fluid loss (N=48) | p-value |
|---|---|---|---|---|
| In hospital treatment | ||||
| Inotrope | 11.5% | 11.9% | 12.5% | 0.982 |
| Vasodilator | 8.2% | 3.6% | 8.3% | 0.375 |
| Thiazide diuretic | 22.9% | 16.3% | 11.6% | 0.229 |
| Total study drug loop diuretic (mg) † | 509 (310–829) | 483 (288–735) | 436 (226–733) | 0.448 |
| Total open label loop diuretic (mg)† | 0 (0–53) | 0 (0–75) | 0 (0–125) | 0.742 |
| Total loop diuretic (mg)† | 598 (383–892) | 570 (325–870) | 536 (340–766) | 0.571 |
| In hospital findings/outcomes | ||||
| Fluid intake (ml)† | 4113 ± 1319 | 4270 ± 1430 | 4343 ± 1809 | 0.740 |
| Fluid output (ml)† | 9134 ± 3409 | 8474 ± 2772 | 5967 ± 2116 | 0.000* |
| Ratio of intake to output† | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.7 ± 0.2 | 0.000* |
| Net fluid output (ml)† | 5021 ± 3221 | 4204 ± 2528 | 1624 ± 1503 | 0.000* |
| Weight loss (kg)† | 4.9 ± 3.0 | 1.3 ± 1.5 | 3.1 ± 7.3 | 0.000* |
| Congestion free at 24 hours | 3.3% | 1.2% | 2.1% | 0.616 |
| Congestion free at 48 hours | 7.6% | 6.0% | 6.4% | 0.905 |
| Congestion free at 72 hours | 13.0% | 6.4% | 14.6% | 0.266 |
| Patients’ global assessment of symptoms† | 4408 ± 1394 | 4166 ± 1510 | 4065 ± 1260 | 0.255 |
| Treatment failure† | 35.2% | 40.5% | 41.7% | 0.644 |
| Worsening or persistent HF | 23.8% | 29.8% | 25.0% | 0.619 |
| Length of stay (days) | 5 (3–9) | 6 (4–9) | 6 (3–10) | 0.108 |
| Change in laboratory parameters from randomization to 72 hours | ||||
| NTpro-BNP (pg/ml) | −674 (−2580 to 27) | −957 (−2836 to 264) | −994 (−2581 to −216) | 0.847 |
| Creatinine (mg/dl) | 0.4 ± 0.3 | 0.0 ± 0.3 | 0.1 ± 0.3 | 0.166 |
| Increase in creatinine ≥ 0.3 mg/dl | 12.3% | 17.9% | 27.1% | 0.066 |
| Cystatin-C (mg/L) | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.2 ± 0.4 | 0.678 |
| eGFR (ml/min/1.73m2) | −2.2 ± 11.3 | −1.1 ± 10.6 | −3.1 ± 12.9 | 0.501 |
| Blood urea nitrogen (mg/dL) | 0.9 ± 10.1 | 3.2 ± 13.0 | 5.5 ± 9.1 | 0.022* |
| Serum sodium (mmol/L) | 0.1 ± 3.2 | 0.3 ± 3.4 | −1.0 ± 3.6 | 0.130 |
| Medications (Discharge) | ||||
| β-Blocker | 75.2% | 86.7% | 78.3% | 0.129 |
| ACE inhibitor or ARB | 59.5% | 59.0% | 60.9% | 0.979 |
| Digoxin | 32.2% | 44.6% | 30.4% | 0.134 |
| Thiazide diuretic | 14.8% | 12.3% | 16.3% | 0.814 |
| Loop diuretic dose (mg) | 160 (80–200) | 160 (80–240) | 160 (80–160) | 0.546 |
| Aldosterone antagonist | 35.5% | 37.3% | 32.7% | 0.865 |
| Discharge Physical Examination findings | ||||
| Heart rate (beats/min) | 78 ± 16 | 76.5 ± 13.0 | 77.0 ±11.5 | 0.900 |
| Systolic blood pressure (mmHg) | 109 ± 15 | 112 ± 19 | 113 ± 17 | 0.629 |
| Jugular venous distention | 15.4% | 16.0% | 29.2% | 0.305 |
| Edema | 42.6% | 39.2% | 19.6% | 0.628 |
NTpro-BNP: N terminal pro B-type natriuretic peptide, eGFR: Estimated glomerular filtration rate. ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker.
variable ascertained from randomization to 72 hours.
Significant p value.
Validation in the ESCAPE and Penn Cohorts
Data on change in weight and fluid status were missing in a similar proportion of the ESCAPE trial, but significantly greater percentage of the observational Penn cohort (Table 2). Overall, findings were similar to DOSE with a modest correlation between fluid and weight loss, wide 95% limits of agreement, large relative disagreement, and a bias toward greater fluid than weight loss (Table 2). The correlation between measures of hemoconcentration and fluid and weight loss was higher for change in weight than fluid loss, particularly for change in albumin and total protein (Table 5).
Table 5.
Correlation of measures of hemoconcentration with fluid and weight loss
| % Change in Hematocrit | % Change in Hemoglobin | % Change in Albumin | % Change in Total Protein | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| ESCAPE† | ||||||||
| Net Fluid Output | 0.133 | 0.022* | 0.121 | 0.039* | 0.158 | 0.058 | 0.171 | 0.052 |
| Weight Change | 0.140 | 0.021* | 0.132 | 0.030* | 0.231 | 0.006* | 0.249 | 0.005* |
| PENN | ||||||||
| Net Fluid Output | 0.108 | 0.008* | 0.118 | 0.003* | -- | -- | -- | -- |
| Weight Change | 0.235 | <0.001* | 0.219 | <0.001* | -- | -- | -- | -- |
data on total protein and albumin were available in n=144 patients in the ESCAPE trial.
Significant p value.
A greater number of differences between patients with relative disagreement between fluid and weight loss was found in ESCAPE and particularly in the observational Penn cohort where fluid and weight losses were not ascertained as part of a research protocol (Supplementary Tables 1–4). In both cohorts the general trend emerged for greater baseline congestion in the group with agreement between fluid and weight loss, more intense in-hospital treatment, but at the time of discharge measures of adequacy of decongestion were either not different across groups or superior in the groups with concordant information on fluid and weight loss (Supplementary Tables 1–4). Many of these differences appeared to be driven by the group with greater weight than fluid loss (Supplementary Tables 1–4).
The incidence of death or rehospitalization in the ESCAPE cohort was not different between groups with greater or less than 50% discrepancy in fluid and weight loss (adjusted p=0.56). However, in the Penn cohort, the risk of death was significantly different between groups (adjusted p=0.023) which was primarily driven by worse outcomes in patients with 50% greater weight than fluid loss (adjusted HR=1.5, 95% CI 1.0 to 2.2, p=0.036).
Discussion
The principal finding of this analysis is that the correlation and agreement between net fluid balance and weight loss in the setting of treatment for acute decompensated heart failure is substantially lower than expected. It is widely acknowledged by clinicians that care for heart failure patients that it is challenging to obtain accurate data on fluid and weight loss. Although it is impossible to determine from this analysis how much of this discrepancy is driven by fluid vs. weight loss, in all likelihood it is both. The limitations to ascertainment of accurate net fluid balances are well known and consist of factors such as unrecorded intake, episodes of incontinence, lack of adherence with urine collection by patients/staff, insensible losses, unaccounted stool, and fluid consumed in the form of food (i.e., fruit). However, daily weights are also challenging to obtain accurately. This error takes the form of not weighing patients on the same scale, weighing different times in the day and/or in relation to meals/urination/defecation, use of bed scales, and different clothing or devices (i.e., telemetry boxes) between weighing. Given the large number of potential sources of error, it is not surprising that such large discrepancies were evident across the cohorts. Furthermore, in each patient the predominant factor causing inaccuracy is likely different. Given that identifying the source of error is often times not straightforward, and the fact that it is challenging even in expert hands to monitor day to day diuretic progress with improvement in symptoms or physical examination findings, our inability to accurately monitor diuretic progress is a major problem.
The implications of these findings for clinical practice are relatively straight-forward; clinicians should be cognizant of the limitations inherent to fluid and weight loss and strive to diligently obtain both parameters then evaluate on a case by case basis how to apply the data toward treatment decisions in individual patients. The ramifications of these findings for clinical trial endpoints present more of a challenge. The limited correlation/agreement between fluid and weight loss, parameters which essentially are measuring the same signal, indicates that one or both of the metrics is incorrect in a substantial percentage of cases. Notably, the 95% limits of agreement spanned a ~4–5 fold larger amount of fluid/weight loss than the average fluid/weight loss of the inpatient acute decompensated heart failure populations. In addition to the fact that it is obviously unacceptable for a clinical trial endpoint not to accurately measure the signal of interest, the increased signal to noise ratio introduced by the error inherent to these metrics will substantially increase the required sample size for these studies. Furthermore, it is plausible that various acute decompensated heart failure interventions could differentially influence fluid vs. weight loss. For example, tolvaptan is known to increase thirst potentially leading to underestimation of fluid intake thus biasing towards a greater recorded net fluid loss.14
As we move forward toward better defining optimal metrics of diuresis and decongestion, an important consideration is that the majority of the available surrogates for diuresis/decongestion all measure slightly different aspects of physiology. Importantly, many of these metrics can be considered more as exposure variables than true endpoints. For example, a weight loss of 5 lbs per day for 4 days may represent either significant under or over treatment if we knew them to be 50 lbs vs. 15 lbs volume overloaded respectively. However, in both sceneries, 5 lbs per day may have represented an ideal diuresis on hospital day 1. Moreover, due to the complex physiology of body fluid homeostasis, parameters such as hemoconcentration and cardiac filling pressures represent only a snapshot in time of one dimension of volume overload.15 For example, a patient with acute myocardial infarction can be euvolemic but with a massively elevated pulmonary capillary wedge pressure whereas a critically ill patient with sepsis can have massive volume overload but low cardiac filling pressures and blood volume. Furthermore, both blood volume and filling pressures will be only transiently improved if significant extravascular volume overload has not yet equilibrated. As such, an ideal marker of true euvolemia will need to incorporate multiple parameters which describe physiology such as blood volume, filling pressures, extra cellular fluid volume, plasma refill rate, and arterial and venous tone. Furthermore, it will be important in planning and interpreting clinical trials to be cognizant if the strategy being tested is to improve the rate/safety of the exposure (i.e., rate of fluid removal, rapidity with which symptoms are improved, or lack of increase in creatinine at a specific time point) or the very different endpoint of bringing a patient to true euvolemia. While we do not know whether it is a biological or a logistical variability in fluid and weight loss driving the discrepancy observed in this study, the true challenge will be not to gauge treatment success on the degree of change but rather to the ultimate target of euvolemia.
Limitations
The data sources for this study consisted of post-hoc analysis of clinical trial populations and a retrospective chart review of a single center of hospitalized acute decompensated heart failure patients. Although the fact that three distinct populations were used with relatively consistent results across the cohorts, the degree of generalizability of these findings to the general heart failure population is unclear. However, given that the populations consisted of clinical trials of acute decompensated heart failure or patients at tertiary care centers with dedicated heart failure programs, results are unlikely to be substantially better in general practice. The current data present correlation and relative agreement between the metrics. However, with the available data and lack of gold standard it is impossible to determine exactly where the errors are coming from and which metric is superior.
Conclusion
Despite essentially measuring the same signal, fluid and weight loss commonly have limited correlation and agreement to clinically significant degrees. Clinicians and researchers alike should be cognizant of the substantial limitations inherent to fluid and weight loss when caring for patients or designing clinical trials of decongestive therapies.
Supplementary Material
Clinical significance.
During the treatment of acute decompensated heart failure, fluid and weight loss appear to have surprisingly limited correlation and agreement
This discrepancy was consistently found across 3 diverse heart failure populations, including a randomized clinical trial of loop diuretic therapy
Patient and in-hospital treatment characteristics could not explain the disagreement between fluid and weight loss
Awareness of these limitations is critical for both patient care and research
Acknowledgments
This manuscript was prepared using ESCAPE and DOSE research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ESCAPE or DOSE study investigators or the NHLBI. This work was supported in part by NIH Grants, 1K23HL114868, L30HL115790 (JT) and K24DK090203 (CP):
Footnotes
Author statements: All authors report no relevant conflicts of interest and had aces’ to the data and a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Mentz RJ, Kjeldsen K, Rossi GP, Voors AA, Cleland JG, Anker SD, Gheorghiade M, Fiuzat M, Rossignol P, Zannad F, Pitt B, O’Connor C, Felker GM. Decongestion in acute heart failure. European journal of heart failure. 2014;16:471–482. doi: 10.1002/ejhf.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, Braunwald E, O’Connor CM, Felker GM. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circulation Heart failure. 2013;6:240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felker GM, O’Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circulation Heart failure. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah MR, O’Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 9.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, Investigators E, Coordinators ES. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA: the journal of the American Medical Association. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 10.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WH. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circulation Heart failure. 2014;7:261–270. doi: 10.1161/CIRCHEARTFAILURE.113.000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term Clinical Effects of Tolvaptan, an Oral Vasopressin Antagonist, in Patients Hospitalized for Heart Failure: The EVEREST Clinical Status Trials. JAMA: The Journal of the American Medical Association. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 15.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–524. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.