Abstract
This review examines important robust methods for sustained, steady state, in vitro culture. To achieve ‘physiologically relevant’ tissues in vitro additional complexity must be introduced to provide suitable transport, cell signaling, and matrix support for cells in 3D environments to achieve stable readouts of tissue function. Most tissue engineering systems draw conclusions on tissue functions such as responses to toxins, nutrition or drugs based on short term outcomes with in vitro cultures (2–14 days). However, short term cultures limit insight with physiological relevance, as the cells and tissues have not reached a steady state.
Keywords: long term culture, tissue engineering, microfluidics, two dimensional culture, three dimensional culture, bioreactors
Defining ‘physiological relevance’ in tissue engineering approaches
The goal of tissue engineering is to generate living tissue constructs in vitro that are morphologically and functionally similar to native tissue. Growing physiologically relevant tissues requires multidisciplinary research where the resulting tissues can be used for the study of human development and disease, to test the efficacy and toxicity of compounds and treatments, and for regenerative medicine applications. In tissue engineering reports, there are many terms that are commonly used to describe outcome measurements of these tissues including: ‘physiological relevance’, ‘mature’ and ‘stable.’ While all of these terms imply that the in vitro tissues behave in a similar manner to in vivo tissues, they may not describe essential details accurately, unless the terms are properly defined for each case.
To make a general, broad definition, ‘physiological relevance’ is the characteristic of (or corresponding to) healthy or normal biological functioning. However, in different situations this will mean different things related to tissue engineering. For instance, if the goal of the study is to screen drug candidates during preclinical drug development for liver treatment, recreating general cellular functions (oxygen uptake, amino acid metabolism and substrate consumption) and liver-specific functions (drug-metabolizing capacities and the production of liver-specific metabolites) can qualify as physiologically relevant [1]. However, for implantation in a patient suffering from liver failure, the liver will have to additionally contain bile ducts, a functional vascular network and a hepatic microarchitecture, as well as have a substantial regenerative capacity, to be considered physiologically relevant [2].
In the same context, physiologically relevant tissues should contain ‘mature’ cells specific to the tissue and goal of the study. However, this brings up the question – what is a mature cell? Each tissue contains different cell types that vary depending on the tissue and the state of maturation of that tissue. Therefore, a ‘mature cell’ can be defined as a cell that exhibits normal biological functions in the ‘developed’ form of the tissue. ‘Developed’ in this case refers to the stage of the desired tissue, which can be embryonic, young, aged, diseased, etc. depending on the goals of the study.
After establishing the targeted or required ‘mature’ status of cells within the tissue it is important to establish when the tissue has become ‘stable’. Importantly, having mature cells does not mean the tissue is stable, as the tissue could still be adjusting, expanding, and forming. Therefore, stability can be defined as a tissue that is not changing with time. This can be determined by tracking material properties [3], matrix content [4], or by other markers of function such as secreted proteins [5–8] or endogenous signals [9, 10]. A homeostatic, ‘stable’ tissue is essential for tissue engineering as a baseline for in vitro studies of the efficacy and toxicity of compounds or to maintain phenotype upon implantation for regenerative applications. It is important to note the goals of the study, however, in some disease states, such as tumors, ‘stable’ tissues would not be the goal.
In this review we describe strategies for improving the physiological relevance of tissue engineered constructs, acknowledging that ‘physiological relevance’ will vary in definition in different contexts. We will touch upon some of the more common strategies for forming ‘stable’ biological functions with ‘mature’ cells that are more in line with in vivo function, with a specific focus on the temporal component of culturing engineered tissues in vitro.
Strategies for improving the physiological relevance of long term cultures
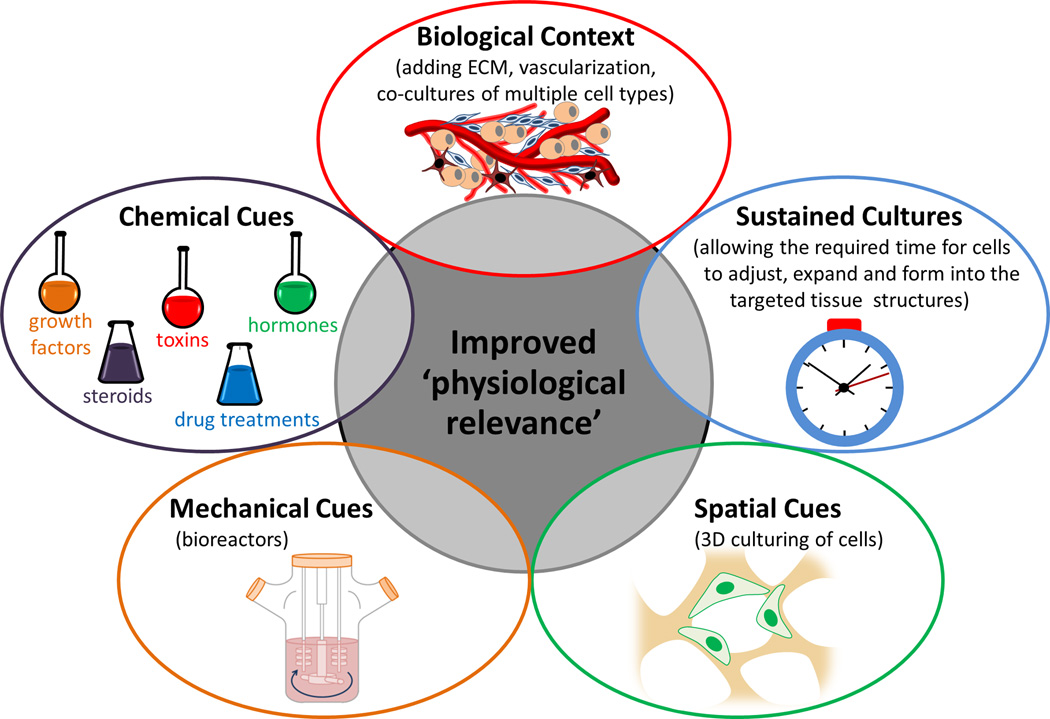
While the endpoint criteria are specific to the tissue of interest and the desired application common strategies to improve the physiological relevance of tissues (Figure 1) include recapitulating: biological context (such as the extracellular matrix, vascularization and cell types), chemical and mechanical cues (through the use of reagents and bioreactors) and incorporating spatial cues (by culturing cells in 3D). All of these strategies require the optimization of culture conditions in an attempt to form mature, stable tissues.
Figure 1.
To improve the 'physiological relevance' of engineered tissues biological context (extracellular matrix, vascularization and cell types), chemical cues, mechanical cues (bioreactors), spatial cues (culturing cells in 3D), and temporal timing of cultures should be considered.
Recapitulating biological context
Each tissue exhibits variability in the amount and type of extracellular matrix components [11, 12]. Therefore, for the in vitro environment the cells should be carefully considered for each tissue to mimic the tissue content and properties. Biomaterial scaffolds predominately consist of ceramics (examples: hydroxyapatite or tri-calcium phosphate), synthetic polymers (examples: polystyrene, poly-L-lactic acid, polyglycolic acid, poly-D,L-lactic-co-glycolic acid), or natural polymers (examples: collagen, alginate, silk) with varying physicochemical properties, architecture, and degradability [13]. In particular, the porosity, pore dispersal, surface area, mechanical properties, and surface chemistry influence the attachment, migration, proliferation, and production of extracellular matrix by the seeded cells within the scaffold. Additionally, to mimic other aspects of the ECM the process can be aided with a hydrogel (examples: Matrigel, collagen), or the hydrogel can be used as a standalone 3D matrix lacking the structural integrity of a more robust, rigid porous scaffold.
One of the major challenges of generating matrix-rich, dense tissues, however, is the limited mass transfer distances for nutritional supply and waste removal. To address this issue, tissue vasculature (which provides and removes nutrients in situ) can be recreated (Box 1). Moreover, to recapitulate the biological context, cellular interactions within a tissue must be considered to help maintain tissue specificity and homeostasis which is fostered through cell-cell signaling. Enhanced differentiation and survival has been achieved in many organ systems by co-culturing relevant cell types, for example: skin [14], neural tissue [15, 16], bone [17], and liver [18]. Co-cultures lead to increased extracellular matrix deposition over mono-cultures, including fibronectin deposits in glomerular tissue [19] and collagen deposition and mineralization in bone tissue constructs [20]. Improved function has been demonstrated by co-cultures including beating cardiomyocytes, which increased fluctuations in intracellular calcium ion concentrations not achieved in mono-cultures [21]. Additionally, proper morphology has been observed in co-cultures for cardiomyocytes [21], endothelial cells [18], and epithelial cells [19] not observed when the cells were cultured individually. Improvements in vascular structures can also be achieved with co-cultures over mono-cultures [17, 18, 22]. While co-culture systems enhance physiological relevance, they increase the complexity of the culture system and require special design considerations. For instance media components, ratios of cell types, and timing of differentiation, need to be optimized to obtain proper tissue formation. Additionally, differential labeling of each cell type is helpful to evaluate cellular interactions and contributions [23].
Box 1 – Recreating tissue vasculature.
Vasculature can be achieved by creating interconnected, endothelial lined, channels [55–58] or by allowing cells to re-create their own microvasculature [17, 18, 22, 59]. The crosstalk between endothelial cells and perivascular/stromal cells [22] and bulk organ cells such as osteoblasts [17] or hepatocytes [18], can be used to control the formation and function of blood vessels with intact barrier functions and 3D architectures and biochemical markers similar to in vivo vasculature. Moreover, comparing different mural cells, mechanical matrix characteristics, and bioactive components demonstrated that there is a dynamic interplay between these factors and the resulting endothelial network assembled in cultures [59]. While these systems provide increased physiological relevance they are more complex and require optimization of different media components. Additional design concerns also should be considered such as the importance of lymphatic drainage in vascularized systems [60] and vascular stabilizing and destabilizing mechanical stresses [61].
Alternatively, as an artificial substitute to support high-density cell growth, ‘pseudovascularization’ through hollow fiber membrane bioreactors has shown promising long term results [1, 62]. The hollow fibers act as the blood vessels transferring culture medium throughout the tissue engineered constructs while the bulk system can be extracellular matrix or other scaffold materials with the seeded cells. These fibers can also be designed to degrade [63] in order to allow remodeling once the tissue has been implanted. While this method is simpler than re-creating endothelial based vascular structures and supports diffusion of nutrients, it does not recreate the cellular interactions between vascular cells and the bulk organ.
Finally, the cell types chosen can affect outcomes. Stem cells, primary cells, immortalized cell lines, or modifying the gene expression of cells for a desired phenotype can be considered. Stem cells are undifferentiated cells that have the potential to differentiate into specialized cells [24]. Primary cells on the other hand are already differentiated cells obtained directly from a specific tissue [25]. They have a limited lifespan in culture and eventually undergo senescence and stop proliferating [25]. Immortalized cells in contrast proliferate and evade cellular senescence [25] and are easier to work with, but provide questionable relevance to in vivo functions.
Chemical and mechanical cues
Cells require chemical cues, fluid flow and mechanical inputs for proper signaling, nutrient supply and mechanotransduction in vivo. Chemical cues such as growth factors drive proliferation, differentiation, or senescence and are highly dependent on cell type. For instance, the TGFβ growth factor family results in diverse and sometimes contradictory roles in different cellular systems [26, 27]. Therefore, careful consideration for chemical additives is important for each engineered tissue.
Bioreactors play a significant role in enhancing the supply of chemical factors and mechanical signals to engineered tissues. They can improve the quality of engineered tissues, automate and standardize tissue manufacturing, control size and shape, establish proper nutrient and metabolite transport via improved mass transfer, and generate more homogenous cell distributions [28–33]. However, bioreactors must be designed to mimic the mechanical cues the cells experience in situ. For instance, articular cartilage is often studied in hydrostatic bioreactors which mimic hydrostatic loading from articulating bones [34], whereas osteogenic differentiation is often studied with perfusion which mimics the interstitial fluid movement through lacunae (caused by bone being loaded in compression and tension) [35]. Additionally, the duration and frequency of the applied stress should be considered, such as fluid flow in bioreactor systems. For cells that experience flow in situ, continuous flow is an appropriate choice. As an example, osteoblasts in continuous flow bioreactors increase cell numbers and efficiency of differentiation over static controls [36]. However, sometimes constant flow can lead to low viability and a heterogeneous populations, whereas periodic flow appears to improve results [37]. Periodic flow enables shear sensitive cells (human embryonic stem cells for example [38]) to withstand the flow, and allows for long static incubation periods where secreted factors can accumulate locally. While mechanotransduction is thought to be an important cue driving development of tissue in vivo [39], directing the differentiation of cells with biomechanical cues requires optimization of the type of stimulus, the temporal component of the applied stimulus, the insertion of rest (static) periods (if necessary), and the magnitude of the stress itself, which all effect cellular responses [35].
Spatial cues
The classic growth of cells on tissue culture plastic and glass 2D surfaces does not reflect the complexity of tissue specific architecture and signaling (biological and mechanical) experienced in situ. Many reviews have focused on the striking differences between 2D and 3D culture conditions [40–43], emphasizing the major impact that 3D cultures have on: drug screening outcomes, cell shape, cell-cell interactions, and cellular interactions with their matrix. More complex 3D drug screening in vitro not only decreases the use of laboratory animals, but can also improve toxicology screening, as it is more similar to the human in vivo condition. Likewise, cell shape in 3D matrices is more similar to their in vivo environment resulting in tissue specific signaling not found in 2D cultures. 3D culture also results in proper cell-cell interactions, as opposed to cell overgrowth, contact inhibition, and dedifferentiation often encountered on planar surfaces. Finally, 3D systems improve cellular interactions with their matrix; including cell adhesion, mechanotransduction, force production and cell migration.
Temporal component of tissue engineering
An often overlooked aspect of physiological relevance is the timeframe in which tissue-equivalent structures develop in vitro. Short term cultures (2–14 days) often do not result in mature tissues with stable functions or markers [3, 4, 44, 45]. Cultivation time in vitro is required to allow the cells to adjust, expand and form into the targeted tissue-like structures; a process that can take weeks depending on the tissue. For example, chondrocytes require 8 weeks to achieve stable mechanical properties of cartilage [3], while skin can take 12 weeks to reach epidermal homeostasis [4]. Longer time frames in culture enable the cells to secrete their own extracellular matrix and remodel biomaterial scaffolds. However, when cultures are extended for longer periods of time they predictably run into many challenges (Box 2).
Box 2 – Challenges of extended culture periods.
In vitro tissue cultures inevitably encounter challenges when they are extended for longer periods of time. Cells often proliferate excessively and become over confluent, altering signaling patterns from contact inhibition [64]. Extended time in culture can also cause cells to de-differentiate [65, 66]. Viability is another issue, as longer term culture increases the risk of contamination [67]. Additionally, necrotic centers can develop, as cells proliferate on the edge of constructs while the cells in the center have a reduced nutrient supply and can become hypoxic [68]. This is especially apparent with metabolically active cardiac [69], hepatic [5, 70], and pancreatic [71] cells, and larger tissue constructs (> 1 mm depending on the diffusion coefficient of the tissue [72]). Hypoxic environments also effect tissue formation, such as with cartilage, intervertebral disc, and cornea, where matrix production is highly dependent on oxygen tension [73]. Long term cultures also increase the resources needed to maintain cultures. Moreover, long term monitoring of the same tissue construct at multiple time points is essential to decrease heterogeneous results.
Without the proper timeframe the dynamic state of tissues can result in deviations from the goal of the intended studies and targeted outcomes. For instance, if the goal is to test the response or toxicity to a drug then it will be difficult to discern the effect of the drug versus the noise due to the dynamics of the system [44]. However, it should be noted that the dynamic (fluctuating) state of developing tissues in vitro over time is different than a dynamic response to an experimental condition, which would be expected in response to drug treatments in the case of pathological studies of a disease. Thus, if these tissues are to be used for drug studies or to create disease models in vitro, long culture periods will often be required to enable proper maturation of the tissue.
For clinical applications aimed at restoring tissue function due to organ failure or large tissue defects due to trauma or surgery, long term pre-culturing of tissue constructs may be necessary to generate an adequate mass of differentiated tissue to implant in vivo. For instance, to generate fully differentiated cartilaginous tissue in long-term in vitro culture, 12 weeks was necessary to maintain phenotype and stable structure when implanting cartilaginous constructs in vivo [46]. For bone applications, increasing pre-cultivation time from 24 hours to 14 days before implantation in vivo resulted in increased compact connective tissue formation and homogenous host microvessels throughout the scaffolds [47]. Likewise, muscle constructs had significantly increased percentages of vascular volume and myoblast survival over time when pre-cultivation of tissues in vitro was extended from 4 to 7 days [48]. For neural applications, pre-culturing neural progenitor cells for a week increased survival of the transplanted cells and resulted in smaller defects compared with no pre-culturing [49]. Based on the range of preconditioning times the required time frame will vary depending on the tissue type linked to the metabolic level of the tissue, along with challenges of integration to native tissues in vivo (e.g., re-vascularization, transport limitations).
What element does time play in the development of tissues and their differentiation?
Different in vitro tissue platforms offer variable utility for “long term culture” (Box 3). Additionally, the differentiation of cells is affected by the length of culture. Stem cells are an attractive option to form patient-specific tissue engineered constructs and have been successfully incorporated in many long term tissue models (40 days [50], 45 days [45], 50 days [51], 12 weeks [46]). However, stem cells can be unstable in long term culture. For example, chondrogenic [3, 52, 53] and osteogenic advantages (6 weeks, [36]) for primary chondrocytes and osteoblasts, respectively, over mesenchymal stem cells (MSCs), was reported in long term culture. MSCs lagged in chondrogenesis, had decreased viability, generated tissues with lower mechanical properties, and had decreased matrix production compared to the primary chondrocytes, ultimately limiting their utility for in functional cartilage repair (112 days [3], 70 days [52]). Furthermore, human MSCs had lower differentiation potential in long term culture (6 weeks) than neonatal and adult chondrocytes, with donor age being an additional factor; adult chondrocytes maintained phenotype better in long term culture compared to neonatal cells [53]. The contrast between primary cells and immortalized cell lines is also evident in longer culture periods. For instance, in a 3D model immortalized fibroblasts proliferated excessively and displayed heterogeneous and random increases in thickness of 3D layers, while normal human primary dermal fibroblasts demonstrated consistent DNA levels and maintained consistent thickness for layered structures [54]. Primary cells on the other hand have the the disadvantage of donor-to-donor variability [1] and therefore are not well characterized, making it more difficult to compare outcomes between experiments. Additionally, these cells are often time-consuming to handle, difficult to obtain in large numbers, dedifferentiate in culture, and require highly specific media and supplements. The highly variable nature of tissue platforms and cell types makes non-destructive data point monitoring invaluable in evaluating suitable culture times to establish mature tissues (Box 4).
Box 3 - Different tissue engineering platforms for long term culture.
2D microfluidic platforms have been proposed for cellular scale tissue engineering approaches, including components of tissues where planar surfaces are relevant (i.e., tight junctions, endothelialization, barrier functions), since they have a high surface area to volume ratio, result in low consumption of reagents, have increased sensitivity of measurements, and display high spatio-temporal control [74, 75]. The small size of microfluidic devices makes them well suited for portable applications such as toxicity sensors [76]. Long term 2D tissue engineered systems have been cultured in microfluidic platforms (Supplementary Table 1) including intestine [77], liver [78, 79], skeletal muscle [80, 81], vasculature [82] and neural networks [83]. However, cultures in 2D microfluidic devices are functional for approximately 11 days (an average for the studies in Supplementary Table 1).
Three dimensional tissue engineering approaches are another platform [40–43], and have been explored combining the advantages of microfluidic manufacturing with increased tissue complexity [84–89]. When microfluidic technology was combined with 3D cultures there is an improvement in culture time achieved over 2D microfluidic conditions (an average 19 days for the studies in Supplementary Table 1). For instance, 3D artificial liver modular devices extended hepatocyte culture for 90 days, meeting criteria for FDA toxicity testing [5, 6]. As platforms are developed further, 3D microfluidic devices should become increasingly useful as a bridge between microfluidic technologies and large scale three dimensional tissue cultures.
Finally, the longest duration systems (Figure i) have been demonstrated in larger 3D tissue engineered systems (averaging the static platforms and 3D bioreactor platforms summarized in Supplementary Tables 2 and 3, respectively, indicates approximately 8 weeks for the average culture period). 3D bioreactor systems have increased cell viability, tissue organization, and the distribution of cells throughout 3D matrices at extended time points of cultivation (Supplementary Table 3), including : (direct) perfusion bioreactors (6 weeks, [36]), (indirect) hollow fiber perfusion bioreactors (3 weeks, [1]), perfusion bioreactors with mechanical stimulation (67 days, [90]), rotating wall vessel bioreactors (9 weeks, [91]), spinner flasks (6 months, [7]), orbital shakers (16 weeks, [3]) and cyclic hydrostatic pressure (21 days, [92]).
Box 4 – Non-invasive methods to characterize cells are necessary for long term cultures.
Nondestructive data points are essential in long term cultures to monitor the same construct throughout growth to help determine when tissues have reached homeostasis. Non-invasive monitoring of tissues helps to control the number of samples required to track the progress of differentiation and maturation of the tissue. Nondestructive outcomes include tracking secreted factors, morphological change in the cells and matrices, metabolic state and many related outcomes. Choosing which secreted factors are appropriate depends on the tissue or organ system being evaluated and the goals of the study. For instance, to monitor hepatic function in long term culture (90 days) supernatant samples were collected to analyze albumin secretion and urea synthesis [5, 6]. On the other hand, adipose differentiation was analyzed over a 6 month period by secretion of leptin and glycerol [7], while bone differentiation over a 46 day period was assessed by the presence of osteocalcin in the supernatant [8]. Additionally, glucose consumption (4 weeks [93], 10 weeks [94]), and lactate released [94] in the culture medium were non-invasive methods used to track cell metabolism over long culture periods.
Non-invasive imaging techniques can also be utilized to track tissue differentiation. 2D microfluidic systems are relatively easy to monitor, as monolayers of cells are often cultured directly on a glass cover slide. This approach can be reproduced with 3D scaffolds, for instance, by entrapping a porous polymer matrix in polydimethylsiloxane and bonding it to a glass coverslip for visualization with a confocal microscope [95]. This platform could be paired with non-destructive dyes or endogenous markers. Noninvasive magnetic resonance microscopy was used to track changes in collagen structure and mineral content to monitor in a 9 week study [62]. Morphological, biochemical, and tissue organizational parameters can be acquired from endogenous sources of contrasts, such as signals emitted in the two photon spectra. As an example, metabolic activity was tracked via two photon fluorescence imaging linked to adipose tissue engineered systems over a 6 month period to quantify redox ratios [9] and the formation of lipid droplets in a 9 week study of 3D adipose tissue [10]. Additionally, micro-fabrication techniques can enhance non-destructive data point collection. To help with tracking over time, devices can register time-lapse images of the same imaging area [96]. Multiple electrode arrays have also been formed on a chip for studying neural activity over a 4 week period [97].
Concluding remarks and future perspectives
To achieve the goals of tissue engineering a multidisciplinary approach that integrates engineering and biological methodologies is necessary. As more tissue engineering approaches are explored, it will be important to consider the appropriate time frame needed to reach the desired outcomes, which will be specific to each tissue and project goal (e.g., toxicity, drug response, disease formation, nutritional impact, etc.). Long term cultures will not always be practical or the most appropriate choice, therefore, choosing the right tissue platform to address the appropriate questions is essential. Microfluidic systems offer short term options and can address many in vitro challenges of interest (Supplementary Tables 1), including applications where portability, small scale approaches are needed and acute responses to toxicants or drugs are sought. However, long term sustained 3D in vitro cultures will be critical for tissue engineering of larger tissue constructs for regeneration and complex organ level studies, as well as to assess chronic drug outcomes, disease development and many related topics (Supplementary Tables 2 and 3). With proper timeframes, stable tissues can be formed and studied to match project goals. To achieve long term cultures, more research into combining micro-fabrication techniques with 3D systems will improve the longevity and usefulness of microfluidic systems. In larger scale applications, perfused vasculature will need to be combined with 3D matrices and other bioreactor technologies, including tracking with nondestructive analysis techniques. Co-culturing of multiple cell types will also be needed to generate more relevant tissues. All aspects of these needs will require continued advances to bring these systems to maturity to help focus making this is an area rich in opportunities for innovation.
Supplementary Material
Figure i.
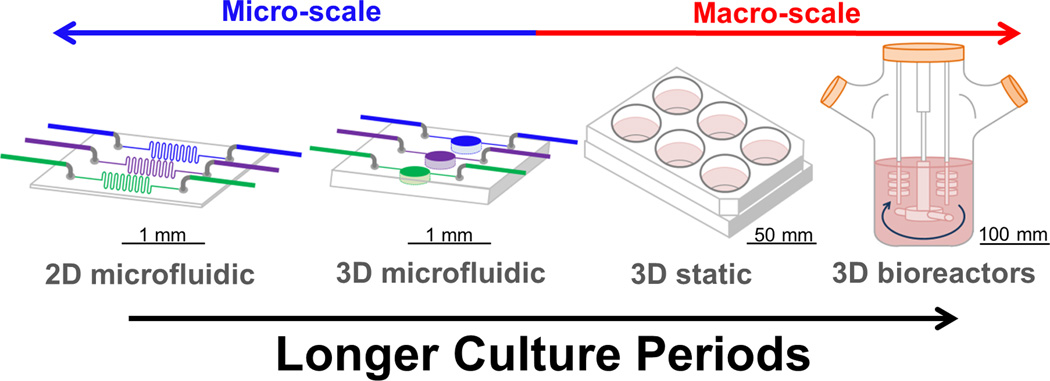
Long term culture in different in vitro platforms. To achieve greater culture duration in vitro; the increased complexity offered by 3D systems over 2D systems, along with larger tissue constructs with enhanced perfusion are required.
Highlights.
Enhance physiological relevance by using bioreactors, 3D co-cultures, vascularization
Long term culture may be required to generate stable, physiologically relevant tissues
Nondestructive data points are essential in long term culture for monitoring the same construct
Acknowledgments
The authors would like to thank Karolina Chwalek, Erica Palma and Dana Cairns for help in editing this document. We also thank the NIH Tissue Engineering Resource Center (P41 EB002520) for supporting our tissue engineering studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mueller D, Tascher G, Muller-Vieira U, Knobeloch D, Nuessler AK, Zeilinger K, Noor F. In-depth physiological characterization of primary human hepatocytes in a 3D hollow-fiber bioreactor. Journal of tissue engineering and regenerative medicine. 2011;5:e207–e218. doi: 10.1002/term.418. [DOI] [PubMed] [Google Scholar]
- 2.Caralt M, Velasco E, Lanas A, Baptista PM. Liver bioengineering: from the stage of liver decellularized matrix to the multiple cellular actors and bioreactor special effects. Organogenesis. 2014;10:250–259. doi: 10.4161/org.29892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell MJ, Fisher MB, Huang AH, Shin JI, Farrell KM, Mauck RL. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. Journal of biomechanics. 2013 doi: 10.1016/j.jbiomech.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark HJ, Boehnke K, Mirancea N, Willhauck MJ, Pavesio A, Fusenig NE, Boukamp P. Epidermal homeostasis in long-term scaffold-enforced skin equivalents. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 2006;11:93–105. doi: 10.1038/sj.jidsymp.5650015. [DOI] [PubMed] [Google Scholar]
- 5.Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, Okano T, Seki M. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials. 2012;33:8304–8315. doi: 10.1016/j.biomaterials.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 6.Giri S, Braumann UD, Giri P, Acikgoz A, Scheibe P, Nieber K, Bader A. Nanostructured self-assembling peptides as a defined extracellular matrix for long-term functional maintenance of primary hepatocytes in a bioartificial liver modular device. International journal of nanomedicine. 2013;8:1525–1539. doi: 10.2147/IJN.S33589. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Bellas E, Marra K, Kaplan DLP. Sustainable three-dimensional tissue model of human adipose tissue. Tissue engineering. Part C, Methods. 2013;19:745–754. doi: 10.1089/ten.tec.2012.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materna T, Rolf HJ, Napp J, Schulz J, Gelinsky M, Schliephake H. In vitro characterization of three-dimensional scaffolds seeded with human bone marrow stromal cells for tissue engineered growth of bone: mission impossible? A methodological approach. Clinical oral implants research. 2008;19:379–386. doi: 10.1111/j.1600-0501.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 9.Quinn KP, Bellas E, Fourligas N, Lee K, Kaplan DL, Georgakoudi I. Characterization of metabolic changes associated with the functional development of 3D engineered tissues by non-invasive, dynamic measurement of individual cell redox ratios. Biomaterials. 2012;33:5341–5348. doi: 10.1016/j.biomaterials.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang T, Zimmerley MS, Quinn KP, Lamarre-Jouenne I, Kaplan DL, Beaurepaire E, Georgakoudi I. Non-invasive monitoring of cell metabolism and lipid production in 3D engineered human adipose tissues using label-free multiphoton microscopy. Biomaterials. 2013;34:8607–8616. doi: 10.1016/j.biomaterials.2013.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladson CL. The extracellular matrix of gliomas: modulation of cell function. Journal of neuropathology and experimental neurology. 1999;58:1029–1040. doi: 10.1097/00005072-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Culav EM, Clark CH, Merrilees MJ. Connective tissues: matrix composition and its relevance to physical therapy. Physical therapy. 1999;79:308–319. [PubMed] [Google Scholar]
- 13.O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today. 2011;14:88–95. [Google Scholar]
- 14.Ikuta S, Sekino N, Hara T, Saito Y, Chida K. Mouse epidermal keratinocytes in three-dimensional organotypic coculture with dermal fibroblasts form a stratified sheet resembling skin. Bioscience, biotechnology, and biochemistry. 2006;70:2669–2675. doi: 10.1271/bbb.60266. [DOI] [PubMed] [Google Scholar]
- 15.Gingras M, Beaulieu MM, Gagnon V, Durham HD, Berthod F. In vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia. 2008;56:354–364. doi: 10.1002/glia.20617. [DOI] [PubMed] [Google Scholar]
- 16.Majumdar D, Gao Y, Li D, Webb DJ. Co-culture of neurons and glia in a novel microfluidic platform. Journal of neuroscience methods. 2011;196:38–44. doi: 10.1016/j.jneumeth.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, Rommens PM. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–4226. doi: 10.1016/j.biomaterials.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Kasuya J, Sudo R, Mitaka T, Ikeda M, Tanishita K. Hepatic stellate cell-mediated three-dimensional hepatocyte and endothelial cell triculture model. Tissue engineering. Part A. 2011;17:361–370. doi: 10.1089/ten.TEA.2010.0033. [DOI] [PubMed] [Google Scholar]
- 19.Wang PC, Takezawa T. Reconstruction of renal glomerular tissue using collagen vitrigel scaffold. Journal of bioscience and bioengineering. 2005;99:529–540. doi: 10.1263/jbb.99.529. [DOI] [PubMed] [Google Scholar]
- 20.Hayden RS, Quinn KP, Alonzo CA, Georgakoudi I, Kaplan DL. Quantitative characterization of mineralized silk film remodeling during long-term osteoblast-osteoclast co-culture. Biomaterials. 2014;35:3794–3802. doi: 10.1016/j.biomaterials.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain A, Collins G, Yip D, Cho CH. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnology and bioengineering. 2013;110:637–647. doi: 10.1002/bit.24727. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab on a chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 23.Kang JH, Gimble JM, Kaplan DL. In vitro 3D model for human vascularized adipose tissue. Tissue engineering. Part A. 2009;15:2227–2236. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JH, Zhou LB, Pan SY. A brief review of recent advances in stem cell biology. Neural Regen Res. 2014;9:684–687. doi: 10.4103/1673-5374.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Darnell J. Molecular Cell Biology. 5 edn. W.H. Freeman and Company; 2004. pp. 236–240. [Google Scholar]
- 26.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 27.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 28.Gaspar DA, Gomide V, Monteiro FJ. The role of perfusion bioreactors in bone tissue engineering. Biomatter. 2012;2:167–175. doi: 10.4161/biom.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabvuure N, Hindocha S, Khan WS. The role of bioreactors in cartilage tissue engineering. Current stem cell research & therapy. 2012;7:287–292. doi: 10.2174/157488812800793018. [DOI] [PubMed] [Google Scholar]
- 30.Salter E, Goh B, Hung B, Hutton D, Ghone N, Grayson WL. Bone tissue engineering bioreactors: a role in the clinic? Tissue engineering. Part B, Reviews. 2012;18:62–75. doi: 10.1089/ten.TEB.2011.0209. [DOI] [PubMed] [Google Scholar]
- 31.Oragui E, Nannaparaju M, Khan WS. The role of bioreactors in tissue engineering for musculoskeletal applications. The open orthopaedics journal. 2011;5(Suppl 2):267–270. doi: 10.2174/1874325001105010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends in biotechnology. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Sistino JJ. Bioreactors for tissue engineering--a new role for perfusionists? The Journal of extra-corporeal technology. 2003;35:200–202. [PubMed] [Google Scholar]
- 34.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. The Journal of the American Academy of Orthopaedic Surgeons. 2013;21:303–311. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy RJ, O'Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue engineering. Part B, Reviews. 2010;16:587–601. doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- 36.Rath SN, Strobel LA, Arkudas A, Beier JP, Maier AK, Greil P, Kneser U. Osteoinduction and survival of osteoblasts and bone-marrow stromal cells in 3D biphasic calcium phosphate scaffolds under static and dynamic culture conditions. Journal of cellular and molecular medicine. 2012;16:2350–2361. doi: 10.1111/j.1582-4934.2012.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giulitti S, Magrofuoco E, Prevedello L, Elvassore N. Optimal periodic perfusion strategy for robust long-term microfluidic cell culture. Lab on a chip. 2013;13:4430–4441. doi: 10.1039/c3lc50643f. [DOI] [PubMed] [Google Scholar]
- 38.Korin N, Bransky A, Dinnar U, Levenberg S. Periodic "flow-stop" perfusion microchannel bioreactors for mammalian and human embryonic stem cell long-term culture. Biomedical microdevices. 2009;11:87–94. doi: 10.1007/s10544-008-9212-5. [DOI] [PubMed] [Google Scholar]
- 39.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 40.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. Journal of cell science. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breslin S, O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug discovery today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nature reviews. Molecular cell biology. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 43.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. Journal of cell science. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DesRochers TM, Suter L, Roth A, Kaplan DL. Bioengineered 3D Human Kidney Tissue, a Platform for the Determination of Nephrotoxicity. PloS one. 2013;8 doi: 10.1371/journal.pone.0059219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrahamsson CK, Yang F, Park H, Brunger JM, Valonen PK, Langer R, Freed LE. Chondrogenesis and mineralization during in vitro culture of human mesenchymal stem cells on three-dimensional woven scaffolds. Tissue engineering. Part A. 2010;16:3709–3718. doi: 10.1089/ten.tea.2010.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu K, Zhou GD, Liu W, Zhang WJ, Cui L, Liu X, Cao Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–2192. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Ghanaati S, Unger RE, Webber MJ, Barbeck M, Orth C, Kirkpatrick JA, Kirkpatrick CJ. Scaffold vascularization in vivo driven by primary human osteoblasts in concert with host inflammatory cells. Biomaterials. 2011;32:8150–8160. doi: 10.1016/j.biomaterials.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 48.Tilkorn DJ, Bedogni A, Keramidaris E, Han X, Palmer JA, Dingle AM, Mitchell GM. Implanted myoblast survival is dependent on the degree of vascularization in a novel delayed implantation/prevascularization tissue engineering model. Tissue engineering. Part A. 2010;16:165–178. doi: 10.1089/ten.TEA.2009.0075. [DOI] [PubMed] [Google Scholar]
- 49.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, Greenberg DA. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao F, Ma T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnology and bioengineering. 2005;91:482–493. doi: 10.1002/bit.20532. [DOI] [PubMed] [Google Scholar]
- 51.Neuss S, Stainforth R, Salber J, Schenck P, Bovi M, Knuchel R, Perez-Bouza A. Long-term survival and bipotent terminal differentiation of human mesenchymal stem cells (hMSC) in combination with a commercially available three-dimensional collagen scaffold. Cell transplantation. 2008;17:977–986. doi: 10.3727/096368908786576462. [DOI] [PubMed] [Google Scholar]
- 52.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Saha S, Kirkham J, Wood D, Curran S, Yang XB. Informing future cartilage repair strategies: a comparative study of three different human cell types for cartilage tissue engineering. Cell and tissue research. 2013;352:495–507. doi: 10.1007/s00441-013-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chetprayoon P, Kadowaki K, Matsusaki M, Akashi M. Survival and structural evaluations of three-dimensional tissues fabricated by the hierarchical cell manipulation technique. Acta biomaterialia. 2013;9:4698–4706. doi: 10.1016/j.actbio.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan JP, Delnero PF, Zheng Y, Verbridge SS, Chen J, Craven M, Stroock AD. Formation of microvascular networks in vitro. Nature protocols. 2013;8:1820–1836. doi: 10.1038/nprot.2013.110. [DOI] [PubMed] [Google Scholar]
- 57.Schimek K, Busek M, Brincker S, Groth B, Hoffmann S, Lauster R, Horland R. Integrating biological vasculature into a multi-organ-chip microsystem. Lab on a chip. 2013;13:3588–3598. doi: 10.1039/c3lc50217a. [DOI] [PubMed] [Google Scholar]
- 58.Tiruvannamalai-Annamalai R, Armant DR, Matthew HW. A glycosaminoglycan based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues. PloS one. 2014;9:e84287. doi: 10.1371/journal.pone.0084287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chwalek K, Tsurkan MV, Freudenberg U, Werner C. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Scientific reports. 2014;4:4414. doi: 10.1038/srep04414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong KH, Truslow JG, Khankhel AH, Chan KL, Tien J. Artificial lymphatic drainage systems for vascularized microfluidic scaffolds. Journal of biomedical materials research. Part A. 2013;101:2181–2190. doi: 10.1002/jbm.a.34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tien J. Microfluidic approaches for engineering vasculature. Current Opinion in Chemical Engineering. 2014;3:36–41. [Google Scholar]
- 62.Chesnick IE, Avallone FA, Leapman RD, Landis WJ, Eidelman N, Potter K. Evaluation of bioreactor-cultivated bone by magnetic resonance microscopy and FTIR microspectroscopy. Bone. 2007;40:904–912. doi: 10.1016/j.bone.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang N, Nichols HL, Tylor S, Wen X. Fabrication of nanocrystalline hydroxyapatite doped degradable composite hollow fiber for guided and biomimetic bone tissue engineering. Materials Science and Engineering: C. 2007;27:599–606. [Google Scholar]
- 64.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell: Extracellular Control of Cell Division, Cell Growth, and Apoptosis. Garland Science. 2002 [Google Scholar]
- 65.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PloS one. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dallo SF, Baseman JB. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microbial pathogenesis. 2000;29:301–309. doi: 10.1006/mpat.2000.0395. [DOI] [PubMed] [Google Scholar]
- 68.Kellner K, Liebsch G, Klimant I, Wolfbeis OS, Blunk T, Schulz MB, Gopferich A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnology and bioengineering. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 69.Radisic M, Vunjak-Novakovic G. Cardiac tissue engineering. J. Serb. Chem. Soc. 2005;70:541–556. [Google Scholar]
- 70.Uygun BE, Yarmush ML, Uygun K. Application of whole-organ tissue engineering in hepatology. Nature reviews. Gastroenterology & hepatology. 2012;9:738–744. doi: 10.1038/nrgastro.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato Y, Endo H, Okuyama H, Takeda T, Iwahashi H, Imagawa A, Inoue M. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. The Journal of biological chemistry. 2011;286:12524–12532. doi: 10.1074/jbc.M110.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Androjna C, Gatica JE, Belovich JM, Derwin KA. Oxygen diffusion through natural extracellular matrices: implications for estimating "critical thickness" values in tendon tissue engineering. Tissue engineering. Part A. 2008;14:559–569. doi: 10.1089/tea.2006.0361. [DOI] [PubMed] [Google Scholar]
- 73.Scott JE. Oxygen and the connective tissues. Trends in biochemical sciences. 1992;17:340–343. doi: 10.1016/0968-0004(92)90307-u. [DOI] [PubMed] [Google Scholar]
- 74.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Polacheck WJ, Li R, Uzel SG, Kamm RD. Microfluidic platforms for mechanobiology. Lab on a chip. 2013;13:2252–2267. doi: 10.1039/c3lc41393d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curtis TM, Widder MW, Brennan LM, Schwager SJ, van der Schalie WH, Fey J, Salazar N. A portable cell-based impedance sensor for toxicity testing of drinking water. Lab on a chip. 2009;9:2176–2183. doi: 10.1039/b901314h. [DOI] [PubMed] [Google Scholar]
- 77.Kimura H, Yamamoto T, Sakai H, Sakai Y, Fujii T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab on a chip. 2008;8:741–746. doi: 10.1039/b717091b. [DOI] [PubMed] [Google Scholar]
- 78.Tan GD, Toh GW, Birgersson E, Robens J, van Noort D, Leo HL. A thin-walled polydimethylsiloxane bioreactor for high-density hepatocyte sandwich culture. Biotechnology and bioengineering. 2013;110:1663–1673. doi: 10.1002/bit.24822. [DOI] [PubMed] [Google Scholar]
- 79.De Bartolo L, Salerno S, Morelli S, Giorno L, Rende M, Memoli B, Drioli E. Long-term maintenance of human hepatocytes in oxygen-permeable membrane bioreactor. Biomaterials. 2006;27:4794–4803. doi: 10.1016/j.biomaterials.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 80.Tourovskaia A, Figueroa-Masot X, Folch A. Long-term micropatterned cell cultures in heterogeneous microfluidic environments. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2004;4:2675–2678. doi: 10.1109/IEMBS.2004.1403768. [DOI] [PubMed] [Google Scholar]
- 81.Tourovskaia A, Figueroa-Masot X, Folch A. Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab on a chip. 2005;5:14–19. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 82.Chen H, Cornwell J, Zhang H, Lim T, Resurreccion R, Port T, Nordon RE. Cardiac-like flow generator for long-term imaging of endothelial cell responses to circulatory pulsatile flow at microscale. Lab on a chip. 2013;13:2999–3007. doi: 10.1039/c3lc50123j. [DOI] [PubMed] [Google Scholar]
- 83.Kanagasabapathi TT, Massobrio P, Tedesco M, Martinoia S, Wadman WJ, Decre MM. An experimental approach towards the development of an in vitro cortical-thalamic co-culture model. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2011;2011:648–651. doi: 10.1109/IEMBS.2011.6090144. [DOI] [PubMed] [Google Scholar]
- 84.Yu L, Chen MC, Cheung KC. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab on a chip. 2010;10:2424–2432. doi: 10.1039/c004590j. [DOI] [PubMed] [Google Scholar]
- 85.Chen MC, Gupta M, Cheung KC. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomedical microdevices. 2010;12:647–654. doi: 10.1007/s10544-010-9417-2. [DOI] [PubMed] [Google Scholar]
- 86.Chen SY, Hung PJ, Lee PJ. Microfluidic array for three-dimensional perfusion culture of human mammary epithelial cells. Biomedical microdevices. 2011;13:753–758. doi: 10.1007/s10544-011-9545-3. [DOI] [PubMed] [Google Scholar]
- 87.Ziolkowska K, Stelmachowska A, Kwapiszewski R, Chudy M, Dybko A, Brzozka Z. Long-term three-dimensional cell culture and anticancer drug activity evaluation in a microfluidic chip. Biosensors & bioelectronics. 2013;40:68–74. doi: 10.1016/j.bios.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 88.Jang K, Sato K, Igawa K, Chung UI, Kitamori T. Development of an osteoblast-based 3D continuous-perfusion microfluidic system for drug screening. Analytical and bioanalytical chemistry. 2008;390:825–832. doi: 10.1007/s00216-007-1752-7. [DOI] [PubMed] [Google Scholar]
- 89.Abaci HE, Gledhill K, Guo Z, Christiano AM, Shuler ML. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab on a chip. 2014 doi: 10.1039/c4lc00999a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seidel JO, Pei M, Gray ML, Langer R, Freed LE, Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445–458. [PubMed] [Google Scholar]
- 91.Lin HJ, O'Shaughnessy TJ, Kelly J, Ma W. Neural stem cell differentiation in a cell-collagen-bioreactor culture system. Brain research. Developmental brain research. 2004;153:163–173. doi: 10.1016/j.devbrainres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Puetzer J, Williams J, Gillies A, Bernacki S, Loboa EG. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose- and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue engineering. Part A. 2013;19:299–306. doi: 10.1089/ten.tea.2012.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie Y, Hardouin P, Zhu Z, Tang T, Dai K, Lu J. Three-dimensional flow perfusion culture system for stem cell proliferation inside the critical-size beta-tricalcium phosphate scaffold. Tissue engineering. 2006;12:3535–3543. doi: 10.1089/ten.2006.12.3535. [DOI] [PubMed] [Google Scholar]
- 94.Dal Pra I, Chiarini A, Boschi A, Freddi G, Armato U. Novel dermo-epidermal equivalents on silk fibroin-based formic acid-crosslinked three-dimensional nonwoven devices with prospective applications in human tissue engineering/regeneration/repair. International journal of molecular medicine. 2006;18:241–247. [PubMed] [Google Scholar]
- 95.Danmark S, Gladnikoff M, Frisk T, Zelenina M, Mustafa K, Russom A, Finne-Wistrand A. Development of a novel microfluidic device for long-term in situ monitoring of live cells in 3-dimensional matrices. Biomedical microdevices. 2012;14:885–893. doi: 10.1007/s10544-012-9668-1. [DOI] [PubMed] [Google Scholar]
- 96.Hanson L, Cui L, Xie C, Cui B. A microfluidic positioning chamber for long-term live-cell imaging. Microscopy research and technique. 2011;74:496–501. doi: 10.1002/jemt.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berdichevsky Y, Sabolek H, Levine JB, Staley KJ, Yarmush ML. Microfluidics and multielectrode array-compatible organotypic slice culture method. Journal of neuroscience methods. 2009;178:59–64. doi: 10.1016/j.jneumeth.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.