Abstract
There is an increasing recognition of clinical overlap in patients presenting with epilepsy and autism spectrum disorder (ASD), and a great deal of new information is available regarding the genetic causes of both disorders. Several biological pathways appear to be involved in both disease processes, including gene transcription regulation, cellular growth, synaptic channel function, and maintenance of synaptic structure. We review several genetic disorders where ASD and epilepsy frequently co-occur, and we discuss the screening tools available to practicing neurologists and epileptologists to help determine which patients should be referred for formal ASD diagnostic evaluation. Finally, we make recommendations regarding the workflow of genetic diagnostic testing available for children with both ASD and epilepsy.
Keywords: ASD, autism, epilepsy
Introduction
Autism spectrum disorders (ASDs) and epilepsies are both heterogeneous conditions that frequently coexist with other developmental disabilities including developmental delay, intellectual disability and behavioral impairments [1]. The co-occurrence of ASDs and epilepsies has long been recognized [2–5]. With the discovery of overlapping molecular causes of both disorders, some have proposed shared etiologic mechanisms [6]. We are just beginning to understand how the two conditions are interconnected.
Identifying these relationships is complicated by the complexity of ASDs and epilepsies, evolving diagnostic criteria, [7] changing classification schemas, [8,9] and a culture among researchers within each disorder that may discourage investigation of shared mechanisms. This separation of inquiry of ASDs from epilepsies is seen at the level of National Institutes of Health, where proposals addressing ASD are traditionally reviewed by the National Institute of Mental Health or Child Health and Development while those addressing epilepsy are reviewed by the National Institute of Neurological Disorders and Stroke, and proposals addressing both may struggle to find a receptive study section. Still, a number of recent advances in our biological knowledge underline the value of screening for the coexistence of these common developmental disorders [10].
Biology
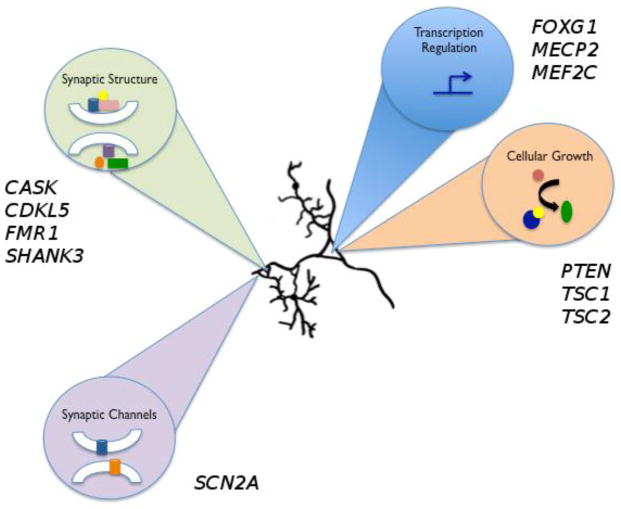
Knowledge of genomic copy number and single gene causes of both ASDs and epilepsy [11,12,13] allows us to identify the biologic processes perturbed in these developmental disorders. As will be explored here, processes with shared involvement in ASDs and epilepsies include gene transcriptional regulation, cellular growth and proliferation, and synapse development, stability, and function (Figure 1).
Figure 1.
Overview of biological pathways common to autism and epilepsy. At least four biological pathways important in neuronal development and function are implicated by involvement of several genes in autism and epilepsy pathogenesis. These pathways include transcriptional regulation (FOXG1, MECP2, MEF2C), cellular growth (PTEN, TSC1, TSC2), synaptic channels (SCN2A), and synaptic structure (CASK, CDKL5, FMR1, SHANK3).
Case definitions of ASD
Autism spectrum disorders (ASDs) are characterized by two core features: (1) deficits in social behaviors and communication and (2) restricted interests and repetitive patterns of behavior [7]. The overall prevalence of ASD is estimated to be 14.7 per 1,000 (1 in 68) children, varying from 5.7 to 21.9 per 1,000 among the CDC-established Autism and Developmental Disabilities Monitoring (ADDM) network sites [14]. ASDs typically manifest before the age of 3 years and are persistent. The heterogeneous phenotypic profile of ASDs has made categorization difficult. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition [7] substantially revised previous classification systems by merging formerly separate diagnostic entities (autistic disorder, Asperger’s disorder, pervasive developmental disorder not otherwise specified) into a single dimension, ASD. This approach may help identify subgroups based on quantity or quality of symptoms or patterns of abnormalities [15]. Additionally, Social Communication Disorder has been added as a new diagnostic category that describes patients with deficits in social communication without demonstrating repetitive behaviors or restricted interests [7].
Numerous primary genetic causes for ASD have been identified [16]. However, historical environmental associations such as fetal valproate and thalidomide exposure suggest multifactorial etiologies may play a role [17,18,19]. The prevalence of epilepsy among children with ASD and vice versa remains unclear. An approximate 16% co-occurrence of epilepsy and ASD was reported based on ADDM network data from 2002 [20]. Other reports estimate that approximately 20–25% children with ASD have epilepsy [21]. A recent population-based study found 44% of children with ASD received a subsequent diagnosis of epilepsy, and 54% of children with epilepsy received a subsequent diagnosis of ASD [22]. Age of onset for epilepsy in ASD is bimodally distributed, with a peak in early childhood (age 2–5 years) and a larger peak in adolescence [23]. Intellectual disability (ID) is a risk factor for epilepsy in ASD: the rate of epilepsy is approximately three times greater in people who have both ASD and ID than in people who have ASD but not ID [24]. Older age, female sex, poor language abilities, and history of regression are most commonly reported as other possible risk factors but are not clinically predictive once adjusted for IQ [25].
Case definition of epilepsy and epilepsy classifications
Epilepsy is defined as the occurrence of more than two unprovoked seizures due to sudden, disorderly, and excessive neuronal discharge [26]. The classification of epilepsies has undergone a change in recent years, moving away from broad schema unrelated to underlying biology (the classical idiopathic, cryptogenic, symptomatic terms) [8, 27], with the recognition that all epilepsy is likely symptomatic of something. More recent efforts have focused on linking classification to the underlying genetic neurobiology, as these mechanisms are discovered [28]. It is likely that the classifications of epilepsy will undergo further revision as these mechanisms are further discerned.
Genetic syndromes in which ASD and epilepsy co-occur
Several conditions caused by genomic copy number variation or mutations in single genes have been associated with both ASD and epilepsy, many of which are summarized in Table 1 and reviewed briefly below.
Table 1.
Single gene and genomic copy number regions commonly associated with autism and epilepsy
| Gene or genomic region | Associated syndrome | Key features |
|---|---|---|
| 15q11-q13 | Chromosome 15q11–13 duplication syndrome | Autism, intellectual disability, ataxia, seizures, developmental delays, and behavioral problems *Deletion of this region is associated with Angelman/Prader- Willi Syndromes |
| Chromosome 21 | Down Syndrome | Distinct facial dysmorphisms, intellectual disability, congenital anomalies and medical comorbidities. |
| 22q13.3 SHANK3 | Phelan-McDermid syndrome | Neonatal hypotonia, global developmental delay, absent to severely delayed speech and autistic behavior and minor dysmorphic features |
| FMR1 | Fragile X Syndrome | Moderate to severe intellectual disability, macroorchidism, and distinct facial features (long face, large ears, and prominent jaw). |
| TSC1/2 | Tuberous sclerosis | Multisystem disorder characterized by hamartomas (brain, heart, lungs, kidneys and skin). |
| PTEN | PTEN-related disorders | Hamartoma syndromes and malignancies (breast, thyroid, endometrial). Macrocephaly and ASD has been reported in children with PTEN mutations. |
| MECP2 | MECP2-related disorder | Severe neurodevelopmental disorder characterized by arrest of development between 6 and 18 months of age, regression of skills, loss of speech, stereotypic hand movements, microcephaly, seizures, and intellectual disability. |
| CDKL5 | CDKL5-related disorder | X-linked dominant condition characterized by early onset of seizures, severe global developmental delay and postnatal microcephaly. Other features include subtle dysmorphic facial features, sleep disturbances, gastrointestinal problems, stereotypic hand movements and intellectual disability. |
| FOXG1 | FOXGI-related disorders | Severe neurodevelopmental disorder with features of classic Rett syndrome but earlier onset in the first months of life |
| MEF2C | MEF2C-related disorder | Severe neurodevelopmental disorder characterized by intellectual disability, epilepsy and stereotypic movements. |
| CASK | CASK-related disorders | Characterized by a distinct malformation phenotype in females involving postnatal microcephaly and pontine and cerebellar hypoplasia, developmental delay, growth retardation and eye abnormalities. |
| SCN2A | SCN2A-related disorders | Autosomal dominant seizure disorder characterized by infantile onset of refractory seizures. |
Genomic Disorders
Duplication of maternally inherited chromosome 15q11-q13 syndrome
Reciprocal duplications of the maternally inherited copy of chromosome 15q11-q13 region are the most frequently reported chromosomal aberration in individuals with ASDs (0.5 – 3%) [29]. Deletions spanning this region represent the most common mechanism for Prader–Willi and Angelman syndromes. Descriptions of the neurobehavioral phenotype associated with duplications of maternal 15q11q13 have emphasized the variability in presentation and frequent co-occurrence of intellectual disability [20].
Patients with duplications of maternal 15q11q13 had a high incidence of infantile spasms [31]. Lennox-Gastaut syndrome has been reported as well [32]. The location of several genes encoding GABA receptor subunits within the duplicated 15q11q13 region (GABRA5, GABRB3, GABRG3) has led to the hypothesis that dysregulation of inhibitory synapses mediates pathogenesis of the epilepsy and ASD phenotypes seen in this disorder [33].
Trisomy 21 (Down Syndrome)
Down Syndrome (DS) results from an extra copy of chromosome 21. DS is characterized by distinct facial dysmorphisms, intellectual disability and associated congenital anomalies. While individuals with DS were generally described as friendly and socially inclined [34], it has been estimated that 5–9% of people with DS meet criteria for ASD [35–38]. Diagnosing ASD in children with DS remains a challenge due to comorbid intellectual disability. In a comparison between twenty children with trisomy 21 with and without ASD, those with ASD were found to have significantly more impaired language abilities, adaptive behavior and cognition [39]. Children with co-occurring DS and ASD may have an overall decrease in brain function as well as an increased risk for seizures [39].
The prevalence of epilepsy in patients with DS is approximately 8–13% [40,41]. Multiple seizure types have been reported in patients with DS, including progressive myoclonic epilepsy associated with dementia [42], infantile spasms [43], and Lennox-Gastaut syndrome with reflex seizures [44]. Reports of developmental outcome of children with a history of infantile spasms and DS has been mixed, with some reporting better than expected outcome [45] and others noting high prevalence of ASDs and less favorable outcome [46]. The reason for such variability in epilepsy and developmental outcome in children with trisomy 21 is unclear.
The effects of DS on brain development remain complicated and uncertain but there is an increased interest in the role of dual-specificity tyrosine phosphorylation-regulated kinase, DYRK1A, activity [47]. In mouse models, Dyrk1A has been shown to play important roles in cell cycle control [48] and synaptic plasticity [49]. Additionally, research whole exome sequencing has identified mutations in DYRK1A in several children with ASD and microcephaly [16].
Other copy number variants (CNV)
Certain pathogenic copy number variants are highly associated with ASD and epilepsy [12,13]. Most recent data support a model in which the severity of the neurodevelopmental disease increases with increasing genomic region affected [50]. Deletions of 15q11.2, 16p11.2 and duplication of 16p13.11 have been detected with high frequency in individuals with ASD [51]. However, the penetrance of these regions of genomic variation varies and the characterization of the pathogenicity of these events is, at times, a challenge [11]. It is not uncommon for a deletion in one of these regions to be inherited from normal parents or to be present in an unaffected or mildly affected sibling. A possible mechanism for ASD/epilepsy associated with these CNVs is a second mutation on the non-deleted allele [52].
Phelan-McDermid syndrome / SHANK3 deletion
Deletion of 22q13.3 containing the SHANK3 gene has been associated with early hypotonia, developmental and speech delay, autism-like behaviors, lymphedema, and dysmorphic features [53,54]. Other complications include gastroesophageal reflux, kidney problems, and skin rashes [54]. The prevalence of epilepsy in patients with 22q13.3 deletion is not known. Some have reported a benign course of generalized tonic-clonic or myoclonic seizures with typical EEG features [55]. A larger series found seizures to be three times more common when the de novo deletion occurred on the maternally rather than paternally inherited chromosome 22 [54].
SHANK3 encodes a scaffolding protein found in the postsynaptic density, that regulates the expression of metabotropic glutamate receptor 5 (mGluR5) [56]. Shank3 also plays a role in the regulation of AMPA receptors recycling and synaptic long-term potentiation [57], and interacts with the voltage-gated potassium channel Kvβ2 within the postsynaptic density [58]. Mice deficient in Shank3 display autistic behavior and have abnormalities in striatal synapses and corticostriatal circuits [59,60]. Deletions of SHANK1 [61] and mutations in SHANK2 have also been reported in patients with ASD [62].
Single gene disorders
Fragile X syndrome
Fragile X syndrome (FRX) is the most common inherited form of intellectual disability with an estimated prevalence of 1 in 4000 males [63]. FRX occurs when a triplet repeat (CGG) expansion leads to inactivation of the FMR1 gene resulting in loss of FMRP expression. FMRP is an RNA-binding protein, localizing to dendritic ribosomes and likely plays a role in synaptic remodeling, required for normal learning and memory [64]. Physical features include prominent ears, long face, macrocephaly and macroorchidism. The cognitive profile includes hyperactivity, anxiety, tactile defensiveness, gaze avoidance and socialization difficulties [65]. FRX has been considered the principle monogenic disorder associated with ASD [63,65]. Reciprocal social interaction and adaptive socialization (as measured by ADI-R) were identified as the core autistic behaviors among a study cohort of FRX individuals, irrespective of intellectual disability [63].
Epilepsy is reported in approximately 10–20% of FRX individuals [64]. Seizure patterns in FRX typically resemble benign focal epilepsy of childhood (BFEC). In a review of 13 individuals with FRX and seizures, 10 were reported to have abnormal EEGs and 6 of these EEG studies showed centrotemporal spikes typical of BFEC [64]. Additionally, 23% individuals with FRX without clinical seizures demonstrated centrotemporal spikes on EEG [64]. It has been proposed that a voltage-gated inward current, ImGluR(V), mediates epileptogenesis by activation of the mGluR5 receptor [66]. The induction of ImGluR(V) may lead to global neuronal changes, rather than synapse-specific events [66]. The activation of mGluR5 across multiple synapses in the setting of poor FMRP translational control leads to heightened electrical excitability [66].
Pathogenic expansion and hypermethylation of a CGG triplet repeat in the 5’ untranslated region of FMR1 results in transcriptional silencing [67,68]. The gene product, FMRP, is an RNA-binding protein [69], and its loss of function in several animal models is associated with a host of downstream effects on neurons. These include dysregulation of NeuroD1 expression in the rat [70], disrupted trans-synaptic signaling in Drosophila [71] and reduction of neuronal long-term potentiation and enhanced long-term depression in zebrafish [72]. In the FRX mouse model, dysregulation of excitatory synaptic formation [73], reduction in expression of specific GABA receptor subunits [74], and N-methyl-D-aspartate (NMDA) receptors [75] have been reported.
Tuberous sclerosis complex
Tuberous sclerosis complex (TSC) is a multisystem disorder characterized by hamartomas of the brain, heart, lungs, kidneys and skin and results from mutations in TSC1 and TSC2 [76]. Their protein products, hamartin and tuberin respectively, bind together and form a protein complex involved in the regulation of the mammalian target of rapamycin (mTOR). The loss of TSC function results in increased Rheb activity and subsequent hyperactivity in mTOR, ultimately leading to disinhibition of protein synthesis and cell growth [77]. Neurologic manifestations of TSC include epilepsy [78], intellectual disability [79] and ASD [80], as well as the specific brain malformations and cortical tubers [81], subependymal nodules and subependymal giant cell astrocytomas [82], and increasing recognition for a role in focal cortical dysplasias [83–85].
Epilepsy occurs in more than 80–90% of patients with TSC [85,86]. Seizure type varies but is often progressive and refractory to pharmacologic treatment. Infantile spasms occur in approximately 20–38% of TSC patients [78] and are generally associated with a poorer prognosis [87]. Patients with intractable epilepsy are often treated with resection, especially if a single tuber is thought to be the epileptogenic focus [88]. There is increasing interest in the use of compounds to disrupt the mTOR pathway in epileptogenesis [89] and suggested mTOR inhibitors as antiepileptogenic therapy [90,91].
ASD is estimated to be present in 20–60% of individuals with TSC and is about equally common in males and females in this population [92]. Intellectual disability, infantile spasms and presence of temporal lobe lesions were initially reported as risk factors for ASD in individuals with TSC but have not been consistently supported [93, 94].
PTEN
PTEN is a tumor suppressor gene that encodes a phosphatase affecting G1 cell cycle arrest and inhibiting the PI3K/AKT/mTOR pathway [95]. Germline mutations of PTEN are associated with four known hamartoma syndromes: Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome (BRRS), Proteus syndrome and Proteus-like conditions [96]. Somatic mutations are reported in varying malignancies, most notably breast, thyroid, and endometrial cancers [96]. Macrocephaly and ASD have been reported in children with germline PTEN mutations [97,98]. PTEN-related ASD is therefore emerging as one of a group of megalencephaly disorders associated with dysregulation of the PI3K-AKT-mTOR pathway [99].
Seizures have been reported in patients with PTEN mutations [100,101], including a number with focal cortical dysplasia [102–104]. Pten knockout mice are known to have seizures [105] that can be suppressed with the mTOR pathway inhibitor rapamycin [106]. Epilepsy appears to be a part of the phentoype for many of the megalencephaly disorders associated with dysregulation of the PI3K-AKT-mTOR pathway [107,108], but the exact roles of mutations in specific genes in this pathway related to seizures and ASDs remains to be clarified.
MECP2-related disorder (formerly Rett syndrome)
MECP2-related disorder predominantly affects females and is characterized by intellectual disability, postnatal microcephaly, loss of spoken language and stereotypic hand movements. Onset of symptoms and regression typically occur at 6 to 18 months of age after a period of apparently normal development [109]. Individuals with MECP2-related disorder demonstrate autistic symptoms [110,111] as well as distinct features that include respiratory rhythm abnormalities, gait impairment, and cardiac complications [112,113].
Among individuals with MECP2 deficiency, 50–90% are reported to have seizures [114–116]. Seizure type is variable, age of onset is rarely before 2 years of age [116] and severity of seizures appears to decline after adolescence [115]. Specific MECP2 mutations (p.T158M and p.R106W) were more highly associated with epilepsy [116].
MECP2 is primarily a transcriptional activator during brain development [117]. The consequences of mutations in MECP2 include abnormal downstream regulation of multiple gene targets, and loss of MECP2 function reduces GABAergic transmission [118] and impaired glutamatergic drive in specific populations of inhibitory interneurons [119]. There is evidence from mouse models that restoration of gene function reversed some of the neurodevelopmental deficits even after symptoms had emerged [120].
CDKL5-related disorder
CDKL5-related disorder is an X-linked condition characterized by early onset of epilepsy, usually infantile spasms, and severe neurodevelopmental outcome with postnatal microcephaly, absent spoken language, and hand stereotypies that are reminiscent of MECP2-related disorder [121]. Although girls with CDKL5 mutations share some ASD features (abnormal social interactions, repetitive movements, and absent speech), the concomitant developmental disability and the epilepsy phenotype [122,123] are much greater than that typically seen in children with classical forms of ASD.
While CDKL5-related disorder was first described in 2004 [124], and its function as a serine-threonine kinase is well characterized, the developmental role of the protein was not known until recently. CDKL5 interacts with NGL-1 and PSD95 (key candidates in ASD pathogenesis in their own right), in glutamatergic post-synapses [125], during dendrite spine development [126], including an important role stabilizing the post-synaptic membrane [118].
FOXG1-related disorders
Children with duplications of FOXG1 on chromosome 14q12 frequently present with infantile spasms [127–129]. Patients commonly respond to adrenocorticotropin therapy with remission of the epileptic spasms and normalization of the EEG [130,131], but have long-term developmental disability that includes autistic features [132]. In contrast, children with deletions of 14q12 that include FOXG1 or intragenic loss-of-function mutations have a disorder of postnatal microcephaly, hypoplasia of the anterior corpus callosum, severe language and motor impairment, and a choreiform movement disorder [133–134]. The mean age of epilepsy onset for children with deletions/loss-of-function mutations of FOXG1 is 22 months, compared to epilepsy onset at 7 months in children with duplications [132].
FOXG1 is a brain-specific transcriptional repressor protein that regulates dorsal-ventral patterning [135] and neurogenesis [136]. Overexpression of FOXG1 in the developing forebrain is associated with thickening of the neuroepithelium [137], and more recent evidence supports a role for class switching in neuroprogenitor cells [138]. However, the mechanisms by which changes in copy number in this gene leads to epilepsy and the associated developmental disabilities are not known.
MEF2C-related disorder
Patients with loss-of-function mutations and deletions of MEF2C on chromosome 5q14.3 were first described with severe intellectual disability, epilepsy, and stereotypic movements [139]. Further characterization of the phenotype includes children with autistic features [140,141] with some overlap noted with features found in MECP2-related disorder. In most patients head size and brain morphology are normal.
The epilepsy found in individuals with MEF2C-related disorder can be variable, with 20% presenting with infantile spasms, 33% with infant-onset myoclonic epilepsy, 24% with childhood-onset generalized epilepsy, and 23% having no epilepsy [142]. The reason for this observed clinical variability in epilepsy type and severity is unclear, but appears to be independent of mutational class, although subjects with partial MEF2C deletions were less likely to have epilepsy [142].
Mef2c plays several roles during brain development, and is a marker of cortical lamination driven by Tbr1 [143]. Mef2c expression is also diminished in Arx and Dlx1/2 deficient mice [144] indicating a complex role during both dorsal glutamatergic and ventral GABAergic development [142]. Finally, Mef2c recognizes a binding site called the synaptic activity-response element (SARE) that activates a series of genes important for synaptic development and function [145].
CASK-related disorders
Mutations affecting CASK were first described in primarily female patients with severe microcephaly and pontocerebellar hypoplasia [146]. Males affected with intellectual disability and oculomotor abnormalities were later described [147]. Absent spoken language and autistic behaviors are described, particularly in girls on the milder spectrum of microcephaly [148].
CASK encodes a calcium/calmodulin-dependent serine protein kinase expressed in the brain [149]. CASK has a role in synapse formation, synapse function and cortical development. The core clinical features in females with CASK mutations includes a distinct malformation phenotype involving postnatal microcephaly and pontine and cerebellar hypoplasia, developmental delay, growth retardation, eye abnormalities and a pattern of facial dysmorphisms [148]. Hypomorphic CASK alleles in male patients appear to cause a milder phenotype, presumably due to a smaller disruption of protein structure and function [150]. However, CASK abnormalities have been reported in male patients with Ohtahara syndrome and severe phenotypic features consistent with previously reported CASK mutations [151]. Nearly all female patients have moderate or severe impairment in intellectual development. Language is generally impaired or absent as well. Behaviors such as hand stereotypies and self-biting are commonly seen. Data are unavailable on ASD prevalence in this population. ASD diagnosis is likely confounded by the severity of impairment and intellectual disability. Epilepsy is reported in more than half of female patients with variable age of onset and seizure type [150].
CASK is an example of a gene that plays multiple roles during brain development. Through interactions in the nucleus with the early cortical patterning proteins RELN and TBR1, CASK plays a role in neuronal migration [149]. CASK additionally plays an important role in post-synaptic structural support [149, 150].
SCN2A-related disorders
Deletion of chromosome 2q24.2q24.3 containing SCN2A was first reported in a child with autistic features and intellectual disability [152]. Then, nonsense mutations in SCN2A were discovered in two children with ASD using whole exome sequencing [153]. At the same time, several children were identified with a spectrum of severe early life epilepsies including Ohtahara syndrome [154–156] malignant migrating partial seizures of infancy [157], and infantile spasms [158–159] with mutations in SCN2A. Other children with SCN2A mutations have been reported with benign neonatal-infantile epilepsy [160] and generalized epilepsy with febrile seizures plus, and is an infrequent cause of Dravet syndrome [161]. While genotype phenotype correlations have been challenging in SCN2A-related epilepsies, there is emerging evidence that missense mutations resulting in more chemically dissimilar amino acid substitutions correlate with worse disease, and that truncating mutations are associated with the most severe phenotypes [162].
SCN2A encodes the voltage-gated sodium channel Na(v)1.2 predominantly expressed in excitatory neurons, and it is unclear how loss-of-function mutations can result in hyperexcitability [163–164]. Less clear is the mechanism by which ASD symptoms result.
Epilepsy syndromes with ASD as frequent neurodevelopmental sequelae
Evidence suggests children with ASD who have epilepsy may have seizures that do not fulfill criteria for specific named electroclinical syndromes [165]. However, several specific epilepsy syndromes appear to be risk factors for later diagnosis of ASD. These include infantile spasms and Lennox-Gastaut syndrome. More recently, overlap has been observed clinically with continuous slow waves during sleep (CSWS) and Landau-Kleffner syndrome and ASD [166].
Infantile spasms
Infantile spasms are a form of epilepsy associated with an EEG pattern of hypsarrythmia and characterized by epileptic spasms that occur before 2 years of age [167]. Infantile spasms are genetically heterogeneous and are associated with abnormalities in several brain developmental pathways [168]. The prevalence of ASD among children with a history of infantile spasms has not been reported consistently, but an association between the two is clearest in tuberous sclerosis [169] and duplications of FOXG1 [132].
Lennox-Gastaut syndrome
Lennox-Gastaut syndrome (LGS) is a childhood-onset epilepsy phenotype characterized by electroclinical features of diffuse slow spike waves and generalized paroxysmal fast activity in sleep. Little literature exists on the prevalence of ASD or the behavioral spectrum in LGS, although ASD has been reported [170–172] and LGS has occurred in patients with duplications of maternal 15q11q13 [32,173].
Landau-Kleffner syndrome / Continuous Spikes and Slow Waves during Slow Sleep
Landau-Kleffner syndrome (LKS) is an epilepsy-aphasia syndrome of unknown etiology characterized by language regression and characteristic continuous spike and waves during slow wave sleep on EEG [174–175]. As LKS became increasingly recognized, several children who had been diagnosed with ASD were noted to have a predominant language deficit [176]. Stereotypies and withdrawal are also common in LKS, but it is not clear that these children also have deficits in social reciprocity [177]. The association may be more related to severe receptive language deficit [175]. Studies have detected copy number variants in LKS patients that have also been associated with ASD [178], and most recently mutations in GRIN2A have been identified in patients with epilepsy-aphasia phenotypes [179–181].
ASD Screening and Diagnosis
Due to the association between seizures and ASD, it is important for epileptologists to recognize when and how to screen for ASD and appropriately refer for diagnostic evaluation. Although ASD persists across the lifespan, timely detection and intervention can alleviate symptoms [182]. We have described several syndromes in which epilepsy and ASD co-occur at a rate that warrants direct referral for an evaluation of ASD and other developmental disorders. Other situations warrant ASD screening in children with or without the genomic syndromes reviewed here. The American Academy of Pediatrics (AAP) recommends ASD screening for all 18- and 24-month old children who either have a sibling with ASD and a caregiver who expresses concerns about ASD symptoms, or who have concerns expressed by multiple caregivers and providers [183]. Screening also should occur if the child has no babbling at 12 months, no single words by 16 months, no spontaneous phrases by 24 months, or loss of social or language skills at any age [184]. Signs that may call for screening of older children and youth include disinterest in back-and-forth interactions with peers; problems with “reading” common social cues or interpreting nonliteral speech (e.g., sarcasm or metaphors); lack of understanding of the perspective of others; inability to engage in social chat or conversations, or highly rigid, perseverative, or repetitive patterns of behavior [183]. Screening usually involves administering a brief rating scale to the parent. For toddlers, the best-established screening instrument currently is the Modified Checklist for Autism in Toddlers – Revised with Follow-up (M-CHAT-R/F) [185–186]. The M-CHAT-R/F contains a 20-item, yes/no rating scale and a brief follow-up interview if three or more items on the rating scale indicate that the child is at risk for ASD. For children age 4 years and older, the most extensively validated screening instrument is the Social Communication Questionnaire, SCQ, which is a 40-item, yes/no rating scale [187]. If children at risk for ASD have a negative screen, the AAP recommends counseling parents on how to recognize ASD symptoms and scheduling a follow-up evaluation [183]. If children have a positive screen, they should be referred for a comprehensive diagnostic evaluation.
The diagnostic evaluation for ASD ideally includes a clinical evaluation by a specialist (child neurologist, developmental behavioral pediatrician, child psychiatrist, clinical psychologist) who has expertise in ASD, a review of findings from developmental tests, a detailed medical and developmental history, referrals for additional testing as indicated by the assessment (e.g., genotyping for children with intellectual disability or dysmorphic features), and administration of a standardized diagnostic instrument. The most commonly used diagnostic instrument in clinical practice is the Autism Diagnostic Observation Schedule – Second Edition (ADOS-2) [188]. The ADOS-2 is a series of structured and semi-structured tasks, approximately 30–60 minutes in duration, involving social and communicative interaction between the examiner and the patient. Behaviors are assigned to predetermined observational categories that are subsequently used to produce a quantitative score [187]. The Autism Diagnostic Interview – Revised (ADI-R) is a companion standardized, 2–3 hour interview for caregivers of individuals with ASD that provides a diagnostic algorism for autism based on ICD-10 and DSM-IV [188,189]. The ADOS-2 and ADI-R are widely regarded as the best-established tools for diagnosing ASD [190]. Table 2 summarizes the commonly used screening and diagnostic tools for ASD.
Table 2.
Features of available autism diagnostic and screening tools.
| Name | Purpose | Completed by | Description | Time (minutes) | Strengths/Weaknesses |
|---|---|---|---|---|---|
| Autism Diagnostic Observation Scale (ADOS) | Diagnostic | Clinician | Series of structured and semi- structured tasks involving social and communicative interaction. | 60 | Requires training Time for administration Widely used in research and clinical settings |
| ADI-R | Diagnostic | Clinician | Clinical interview that probes for autism symptoms and behaviors. | 120–180 | Standard tool used clinically and in research Time constraint limits clinical use |
| Childhood Autism Rating Scale (CARS) | Diagnostic | Clinician | 15-item direct- observation tool for children over 2 years old that includes items drawn from five prominent systems for diagnosis. | 20–30 | Not appropriate for infants or toddlers Requires training |
| Gilliam Autism Rating Scale (GARS-3) | Diagnostic | Clinician | 56-item assessment for use in 3 to 22 years old grouped into 6 subscales including restrictive and repetitive behaviors, social interaction and communication, emotional responses, cognitive style, and maladaptive speech. | 5–10 | Gives severity estimation 44 new items recently added to GARS-3; requires training for administration and interpretation Demonstrated good reliability and validity |
| Ages and Stages Questionnaires (ASQ) | Screening | Caregiver | 19 age-specific questionnaires (4 – 60 months) for use as developmental and social- emotional screening tool. | 10–15 | Requires little training Fast administration Available in several languages Only tool that links to developmental milestones |
| Communication and Symbolic Behavior Developmental Profile (CSBS DP) Infant Toddler Checklist (ITC) | Screening | Caregiver | 24-item questionnaire designed to identify at-risk children between 6 and 24 months of age based on communication and symbolic abilities. | 5–10 | Fast administration High sensitivity and specific to identify infants and toddlers with developmental delay Does not discriminate between ASD and other communication disorders |
| Parents’ Evaluation of Developmental Status (PEDS) | Screening | Caregiver | 10 single-response form for all ages designed to elicit and address parents' concerns about their child's development and health. | 2–10 | Quick administration Available in multiple languages Low Readability |
| Modified Checklist for Autism in Toddlers (MCHAT-R/F) | Screening | Caregiver | 20-item yes/no questions appropriate for ages 16 to 30 months assesses risk of ASD. Includes structured follow-up questions for children at medium risk prior to referral for diagnostic evaluation. | 5–10 | Validated first-tier screen Available in multiple languages Ease of administration and scoring |
| Screening Tool for Autism in Toddlers and Young Children (STAT) | Screening | Clinician | Interactive tool designed as second-level screen for children with suspected developmental concern. 12 observed activities that assess behaviors in 4 social- communicative domains. | 20 | Requires training Restricted to ages 24–35 months Not validated as a first-tier screen Language comprehension is not required |
| Social Communication Question ;formerly Autism Screening Questionnaire (SCQ) | Screening | Caregiver | Parent-report screen consisting of 40 yes-no questions designed to identify children at risk for ASD from general population. | 5–10 | Fast administration Well-validated Less valid in young children with limited language |
The diagnosis of ASD is difficult in the context of intellectual disability. Although these standardized diagnostic tools are available to assist in making an ASD diagnosis, they need to be used as part of a broader evaluation by a clinician with expertise in ASD. The sensitivity of the ADOS-2 is high (.91-.97), but specificity is lower (.50–.94), particularly for children with ID or minimal verbal skills [191]. ID is common in many of the syndromes reviewed in this article, and there is considerable phenotypic overlap between ASD and ID, making differential diagnosis difficult [192]. In the absence of identified risk factors for ASD, it may be most efficient to begin by conducting a general developmental screen before administering a screen for ASD. Many children with epilepsy will not meet criteria for an ASD diagnosis but are likely to have other developmental concerns. For example, one study of children with epilepsy found a high percentage of positive screens for ASD using the SCQ (15%), M-CHAT (58%) and the ASQ (82%). Positive screening results were associated with ASD diagnosis in only 8% of patients with a positive M-CHAT and 57% of children with a positive SCQ, but a much higher percentage (20% of all children with positive screens) warranted referral for other services such as psychiatric, psychological, or educational services [193]. The frequency of referrals for services confirms the importance of developmental screening, but the high rate of false positive screens for ASD suggests that routine screening for ASD in all children with epilepsy may not be optimal [194].
Conclusion
The co-occurrence of ASD and epilepsy is well recognized but the mechanisms behind this association remain unclear [2–5]. Many of the reported series have small numbers of patients and have inconsistent and varying conclusions [25,195,196]. Low IQ is a well-established risk factor for ASD in children with epilepsy [25]. Developments in our ability to detect pathogenic genomic variations and single gene associations with ASDs and epilepsies have led to a better understanding of their shared biological processes and mechanisms. These pathways include, but are not limited to, gene transcriptional regulation, cellular growth, and synapse development, stability, and function.
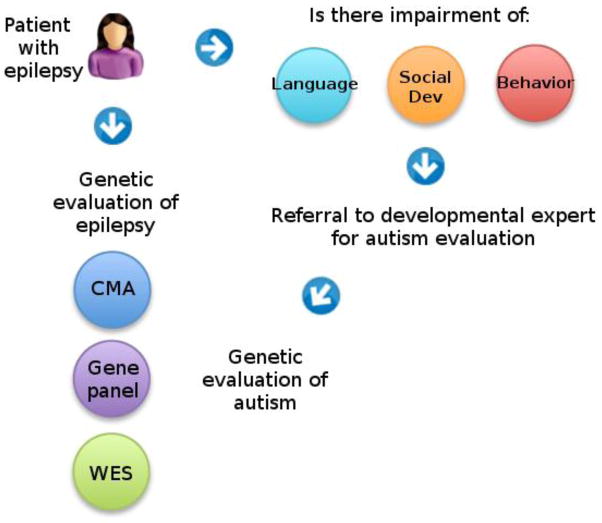
ASD and epilepsies are often co-morbid with varying degrees of developmental delay, learning disability, intellectual disability and behavior problems that confound the diagnosis of ASD and will likely remain a persistent clinical challenge. It is important to recognize the key features of ASD (deficits in social behaviors and communication, restricted interests and repetitive patterns of behavior), when to screen and when to refer for more diagnostic evaluation. The evaluation of children with epilepsy who are at risk for ASD involves coordinated genetic and behavioral testing strategies, illustrated in Figure 2. The clinical genetic testing strategy for both epilepsies and ASD are similar and involve sequential use of chromosomal microarray (CMA), followed by targeted next-generation sequencing gene panels, and if those are normal, whole exome sequencing.
Figure 2.
Suggested workflow for evaluation of epilepsy patients who may be at risk for autism. A patient with epilepsy should be screened for impairment in language, social development, and/or behavior. If warranted, the patient should be referred to a trained expert in autism diagnosis. If a diagnosis of autism is confirmed, or if significant other developmental concerns exist (i.e. intellectual disability), genetic evaluation is appropriate. The clinical genetic evaluation for autism and epilepsy may have overlap, and may be tailored by recognition of conditions detailed in this paper where autism and epilepsy overlap. Current clinical genetic evaluation includes sequentially chromosomal microarray (CMA), autism and epilepsy next-generation sequencing gene panels, and if necessary, whole exome sequencing (WES).
CMA has emerged as a powerful genetic tool in many patient populations, including individuals with ASDs with a reported overall diagnostic yield of 10% [197,198,199]. Certain selection factors such as dysmorphic features, intellectual disability and family history of ASD can increase diagnostic yield [200–203]. Children with ASD with abnormal features on physical exam are 10 times more likely to have a diagnosable genetic condition than those with normal phenotypic appearance [201]. Other clinical considerations include family history, micro- and macrocephaly, abnormal finger digit ratios and cognitive impairment [202]. CMA has demonstrated the highest diagnostic yield (66.7%) in patients with intellectual disability, ASD and dysmorphic features, supporting its use as the first-tier diagnostic genetic test in this subgroup [199,203].
Genetic testing for single gene disorders such as Fragile X should be routinely performed for males with ASD. MECP2 sequencing should be performed for all females with ASD and MECP2 duplication testing should be performed in males with a suggestive phenotype. PTEN testing is recommended in individuals with significant macrocephaly (> 2.5 SD above mean). More recently, the availability of next-generation sequencing panels means that in many cases multiple genes can be evaluated simultaneously with one test, at reduced cost overall. The estimated yield of diagnostic whole exome sequencing in the clinical setting is at least 25% [204], and should be considered if CMA and more targeted gene panel sequencing are normal. Advances in this area have led to identification and discovery of many new de novo mutations in ASD [16,153,205]. Continued research focused on children with epilepsy and ASD will likely yield further knowledge with insight into new therapies.
Highlights.
ASD and epilepsies commonly co-occur.
A number of new genetic discoveries suggest shared biology for both disorders.
Several screening and diagnostic tools are available to clinicians.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Mental Health under award numbers R01 MH084870 and R01 HD073975 (to TS) and the National Institute of Neurologic Disorders and Stroke under award number K08NS078054 (to ARP).
Footnotes
Disclosures
The authors have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salpekar JA, Mishra G. Key Issues in Addressing the Comorbidity of Attention Deficit Hyperactivity Disorder and Pediatric Epilepsy. Epilepsy Behav. 2014;37C:310–5. doi: 10.1016/j.yebeh.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Taft LT, Cohen HJ. Hypsarythmia and infantile autism: a clinical report. J Autism Child Schizophr. 1971;3:327–36. doi: 10.1007/BF01557352. [DOI] [PubMed] [Google Scholar]
- 3.Knobloch H, Pasamanick B. Some etiologic and prognostic factors in early infantile autism and psychosis. Pediatrics. 1975;2:182–91. [PubMed] [Google Scholar]
- 4.Deykin EY, MacMahon B. The incidence of seizures among children with autistic symptoms. Am J Psychiatry. 1979;10:1310–2. doi: 10.1176/ajp.136.10.1310. [DOI] [PubMed] [Google Scholar]
- 5.Riikonen R, Amnell G. Psychiatric disorders in children with earlier infantile spasms. Dev Med Child Neurol. 1981;6:747–60. doi: 10.1111/j.1469-8749.1981.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 6.Brooks-Kayal A. Epilepsy and autism spectrum disorders: Are there common developmental mechanisms? Brain and Dev. 2010;32:731–8. doi: 10.1016/j.braindev.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: 2013. [Google Scholar]
- 8.Berg AT, Millichap JJ. The 2010 revised classification of seizures and epilepsy. Continuum (Minneap Minn) 2013 Jun;19(3 Epilepsy):571–97. doi: 10.1212/01.CON.0000431377.44312.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010 Apr;51(4):676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 10.Eom S, Fisher B, Dezort C, Berg AT. Routine developmental, autism, behavioral, and psychological screening in epilepsy care settings. Dev Med Child Neurol. 2014 Nov;56(11):1100–5. doi: 10.1111/dmcn.12497. [DOI] [PubMed] [Google Scholar]
- 11.Mefford HC, Yendle SC, Hsu C, Cook J, Geraghty E, McMahon JM, et al. Rare copy number variants are an important cause of epileptic encephalopathies. Ann Neurol. 2011 Dec;70(6):974–85. doi: 10.1002/ana.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014 May 1;94(5):677–94. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson H, Shen Y, Avallone J, Sheidley BD, Pinsky R, Bergin AM, et al. Copy number variation plays an important role in clinical epilepsy. Ann Neurol. 2014 Jun;75(6):943–58. doi: 10.1002/ana.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Developmental Disabilities Monitoring Network Surveillance Year 2010. Centers for Disease Control and Prevention. MMWR Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- 15.Grzadzinski R, Huerta M, Lord C. DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol Autism. 2013 May 15;4(1):12. doi: 10.1186/2040-2392-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012 Dec 21;338(6114):1619–22. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicol Teratol. 2013;36:47–56. doi: 10.1016/j.ntt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011 Nov;68(11):1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23(2–3):189–99. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Levy SE, Giarelli E, Lee LC, Schieve LA, Kirby RS, Cunniff C, et al. Autism Spectrum Disorder and Co-occurring Developmental, Psychiatric, and Medical Conditions Among Children in Multiple Populations of the United States. J Dev Behav Pediatr. 2010 May;31(4):267–75. doi: 10.1097/DBP.0b013e3181d5d03b. [DOI] [PubMed] [Google Scholar]
- 21.Woolfenden S, Sarkozy V, Ridley G, Coory M, Williams K. A systematic review of two outcomes in autism spectrum disorder – epilepsy and mortality. Dev Med Child Neurol. 2012 Apr;54(4):306–12. doi: 10.1111/j.1469-8749.2012.04223.x. [DOI] [PubMed] [Google Scholar]
- 22.Jokiranta E, Sourander A, Suominen A, Timonen-Soivio L, Brown AS, Sillanpää M. Epilepsy among children and adolescents with autism spectrum disorders: a population-based study. J Autism Dev Disord. 2013 Oct;44(10):2547–57. doi: 10.1007/s10803-014-2126-6. [DOI] [PubMed] [Google Scholar]
- 23.Tuchman R, Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep. 2011 Aug;11(4):428–34. doi: 10.1007/s11910-011-0195-x. [DOI] [PubMed] [Google Scholar]
- 24.Amiet C, Gourfinkel-An I, Bouzamondo A, Todjman S, Baulac M, Lechat P, et al. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry. 2008 Oct 1;64(7):577–82. doi: 10.1016/j.biopsych.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, et al. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS One. 2013 Jul 4;8(7):e67797. doi: 10.1371/journal.pone.0067797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams, et al. Principles of Neurology. 6. p. P313. [Google Scholar]
- 27.Berg AT, Scheffer IE. New concepts in classification of the epilepsies: entering the 21st century. Epilepsia. 2011 Jun;52(6):1058–62. doi: 10.1111/j.1528-1167.2011.03101.x. [DOI] [PubMed] [Google Scholar]
- 28.Paciorkowski AR, Thio LL, Dobyns WB. Genetic and biologic classification of infantile spasms. Pediatric Neurol. 2011 Dec;45(6):355–67. doi: 10.1016/j.pediatrneurol.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Depienne C, Moreno-De-Luca D, Heron D, Bouteiller B, Gennetier A, Delorme R, et al. Screening for genomic rearrangements and methylation abnormalities of the 15q11-q13 region in autism spectrum disorders. Biol Psychiatry. 2009 Aug 15;66(4):349–59. doi: 10.1016/j.biopsych.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Bolton PF, Dennis NR, Browne CE, Thomas NS, Veltman MW, Thompson RJ, et al. The phenotypic manifestations of interstitial duplications of proximal 15q with special reference to the autistic spectrum disorders. Am J Med Genet. 2001 Dec 8;105(8):675–85. doi: 10.1002/ajmg.1551. [DOI] [PubMed] [Google Scholar]
- 31.Conant KD, Finucane B, Cleary N, Martin A, Muss C, Delany M, et al. A survey of seizures and current treatments in 15q duplication syndrome. Epilepsia. 2014 Mar;55(3):396–402. doi: 10.1111/epi.12530. [DOI] [PubMed] [Google Scholar]
- 32.Orrico A, Zollino M, Galli L, Buoni S, Marangi G, Sorrentino V. Late-onset Lennox-Gastaut syndrome in a patient with 15q11.2-q13.1 duplication. Am J Med Genet A. 2009 May;149A(5):1033–5. doi: 10.1002/ajmg.a.32785. [DOI] [PubMed] [Google Scholar]
- 33.Paciorkowski AR, Thio LL, Rosenfeld JA, Gajecka M, Gurnett CA, Kulkarni S, et al. Copy number variants and infantile spasms: evidence for abnormalities in ventral forebrain development and pathways of synaptic function. Eur J Hum Genet. 2011 Dec;19(12):1238–45. doi: 10.1038/ejhg.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starr EM, Berument Sk, Tomlins M, Papanikolaou K, Rutter M. Brief report: autism in individuals with Down syndrome. J Autism Dev Disord. 2005 Oct;35(5):665–73. doi: 10.1007/s10803-005-0010-0. [DOI] [PubMed] [Google Scholar]
- 35.Ghaziuddin M, Tsai LY, Ghaziuddin N. Autism in Down's syndrome: presentation and diagnosis. J Intellect Disabil Res. 1992 Oct;36( Pt 5):449–56. doi: 10.1111/j.1365-2788.1992.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 36.Kent L, Evans J, Paul M, Sharp M. Comorbidity of autistic spectrum disorders in children with Down syndrome. Dev Med Child Neurol. 1999 Mar;41(3):153–8. doi: 10.1017/s001216229900033x. [DOI] [PubMed] [Google Scholar]
- 37.Turk J. Society for the Study of Behavioural Phenotypes: 2nd Symposium Abstracts. Oxford: SSBP; 1992. Children with Down’s syndrome and Fragile X syndrome: a comparison study. [Google Scholar]
- 38.Rasmussen P, Börjesson O, Wentz E, Gillberg C. Autistic disorders in Down syndrome: background factors and clinical correlates. Dev Med Child Neurol. 2001 Nov;43(11):750–4. doi: 10.1017/s0012162201001372. [DOI] [PubMed] [Google Scholar]
- 39.Molloy CA, Murray DS, Kinsman A, Castillo H, Mitchell T, Hickey FJ, et al. Differences in the clinical presentation of Trisomy 21 with and without autism. J Intellect Disabil Res. 2009 Feb;53(2):143–51. doi: 10.1111/j.1365-2788.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 40.Pueschel SM, Louis S, McKnight P. Seizure disorders in Down syndrome. Arch Neurol. 1991 Mar;48(3):318–20. doi: 10.1001/archneur.1991.00530150088024. [DOI] [PubMed] [Google Scholar]
- 41.Arya R, Kabra M, Gulati S. Epilepsy in children with Down syndrome. Epileptic Disord. 2011 Mar;13(1):1–7. doi: 10.1684/epd.2011.0415. [DOI] [PubMed] [Google Scholar]
- 42.d’Orsi G, Specchio LM. Progressive myoclonus epilepsy in Down syndrome patients with dementia. J Neurol. 2014 Aug;261(8):1584–97. doi: 10.1007/s00415-014-7376-x. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg-Stern H, Strawsburg RH, Patterson B, Hickey F, Bare M, Gadoth N, et al. Seizure frequency and characteristics in children with Down syndrome. Brain Dev. 2001 Oct;23(6):375–8. doi: 10.1016/s0387-7604(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 44.Ferlazzo E, Adjien CK, Guerrini R, Calarese T, Crespel A, Elia M, et al. Lennox-Gastaut syndrome with late-onset and prominent reflex seizures in trisomy 21 patients. Epilepsia. 2009 Jun;50(6):1587–95. doi: 10.1111/j.1528-1167.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 45.Stafstrom CE, Konkol RJ. Infantile spasms in children with Down syndrome. Dev Med Child Neurol. 1994 Jul;36(7):576–85. doi: 10.1111/j.1469-8749.1994.tb11894.x. [DOI] [PubMed] [Google Scholar]
- 46.Sanmaneechai O, Sogawa Y, Silver W, Ballaban-Gil K, Moshé SL, Shinnar S. Treatment outcomes of West syndrome in infants with Down syndrome. Pediatr Neurol. 2013 Jan;48(1):42–7. doi: 10.1016/j.pediatrneurol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Park J, Yang EJ, Yoon JH, Chung KC. Dyrk1A overexpression in immortalized hippocampal cells produces the neuropathological features of Down syndrome. Mol Cell Neurosci. 2007 Oct;36(2):270–9. doi: 10.1016/j.mcn.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Soppa U, Schumacher J, Florencio Ortiz V, Pasqualon T, Tejedor FJ, Becker W. The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation. Cell Cycle. 2014 Jul 1;13(13):2084–100. doi: 10.4161/cc.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souchet B, Guedj F, Sahún I, Duchon A, Daubigney F, Badel A. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis. 2014 Sep;69:65–75. doi: 10.1016/j.nbd.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 50.Girirajan S, Campbell CD, Eichler EE. Human copy number variation and complex genetic disease. Annu Rev Genet. 2011;45:203–26. doi: 10.1146/annurev-genet-102209-163544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008 Feb 15;17(4):628–38. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 52.Girirajan S, Rosenfeld JA, Cooper GM, Antonacci G, Siswara P, Itsara A, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010 Mar;42(3):203–9. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manning MA, Cassidy SB, Clericuzio C, Cherry AM, Schwartz S, Hudgins L, et al. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. 2004 Aug;114(2):451–7. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- 54.Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen CF, Rollins JD, et al. Clinical and genomic evaluation of 201 patients with Phelan-McDermid syndrome. Hum Genet. 2014 Jul;133(7):847–59. doi: 10.1007/s00439-014-1423-7. [DOI] [PubMed] [Google Scholar]
- 55.Figura MG, Coppola A, Bottitta M, Calabrese G, Grillo L, Luciano D, et al. Seizures and EEG pattern in the 22q13.3 deletion syndrome: Clinical report of six Italian cases. Seizure. 2014 Oct;23(9):774–9. doi: 10.1016/j.seizure.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011 Oct 7;286(40):34839–50. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raynaud F, Janossy A, Dahl J, Bertaso F, Perroy J, Varrault A, et al. Shank3-Rich2 interaction regulates AMPA receptor recycling and synaptic long-term potentiation. J Neurosci. 2013 Jun 5;33(23):9699–715. doi: 10.1523/JNEUROSCI.2725-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proepper C, Putz S, Russell R, Boeckers TM, Liebau S. The Kvβ2 subunit of voltage-gated potassium channels is interacting with ProSAP2/Shank3 in the PSD. Neuroscience. 2014 Mar 7;261:133–43. doi: 10.1016/j.neuroscience.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 59.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011 Apr 28;472(7344):437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herbert MR. SHANK3, the synapse, and autism. N Engl J Med. 2011 Jul 14;365(2):173–5. doi: 10.1056/NEJMcibr1104261. [DOI] [PubMed] [Google Scholar]
- 61.Sato D, Lionel AC, Leblond CS, Prasad A, Pinto D, Walker S. SHANK1 deletions in males with autism spectrum disorder. Am J Hum Genet. 2012 May 4;90(5):879–87. doi: 10.1016/j.ajhg.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010 Jun;42(6):489–91. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 63.Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet A. 2004 Sep 1;129A(3):225–34. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 64.Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol. 2002 Nov;44(11):724–8. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- 65.Hagerman RJ, Hagerman PJ. Fragile X syndrome: diagnosis, treatment, and research. 3. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- 66.Bianchi R, Chuang SC, Zhao W, Young SR, Wong RK. Cellular plasticity for group I mGluR-mediated epileptogenesis. J Neurosci. 2009 Mar 18;29(11):3497–507. doi: 10.1523/JNEUROSCI.5447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 68.Verheij C, Bakker CE, de Graaff E, Keulemans K, Willemsen R, Verkerk AJ. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993 Jun 24;363(6431):722–4. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- 69.Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993 Oct 22;262(5133):563–6. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- 70.Jeon SJ, Kim JW, Kim KC, Han SM, Go HS, Seo JE, et al. Translational regulation of NeuroD1 expression by FMRP: involvement in glutamatergic neuronal differentiation of cultured rat primary neural progenitor cells. Cell Mol Neurobiol. 2014 Mar;34(2):297–305. doi: 10.1007/s10571-013-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedman SH, Dani N, Rushton E, Broadie K. Fragile X mental retardation protein regulates trans-synaptic signaling in Drosophila. Dis Model Mech. 2013 Nov;6(6):1400–13. doi: 10.1242/dmm.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng MC, Yang YL, Lu KT. Behavioral and synaptic circuit features in a zebrafish model of fragile X syndrome. PLoS One. 2013;8(3):e51456. doi: 10.1371/journal.pone.0051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zang T, Maksimova MA, Cowan CW, Bassel-Duby R, Olson EN, Huber KM. Postsynaptic FMRP bidirectionally regulates excitatory synapses as a function of developmental age and MEF2 activity. Mol Cell Neurosci. 2013 Sep;56:39–49. doi: 10.1016/j.mcn.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong A, Zhang A, Ke Y, El Idrissi A, Shen CH. Downregulation of GABA(A) β subunits is transcriptionally controlled by Fmr1p. J Mol Neurosci. 2012 Feb;46(2):272–5. doi: 10.1007/s12031-011-9531-5. [DOI] [PubMed] [Google Scholar]
- 75.Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2012 Feb;22(2):241–54. doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- 76.Northrup H, Koenig MK, Au KS. Tuberous Sclerosis Complex in GeneReviews. Seattle (WA): University of Washington, Seattle; 1993–2014. [PubMed] [Google Scholar]
- 77.Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet. 2003 Jan;67(Pt 1):87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 78.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010 Jul;51(7):1236–41. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Eeghen AM, Chu-Shore CJ, Pulsifer MB, Camposano SE, Thiele EA. Cognitive and adaptive development of patients with tuberous sclerosis complex: a retrospective, longitudinal investigation. Epilepsy Behav. 2012 Jan;23(1):10–5. doi: 10.1016/j.yebeh.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Numis AL, Major P, Montenegro MA, Muzykewicz KA, Pulsifer MB, Thiele EA. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011 Mar 15;76(11):981–7. doi: 10.1212/WNL.0b013e3182104347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu-Shore CJ, Major P, Montenegro M, Thiele E. Cyst-like tubers are associated with TSC2 and epilepsy in tuberous sclerosis complex. Neurology. 2009 Mar 31;72(13):1165–9. doi: 10.1212/01.wnl.0000345365.92821.86. [DOI] [PubMed] [Google Scholar]
- 82.Torres OA, Roach ES, Delgado MR, Sparagana SP, Sheffield E, Swift D. Early diagnosis of subependymal giant cell astrocytoma in patients with tuberous sclerosis\. [DOI] [PubMed] [Google Scholar]
- 83.Jahodova A, Krsek P, Kyncl M, Jezdik P, Kudr M, Komarek V. Distinctive MRI features of the epileptogenic zone in children with tuberous sclerosis. Eur J Radiol. 2014 Apr;83(4):703–9. doi: 10.1016/j.ejrad.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 84.Liu J, Reeves V, Michalak Z, Coppala A, Diehl B, Sisodiya SM, et al. Evidence for mTOR pathway activation in a spectrum of epilepsy-associated pathologies. Acta Neuropathol Commun. 2014 Jul 8;2:71. doi: 10.1186/2051-5960-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotulska K, Jurkiewicz E, Donmańska-Pakiela D, Grajkowska W, Mandera M, Borkowska J. Epilepsy in newborns with tuberous sclerosis complex. Eur J Paediat Neurol. 2014 Jul 5; doi: 10.1016/j.ejpn.2014.06.009. pii: S1090-3798(14)00113-5. [DOI] [PubMed] [Google Scholar]
- 86.Sparangana SP, Roach ES. Tuberous sclerosis complex. Curr Opin Neurol. 2000 Apr;13(2):115–9. doi: 10.1097/00019052-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 87.Muzykewicz DA, Costello DJ, Halpern EF, Thiele EA. Infantile spasms in tuberous sclerosis complex: prognostic utility of EEG. Epilepsia. 2009 Feb;50(2):290–6. doi: 10.1111/j.1528-1167.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 88.Krsek P, Jahodova A, Kyncl M, Kudr M, Komarek V, Jezdik P, et al. Predictos of seizure-free outcome after epilepsy surgery for pediatric tuberous sclerosis complex. Epilepsia. 2013 Nov;54(11):1913–21. doi: 10.1111/epi.12371. [DOI] [PubMed] [Google Scholar]
- 89.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009 May 27;29(21):6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong M. A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev Neurother. 2013 Jun;13(6):657–69. doi: 10.1586/ern.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardamone M, Flanagan D, Mowat D, Kennedy SE, Chopra M, Lawson JA. Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. J Pediatr. 2013 May;164(5):1195–200. doi: 10.1016/j.jpeds.2013.12.053. [DOI] [PubMed] [Google Scholar]
- 92.Hunt A, Shepherd C. A prevalence study of autism in tuberous sclerosis. J Autism Dev Disord. 1993 Jun;23(2):323–39. doi: 10.1007/BF01046223. [DOI] [PubMed] [Google Scholar]
- 93.Smalley S. Autism and tuberous sclerosis. J Autism Dev Disord. 1998 Oct;28(5):407–14. doi: 10.1023/a:1026052421693. [DOI] [PubMed] [Google Scholar]
- 94.Weber AM, Egelhoff JC, McKellop JM, Franz DN. Autism and the cerebellum: evidence from tuberous sclerosis. J Autism Dev Disord. 2000 Dec;30(6):511–7. doi: 10.1023/a:1005679108529. [DOI] [PubMed] [Google Scholar]
- 95.Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet. 2013 May;163C(2):114–21. doi: 10.1002/ajmg.c.31364. [DOI] [PubMed] [Google Scholar]
- 96.Eng C. GeneReviews. Seattle (WA): University of Washington, Seattle; 1993–2014. PTEN hamartoma tumor syndrome. [PubMed] [Google Scholar]
- 97.Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005 Apr;42(4):318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herman GE, Butter E, Enrile B, Pastore M, Prior TW, Sommer A. Increasing knowledge of PTEN germline mutations: two additional patients with autism and macrocephaly. Am J Med Genet A. 2007 Mar 15;143A(6):589–93. doi: 10.1002/ajmg.a.31619. [DOI] [PubMed] [Google Scholar]
- 99.Mirzaa GM, Poduri A. Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology. Am J Med Genet C Semin Med Genet. 2014 Jun;166C(2):156–72. doi: 10.1002/ajmg.c.31401. [DOI] [PubMed] [Google Scholar]
- 100.Conti S, Condò M, Posar A, Mari G, Resta N, Renieri A, et al. Phosphatase and tensin homolog (PTEN) gene mutations and autism: literature review and a case report of a patient with Cowden syndrome, autistic disorder, and epilepsy. J Child Neurol. 2012 Mar;27(3):392–7. doi: 10.1177/0883073811420296. [DOI] [PubMed] [Google Scholar]
- 101.Marchese M, Conti V, Valvo G, Moro F, Muratori G, Tancredi R, et al. Autism-epilepsy phenotype with macrocephaly suggests PTEN, but not CLIALCAM, genetic screening. BMC Med Genet. 2014 Feb 27;15:26. doi: 10.1186/1471-2350-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elia M, Amato C, Bottitta M, Grillo L, Calabrese G, Esposito M, et al. An atypical patient with Cowden syndrome and PTEN gene mutation presenting with cortical malformation and focal epilepsy. Brain Dev. 2012 Nov;34(10):873–6. doi: 10.1016/j.braindev.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 103.Cheung KM, Lam CW, Chan TK, Siu WK, Yong L. Atypical focal cortical dysplasia in a patient with Cowden syndrome. Hong Kong Med J. 2014 Apr;20(2):165–7. doi: 10.12809/hkmj133863. [DOI] [PubMed] [Google Scholar]
- 104.Child ND, Cascino GD. Mystery case: Cowden syndrome presenting with partial epilepsy related to focal cortical dysplasia. Neurology. 2013 Sep 24;81(13):e98–9. doi: 10.1212/WNL.0b013e3182a55ef0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001 Dec;29(4):396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- 106.Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, et al. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011 Nov;52(11):2065–75. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mirzaa G, Dodge NN, Glass I, Day C, Gripp K, Nicholson L, et al. Megalencephaly and perisylvian polymicrogyria with postaxial polydactyly and hydrocephalus: a rare brain malformation syndrome associated with mental retardation and seizures. Neuropediatrics. 2004 Dec;35(6):353–9. doi: 10.1055/s-2004-830497. [DOI] [PubMed] [Google Scholar]
- 108.Mirzaa GM, Parry DA, Fry AE, Giamanco KA, Schwartzentruber J, Vanstone M, et al. De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat Genet. 2014 May;46(5):510–5. doi: 10.1038/ng.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Percy AK. Rett syndrome: clinical correlates of the newly discovered gene. Brain Dev. 2001;23(Suppl 1):S202–5. doi: 10.1016/s0387-7604(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 110.Young DJ, Bebbington A, Anderson A, Ravine D, Ellaway C, Kulkarni A. The diagnosis of autism in a female: could it be Rett syndrome? Eur J Pediatr. 2008 Jun;167(6):661–9. doi: 10.1007/s00431-007-0569-x. [DOI] [PubMed] [Google Scholar]
- 111.Neul JL. The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin Neurosci. 2012 Sep;14(3):253–62. doi: 10.31887/DCNS.2012.14.3/jneul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramirez JM, Ward CS, Neul JL. Breathing challenges in Rett syndrome: lessons learned from humans and animal models. Respir Physiol Neurobiol. 2013 Nov 1;189(2):280–7. doi: 10.1016/j.resp.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Felice C, Maffei S, Signorini C, Leoncini S, Lunghetti S, Valacchi G. Subclinical myocardial dysfunction in Rett syndrome. Eur Heart J Cardiovasc Imaging. 2012 Apr;13(4):339–45. doi: 10.1093/ejechocard/jer256. [DOI] [PubMed] [Google Scholar]
- 114.Dolce A, Ben-Zeev B, Naldu S, Kossoff EH. Rett syndrome and epilepsy: and update for child neurologists. Pediatr Neurol. 2013 May;48(5):337–45. doi: 10.1016/j.pediatrneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 115.Nissenkorn A, Gak E, Vecsler M, Reznik H, Menascu S, Ben Zeev B. Epilepsy in Rett Syndrome – the experience of a national Rett center. Epilepsia. 2010 Jul;51(7):1252–8. doi: 10.1111/j.1528-1167.2010.02597.x. [DOI] [PubMed] [Google Scholar]
- 116.Glaze DG, Percy AK, Skinner S, Motil KJ, Neul JL, Barrish JO, et al. Epilepsy and the natural history of Rett syndrome. Neurology. 2010 Mar 16;74(11):909–12. doi: 10.1212/WNL.0b013e3181d6b852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008 May 30;320(5880):1224–9. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang W, Peterson M, Beyer B, Frankel WN, Zhang ZW. Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014 Feb 12;34(7):2754–63. doi: 10.1523/JNEUROSCI.4900-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calfa G, Li W, Rutherford JM, Pozzi-Miller L. Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice. Hippocampus. 2014 Sep 10; doi: 10.1002/hipo.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurogical defects in a mouse model of Rett syndrome. Science. 2007 Feb 23;315(5818):1143–7. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanefeld F. The clinical pattern of Rett syndrome. Brain Dev. 1985;7(3):320–5. doi: 10.1016/s0387-7604(85)80037-1. [DOI] [PubMed] [Google Scholar]
- 122.Bahi-Buisson N, Nectoux J, Rosas-Vargas H, Milh M, Boddaert N, Girard B, et al. Key clinical features to identify girls with CDKL5 mutations. Brain. 2008 Oct;131(Pt 10):2647–61. doi: 10.1093/brain/awn197. [DOI] [PubMed] [Google Scholar]
- 123.Bahi-Buisson N, Kaminska A, Boddaert N, Rio M, Afenjar A, Gérard M, et al. The three stages of epilepsy in aptients with CDKL5 mutations. Epilepsia. 2008 Jun;49(6):1027–37. doi: 10.1111/j.1528-1167.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 124.Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, Archer H, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004 Dec;75(6):1079–93. doi: 10.1086/426462. Epub 2004 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ricciardi S, Ungaro F, Hambrock M, Rademacher N, Stefanelli G, Brambilla D, et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol. 2012 Sep;14(9):911–23. doi: 10.1038/ncb2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu YC, Li D, Wang L, Lu B, Zheng J, Zhao SL, et al. Palmitoylation-dependent CDKL5-PSD-95 interaction regulates synaptic targeting of CDKL5 and dendritic spine development. Proc Natl Acad Sci U S A. 2013 May 28;110(22):9118–23. doi: 10.1073/pnas.1300003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yeung A, Bruno D, Scheffer IE, Carranza D, Burgess T, Slater HR. 4.45 Mb microduplication in chromosome band 14q12 including FOXG1 in a girl with refractory epilepsy and intellectual impairment. Eur J Med Genet. 2009 Nov-Dec;52(6):440–2. doi: 10.1016/j.ejmg.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 128.Brunetti-Pierri N, Paciorkowski AR, Ciccone R, Della Mina E, Bonaglia MC, Borgatti R, et al. Duplications of FOXG1 in 14q12 are associated with developmental epilepsy, mental retardation, and severe speech impairment. Eur J Hum Genet. 2011 Jan;19(1):102–7. doi: 10.1038/ejhg.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Striano P, Paravidino R, Sicca F, Chiurazzi P, Gimelli S, Coppola A, et al. West syndrome associated with 14q12 duplications harboring FOXG1. Neurology. 2011 May 3;76(18):1600–2. doi: 10.1212/WNL.0b013e3182194bbf. [DOI] [PubMed] [Google Scholar]
- 130.Pontrelli G, Cappelletti S, Claps D, Sirleto P, Ciocca L, Petrocchi S, et al. Epilepsy in patients with duplications of chromosome 14 harboring FOXG1. Pediatr Neurol. 2014 May;50(5):530–5. doi: 10.1016/j.pediatrneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 131.Bertossi C, Cassina M, De Palma L, Vecchi M, Rossato S, Toldo I, et al. 14q12 duplication including FOXG1: is there a common age-dependent epileptic phenotype? Brain Dev. 2014 May;36(5):402–7. doi: 10.1016/j.braindev.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 132.Seltzer LE, Ma M, Ahmed S, Bertrand M, Dobyns WB, Wheless J, et al. Epilepsy and outcome in FOXG1-related disorders. Epilepsia. 2014 Aug;55(8):1292–300. doi: 10.1111/epi.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kortüm F, Das S, Flindt M, Morris-Rosendahl DJ, Goldstein A, Horn D, et al. The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J Med Genet. 2011 Jun;48(6):396–406. doi: 10.1136/jmg.2010.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bahi-Buisson N, Nectoux J, Girard B, Van Esch H, De Ravel T, Boddaert N. Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant. Neurogenetics. 2010 May;11(2):241–9. doi: 10.1007/s10048-009-0220-2. [DOI] [PubMed] [Google Scholar]
- 135.Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008 Sep;9(9):678–85. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brancaccio M, Pivetta C, Granzotto M, Filippis C, Mallamaci A. Emx2 and Foxg1 inhibit gliogenesis and promote neuronogenesis. Stem Cells. 2010 Jul;28(7):1206–18. doi: 10.1002/stem.443. [DOI] [PubMed] [Google Scholar]
- 137.Ahlgren S, Vogt P, Bronner-Fraser M. Excess FoxG1 causes overgrowth of the neural tube. J Neurobiol. 2003 Dec;57(3):337–49. doi: 10.1002/neu.10287. [DOI] [PubMed] [Google Scholar]
- 138.Kumamoto T, Toma K, Gunadi, McKenna WL, Kasukawa T, Katzman S, et al. Foxg1 coordinates the switch from nonradially to radially migrating glutamatergic subtypes in the neocortex through spatiotemporal repression. Cell Rep. 2013 Mar 28;3(3):931–45. doi: 10.1016/j.celrep.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Le Meur N, Holder-Espinasse M, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, et al. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet. 2010 Jan;47(1):22–9. doi: 10.1136/jmg.2009.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nowakowska BA, Obersztyn E, Szymaskańska K, Bekiesińska-Figatowaska M, Xia Z, Ricks CB, et al. Severe mental retardation, seizures, and hypotonia due to deletions of MEF2C. Am J Med Genet B Neuropsychiatr Genet. 2010 Jul;153B(5):1042–51. doi: 10.1002/ajmg.b.31071. [DOI] [PubMed] [Google Scholar]
- 141.Novara F, Beri S, Giorda R, Ortibus E, Nageshappa S, Darra F, et al. Refining the phenotype associated with MEF2C haploinsufficiency. Clin Genet. 2010 Nov;78(5):471–7. doi: 10.1111/j.1399-0004.2010.01413.x. [DOI] [PubMed] [Google Scholar]
- 142.Paciorkowski AR, Traylor RN, Rosenfeld JA, Hoover JM, Harris CJ, Winter S, et al. MEF2C Haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics. 2013 May;14(2):99–111. doi: 10.1007/s10048-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RA, Beyer RP, et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13129–34. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labosky PA, Golden JA. Identification of Arx transcriptional targets in the developing basal forebrain. Hum Mol Genet. 2008 Dec 1;17(23):3740–60. doi: 10.1093/hmg/ddn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez-Tornos FM, San Aniceto I, Cubelos B, Nieto M. Enrichment of conserved synaptic activity-responsive element in neuronal genes predicts a coordinated response of MEF2, CREB and SRF. PLoS One. 2013;8(1):e53848. doi: 10.1371/journal.pone.0053848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Christian SL, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008 Sep;40(9):1065–7. doi: 10.1038/ng.194. [DOI] [PubMed] [Google Scholar]
- 147.Hackett A, Tarpey PS, Licata A, Cox J, Whibley A, Boyle J, et al. CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes. Eur J Hum Genet. 2010 May;18(5):544–52. doi: 10.1038/ejhg.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moog U, Kutsche K, Kortüm F, Chilian B, Bierhals T, Apeshiotis N, et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011 Nov;48(11):741–51. doi: 10.1136/jmedgenet-2011-100218. [DOI] [PubMed] [Google Scholar]
- 149.Hsueh YP. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem. 2006;13(16):1915–27. doi: 10.2174/092986706777585040. [DOI] [PubMed] [Google Scholar]
- 150.Takanashi J, Okamoto N, Yamamoto Y, Hayashi S, Arai H, Takahashi Y, et al. Clinical and radiological features of Japanese patients with a severe phenotype due to CASK mutations. Am J Med Genet A. 2012 Dec;158A(12):3112–8. doi: 10.1002/ajmg.a.35640. [DOI] [PubMed] [Google Scholar]
- 151.Saitsu H, Kato M, Osaka H, Moriyama N, Horita H, Nishiyama K, et al. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia. 2012 Aug;53(8):1441–9. doi: 10.1111/j.1528-1167.2012.03548.x. [DOI] [PubMed] [Google Scholar]
- 152.Chen CP, Lin SP, Chern SR, Chen YJ, Tsai FJ, Wu PC, et al. Array-CGH detection of a de novo 2.8 Mb deletion in 2q24.2-->q24.3 in a girl with autistic features and developmental delay. Eur J Med Genet. 2010 Jul-Aug;53(4):217–20. doi: 10.1016/j.ejmg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 153.Sanders SJ, Murtha MT, CUpta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012 Apr 4;485(7397):237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakamura K, Kato M, Osaka H, Yamashita S, Nakagawa E, Haginoya K, et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 2013 Sep 10;81(11):992–8. doi: 10.1212/WNL.0b013e3182a43e57. [DOI] [PubMed] [Google Scholar]
- 155.Touma M, Joshi M, Connolly MC, Grant PE, Hansen AR, Khwaja O, et al. Whole genome sequencing identifies SCN2A mutation in monozygotic twins with Ohtahara syndrome and unique neuropathologic findings. Epilepsia. 2013 May;54(5):e81–5. doi: 10.1111/epi.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martin HC, Kim GE, Pagnamenta AT, Murakami Y, Carvill GL, Meyer E, et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet. 2014 Jun 15;23(12):3200–11. doi: 10.1093/hmg/ddu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dhamija R, Wirrell E, Falcao G, Kirmani S, Wong-Kisiel LC. Novel de novo SCN2A mutation in a child with migrating focal seizures of infancy. Pediatr Neurol. 2013 Dec;49(6):486–8. doi: 10.1016/j.pediatrneurol.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 158.Ogiwara I, Ito K, Sawaishi Y, Osaka H, Mazaki E, Inoue I, et al. De novo mutations of voltage-gated sodium channel alphaII gene SCN2A in intractable epilepsies. Neurology. 2009 Sep 29;73(13):1046–53. doi: 10.1212/WNL.0b013e3181b9cebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sudaram SK, Chugani HT, Tiwari VN, Hug AH. SCN2A mutation is associated with infantile spasms and bitemporal glucose hypometabolism. Pediatr Neurol. 2013 Jul;49(1):46–9. doi: 10.1016/j.pediatrneurol.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herlenius E, Heron SE, Grinton BE, Keay D, Scheffer IE, Mulley JC, et al. SCN2A mutations and benign familial neonatal-infantile seizures: the phenotypic spectrum. Epilepsia. 2007 Jun;48(6):1138–42. doi: 10.1111/j.1528-1167.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 161.Shi X, Yasumoto S, Nakagawa E, Fukasawa T, Uchiya S, Hirose S. Missense mutation of the sodium channel gene SCN2A causes Dravet syndrome. Brain Dev. 2009 Nov;31(10):758–62. doi: 10.1016/j.braindev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 162.Brunklaus A, Ellis R, Reavey E, Semsarian C, Zuberi SM. Genotype phenotype associations across the voltage-gated sodium channel family. J Med Genet. 2014 Oct;51(10):650–8. doi: 10.1136/jmedgenet-2014-102608. [DOI] [PubMed] [Google Scholar]
- 163.Kile KB, Tian N, Durand DM. Scn2a sodium channel mutation results in hyperexcitability in the hippocampus in vitro. Epilepsia. 2008 Mar;49(3):488–99. doi: 10.1111/j.1528-1167.2007.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lossin C, Shi X, Rogawski MA, Hirose S. Compromised function in the Na(v)1.2 Dravet syndrome mutation R1312T. Neurobiol Dis. 2012 Sep;47(3):378–84. doi: 10.1016/j.nbd.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 165.Francis A, Msall M, Obringer E, Kelley K. Children with autism spectrum disorder and epilepsy. Pediatr Ann. 2013 Dec;42(12):255–60. doi: 10.3928/00904481-20131122-10. [DOI] [PubMed] [Google Scholar]
- 166.Deonna T, Roulet-Perez E. Early-onset acquired epileptic aphasia (Landau-Kleffner syndrome, LKS) and regressive autistic disorders with epileptic EEG abnormalities: the continuing debate. Brain Dev. 2010 Oct;32(9):746–52. doi: 10.1016/j.braindev.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 167.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004 Nov;45(11):1416–28. doi: 10.1111/j.0013-9580.2004.02404.x. [DOI] [PubMed] [Google Scholar]
- 168.Paciorkowski AR, Thio LL, Dobyns WB. Genetic and biologic classification of infantile spasms. Pediatr Neurol. 2011 Dec;45(6):355–67. doi: 10.1016/j.pediatrneurol.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bolton PF, Park RJ, Higgins JN, Griggiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002 Jun;125(Pt 6):1247–55. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 170.Boyer JP, Deschatrette A, Delwarde M. Convulsive autism? Apropos of 9 cases of primary autism associated with the Lennox-Gastaut syndrome. Pediatrie. 1981 Jul-Aug;36(5):353–68. [PubMed] [Google Scholar]
- 171.Septien L, Giroud M, Sautreaux JL, Brenot M, Marin A, Dumas R, et al. Effects of callosotomy in the treatment of intractable epilepsies in children on psychiatric disorders. Encephale. 1992 Mar-Apr;18(2):199–202. [PubMed] [Google Scholar]
- 172.Besag FM. Behavioral aspects of pediatric epilepsy syndromes. Epilepsy Behav. 2004 Feb;5( Suppl 1):S3–13. doi: 10.1016/j.yebeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 173.Rocha J, Guerra C, Oliveira R, Dória S, Rego R, Rosas MJ. Late-onset Lennox-Gastaut syndrome as a phenotype of 15q11.1q13.3 duplication. Epileptic Disord. 2012 Jun;14(2):159–62. doi: 10.1684/epd.2012.0502. [DOI] [PubMed] [Google Scholar]
- 174.Laundau WM, Kleffner FR. Syndrome of acquired aphasia with convulsive disorder in children. Neurology. 1957;7:523–30. doi: 10.1212/wnl.7.8.523. [DOI] [PubMed] [Google Scholar]
- 175.Deonna T, Roulet-Perez E. Early-onset acquired epileptic aphasia (Landau-Kleffner syndrome, LKS) and regressive autistic disorders with epileptic EEG abnormalities: the continuing debate. Brain Dev. 2010 Oct;32(9):746–52. doi: 10.1016/j.braindev.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 176.Billard C, Fluss J, Pinton F. Specific language impairment versus Landau-Kleffner syndrome. Epilepsia. 2009 Aug;50( Suppl 7):21–4. doi: 10.1111/j.1528-1167.2009.02213.x. [DOI] [PubMed] [Google Scholar]
- 177.Stefanatos G. Changing perspectives on Landau-Kleffner syndrome. Clin Neuropsychol. 2011 Aug;25(6):963–88. doi: 10.1080/13854046.2011.614779. [DOI] [PubMed] [Google Scholar]
- 178.Lesca G, Rudolf G, Labalme A, Hirsch E, Arzimanoglou A, Genton P, et al. Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: genomic dissection makes the link with autism. Epilepsia. 2012 Sep;53(9):1526–38. doi: 10.1111/j.1528-1167.2012.03559.x. [DOI] [PubMed] [Google Scholar]
- 179.Carvill GL, Regan BM, Yendle SC, O’Roak BJ, Lozovaya N, Bruneau N, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013 Sep;45(9):1073–6. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet. 2013 Sep;45(9):1067–72. doi: 10.1038/ng.2728. [DOI] [PubMed] [Google Scholar]
- 181.Lesca G, Rudolf G, Bruneau N, Lozovaya N, Labalme A, Boutry-Kryza N, et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat Genet. 2013 Sep;45(9):1061–6. doi: 10.1038/ng.2726. [DOI] [PubMed] [Google Scholar]
- 182.Tuchman R, Alessandri M, Cuccaro M. Autism spectrum disorders and epilepsy: moving towards a comprehensive approach to treatment. Brain Dev. 2010 Oct;32(9):719–30. doi: 10.1016/j.braindev.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Johnson CP, Myers SM American Academy of Pediatrics Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007 Nov;120(5):1183–215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 184.Filipek PA, Accardo PJ, Baranek GT, Cook EH, Jr, Dawson G, Gordon B, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999 Dec;29(6):439–84. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 185.Robins DL, Casagrande K, Barton M, Chen CM, Dumont-Mathieu T, Fein D. Validation of the modified checklist for Autism in toddlers, revised with follow-up (M-CHAT-R/F) Pediatrics. 2014 Jan;133(1):37–45. doi: 10.1542/peds.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001 Apr;31(2):131–44. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- 187.Eaves LC, Wingert HD, Ho HH, Mickelson EC. Screening for autism spectrum disorders with the social communication questionnaire. J Dev Behav Pediatr. 2006 Apr;27(2 Suppl):S95–S103. doi: 10.1097/00004703-200604002-00007. [DOI] [PubMed] [Google Scholar]
- 188.Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord. 2014 Aug;44(8):1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lord C, Rutter M, DiLavore P, et al. The Autism Diagnostic Observation Schedule. 2. Western Psychological Publishing; 2012. [Google Scholar]
- 190.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994 Oct;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 191.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007 Apr;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 192.Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852–873. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- 193.Eom S, Fisher B, Dezort C, Berg A. Routine developmental, autism, behavioral, and psychological screening in epilepsy care settings. Dev Med and Child Neurol. 2014;56(11):1100–5. doi: 10.1111/dmcn.12497. [DOI] [PubMed] [Google Scholar]
- 194.Fodstad JC, Dunn DW. Screening for autism spectrum disorders in children with epilepsy: where to begin… and where to go? Dev Med Child Neurol. 2014 Nov;56(11):1038–9. doi: 10.1111/dmcn.12516. [DOI] [PubMed] [Google Scholar]
- 195.Charman T, Gotham K. Measurement Issues: Screening and diagnostic instruments for autism spectrum disorders - lessons from research and practice. Child Adolesc Ment Health. 2013 Feb 1;18(1):52–63. doi: 10.1111/j.1475-3588.2012.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Berg AT, Plioplys S. Epilepsy and autism: is there a special relationship? Epilepsy Behav. 2012 Mar;23(3):193–8. doi: 10.1016/j.yebeh.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schaefer GB, Mendelsohn NJ. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet in Med. 2013 Mar;15:399–407. doi: 10.1038/gim.2013.32. [DOI] [PubMed] [Google Scholar]
- 198.Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Battaglia A, Viola D, Bernardini L, Novelli A, et al. Confirmation of chromosomal microarray as a first-tire clinical diagnostic test for individuals with developmental delay, intellectual disability, autism spectrum disorders and dysmorphic features. European Journal of Paediatric Neurology. 2013 Nov 17;6:589–599. doi: 10.1016/j.ejpn.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 200.Schaefer GB, Starr L, Pickering D, Skar G, Dehaai K, Sanger WG. Array comparative genomic hybridization findings in a cohort referred for an autism evaluation. J Child Neurol. 2010;25:1498–1503. doi: 10.1177/0883073810370479. [DOI] [PubMed] [Google Scholar]
- 201.Miles JH, Hillman RE. Value of a clinical morphology examination in autism. Am J Med Genet. 2000;91:245–253. [PubMed] [Google Scholar]
- 202.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 203.Jacquemont ML, Sanlaville D, Redon R, et al. Array-based comparative genomic hybridisation identifies high frequency of cryptic chromosomal rearrangements in patients with syndromic autism spectrum disorders. J Med Genet. 2006;43:843–849. doi: 10.1136/jmg.2006.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013 Oct 17;369(16):1502–11. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.O’Roak BK, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012 May 10;485:246–252. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]