SUMMARY
Slow-growing and pathogenic Mycobacterium spp. are characterized by the presence of galactosamine (GalN) that modifies the interior branched arabinosyl residues of the arabinogalactan (AG) that is a major heteropolysaccharide cell wall component. The availability of null mutants of the polyprenyl-phospho-N-acetylgalactosaminyl synthase (Rv3631, PpgS) and the (N-acetyl-) galactosaminyl transferase (Rv3779) of Mycobacterium tuberculosis (Mtb) has provided a means to elucidate the role of the GalN substituent of AG in terms of host-pathogen interactions. Comparisons of treating human peripheral blood monocyte-derived dendritic cells (hPMC-DCs) with wild-type, Rv3631 and Rv3779 mutant strains of Mtb revealed increased expression of DC maturation markers, decreased affinity for a soluble DC-SIGN probe, reduced IL-10 secretion and increased TLR-2-mediated NF-κB activation among GalN-deficient Mtb strains compared to GalN-producing strains. Analysis of surface expression of a panel of defined or putative DC-SIGN ligands on both WT strains or either Rv3631 or Rv3779 mutant did not show significant differences suggesting that the role of the GalN substituent of AG may be to modulate access of the bacilli to immunologically-relevant receptor domains on DCs or contribute to higher ordered pathogen associated molecular pattern (PAMP)/pattern recognition receptor (PRR) interactions rather than the GalN-AG components having a direct immunological effect per se.
1. Introduction
Tuberculosis is a major global cause of death. It is estimated that one third of the world’s population is infected with Mtb causing approximately 1.4 million deaths per year. The primary host target for Mtb is the human macrophage with survival of the bacterium within phagosomes. Innate immunity is implemented by the activated macrophage and is the initial and primary response against newly acquired Mtb. However it is the subsequent recruitment of T cells by host dendritic cells (DCs) that determines quality and extent of cell-mediated adaptive immunity. This development of an adaptive immune response ultimately provides protection against mycobacterial infections and/or restricts its dissemination within the host. Immature DCs are present throughout peripheral tissue in effect acting a sentinels guarding against pathogens that bode harmful intent to the host. DCs are among the first host cells that are encountered by Mtb and their precise activation and differentiation occurs through a vast network of receptors (termed pattern recognition receptors (PRR)) that engage with cognate ligands expressed on Mtb and other potential pathogens known collectively as pathogen-associated molecular patterns (PAMPs). The outcome of DC activation determines the nature of the adaptive immune responses (Th1, Th2, Treg, Th17 etc.) and is determined by signaling events arising from PRR/PAMP interactions. The crosstalk between these numerous signaling pathways ultimately determines the type of host immune response that is mounted against the pathogen [1]. The activated DCs resulting from a signature encounter with PAMPs translocate cytoplasmic MHC class II molecules to the cell surface for antigen presentation and up-regulate co-stimulatory molecules such as CD80, CD86 and CD40 ensuring activation of naïve T cells through antigen presentation and co-stimulation. Suboptimal DC activation results in lower co-stimulatory and MHC surface expression and can ultimately result in antigen-specific T cell anergy. Pathogens have evolved numerous mechanisms to thwart PRR/PAMP interactions in order to evade host immunity and allow for establishment of infection.
The cell envelope of Mtb is a complex structure comprising an inner membrane, a cell wall core composed of three-covalently-bound macromolecules (peptidoglycan, arabinogalactan (AG) and mycolic acids), an outer membrane (or mycomembrane) and a loosely attached polysaccharide and protein-containing capsule-like structure [2]. Various non-covalently bound lipids, glycolipids and lipoglycans, in particular phosphatidyl-myo-inositol mannosides (PIMs), lipomannan (LM) and lipoarabinomannan (LAM) populate the inner and outer membranes [2]. This collective assemblage of glycolipids, polysaccharides and lipoglycans exhibit a broad range of immunomodulatory activities that are implicated in the pathogenesis of tuberculosis [3–6].
Galactosamine (GalN) is a minor covalently bound amino sugar component of Mtb AG that substitutes the C2 position of a portion of the internal 3, 5-branched D-Araf residues in this molecule [7,8]. The GalN residue has been estimated to occur at approximately one residue per entire AG molecule [7–9]. Most intriguingly, a similar sugar residue has been observed to modify the AG of slow-growing pathogenic/opportunistic mycobacteria such as M. avium, M. kansasii, M. bovis, and M. leprae [10] but not that of M. smegmatis, M. neoaurum and M. phlei [9] [7,8] suggestive of the notion that the AG of opportunistic fast-growing Mycobacterium spp. may be devoid of the GalN substituent. This begs several questions as to the significance of the GalN motif on AG of slow growing mycobacteria: Does the GalN motif provide any adaptive advantage to the mycobacteria? Does the GalN motif contribute to the tendency toward pathogenicity? If so, does the GalN substituent in the cell wall of slow-growing pathogenic mycobacteria confer any immune evasive tactics?
We recently reported on the discovery of the biosynthetic pathway for the galactosaminylation of AG [11]. Disruption of the genes encoding either the polyprenyl-phospho-N-acetylgalactosaminyl synthase, Rv3631 or the polyprenyl-phospho-(N-acetyl)galactosaminyl-dependent glycosyltransferase Rv3779 which ultimately transfers the GalN (or N-acetyl-GalN) residue to AG resulted in Mtb H37Rv mutants devoid of GalN substituent on AG. The availability of Mtb Rv3631 and Rv3779 knockout mutants has provided the unique opportunity to explore and elucidate the function(s) of the GalN substituent of AG as it pertains to host immune responses. Specifically, we sought to test the idea that this motif may, in some manner, either directly or indirectly (e.g., by altering the topology and/or composition of the bacterial surface) provide some sort of immune evasive tactic to Mtb. In this study, we compared the ability of wild-type (WT) vs. GalN-deficient Mtb strains to activate human peripheral blood-derived dendritic cells (hPBM-DCs) by measuring their ability to induce DC maturation markers. Since we were working in a human system, we chose to compare expression of maturation markers from hPBM-DCs derived from several blood donors in order to address heterogeneity issues that arise when examining out-bred human populations. In all donors screened, we show that when the GalN motif is present on the AG of Mtb, hPBM-DCs consistently fail to achieve full maturation over 36 hours when compared to DCs treated with GalN-deficient strains. In addition, when analyzing patterns of cytokine secretion, DCs treated with WT Mtb strains producing galactosaminylated AG were stimulated to secrete significantly more IL-10 than their corresponding mutants. When probing intact bacilli with the soluble human chimeric PRR, hDC-SIGN, we observed that the WT strains bound more of the soluble probe than their GalN-deficient counterparts suggestive of a profound difference in either the amounts or accessibility of surface ligands to the DC-SIGN probe. Significant differences were also observed in the ability of WT vs. GalN-deficient strains to activate TLR2-mediated NF-κB translocation with the knock-out mutants showing significantly more activation than their WT counterparts. Collectively, these data suggest that the GalN motif on the AG of Mtb endows the bacillus with an immune evasive tactic and may contribute to virulence.
2. Material and Methods
2.1 Bacterial Strains and Growth Conditions
Mtb H37Rv ATCC 25618 (hereafter renamed H37Rv(a)), Mtb H37Rv TMC102 (hereafter renamed H37Rv(b)), their corresponding isogenic Rv3136 and Rv3779 knock-out mutants and complemented mutant strains were described elsewhere [11,12]. All Mtb strains were grown in either Sauton’s, Middlebrook 7H9 supplemented with 10% OADC and 0.05% Tween 80 or Glycerol-Alanine-Salts (GAS) medium. M. smegmatis strain mc2155 was grown in 7H9-OADC-Tween 80 or LB medium. When required, kanamycin (kan) and hygromycin (hyg) were added to final concentrations of 20 μg/ml and 50 μg/ml, respectively. Cultures were harvested at mid log phase (O.D.600nm = 0.6–0.8) and washed 3 times with PBS. Cell pellets were suspended in PBS at 109 CFU/ml. To safely remove the bacteria from BSL3 containment in order to perform all of the experiments described herein, the suspended bacteria were gamma-irradiated in a JL Shepherd 31–14 machine using a 6000 Ci 137Cs source for 1620 minutes (27 hours) at 1543 rads/minute for a total dosage of 2.5 megarads. Loss of viability was confirmed by AlamarBlue® testing. In some experiments, Mtb bacilli were killed by heat treatment at 90°C for 40 min. M. leprae was harvested from either 9-banded armadillo tissues (liver or spleen or from nu/nu mouse foot pads and characterized and counted by the method of Shepard et al. [13]. Both the M. smegmatis and M. leprae were irradiated identically to the Mtb strains.
2.2 Antibodies
All monoclonal antibodies were obtained through BEI Resources, NIAID, NIH and were produced in cell culture using B cell hybridomas generated by fusion of myeloma cells with immunized mouse splenocytes. The following mouse monoclonal antibodies were used: anti-Mtb LAM, clone CS-35; Anti-Mtb 19 kDa (LpqH), clone IT-19; anti-Mtb DnaK, clone IT-40 (HAT1), clone α-Rv1411c and Anti-Mtb Mpt32 (45/47-kDa antigen or Apa), clone CS-93.
2.3 Procurement of Human Blood
Human whole blood was obtained from the Garth Englund Blood Center at Poudre Valley Hospital which is part of the University of Colorado Health System, Fort Collins, CO. Whole blood was obtained from anonymous donors who have been deemed acceptable for blood donation through screening for Human Immunodeficiency Virus, and Human Hepatitis A, B and C. No blood was donated specifically for this work and was procured from the blood center as a quantity not sufficient (QNS) for clinical application and is otherwise disposed. Hence, no Internal Review Board (IRB) requirement was necessary for the acquisition of these human blood specimens.
2.4 Preparation of Human Peripheral Blood Monocyte Derived-Dendritic Cells (PBM-DC)
Human whole blood (between 200–400 cc) was centrifuged at 400xg for 20 min to separate packed red cells from serum. Donor serum was collected to be used as an autologous serum component in the DC differentiation medium. Reduced red cell whole blood was diluted 2-fold with PBS, layered over Ficoll-Paque® PLUS (GE Healthcare Life Sciences, Pittsburgh, PA) and centrifuged at 400xg. White blood cells (WBCs) were extracted from the interface, washed in Hanks Balanced Salt Solution (HBSS) X 3 and subsequently suspended in RPMI with 10% fetal bovine serum (FBS). Monocytes were either separated by magnetic beads conjugated with CD14 or by adherence to plastic for 2–3 hrs at 37°C. Separated or adherence depleted monocytes were washed, counted and plated at 0.5–1.0×106 cells/ml with medium containing 10% FBS, 5% autologous donor serum, 50 ng/ml recombinant human GM-CSF and 10 ng/ml recombinant human IL-4 (both from R & D Systems™, Minneapolis, MS). Monocytes were allowed to differentiate for 5–7 days (as determined by flow cytometric analysis of HLA-DR and morphological changes upon microscopic inspection). The differentiation medium was replaced every 48–72 hr depending on the condition of the cells and pH of the culture.
2.5 Stimulation of hPBM-DC with mycobacteria
Differentiated monocytes (immature hPBM-DC) were plated at 1×106 cells /ml in 24 well plates and were either untreated or treated with LPS at 25 ng/ml or γ-irradiated WT or GalN-deficient strains of Mtb at an M.O. I. of 50 bacilli per cell. The cultures were allowed to incubate at 37°C for 36 hr or for 12, 24 and 48 hours in cases where maturation kinetics were analyzed.
2.6 Flow cytometric analysis of hPBM-DCs
Following treatment of hPBM-DCs with LPS or mycobacteria, DCs were stained with phycoerythrin (PE)-conjugated anti-human CD-40, FITC-conjugated anti-human CD80, PerCP-eFluor 710®-conjugated anti-human CD86 and eFluor 450®-conjugated anti-human HLA-DR (eBiosciences, San Diego, CA) and analyzed for mean fluorescence intensity on a Becton Dickenson FACS Cantos II flow cytometer. Flow Cytometry Standard (FCS) file data were analyzed using Flowjo® software (Treestar Inc., Ashland, OR).
2.7 Assessment of cytokine secretion in DC cultures
The DC cultures were analyzed using the BD Human Inflammation CBA Kit (Franklin Lakes, NJ) per manufacturer’s recommendation. The kit determines the culture concentration of IL-8, IL-1β, IL-6, IL-10, TNFα, and IL12p70. Supernatants from 1 ml Mtb cell-treated DCs were obtained and centrifuged to remove all sediments. Thereafter, the bead samples were read using a FACsCanto II flow cytometer by using BD Biosciences CBA software. Cytokine levels in each sample were calculated per company protocol by extrapolating the mean fluorescence intensity (MFI) for each sample into the standard curves for every cytokine.
2.8 Preparation of soluble AG
The cell wall core mycolyl-arabinogalactan-peptidoglycan (mAGP) complex was obtained from the residual pellet after lipid and lipoglycan extraction as described [14]. Soluble AG was prepared from mAGP by base treatment as described [15].
2.9 Soluble DC-SIGN-Fc binding Assay
DC-SIGN-Fc consists of the extracellular portion of DC-SIGN (amino acid residues 64–404) fused at the COOH terminus to a human IgG1-Fc fragment [16]. The soluble DC-SIGN adhesion assay was performed as follows: The recombinant DC-SIGN-Fc probe was purchased from R&D Systems (Minneapolis, MN). The bacteria (5×105 CFU) washed three times with PBS containing 1% BSA were suspended in 100 μl of the same buffer containing 10 μM CaCl2 and 10 μl of the reconstituted (per manufacturer’s recommendation) human DC-SIGN-Fc was added. The cells were incubated at 37°C for 45 min followed by washing unbound probe t hree times in PBS/1% BSA. One μl of PE-conjugated anti-human Fc antibody (Affymetrix, eBioscience, San Diego, CA) was added and the tubes were incubated at 4°C for 15 min. The mycobac teria were washed three times with PBS/1% BSA and analyzed for PE fluorescence by flow cytometry.
2.10 Assessment of hTLR2-mediated NF-κB activity using HEK-Blue hTLR2 reporter cells
HEK-Blue™ human TLR2 (hTLR2) cells were purchased from InvivoGen (San Diego, CA). HEK-Blue™ human TLR2 (hTLR2) cells which co-express the TLR2 co-receptor gene CD14 to enhance TLR2 responses are designed for studying stimulation of human TLR2 by monitoring spectrophometrically the activation of NF-κB. Cells were distributed in flat-bottom 96 well plates in 180 μl at 50,000 cells per well and either the TLR2 agonist, Pam3CSK4 (50 ng/ml), the Mtb strains or M. smegmatis mc2155 were added in triplicate wells at MOI of either 3 or 10. The plates were allowed to incubate at 37°C for 6 hours in a humidified inc ubator with 5% CO2 and the assessment of hTLR-mediated NF-κB activity was monitored spectrophotometrically at 612–655 nm.
2.11 Statistical analyses
Statistical analyses was perform using Prism® Software (GraphPad Software, La Jolla, CA). The choice of statistical test was recommended by Dr. Philip Chapman, Experiment Station Statistician, Dept. of Statistics, CSU, Fort Collins, CO. The statistical test for comparison of DC maturation among the human cohorts was the Wilcoxon matched-paired signed rank test. The comparative significance per donor was based on a paired one-tailed statistical analysis since a two-tailed test would consider the possibility of down-regulation of markers from resting DC values (which was not observed in our cohort). Since the average homeostatic, baseline level of expression of maturation markers in resting human immature DCs (iDCs) varies considerably amongst various individuals, we chose to measure the extent of maturation as simply an increase in expression per donor over resting levels. Thus, for each donor, we performed a paired (measuring paired treatment groups) one-tailed test and generated p values in the figures calculated from all donors based on the cumulative p values derived from each donor. Nonetheless, the analysis using either one- or two-tailed tests generated significant p values.
3. Results
3.1 GalN-deficient Mtb mutants stimulate maturation markers on human PBM-DCs (hPBM-DCs) to a significantly greater extent
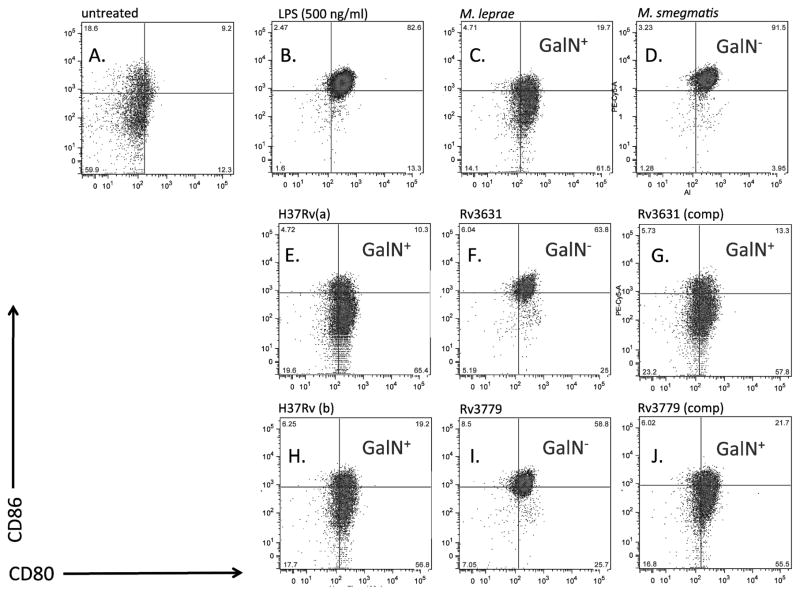
To determine whether the absence of GalN substituent on Mtb AG had any effect on the maturation of human DCs, a variety of hPBM-DCs from several donors were exposed to either the WT Mtb strains (H37Rv ATCC 25618 and H37Rv TMC102; (termed, “H37Rv(a)” or “H37Rv(b)”, respectively, herein) or their corresponding Rv3631 and Rv3779 knock-out mutants [12] [11]. The Rv3631 mutant is deficient in the production of polyprenyl phosphate-linked N-acetylgalactosamine, the activated N-acetylgalactosamine donor used by integral membrane glycosyltransferases of the GT-C superfamily in the transfer of (N-acetyl) galactosamine to their cell envelope acceptor(s) on the periplasmic side of the plasma membrane [11]. AG is, to this date, the only Mtb cell envelope acceptor known to be modified by this substituent, a finding supported by our own investigations that have thus far failed to identify this amino sugar in any other Mtb glycoconjugate, including glycoproteins (data not shown). Yet, since one cannot completely exclude the existence of other GalN and N-acetyl-GalN acceptors in the Mtb envelope, we decided to compare side-by-side the interactions of the Rv3631 and Rv3779 knock-out mutants with human cells since the Rv3779 transferase is likely to be specific for the substrate (AG) onto which it transfers GalN. Although not a perfect control due to the direct or indirect involvement of this enzyme in the mannosylation rate of higher order phosphatidylinositol mannosides (PIMs), lipomannan (LM) and lipoarabinomannan (LAM) [12], the Rv3779 mutant which is expected to specifically be missing a GalN substituent on AG still provided a useful comparison in these experiments. All experiments described in this work were performed using irradiated bacteria (including both the M. smegmatis and M. leprae samples) in order to make direct comparisons between de facto molecular configurations of the bacterial cell envelope and to rule out any differences in DC maturation due to possible differences in bacterial intracellular growth and survival imposed by the mutations. Figure 1 shows dot plots of flow cytometric analysis from one donor indicating the mean fluorescence intensity (MFI) of CD80 (B7.1) and CD86 (B7.2) co-receptor expression and compares resting levels (Panel A) to hPBM-DCs exposed for 36 hrs to LPS (Panel B), M. leprae (Panel C), M. smegmatis (Panel D), the two WT Mtb H37Rv strains ATCC 25618 [(H37Rv(a), Panel E] and TMC102 [H37Rv(b), Panel H] and their respective Rv3631 (Panel F) and Rv3779 (Panel I) knock-out mutants. Additionally, we compared the MFI of CD80 vs. CD86 in hPBM-DCs treated with Rv3631 and Rv3779 expressing the respective WT complementation genes [(Rv3631(comp) and Rv3779)comp)] (Panel G and J). When compared to treatment with LPS, exposure of hPBM-DCs to mycobacteria producing a galactosaminylated AG such as M. leprae or either WT Mtb H37Rv strain failed to optimally stimulate DC maturation as determined by lower comparative expression of either CD80 or CD86. Exposure of the DCs to M. smegmatis that naturally lacks the GalN substituent of AG (among other compositional differences with Mtb) showed levels of expression of CD80/CD86 similar to LPS treatment. Interestingly, disruption of either Rv3631 or Rv3779, rendering the AG devoid of GalN, increased Mtb’s ability to stimulate optimal maturation of the DCs that is more comparable to either LPS- or M. smegmatis-stimulated DC. The increased maturation of hPBM-DCs was abrogated when either the Rv3631 or Rv3779 mutants were complemented with their respective WT gene (Figure 1; compare Panels E, F, G, H, I, and J).
Figure 1. Expression of the co-stimulatory molecules CD80 and CD86 by hPBM-DCs exposed to gamma-irradiated Mtb H37Rv WT, GalN-deficient and complemented mutant strains.
The strains tested are WT Mtb H37Rv(a) and H37Rv(b), the GalN-deficient mutants H37Rv(a)ΔRv3631 (Rv3631) and H37Rv(b)ΔRv3779 (Rv3779), and the complemented mutant strains, Rv3631 (comp) and Rv3779 (comp). Mtb bacilli were added at an MOI of 50 and DCs were incubated with the bacteria at 37°C for 36 hours. h PMB-DC were stained with fluorescent-conjugated antibodies against CD80, and CD86 and analyzed for mean fluorescence intensity by flow cytometry. All bacterial strains were irradiated according to the Materials and Methods section.. The mean fluorescence intensity (MFI) values for this figure are as follows: CD80: Untreated = 232, LPS= 369, M. leprae=195, M. smegmatis=344, H37Rv(a) = 252, Rv3631 = 285, Rv3631(comp) = 212, H37Rv(b) = 231, Rv3779 = 321, Rv3779(comp) = 213; CD86: Untreated = 908, LPS=2321, M. leprae = 728, M. smegmatis = 2143, H37Rv(a) = 1009, Rv3631= 1900, Rv3631(comp) = 1715, H37Rv(b) = 896, Rv3779 = 2113, Rv3779(comp) = 943. This representative of 20 total experiments.
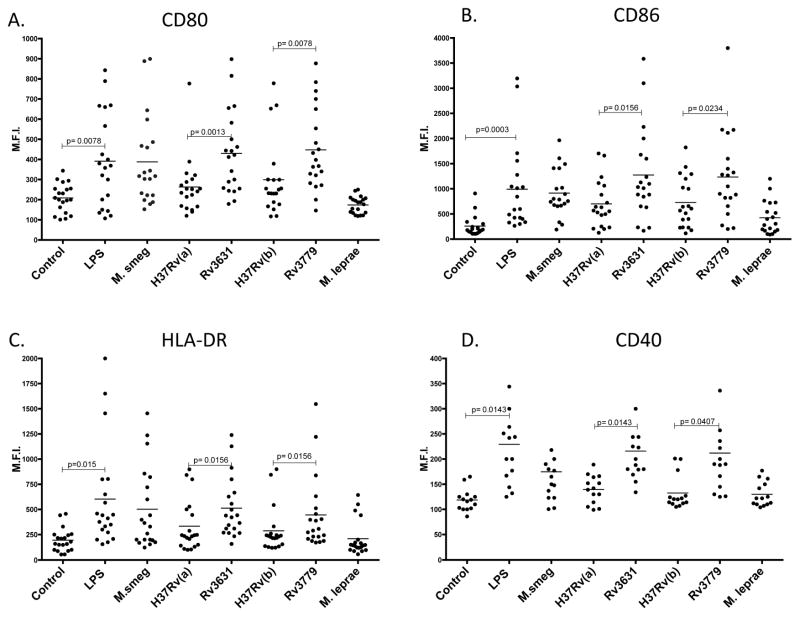
To first determine whether this observation was consistent across a heterogeneous human cohort, we tested DC maturation phenotypes comprising the expression of CD80, CD86, CD40 and a common human major histocompatibility complex (MHC) class II antigen, the human leukocyte antigen (HLA)-DR from 21 different blood donors (14 in the case of CD40). Figure 2 shows that for all donors, the WT Mtb strains were only slightly better than M. leprae in stimulating expression of the selected DC co-stimulatory and MHC molecules and, when compared to either LPS, or M. smegmatis- or either of the Mtb GalN-deficient mutants stimulated overall less expression in treated DCs. There were no significant differences in CD80 expression between all donors’ DCs treated with M. smegmatis and those treated either of the Mtb Rv3631 (p>0.40) or Rv3779 (p>0.40) knock-out mutants, whereas, significant differences were observed between Mtb H37Rv(a) and the Rv3631 knock-out (p<0.01) and Mtb H37Rv(b) and the Rv3779 knock-out (p<0.01) (Figure 2 Panel A). For CD86 expression, both GalN-deficient Mtb mutants generated a DC simulation profile that was significantly comparable to that observed for M. smegmatis (p>0.05 in both cases) with the two mutants having increased expression of CD86 when compared to their respective WT parental strains (Figure 2; Panel B). The same trend was observed with the common human MHC Class II marker HLA-DR and CD40 (14 donors in the case of CD40), which also showed higher levels of surface expression in DCs treated with LPS, M. smegmatis and either Mtb mutant than in the WT Mtb strains. M. leprae failed to stimulate DCs to optimal maturation phenotype as determined by lower expression of all of the maturation markers used in this work and further substantiating previous observations [17]. Experiments performed using mycobacteria grown in either 7H9 or an alternative medium such as GAS medium yielded similar results suggesting that the DC maturation characteristics imposed by these bacteria are not dependent on the type of growth medium used (data not shown). Importantly, we observed similar trends among the cohorts using four separate preparations of each Mtb strain ensuring that the differences observed were not due to some batch-specific issue.
Figure 2. The GalN-deficient Rv3631 and Rv3779 knock-out mutants consistently stimulate better expression and translocation of the common DC maturation markers, CD80, CD86, CD40 and HLA-DR when compared to their GalN-proficient WT parents.
hPBM-DCs were treated and analyzed as in Figure 1. Whole blood was obtained from 20 individuals and hPBM-DCs were prepared and treated with LPS (25 ng/ml), M. smegmatis (M.smeg), WT H37Rv(a), H37Rv(a)ΔRv3631, WT H37Rv(b), H37Rv(b)ΔRv3779 or M. leprae. The figure shows the cumulative results for mean fluorescence intensity values for the human cohort. CD40 plots were limited to 14 donors because this analysis was initiated later in the project. Statistical power is, however, maintained using this lower number of donors. P values were obtained using a paired one-tailed t test that determines the significance between pairwise values among treatment groups (see Material and Methods Statistical section). The horizontal bars represent the mean value measured for each treatment regimen.
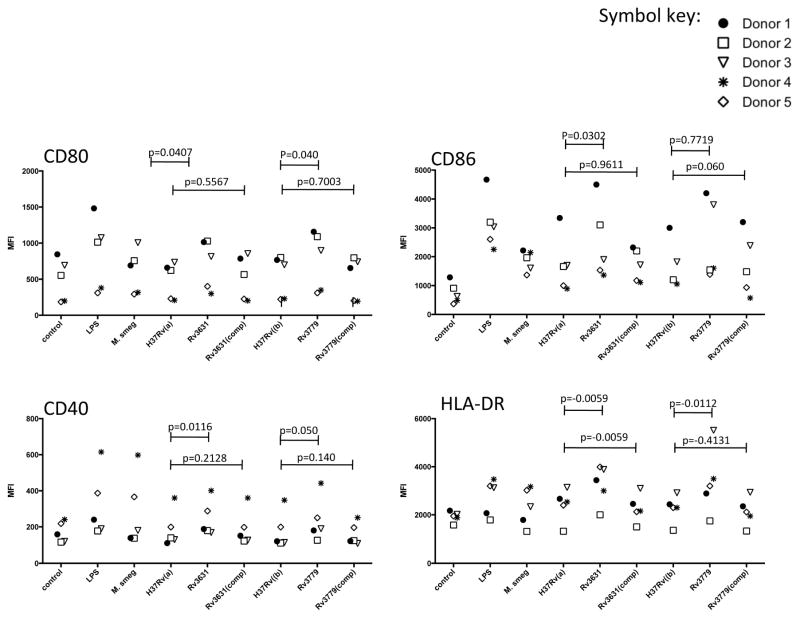
The optimal maturation of hPBM-DCs conferred by the two Mtb knock-out mutants reverted to WT levels upon complementation with WT copies of the Rv3631 and Rv3779 genes. Figure 3 indeed shows that for five separate donors, the highest level of expression among the Mtb strains of the four DC maturation markers is conferred by the Rv3631 and Rv3779 knock-out mutants as determined by optimal expression of CD80, CD86, CD40 and HLA-DR. Both complemented strains have been shown to be proficient in the galactosaminylation of AG [11]. In addition, Figure 3 also illustrates the per donor consistency of the WT vs. mutant and the restoration phenotypes of the complemented strains in terms of the DC maturation marker trend as denoted by the different symbols used for each donor in the Figure.
Figure 3. Genetic complementation of the Rv3631 and Rv3779 knock-out mutants restores hPBM-DC activation to WT levels.
DCs were treated and analyzed as in Figures 1 and 2. Separate blood donor responses are indicated by the various symbols. The significance of pair-wise comparisons between respective WT vs. mutants and WT vs. complemented mutants are indicated by the p values above the spanning lines in the figure.
3.2 The presence of a galN substituent on the AG of Mtb induces hPBM-DCs to secrete higher levels of IL-10
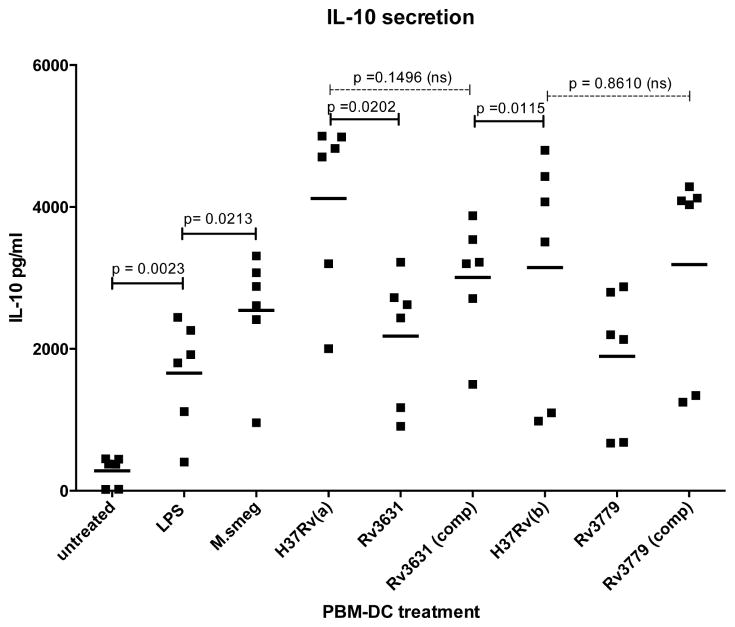
The development of a Th1 type immune response is crucial for an effective control of Mtb infection. Th1 responses are usually determined, in part, by initial secretion of IL-12, TNFα and IFNγ cytokines by antigen presenting cells (APCs) such as DCs. Conversely, Th1 responses are down-regulated by IL-10 as determined the cytokine’s ability to down-regulate expression of Th1 cytokines, MHC class II antigens and co-stimulatory molecules such as CD80/86 on APCs. To determine whether cytokine secretion is modulated and may be linked to the inhibition of DC maturation by Mtb strains harboring a GalN substituent on their AG, we compared levels of IL-8, IL-1β, IL-6, IL-10, TNFα, and IL12p70 in culture supernatants of hPBM-DC treated with the various WT and recombinant Mtb strains. Figure 4 shows for six separate donors that, compared to hPBM-DCs treated with both GalN-deficient mutants, levels of IL-10 secretion were significantly increased in hPBM-DCs that were treated with the WT parental strains. With respect to IL-10 secretion from Mtb-treated DCs, two donors were termed “low responders” as indicated by the low level outliers depicted in Figure 4. Comparative levels of IL-8, IL-1β, TNFα and IL12p70 in culture supernatants showed no significant difference in the DC cultures treated with any Mtb strains (Supplemental Figure S1). Importantly, genetic complementation of the two mutant strains [Rv3631(comp) and Rv3779(comp) partially restored IL-10 secretion (Figure 4). These data suggest that the GalN substituent on AG, in some direct or indirect way, alters Mtb’s interactions with host cell receptors resulting in a reprogramming of the DC maturation profile.
Figure 4. hPBM-DCs from six donors treated with Mtb mutant strains deficient in the galactosaminylation of AG fail to produce IL-10 to the level of the WT parent strains.
DCs were treated as in Figure 1 and supernatants were analyzed for cytokines. The samples were read using a FACsCanto II flow cytometer by using the BD Biosciences CBA software. Cytokine levels in each sample were calculated per company protocol by extrapolating the mean fluorescence intensity for each sample into the standard curves for every cytokine. X-axis labels and p value calculations are the same as in Figures 1–3. A p value of ≤0.05 is considered significant (comparative values above solid spanning lines. Thus the differences between respective WT and mutant IL-10 secretion is considered significant whereas the differences were not significant in the comparison of the WT with their respective complemented mutants (comparative values above the dashed spanning lines). The horizontal bars represent the mean value of the 6 donors measured for each treatment regimen.
3.3 Purified galactosaminylated and non-galactosaminylated AG do not significantly signal hPBM-DC to up-regulate maturation markers
To determine whether the GalN substituent on AG contributes any direct effect on DC maturation, we purified AG from the WT Mtb H37Rv strains (H37Rv(a) and H37Rv(b)) as well as the GalN-deficient Rv3631 and Rv3779 mutants. Treatment of hPBM-DCs with 1, 10 or 100 ng/ml of purified AG from the various Mtb strains, however, failed to stimulate the DCs to express significant levels of maturation markers (Supplemental Figure S2). The extent of MFI with all purified AG molecules were statistically comparable to untreated controls with no significant dose-response between 1, 10 and 100 ng/ml. There was little change between DCs treated for 24 hrs and those treated for 36 hrs (data not shown). Likewise, analysis for both intracellular and secreted cytokines showed no difference between the purified AG-treated DCs and untreated controls (data not shown). These results thus suggest that GalN-modified AG per se does not contribute to the differential DC signaling.
3.4 The GalN substituent of AG does not alter the rate of Mtb endocytosis/phagocytosis by hPBM-DCs
To determine whether the GalN substituent on AG of Mtb affects innate cell endocytosis/phagocytosis. Resting/immature hPBM-DCs were treated with graded increasing doses of irradiated Mtb strains stained with the vital dye PKH26 and ranging from an MOI of 0 to 100 and analyzed for acquisition of red fluorescence by flow cytometry as a function of time. A comparison of the rates of DC uptake of the H37Rv(a) and H37Rv(b) WT strains and their corresponding Rv3631 and Rv3779 mutants was determined by measuring the extent of uptake of red fluorescence by flow cytometry. Both WT strains and their respective GalN-deficient mutants showed similar rates of fluorescence acquisition (Supplemental Figure S3 and data not shown). To verify that uptake of the bacilli was due to phagocytosis - a functioning temperature-dependent metabolic processes - we performed the above experiment at 0°C. Analysis of the uptake of fluorescent bacteria at 0°C showed minimal acquisition that was not time- or M.O.I-dependent suggestive of only extracellular adsorption (Supplemental Figure S3). The extent of bacillary phagocytosis by hPBM-DCs from three donors was thus not affected by the presence of a GalN substituent on AG. These observations suggest that the altered DC maturation imposed by the GalN substituent of AG in Mtb is not due to changes in the ability of the host cell to take up the bacillus but rather due to differences in engagement of particular host cell receptors. In particular, the GalN motif does not seem to enhance or interfere with host receptors that promote phagocytosis.
3.5 DC-SIGN-Fc probe binds to GalN-containing Mtb strains more extensively than GalN-deficient strains
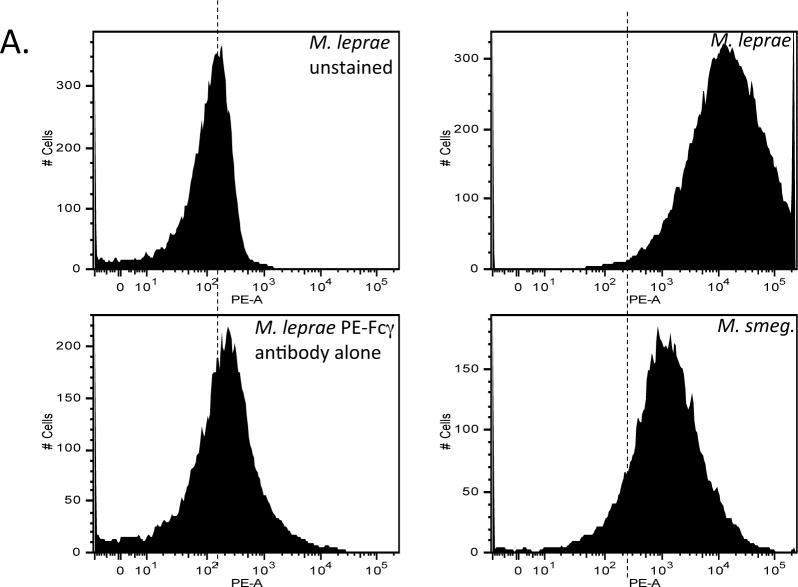
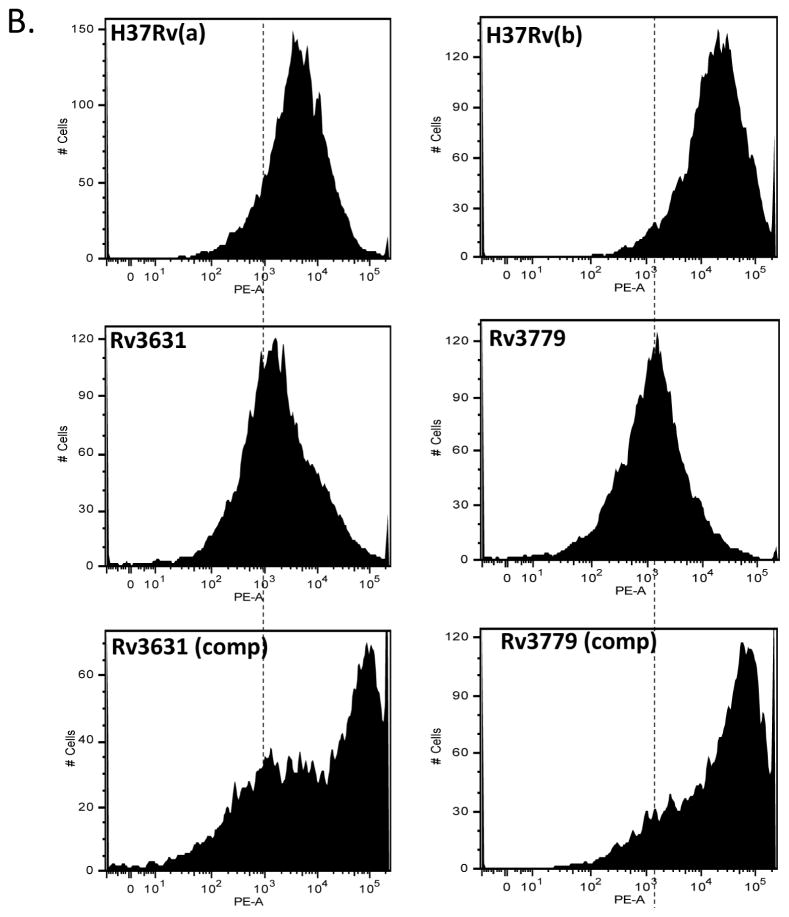
The dendritic cell-specific C-type lectin, dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) is a calcium dependent carbohydrate-binding receptor with specificity, in part, for mannose-containing glycosides and Lewis antigens containing fucose [18] [19]. DC-SIGN, by virtue of interaction with highly mannosylated structures in the Mtb cell wall serves to impair DC maturation and induces the secretion of the anti-inflammatory cytokine IL-10 [20]. Many ligands have been described in mycobacteria including Mtb that are capable of binding to DC-SIGN. In particular it has been shown that binding to mannosylated LAM (ManLAM) from M. bovis BCG and Mtb inhibits IL-12 production through binding to host innate immune receptors such as the Mannose Receptor (MR) [21] as well as DC-SIGN, and such interactions may be restricted to species of the Mtb complex [22]. Infection studies with (human) DC-SIGN transgenic mice revealed that the lectin’s interaction with Mtb may likely serve as a strategy for host protection against Mtb-mediated tissue pathology and thus provide an environment of Mtb persistence as opposed to simply diminishing host immunity and favoring infection [19]. In addition to ManLAM, several other Mtb ligands such as the mannosylated 19 kDa (LpqH) lipoprotein, DnaK, the 45 kDa (alanine/proline-rich antigen, Apa) glycoproteins and hexamannosylated phosphatidyl-myo-inositol mannosides (PIM6) have been proposed to bind DC-SIGN [5,22–24]. We thus sought to determine whether the GalN substituent on AG alters overall interaction of Mtb with DC-SIGN. To determine whether DC-SIGN differentially interacts with GalN-proficient vs. GalN-deficient Mtb strains, we probed the surface of intact irradiated bacteria using a recombinant human DC-SIGN-Fc chimeric probe. Figure 5a shows that M leprae binds significantly more of the chimeric human DC-SIGN probe than M. smegmatis. Both WT Mtb H37Rv strains bound the DC-SIGN probe to a greater extent than their respective Rv3631 and Rv3779 mutants (Fig. 5b and 5c). Interestingly, the two complemented mutants bound the probe considerably better than their corresponding WT parent strain, perhaps as an effect of the increased level of expression of Rv3631 and Rv3779 from multi-copy replicative plasmids in these strains. The binding of the probe to WT Mtb H37Rv(a) is specifically characteristic of C-type lectins since the interaction is blocked by either free yeast mannan or by chelation of Ca++ by EGTA (Supplemental figure S4), Supplemental figure S5 shows a graphical representation of these data). Altogether, results thus suggest that the presence of a GalN substituent on AG may impose an overall altered conformation of the cell surface allowing a more optimized access to DC-SIGN and that more DC-SIGN/ligand engagement may, in part, be responsible for the abrogated hPBM-DC maturation and increased IL-10 response observed when DCs are treated with the WT (GalN-proficient) strains.
Figure 5. Probing of WT, mutant and complemented mutant Mtb bacilli with a soluble human DC-SIGN-Fc chimeric probe.
The γ-irradiated bacteria were incubated for 45 min at 37°C in the presence of the probe followed by washing to remove unbound probe and adding an anti-human Fc antibody conjugated with PE. The samples were washed three times and analyzed by flow cytometry. Panel A: flow cytometry histograms comparing MFI between unstained M. leprae (stained with PE conjugated Fcγ antibody alone, M. smegmatis probed with DC-SIGN-Fc + PE conjugated anti-Fcγ (PE-Fcγ). Dashed line delineates the MFI of unstained M. leprae sample as a reference. Panel B, first column: histograms comparing MFI between WT H37Rv(a), the Rv3631 mutant and the complemented mutant strain [Rv3631 (comp)] probed with hDC-SIGN-Fc followed by PE-α-Fcγ. Panel B, second column: histograms comparing MFI between H37Rv(b), the Rv3779 mutant and the complemented mutant strain [Rv3779 (comp)] probed with hDC-SIGN-Fc followed by PE-α-Fcγ. These histograms are representative of 3 separate experiments that show significant consistency (p > 0.85) between replicate WT, mutant or complemented mutants and significant differences between comparisons of WT and GalN mutants (p < 0.05).
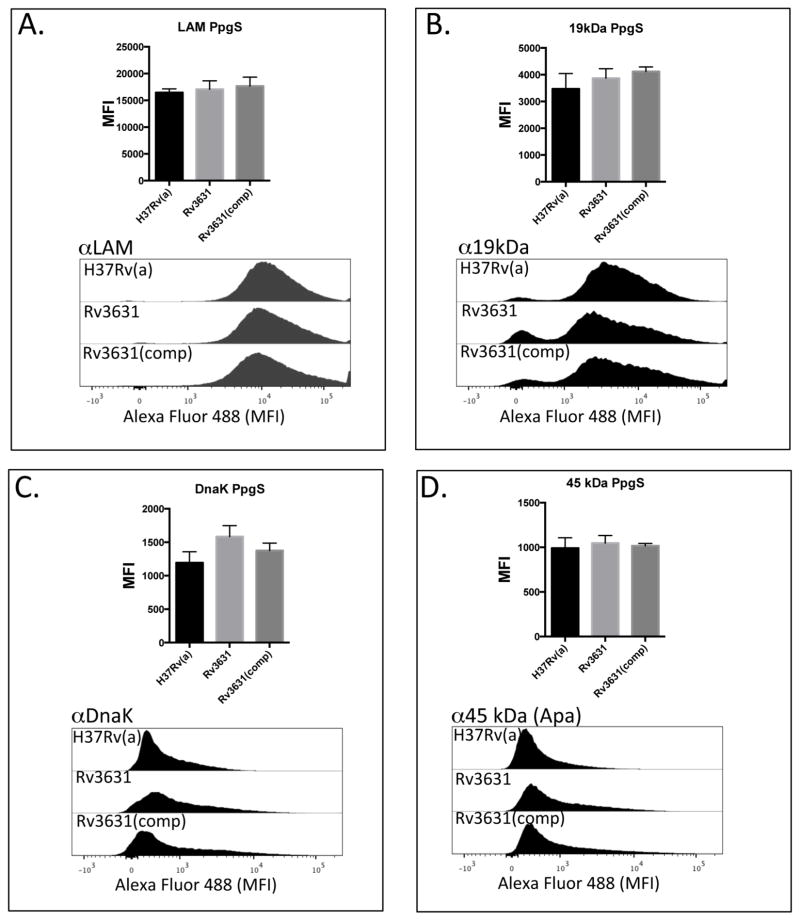
To further examine whether the presence of a GalN motif on AG had any impact on the translocation/expression of selected known DC-SIGN ligands to the cell surface, we probed the surface of intact irradiated WT and mutant Mtb strains with a panel of monoclonal antibodies (MoAbs) specific for known or putative DC-SIGN ligands. Bacteria were labeled with MoAb specific for LAM [20], 19 kDa protein [25], DnaK [24], and the 45–47 kDa antigen (Apa) [26]. Mouse MoAbs bound to Mtb were fluorescently labeled with a secondary Alexa fluor 488-conjugated anti-mouse IgG antibody and the geometric MFI was determined by flow cytometry (Figure 6). Disruption of the Rv3631 or Rv3779 genes did not alter the binding of any of the MoAb tested to the cell surface. Importantly, the CS-35 monoclonal antibody which recognizes the arabinan domain of LAM and arabinomannan bound similarly to the cell surface of the Rv3631 and Rv3779 mutants as well as both WT reference strains, indicating that the previously reported reduced production of LAM in this strain [12] does not significantly contribute to the observed differential DC maturation phenotype. Probing all Mtb strains with a non-specific Alexa Fluor 488-conjugated anti-mouse antibody showed little staining above unstained controls indicative of the specificity of the MoAbs used in this assay (supplemental Figure S6). These results further lend credence to the hypothesis that GalN-modified AG per se does not contribute to the differential DC maturation phenotype or impact the export of APC ligands to the cell surface but, rather alters the topology of the cell surface in a way that significantly impacts Mtb’s interactions with antigen-presenting cells such as DCs.
Figure 6. Surface exposure of DC-SIGN ligands by the WT and GalN-deficient mutants.
WT Mtb strains when compared to their corresponding Rv3631 (Panels A–D) and Rv3779 (Panels EH) GalN-deficient Mtb mutants display similar levels of the DC-SIGN ligands ManLAM, 19 kDa antigen, DnaK, and Apa (45 KDa antigen) as their WT counterparts as determined by the similar extent of MoAb binding to their cell surfaces. Complementation of these mutations ((Rv3631(comp) and Rv3779(comp)) also had no significant effect on surface availability. Intact irradiated Mtb strains were labeled identically with previously titrated MoAbs against the above DC-SIGN ligands followed by extensive washing of unbound MoAb. MoAb-bound bacteria were counterstained with a secondary Alexa-Fluor 488-conjugated anti-mouse IgG, followed by washing and geometric MFI determined by flow cytometry. The bar graph on the top of each histogram set represents the result of a total of 3 separate experiments. (“PpgS” refers to the polyprenyl-phospho-N-acetylgalactosaminyl synthase strain comparisons and “GT” refers to the galactosaminyl transferase strain comparisons.) P values for all experiments were > 0.150.
3.6 The GalN-deficient Mtb mutants stimulate more production of TLR2-mediated NF-κB than their WT parents
TLRs play an important role in Mtb infection. Specifically, TLR2 has been shown to bind to several Mtb lipoproteins such as LprA (Rv1270) [27], LprG (Rv1411c) [28] as well as lipomannan [29] and phosphatidyl-myo-inositol mannosides [30] to induce immune responses. To determine whether the GalN motif intervenes, in any capacity, with TLR2-mediated NF-κB activation, we incubated the various Mtb strains with HEK-Blue™ hTLR cells (InvivoGen, San Diego, CA) expressing the human TLR2 gene and a secreted embryonic alkaline phosphatase (SEAP) reporter gene under control of the IFN-β minimal promoter fused to five NF-κB and AP-1 binding sites. Figure 7 shows that the WT parents and the two complemented mutant strains showed overall less NF-κB activation when compared to their respective Rv3631 and Rv3779 mutants as determined by the level of SEAP secretion from the HEK-Blue™ Cells. These data are supportive of the notion that the presence of a GalN substituent on the AG of Mtb also effectively alters the interaction interface between TLRs and the bacterium.
Figure 7. The Rv3631 and Rv3779 GalN-deficient mutants stimulate more production of TLR2-mediated NF-κB than their WT Mtb parents and complemented mutant strains.
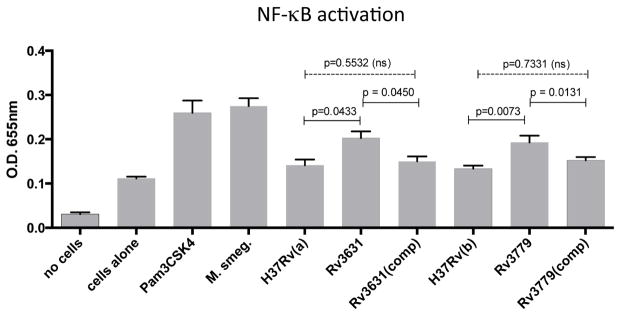
HEK-Blue™ hTLR2 cells were incubated either alone, or with the TLR2 agonist PAM3CSK4 or with the indicated Mtb and M. smegmatis strains at an MOI of 3. Cells and bacteria were cultured in an alkaline phosphatase detection medium for 6 hours and the change in color of the medium due to secreted SEAP was measured at 650 nm on a plate-reading spectrophotometer. Results are shown as the mean + SD of triplicate cultures. P values are indicated on the figure.
4. Discussion
Galactosamine has been recognized as a minor covalently bound amino sugar component of the cell wall of slow growing pathogenic and opportunistic mycobacteria such as Mtb, M. leprae, M. avium, M. bovis BCG but not that of chiefly non-pathogenic and fast growing mycobacteria such as M. smegmatis, M. neoaurum or M. phlei [7–9,31,32]. We recently reported on the identification of two glycosyltransferases, Rv3631 and Rv3779, required for providing and transferring, respectively, the GalN substrate for the modification of AG in Mtb H37Rv [11]. Disruption of either Rv3631 or Rv3779 abolishes the synthesis of the GalN substituent of AG. Currently, non-N-acetylated hexosamine residues have been chiefly described as components of the LPS of some Gram-negative bacteria [33–36] where they play different roles in bacterial physiology, antimicrobial resistance and virulence (see further in the discussion). Since DCs are essential for determining the nature of the adaptive cell-mediated immune responses and ultimately determine the eventual success or failure of a pathogen’s capability to infect and persist in the host, we sought to determine whether the presence of a GalN substituent on the AG of some mycobacterial pathogens impacted a primary step in the human host’s innate immune response, namely, the ability to activate and stimulate DCs to mature. Although DCs are not the primary targets for infection by Mtb, they are critically important in the induction of cellular immune responses that ultimately determine the fate of infection [37]. Induction of innate responses as well as activation of specific T cells is directly related to the state of DC maturation and this induction is directly modulated by the nature of the encounter with the bacillus [21]. Immature DCs (iDC) are highly phagocytic and well equipped for antigen uptake. Microbial products stimulate iDCs to mature and secrete cytokines through interactions with pattern recognition receptors (PRR) such as toll-like receptors (TLRs) and NOD-like receptors (NRLs) [38,39]. Activated DCs are characterized by up regulation of co-stimulatory markers such as CD80, CD86 and CD40 as well as MHC Class II molecules. Since tuberculosis is primarily a human disease we chose to determine whether the GalN substituent on the AG portion of the cell wall has any effect on the activation of hPBM-DCs by assessing the expression of these markers. We also considered the broad heterogeneity in the extent and nature of innate and adaptive immune responses in out-bred human populations. Thus, in order to resolve common trends, we chose to measure DC maturation profiles from 20 different healthy male and female blood donors.
We show here that Mtb devoid of the GalN substituent of AG either by disruption of Rv3631 or Rv3779 induces hPBM-DCs to mature to levels comparable to non-tuberculous mycobacteria that are naturally devoid of this motif. In all of the hPBM-DCs examined in this study, when compared to the WT parent strains, both the Rv3631 and Rv3779 mutants consistently and significantly stimulated up-regulation of all CD80, CD86 and CD40 co-stimulatory molecules as well as HLA-DR MHC class II, indicating that the GalN motif of AG present in Mtb H37Rv may either directly or indirectly abrogate the ability of human DCs to fully mature. Complementation of the mutant strains with WT copies of the Rv3631 and Rv3779 genes mutations restored both the GalN motif on AG as well as the diminished DC activation phenotype (Figure 3).
Cytokine secretion profiles from treated DC culture supernatants were also determined. In all, six human cytokines were measured (IL-8, IL-1β, IL-6, IL-10, TNFα, and IL12p70) in which only IL-10 showed significant differences between GalN-proficient and GalN-deficient strains. In hPBM-DCs from all donors measured, those treated with the WT Mtb parent strains showed significantly higher secretion of IL-10 suggesting that the GalN motif of AG somehow shifts the activation profile of the DCs to one that is more immunosuppressive or anti-inflammatory in nature. This would be an ideal scenario for a pathogen seeking a foothold in a host organism and to minimize host inflammatory-mediated tissue destruction and providing a persistence niche.
When hPBM-DCs were treated with purified AG (up to 1000 ng/ml) derived from the various Mtb strains, there was no significant difference in DC maturation phenotype (Supplemental Figure S2 and data not shown) as determined by surface translocation and expression of Class II, CD80, CD86 and CD40. This suggested that GalN modified AG per se is not the direct molecular determinant responsible for the observed differential DC maturation profiles. Rather, it implies that the GalN motif may somehow, through secondary or tertiary effects, either control the surface access of mycobacterial DC ligands or perhaps the export of these ligands to the cell surface. Comparable amounts of known or suspected DC-SIGN, TLR and MR ligands LAM, 19 kDa, DnaK, and Apa were detected on intact Mtb WT and mutant strains. This was determined by comparable staining with specific antibodies at the bacterial cell surface suggesting that accessibility of the immune receptors to their ligands rather than the proper translocation of these ligands to the cell surface accounts for the observed DC maturation phenotypes.
It has been demonstrated that the DC-specific C-type lectin DC-SIGN is an important receptor on DCs that captures and internalizes M. bovis BCG. Blocking of DC-SIGN using anti-DC-SIGN antibody restricts infection of DCs [20]. ManLAM, for instance, is one of several ligands for DC-SIGN (as well as TLRs) on the surface of mycobacteria [22] [5]. When ManLAM is bound to DC-SIGN on DCs, it abrogates mycobacteria- or LPS-induced DC maturation thereby diminishing the host immune response and potentially promoting infection. We thus chose to probe intact bacteria with a soluble chimeric human DC-SIGN-Fc molecule in order to determine whether the GalN motif somehow modifies the ability of DC-SIGN to bind to mycobacteria. In this study, we showed that the presence of a GalN substituent on AG enhances binding of the DC-SIGN-Fc probe. The enhanced DC-SIGN binding by the WT Mtb strains might also explain the increase in IL-10 secretion. It is also of interest to note that the naturally GalN-proficient M. leprae binds considerably more DC-SIGN probe when compared to the naturally GalN-deficient M. smegmatis although many differences other than GalN in the cell envelope composition of these Mycobacterium species are likely to account for differential interaction with DC-SIGN. We currently do not know whether the GalN motif affects interaction with other DC- and macrophage-resident immunomodulatory PRRs such as mannose receptor (MR). MR (CD206) is C-type lectin PRR that is highly expressed on alveolar macrophages [40]. Mycobacterial stimulation through MR leads to production of anti-inflammatory cytokines IL-4 and IL-13, inhibition of IL-12 and diminished activation of oxidative responses [21]. It is possible that recognition by C-type lectins such as DC-SIGN and MR depends not only on the level of mannosylation between Mtb strains [5] but on their relative accessibility as perhaps determined by GalN-modified AG.
Immature DCs are distributed throughout peripheral tissues and have been conferred the role of sentinel against potentially pathogenic organisms. Upon pathogen recognition and capture, DCs become activated and are poised to process and subsequently present antigenic peptides in the context of MHC II or lipids/lipopeptides in the context of “non-classical” MHC-like molecules such as CD1 and subsequently migrate to secondary lymphoid organs where they encounter naïve T cells. The induction of adaptive immunity critically depends on the nature of pathogen recognition by DCs. The specific nature of the host immune response is ultimately determined by crosstalk between different signaling pathways induced by a variety of PRRs engaging with a variety of pathogen-associated ligands (PAMPs). The GalN motif on mycobacterial AG may represent a molecular configuration that mediates higher-ordered PRR recognition that has been previously unrealized.
The relative restriction of the GalN substituent to pathogenic slow-growing mycobacterial species is intriguing. Basic sugar residues and positively charged primary amines are emerging as significant players in determining host/pathogen responses. Recent work has shown that for Francisella tularensis and subsp., novicida that a GalN motif covalently modifies LPS on the lipid A moiety essentially neutralizing its negative charge and enhancing virulence and thwarting protective immune responses in mice. Mutants lacking the GalN motif on Lipid A were more attenuated and were able to appropriate protective immunity in mice [34]. In Burkholderia cenocepacia, addition of a 4-amino-4-deoxy-L-arabinose (L-Ara4N) to LPS confers extraordinary resistance to antimicrobial peptides such as polymyxin B and melittin [41]. In the Gram-positive bacterium Bacillus thuringienis subsp. israelenis both GalN and glucosamine (GlcN) residues have been reported to be substituents of the glycosylation motif of a glycoprotein toxin proposed to be important to the biological activities of the toxin [42]. GalN has also been identified as a component of a galactose-containing polysaccharide from Streptococcus mutans [43]. In the Gram-negative opportunistic pathogen Acinetobacter baumannii, phosphoethanolamine (pEtN) alterations of Lipid A have been linked to resistance to colistin (Polymyxin E). In addition, is has been shown that the fastidious slow growing Gram-negative commensal bacterium of canine and feline oral flora Capnocytophaga canimorsus has an unusual LPS whose pro-inflammatory properties are related to the core oligosaccharide moiety of the molecule rather than lipid A [44]. The core oligosaccharides contain a 1-phosphoethanolamine (P-EtN) group at the 4′ position of the core in place of the phosphate. This, in effect, increased endotoxicity by dramatically increasing the binding of the C. canimorsus LPS via it’s core to human myeloid differentiation factor 2 (MD-2) most likely through charge screening. It has been suggested that the positive charge from the P-EtN group compensates for the lack of endotoxicity of the C. canimorsus lipid A by forming a stable hexameric (LPS/TLR4/MD-2)2 complex thus enabling the TLR4 complex [45]. In terms of targeting modulatory host receptors, N-acetylglucosamine (GlnNAc) sugar residues within the LPS core of E. coli, Salmonella. enterica and N. gonorrhoeae likely play a significant role in targeting the bacteria to DC-SIGN [46]. Thus, simple amino sugars or charged amines strategically placed within the bacterial landscape interacting with host immune receptors may profoundly affect the virulence of the bacteria.
We thus postulate that a possible mechanism whereby the GalN substituent on AG would serve to permit better access to DC-SIGN would be by an alteration in the overall cell wall charge that serves to alter the bacterial cell surface topology and/or its composition by interfering with the export of surface exposed constituents. While purified AG does not have any differential effect on DC maturation regardless of whether it contains GalN, the altered polysaccharide indeed does so in context with the whole bacterium, suggesting that GalN residing on AG on this portion of the cell wall serves to alter bacterial interactions with DC receptors such as DC-SIGN indirectly. Hence the GalN motif may serve to modulate Mtb innate host cell interactions indirectly through a trans mechanism rather than a more direct interaction with altered AG.
Our observed increased binding of DC-SIGN coupled with the altered TLR-mediated NF-κB activation in GalN-containing Mtb is also intriguing since it has been shown that binding of DC-SIGN by ManLAM modulates TLR activity [47]. This signaling is modified by activating the serine-threonine kinase Raf-1 that, through a phosphorylation event, allows for the acetylation of the p65 subunit of NF-κB. However, TLRs must be activated/engaged to activate nuclear translocation of p65 in order for DC-SIGN to mediate acetylation. Acetylation of p65 prolongs the transcriptional activity of NF-κB and enhances the rate of initiation of transcription of IL-10, which results in increased IL-10 production. Thus PAMP-specific signaling through selective engagement of DC-SIGN as well as crosstalk from other PRRs (such as TLR) is crucial to the induction of pathogen- as well as motif-specific immune responses. Future studies will unravel how alterations in the cell surface of pathogens such as Mtb having GalN motifs decorating their AG might ultimately determine their ability to infect and persist in host organisms.
Supplementary Material
Secretion of TNFα, IL-12p70, IL-8, IL-6 and IL-1β DCs were treated as in Figure 1 and supernatants were analyzed for cytokines. The samples were read using a FACsCanto II flow cytometer by using the BD Biosciences CBA software. Cytokine levels in each sample were calculated per company protocol by extrapolating the mean fluorescence intensity for each sample into the standard curves for every cytokine. X-axis labels and p value calculations are the same as in Figures 1- 4. A p value of ≤0.05 is considered significant. Dotted spanning lines indicate no significance solid spanning lines indicate significance (“ns” is non-significant).
hPBM-DCs derived from donor blood were treated with purified arabinogalactan from either WT or Rv3631 and Rv3779 GalN-deficient Mtb strains for 36 hr followed by analysis of the DCs for the expression of CD80, CD86, HLA-DR and CD40. “ns” are comparisons with p > 0.05.
The WT H37Rv(a) and corresponding Rv3631 mutant strains were stained with the vital membrane dye PKH26 and added at various MOI to hPBM-DCs for the indicated time (X-axis) at 37°C. The figure shows the uptake of these strains at 0°C indicating the requirement for active metabolis m for phagocytosis.
Determination of the specificity of DC-SIGN lectin binding by blocking with 50 μg/ml yeast mannan (Sigma) or 5 mM EGTA. Pretreatment of DCs with either 50 μg/ml of yeast mannan or 5 mM EGTA block the interaction of the DC-SIGN-Fc chimeric probe with the H37Rv(a) strains. This result was the case for all the WT, mutant and complemented strains analyzed in this work.
Graphical representation of overall DC-SIGN binding to WT vs. mutant vs. complemented mutants. The Y-axis is the MFI of the various mycobacterial probed with the soluble chimeric DC-SIGN-Fc probe followed by an anti-human Fc secondary PE-conjugated Ab. *, p ≤ 0.05, ** p ≤ 0.05 and *** p ≤ 0.005
Non antigen specific Alexa fluor-488-conjugated isotype control Ab did not bind to the surface of WT Mtb or their respective Rv3631 and Rv3779 mutants as well as their respective complements. This reveals that the mycobacteria were sufficiently blocked with human serum prior to probing for the DC-SIGN ligand and effectively rules out the possibility of non-specific antibody binding contributing to the MFI of the antigen-specific bacterial surface probing. The MFI for each histogram is denoted in the top right corner of each figure.
Acknowledgments
We thank Dr. Patrick J. Brennan for critical reading of the manuscript and for providing extremely helpful suggestions and to Dr. Phillip L. Chapman for providing expertise statistical advice and analysis to this work.
Funding: This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant AI064798 to MJ. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Angala SK, Belardinelli JM, Huc-Claustre E, Wheat WH, Jackson M. The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol. 2014:1–39. doi: 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilleron M, Jackson M, Nigou J, Puzo G. In: Structure, activities and biosynthesis of the Phosphatidyl-myo-Inositol-based lipoglycans. Daffé M, Reyrat JM, editors. Washington, DC: ASM; 2008. [Google Scholar]
- 5.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neyrolles O, Guilhot C. Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis (Edinb) 2011;91:187–195. doi: 10.1016/j.tube.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee A, Wu SW, Scherman MS, Torrelles JB, Chatterjee D, McNeil MR, Khoo KH. Sequencing of oligoarabinosyl units released from mycobacterial arabinogalactan by endogenous arabinanase: identification of distinctive and novel structural motifs. Biochemistry. 2006;45:15817–15828. doi: 10.1021/bi060688d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhamidi S, Scherman MS, Rithner CD, Prenni JE, Chatterjee D, Khoo KH, McNeil MR. The identification and location of succinyl residues and the characterization of the interior arabinan region allow for a model of the complete primary structure of Mycobacterium tuberculosis mycolyl arabinogalactan. J Biol Chem. 2008;283:12992–13000. doi: 10.1074/jbc.M800222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draper P, Khoo KH, Chatterjee D, Dell A, Morris HR. Galactosamine in walls of slow-growing mycobacteria. Biochem J. 1997;327 (Pt 2):519–525. doi: 10.1042/bj3270519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhamidi S, Scherman MS, Jones V, Crick DC, Belisle JT, Brennan PJ, McNeil MR. Detailed structural and quantitative analysis reveals the spatial organization of the cell walls of in vivo grown Mycobacterium leprae and in vitro grown Mycobacterium tuberculosis. J Biol Chem. 2011;286:23168–23177. doi: 10.1074/jbc.M110.210534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skovierova H, Larrouy-Maumus G, Pham H, Belanova M, Barilone N, Dasgupta A, Mikusova K, Gicquel B, Gilleron M, Brennan PJ, et al. Biosynthetic origin of the galactosamine substituent of Arabinogalactan in Mycobacterium tuberculosis. J Biol Chem. 2010;285:41348–41355. doi: 10.1074/jbc.M110.188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherman H, Kaur D, Pham H, Skovierova H, Jackson M, Brennan PJ. Identification of a polyprenylphosphomannosyl synthase involved in the synthesis of mycobacterial mannosides. J Bacteriol. 2009;191:6769–6772. doi: 10.1128/JB.00431-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepard CC, McRae DH. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968;36:78–82. [PubMed] [Google Scholar]
- 14.Escuyer VE, Lety MA, Torrelles JB, Khoo KH, Tang JB, Rithner CD, Frehel C, McNeil MR, Brennan PJ, Chatterjee D. The role of the embA and embB gene products in the biosynthesis of the terminal hexaarabinofuranosyl motif of Mycobacterium smegmatis arabinogalactan. J Biol Chem. 2001;276:48854–48862. doi: 10.1074/jbc.M102272200. [DOI] [PubMed] [Google Scholar]
- 15.Daffe M, Brennan PJ, McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990;265:6734–6743. [PubMed] [Google Scholar]
- 16.Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Krieger E, Vriend G, Figdor CG, van Kooyk Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J Biol Chem. 2002;277:11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- 17.Murray RA, Siddiqui MR, Mendillo M, Krahenbuhl J, Kaplan G. Mycobacterium leprae inhibits dendritic cell activation and maturation. J Immunol. 2007;178:338–344. doi: 10.4049/jimmunol.178.1.338. [DOI] [PubMed] [Google Scholar]
- 18.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. Eur J Cell Biol. 2010;89:95–101. doi: 10.1016/j.ejcb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 22.Pitarque S, Herrmann JL, Duteyrat JL, Jackson M, Stewart GR, Lecointe F, Payre B, Schwartz O, Young DB, Marchal G, et al. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem J. 2005;392:615–624. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driessen NN, Ummels R, Maaskant JJ, Gurcha SS, Besra GS, Ainge GD, Larsen DS, Painter GF, Vandenbroucke-Grauls CM, Geurtsen J, et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll MV, Sim RB, Bigi F, Jakel A, Antrobus R, Mitchell DA. Identification of four novel DC-SIGN ligands on Mycobacterium bovis BCG. Protein Cell. 2010;1:859–870. doi: 10.1007/s13238-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart GR, Wilkinson KA, Newton SM, Sullivan SM, Neyrolles O, Wain JR, Patel J, Pool KL, Young DB, Wilkinson RJ. Effect of deletion or overexpression of the 19-kilodalton lipoprotein Rv3763 on the innate response to Mycobacterium tuberculosis. Infect Immun. 2005;73:6831–6837. doi: 10.1128/IAI.73.10.6831-6837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satchidanandam V, Kumar N, Jumani RS, Challu V, Elangovan S, Khan NA. The glycosylated Rv1860 protein of mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG. PLoS Pathog. 2014;10:e1004176. doi: 10.1371/journal.ppat.1004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecora ND, Gehring AJ, Canaday DH, Boom WH, Harding CV. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J Immunol. 2006;177:422–429. doi: 10.4049/jimmunol.177.1.422. [DOI] [PubMed] [Google Scholar]
- 28.Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- 29.Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- 30.Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol. 2001;69:1036–1044. [PubMed] [Google Scholar]
- 31.Acharya PV, Goldman DS. Chemical composition of the cell wall of the H37Ra strain of Mycobacterium tuberculosis. J Bacteriol. 1970;102:733–739. doi: 10.1128/jb.102.3.733-739.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Draper P. The walls of Mycobacterium lepraemurium: chemistry and ultrastructure. J Gen Microbiol. 1971;69:313–324. doi: 10.1099/00221287-69-3-313. [DOI] [PubMed] [Google Scholar]
- 33.Sonesson A, Jantzen E, Tangen T, Zahringer U. Chemical characterization of lipopolysaccharides from Legionella feeleii, Legionella hackeliae and Legionella jordanis. Microbiology. 1994;140 (Pt 10):2663–2671. doi: 10.1099/00221287-140-10-2663. [DOI] [PubMed] [Google Scholar]
- 34.Kanistanon D, Hajjar AM, Pelletier MR, Gallagher LA, Kalhorn T, Shaffer SA, Goodlett DR, Rohmer L, Brittnacher MJ, Skerrett SJ, et al. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Ribeiro AA, Guan Z, Raetz CR. Identification of undecaprenyl phosphate-beta-D-galactosamine in Francisella novicida and its function in lipid A modification. Biochemistry. 2009;48:1162–1172. doi: 10.1021/bi802211k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray PJ. Defining the requirements for immunological control of mycobacterial infections. Trends Microbiol. 1999;7:366–372. doi: 10.1016/s0966-842x(99)01567-x. [DOI] [PubMed] [Google Scholar]
- 38.Saraav I, Singh S, Sharma R. Outcome of Mycobacterium tuberculosis and Toll-like receptor interaction: immune response or immune evasion? Immunology and Cell Biology. 2014 doi: 10.1038/icb.2014.52. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Rhebergen AM, Eisenbarth SC. Licensing Adaptive Immunity by NOD-Like Receptors. Front Immunol. 2013;4:486. doi: 10.3389/fimmu.2013.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 41.Hamad MA, Di Lorenzo F, Molinaro A, Valvano MA. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia(dagger) Mol Microbiol. 2012;85:962–974. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- 42.Pfannenstiel MA, Muthukumar G, Couche GA, Nickerson KW. Amino sugars in the glycoprotein toxin from Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1987;169:796–801. doi: 10.1128/jb.169.2.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiu TH. Biosyntheses of galactosyl lipids and polysaccharide in Streptococcus mutans. Biochim Biophys Acta. 1988;963:359–366. doi: 10.1016/0005-2760(88)90302-5. [DOI] [PubMed] [Google Scholar]
- 44.Ittig S, Lindner B, Stenta M, Manfredi P, Zdorovenko E, Knirel YA, dal Peraro M, Cornelis GR, Zahringer U. The lipopolysaccharide from Capnocytophaga canimorsus reveals an unexpected role of the core-oligosaccharide in MD-2 binding. PLoS Pathog. 2012;8:e1002667. doi: 10.1371/journal.ppat.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coats SR, Berezow AB, To TT, Jain S, Bainbridge BW, Banani KP, Darveau RP. The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria. Infect Immun. 2011;79:203–210. doi: 10.1128/IAI.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Snyder S, Feng P, Azadi P, Zhang S, Bulgheresi S, Sanderson KE, He J, Klena J, Chen T. Role of N-acetylglucosamine within core lipopolysaccharide of several species of gram-negative bacteria in targeting the DC-SIGN (CD209) J Immunol. 2006;177:4002–4011. doi: 10.4049/jimmunol.177.6.4002. [DOI] [PubMed] [Google Scholar]
- 47.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Secretion of TNFα, IL-12p70, IL-8, IL-6 and IL-1β DCs were treated as in Figure 1 and supernatants were analyzed for cytokines. The samples were read using a FACsCanto II flow cytometer by using the BD Biosciences CBA software. Cytokine levels in each sample were calculated per company protocol by extrapolating the mean fluorescence intensity for each sample into the standard curves for every cytokine. X-axis labels and p value calculations are the same as in Figures 1- 4. A p value of ≤0.05 is considered significant. Dotted spanning lines indicate no significance solid spanning lines indicate significance (“ns” is non-significant).
hPBM-DCs derived from donor blood were treated with purified arabinogalactan from either WT or Rv3631 and Rv3779 GalN-deficient Mtb strains for 36 hr followed by analysis of the DCs for the expression of CD80, CD86, HLA-DR and CD40. “ns” are comparisons with p > 0.05.
The WT H37Rv(a) and corresponding Rv3631 mutant strains were stained with the vital membrane dye PKH26 and added at various MOI to hPBM-DCs for the indicated time (X-axis) at 37°C. The figure shows the uptake of these strains at 0°C indicating the requirement for active metabolis m for phagocytosis.
Determination of the specificity of DC-SIGN lectin binding by blocking with 50 μg/ml yeast mannan (Sigma) or 5 mM EGTA. Pretreatment of DCs with either 50 μg/ml of yeast mannan or 5 mM EGTA block the interaction of the DC-SIGN-Fc chimeric probe with the H37Rv(a) strains. This result was the case for all the WT, mutant and complemented strains analyzed in this work.
Graphical representation of overall DC-SIGN binding to WT vs. mutant vs. complemented mutants. The Y-axis is the MFI of the various mycobacterial probed with the soluble chimeric DC-SIGN-Fc probe followed by an anti-human Fc secondary PE-conjugated Ab. *, p ≤ 0.05, ** p ≤ 0.05 and *** p ≤ 0.005
Non antigen specific Alexa fluor-488-conjugated isotype control Ab did not bind to the surface of WT Mtb or their respective Rv3631 and Rv3779 mutants as well as their respective complements. This reveals that the mycobacteria were sufficiently blocked with human serum prior to probing for the DC-SIGN ligand and effectively rules out the possibility of non-specific antibody binding contributing to the MFI of the antigen-specific bacterial surface probing. The MFI for each histogram is denoted in the top right corner of each figure.