Abstract
Cargo adaptors sort transmembrane protein cargos into nascent vesicles by binding directly to their cytosolic domains. Recent studies have revealed previously unappreciated roles for cargo adaptors and regulatory mechanisms governing their function. The AP-1 and AP-2 clathrin adaptors switch between open and closed conformations that ensure they function at the right place at the right time. The exomer cargo adaptor plays a direct role in remodeling the membrane for vesicle fission. Several different cargo adaptors functioning in distinct trafficking pathways at the Golgi are similarly regulated through bivalent binding to the Arf1 GTPase, potentially enabling regulation by a threshold concentration of Arf1. Taken together, these studies highlight that cargo adaptors do more than just adapt cargos.
Keywords: Membrane trafficking, Vesicle, Cargo adaptor, GTPase
Cargo adaptors sort proteins into nascent vesicles
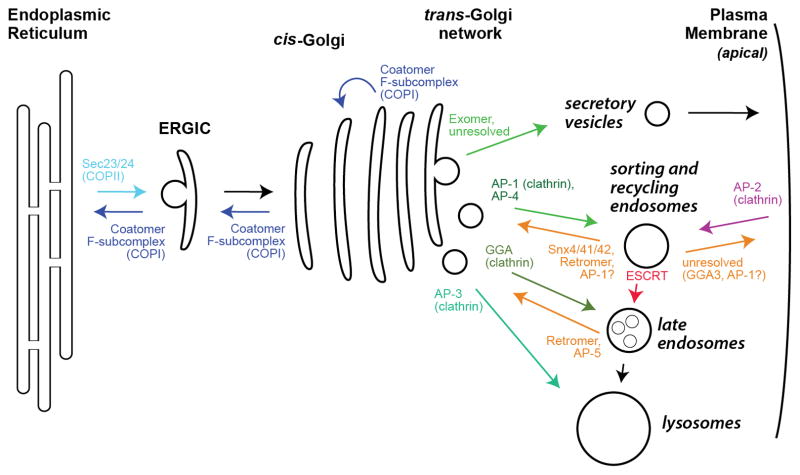
Cargo adaptors play a central role in membrane trafficking by packaging cargo proteins into nascent vesicles. Cargo adaptors bind directly to sorting signals in the cytosolic tails of transmembrane cargos (or transmembrane cargo receptors serving as recognition interfaces for lumenal cargos), concentrating them into vesicles or tubules for transport through the secretory and endocytic pathways of cells [1–3]. At each organelle, a distinct set of cargo adaptors functions (Figure 1).
Figure 1. Overview of cargo adaptor localization.
Shown is a schematic of the secretory and endocytic pathways of a typical eukaryotic cell, highlighting the trafficking pathways controlled by the major cargo adaptors. Traffic is shown to and from the apical plasma membrane. Traffic to the basolateral membrane appears to rely upon similar adaptors used for traffic to the endo-lysosomal system [9]. The precise pathways controlled by many cargo adaptors, especially regarding endocytosis and the endosomal membrane system, remains a subject of debate. Note that Exomer traffics only ~5% of PM proteins in budding yeast, and there is no homolog of Exomer in metazoans, so the sorting mechanism for the bulk of apical PM proteins remains unresolved. ERGIC denotes the ER-Golgi Intermediate Compartment. See also Table 1.
Cargo adaptor recruitment to the appropriate membrane surface is tightly regulated: in addition to binding cargo, cargo adaptors usually bind directly to the membrane, with membrane specificity enforced through interaction with an organelle-specific phosphoinositide lipid and/or a small GTPase [2, 4, 5]. Cargo adaptors usually also bind to the structural scaffold of a vesicle coat (such as clathrin). Different cargo adaptors may link to the same coat scaffold and therefore connect multiple types of cargos to a single vesicle coat, analogous to electric plug adapters; accordingly, over a dozen cargo adaptors are found in higher eukaryotes [6–10] (Table 1).
Table 1.
Primary Eukaryotic Cargo Adaptors
| Cargo adaptor | Primary trafficking pathway | Number of subunits in cargo adaptor a | Coat or scaffold | Primary GTPase and lipid regulator(s) |
|---|---|---|---|---|
| AP-1 | TGN-to-EE, EE/RE-to-PM | 4 | clathrin | Arf1 b, PI(4)P |
| AP-2 | Endocytosis | 4 | clathrin | PI(4,5)P2 |
| AP-3 | TGN-to-lysosome, EE-to-lysosome | 4 | clathrin | Arf1 b |
| AP-4 | TGN-to-EE | 4 | none known | Arf1 b |
| AP-5 | LE-to-TGN | 4 | SPG11/15 | PI(3)P |
| BBSome/IFT complexes | Ciliary trafficking | Unresolved c | Unresolved c | Arl6 (aka BBS3) |
| F-subcomplex (COPI) | Golgi, Golgi-to-ER | 4 | α-COP β′-COP ε-COP (B-subcomplex) |
Arf1 b |
| GGA1 | TGN-to-LE | 1 | clathrin | Arf1 b, PI(4)P |
| GGA2 | TGN-to-LE | 1 | clathrin | Arf1 b, PI(4)P |
| GGA3 | TGN-to-LE, RE-to-PM | 1 | clathrin | Arf1 b, Arf6, PI(4)P |
| ESCRT | MVB (LE) sorting | ESCRT-0: 2 ESCRT-I: 4 ESCRT-II: 4 |
ESCRT-III | PI(3)P |
| Exomer | TGN-to-PM | 4 (dimer of dimers) | none known | Arf1 b |
| Retromer | EE-to-TGN, LE-to-TGN | 3 | Vps5 (Snx1) Vps17 (Snx2) |
Rab7, PI(3)P |
| Sec23/24 (COPII) | ER-to-Golgi | 2 | Sec13/31 | Sar1 |
| Snx4/41/42 | EE-to-TGN, EE/RE-to-PM | 3 | none known | Rab11? |
| TSET | Endocytosis | 4 | TTRAY | none known |
Note that some subunits have multiple paralogs in some organisms, and for some complexes the number of subunits varies among organisms.
Arf1 denotes the paralogous Arf1-5 proteins.
The BBSome has 8 subunits, the IFT-A complex has ~6 subunits, and the IFT-B complex has ~14 subunits. It remains to be determined which subunits are involved in cargo recognition and which subunits are involved in scaffolding.
The role of cargo adaptors in selecting and sorting cargos is well established. Our understanding of membrane trafficking has been expedited by the structural analysis of cargo adaptors and vesicle coat complexes [11, 12], but these proteins represent a challenge to structural biologists due to their large size, flexibility, and multi-subunit architecture. In spite of these obstacles, vast mechanistic data have been gleaned from many structural studies of cargo adaptors which function as part of the COPI, COPII, retromer, and clathrin vesicle coat protein complexes. For example, the structural basis for cargo specificity has been documented for several adaptors through a series of illuminating adaptor/cargo co-crystal structures [13–37].
An unexpected theme has emerged from several recent structural studies on the regulation of cargo adaptors. Although it has been known for some time that certain cargo adaptors regulate the nucleotide status of their regulators and coordinate interactions with vesicle tethering factors [38], it now appears that several cargo adaptors exert an even more active role in the regulation of vesicle biogenesis than previously appreciated. This review will discuss recent studies emphasizing that cargo adaptors do not simply adapt cargos, but also regulate multiple steps of transport vesicle formation.
COPII coat assembly may accommodate different cargo sizes
The formation of COPII vesicles, which sort cargos leaving the ER (Figure 1), is the first trafficking step in the secretory pathway [39]. The structure of the Sec23/Sec24 cargo adaptor bound to the ER-specific Sar1 GTPase revealed the mechanism of membrane recruitment, and was the first structural explanation for cargo adaptor regulation by a GTPase [40]. Other early structural studies of the Sec23/24 complex established the basis for cargo binding and for interaction with the Sec13/Sec31 scaffolding subcomplex [18, 41, 24].
Although the polyhedral cage-like structure of the Sec13/31 scaffold surrounding vesicles is well characterized [42–47], a recent cryo-EM structure of complete COPII complexes assembled on membrane tubules uncovered an alternative cylindrical architecture adopted by this scaffold [48]. This same study also revealed that the Sec23/24 cargo adaptor assembled into a regular lattice on the membrane surface [48]. Taken together, the findings from this and earlier work [43, 44, 49, 46, 47] indicate that the COPII coat can adopt a variety of geometries to accommodate a range of vesicle sizes, with the Sec23/24 adaptor itself playing an unexpected structural role in determining vesicle shape [48].
Membrane and cargo binding activates AP-2 to interact with clathrin
Clathrin serves as the structural scaffold for several types of vesicles budding from the plasma membrane (PM), the trans-Golgi network (TGN), and some types of endosomes [50]. Correspondingly, clathrin interacts with several different cargo adaptor complexes (Table 1). Two of the most thoroughly studied of these are AP-1 and AP-2, which function at the TGN/endosomes and PM, respectively [51] (Figure 1). AP-1 and AP-2 are heterotetrameric complexes consisting of two large subunits, a medium subunit, and a small subunit (γ, β1, μ1, and σ1 for AP-1; α, β2, μ2, and σ2 for AP-2) [52].
The large subunits of the APs possess “appendage” domains that recruit accessory factors assisting in cargo sorting, vesicle scission, and uncoating [50]. The appendages are connected to the core complex through a flexible linker referred to as the “hinge”. The hinge of the β subunit provides the platform for clathrin assembly via an interaction between a motif in the linker – residues LLNLD, referred to as the clathrin box – and clathrin heavy chain [53]. An additional interaction between the appendage domain and clathrin heavy chain has also been reported [23]. These interactions are believed to arrange clathrin trimers properly during formation of the clathrin cage, as it has been known for some time that clathrin cage assembly is stimulated by the AP complexes [54]. Hence, the original name for the AP was “assembly polypeptide”, though AP is now often used to denote “adaptor protein”.
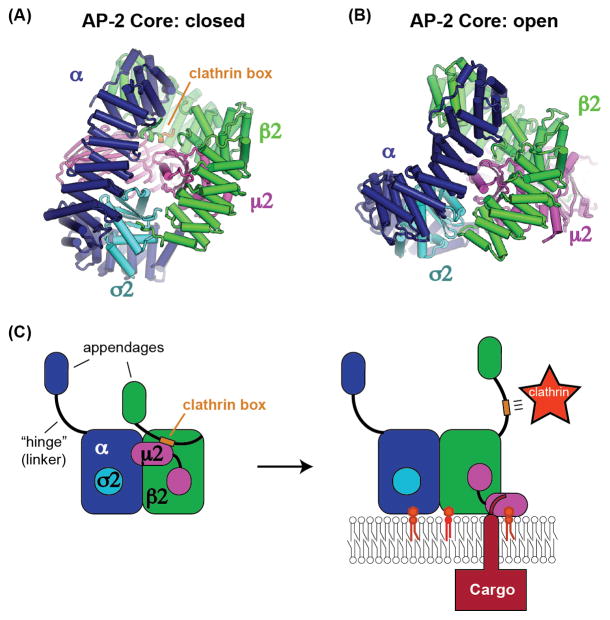
Despite the wealth of information available regarding the interactions between the AP complexes and clathrin, a recent study revealed an unexpected level of regulation controlling this interaction [55]. Surprisingly, a truncated form of the AP-2 β2 subunit bound more tightly to clathrin, and stimulated clathrin cage formation more, than a construct of AP-2 that is essentially intact (lacking only the α appendage and hinge regions). This implies that binding of the AP-2 clathrin box to clathrin is autoinhibited by the AP-2 core. The mechanism for this autoinhibition was provided by a structure demonstrating that the clathrin box motif binds to an interior pocket of the AP-2 core (Figure 2A), sequestering it from binding clathrin [55].
Figure 2. AP-2 binding to cargo and PI(4,5)P2 membranes triggers recruitment of clathrin.
(A) The recent structure of the AP-2 core complex in the closed conformation revealing an autoinhibitory interaction with the clathrin box motif (orange) of the β2 subunit [55]. (B) The structure of the AP-2 core complex in the open conformation demonstrating that the binding site for the clathrin box is no longer present [29], consequently the clathrin box is available to recruit clathrin. (C) Model for activation of AP-2 by binding to cargo and PI(4,5)P2, leading to recruitment of clathrin through the released clathrin box motif [55].
Autoinhibition of clathrin binding is relieved when AP-2 is bound to membranes containing phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), and clathrin binding is further stimulated by addition of a cargo peptide. These results are best explained by previous structural studies establishing that AP-2 adopts two very different conformations: open and closed [56, 29] (an intermediate or “unlatched” conformation has also been observed [25]). The open conformation is stabilized by binding to PI(4,5)P2 and cargo [29, 55]. In the closed conformation, AP-2 cannot bind to cargo [13, 56], and it is this closed conformation that sequesters the clathrin box in the interior of the AP-2 core [55]. In contrast, the structural data indicate that the clathrin box motif is not capable of binding to the open form of the AP-2 core due to steric constraints (Figure 2B). Thus, once AP-2 binds to its cargo on a PI(4,5)P2 containing membrane, the clathrin box is released from the interior of the AP-2 core and becomes available to recruit clathrin [55] (Figure 2C). This study is an elegant example of the power of structure and biochemistry to elucidate an unexpected cell biological mechanism. The implication is that the cargo adaptor itself is regulating clathrin-coated vesicle formation, to ensure that vesicles form at the right place at the right time. It remains to be determined whether this mechanism also applies to AP-1 and other clathrin adaptors, or perhaps to cargo adaptors more broadly.
The COPI cargo adaptor forms a bivalent Arf1 complex
COPI, a.k.a. “coatomer”, is a heptameric complex that sorts retrograde cargos at the Golgi and is regulated by the Arf1 GTPase [57] (Figure 1). The β, γ, δ, and ζ subunits of the “F-subcompex” bear striking resemblance in fold and function to the clathrin AP complexes [58], and are thought to serve the cargo adaptor function for COPI. Unlike the clathrin and COPII vesicle coats, for which cargo adaptor and coat recruitment occur in two distinct steps, the entire coatomer complex, consisting of both the cargo adaptor and “cage-like” subunits, is recruited to the membrane en bloc [59]. Together, these seven subunits pair cargo and small GTPase binding with the machinery necessary for vesicle biogenesis [60].
A structure of the γ/ζ subcomplex bound to Arf1 revealed several important regulatory features [61]. The structure demonstrated that the γ subunit binds directly to Arf1-GTP through contacts with the “switch” regions of Arf1 (Figure 3A) [61], as is typical for effectors of Arf1 and other small GTPases [62, 63]. On the basis of sequence and presumed structural homology between the γ and β subunits, a second Arf1 binding site was identified on the β subunit, thus establishing the Arf1:coatomer stoichiometry as 2:1. These interactions with the two Arf1 molecules, anchored to the membrane through amphipathic helices, permitted the modeling of the COPI cargo adaptor complex bound to the membrane [61].
Figure 3. Bivalent binding to the Arf1 GTPase by the AP-1, COPI, and Exomer cargo adaptors.
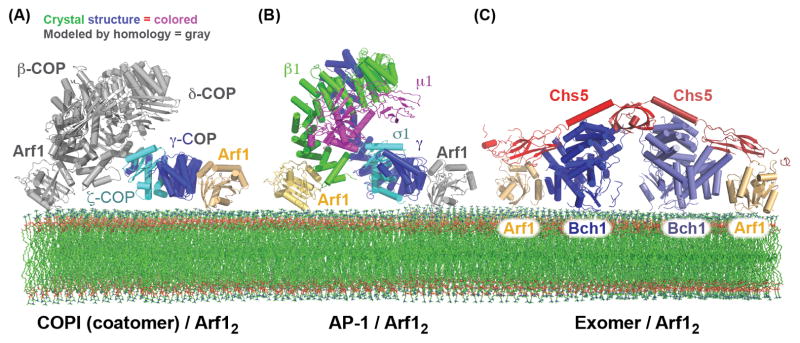
(A) Structural model of the COPI F-subcomplex recruited to the membrane surface by two molecules of Arf1. The Arf1 molecule and portions of the F-subcomplex shown in gray are modeled based on homology to the AP-2 complex, and homology between the β-COP and γ-COP subunits (the observed and modeled Arf1 interactions were confirmed biochemically) [61]. It should be noted that a recent cryo-EM study of the COPI coat suggested that the F-subcomplex may adopt a different conformation than does the AP-2 core [112]. (B) Structural model of the AP-1 core complex recruited to the membrane surface by two molecules of Arf1. The Arf1 molecule shown in gray is modeled based on homology between the β1 and γ subunits (the observed and modeled Arf1 interactions were confirmed biochemically) [71]. (C) Structure of the Exomer/Arf1 complex bound to membranes (all interactions, including with the membrane surface, were confirmed biochemically) [88].
The COPI cargo adaptor sits on the membrane surface in an orientation similar to that of AP-2 (Figure 3A). Correspondingly, the Arf1 binding sites on coatomer are located in similar positions to PI(4,5)P2 binding sites on AP-2. It should also be noted that dimerization of Arf1 itself has been reported to be important for COPI vesicle formation [64]. However, it has not been established whether the Arf1 dimer interface is intact when Arf1 is engaged with its effectors.
The COPI γ/ζ/Arf1 structure additionally provides a potential functional mechanism for the known ability of COPI to stimulate Arf GTPase-activating protein (Arf-GAP) activity [65]. Composite structural modeling indicated that COPI may bind to both Arf1 and to an Arf-GAP protein simultaneously, and therefore may stimulate Arf-GAP activity by “templating” the Arf1/Arf-GAP complex. This interaction, by inactivating Arf1, may stimulate the uncoating process [61].
AP-1 forms a bivalent Arf1 complex that stabilizes its open conformation
AP-1 sorts cargos from the TGN and endosomes (Figure 1), where it is recruited by interactions with Arf1, the lipid PI(4)P, and cargos [66–69]. The first structure of the AP-1 core revealed an architecture similar to that of the closed form of AP-2 [70]. More recently the structure of the open form of AP-1 was elucidated through analysis of an AP-1/Arf1 complex (Figure 3B) [71]. Binding to Arf1 was found to be sufficient to stabilize the open state of AP-1. Similarly to AP-2, the open state of AP-1 adopts a conformation that enables it to simultaneously bind to all three of its ligands: Arf1, cargo, and a PI(4)P-containing membrane. The structure further identified an interaction between the AP-1 β1-subunit and the switch regions of Arf1, similar to the interactions previously established between Arf1 and both the β- and γ-subunits of COPI [61]. A further similarity to the COPI/Arf1 study was the use of homology to delineate an additional Arf1-binding site in the N-terminus of the γ-subunit of AP-1, thus establishing that a single AP-1 complex binds simultaneously to two Arf1 molecules and constructing a plausible model of how the complex binds to the membrane [71] (Figure 3B).
An interesting outcome of this work was the finding that not all Arf1 interaction surfaces on AP-1 are created equal. Whereas the interaction between Arf1 and the β1-subunit enforced the open conformation of AP-1, the equivalent interaction with the AP-1 γ-subunit did not. However, a surprising “backside” Arf1 interaction between a non-switch region of Arf1 and a different portion of the γ-subunit, which would enable a single Arf1 molecule to bridge two separate AP-1 complexes, also stabilized the open form of AP-1. These results lead to a plausible step-wise model of AP-1 membrane recruitment by Arf1, and subsequent activation of AP-1, through the assembly of higher-order Arf1:AP-1 complexes triggering conformational change to the open state. This regulatory mechanism would ensure that AP-1 does not engage cargo until it has been properly recruited to the correct membrane by Arf1 [71]. It is tempting to speculate that this conformation of AP-1 would then be free to recruit clathrin by revealing its clathrin box, in a manner analogous to that described above for AP-2 [55]. Readers interested in learning more details about the conformational changes exhibited by AP-1 and AP-2 are referred to a recent review [72].
Exomer forms a bivalent Arf1 complex and remodels membranes
Structural analyses have provided many insights into cargo adaptor function and regulation. Through functional analysis, these structures have helped generate mechanistic models for a number of transport steps throughout the secretory pathway. However, sorting cargo at the TGN into secretory vesicles destined for the apical PM remains a notable gap in our understanding of membrane trafficking. Although a number of proteins have been implicated in the formation of these vesicles, little is known about the sorting and packaging of cargo in this pathway [73, 9]. The only cargo adaptor known to act directly in this sorting step is the exomer complex, which traffics a subset of cargo from the TGN to the PM in a regulated manner [74–84] (Figure 1). Although exomer lacks an obvious homolog in metazoans (it was originally discovered in budding yeast), it merits investigation as one of our only toeholds on this pathway.
Exomer is a heterotetrameric complex [85] consisting of a homodimer of the core subunit, Chs5, each paired with one member of the four paralogous ChAPs (Chs5-Arf1 binding proteins), Chs6, Bud7, Bch1, and Bch2, which convey cargo specificity. The first structures of the exomer complex, composed of Chs5 and the Chs6 or Bch1 ChAP subunits, revealed that homodimerization of the Chs5 subunit occurs through an unusual N-terminal domain that appears to function as a molecular hinge [85, 86]. A combination of X-ray crystallography, normal mode analysis, and small angle X-ray scattering demonstrated that the exomer complex is capable of constrained flexible motions centered on the Chs5 N-terminal hinge. The exomer hinge motion should not be confused with the “hinge” region of the clathrin cargo adaptors, which is actually a flexible linker.
One surprise from the initial crystal structure was the existence of a structural domain with a fold reminiscent of, but not identical to, the appendage domains found in the AP and COPI complexes [87]. This domain, named the FBE domain (FN3-BRCT domain of exomer), was found to be critical for exomer recruitment to membranes via interaction with Arf1 [85]. Recent structural analysis of a Chs5/Bch1 exomer complex bound to Arf1 [88] (Figure 3C) revealed an interface between the Chs5 FBE domain and a non-switch region of Arf1, as well as another interface between the ChAP (Bch1) and the switch regions of Arf1. In addition to its critical role in membrane recruitment, the FBE domain may also play a regulatory role in vesicle biogenesis, as it interferes with Arf-GAP activity on Arf1 [85]. Interestingly, a recent structure of a BBSome cargo adaptor subunit bound to the Arl6 GTPase also revealed an interaction with a non-switch region of the GTPase [89]. Together with the AP-1 “backside” Arf1 interaction and the exomer FBE-Arf1 interaction, it appears that non-switch GTPase interfaces are somewhat common for cargo adaptors.
The exomer heterotetramer binds to two molecules of Arf1. The symmetrical nature of the structure and the knowledge of how Arf1 binds membranes [90] leads to a model of the complex at the membrane surface (Figure 3C), revealing important electrostatic interactions between the ChAPs and the membrane [85, 88]. The ChAPs interact with their cargos at the membrane via an unresolved mechanism, which, due to the regulated nature of exomer cargo trafficking, may involve competition for cargo with other cargo adaptors [37].
Although the mechanism of membrane fission, the final step of vesicle biogenesis, has been studied extensively for endocytosis [91, 92], fission remains poorly understood for many trafficking steps. A number of proteins and lipid components are known to be important for vesicle fission, including proteins that physically insert into the membrane such as the Sar1 GTPase [93] and epsin [94, 92], proteins that constrict the diameter of the budding vesicle neck such as dynamin [4, 91, 95] or otherwise generate membrane curvature such as the banana-shaped BAR-domain proteins [96, 92, 97], structural protein scaffolds such as the Sec13/31 COPII subcomplex [98], and specific lipids encouraging membrane deformation by altering the biophysical properties of the bilayer [99, 100]. In fact, multiple factors are important for driving and regulating fission of a nascent vesicle or membrane tubule [101–104].
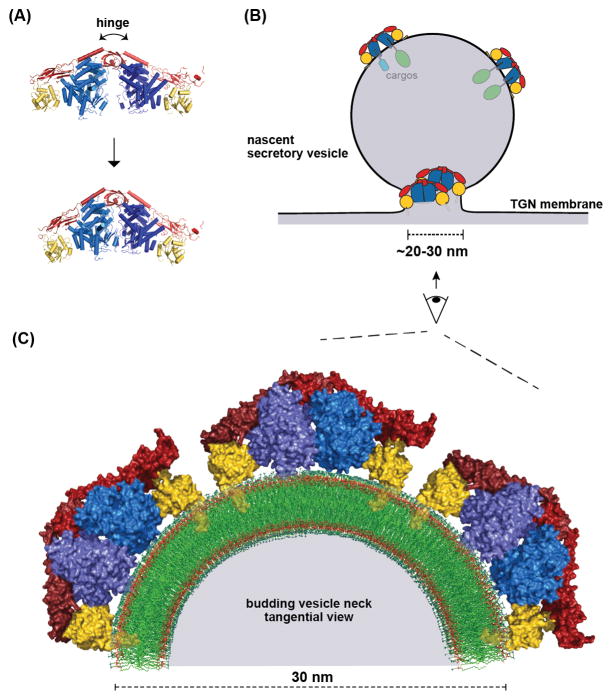
Structural analysis of the membrane-binding surface of the exomer complex revealed a potential membrane-insertion element [88]. Accordingly, exomer was found to cooperate with Arf1 to drive membrane fission in vitro, and the exomer membrane insertion element was important for this activity both in vitro and in vivo. Arf1 (and the related Sar1 GTPase) can remodel membranes via an N-terminal amphipathic helix [93, 105–108], and exomer appears to amplify this intrinsic capability of Arf1. The ability of exomer to bind and orient two Arf1 molecules, on both flat and highly curved membrane surfaces (such as a Golgi membrane and at the neck of a budding vesicle), likely arises from the hinge-motion afforded by the Chs5 N-terminal domain [86, 88] (Figure 4). Thus, unlike most other cargo adaptors, exomer appears to participate directly in membrane remodeling and fission [88].
Figure 4. The exomer cargo adaptor remodels the membrane.
(A) Normal mode analysis was used to model the hinge motion of the Exomer/Arf1 complex [88]. The hinge motion of the exomer complex has been established [86]. (B) Schematic of the dual roles of exomer in biogenesis of a secretory vesicle: cargo sorting and membrane remodeling. (C) Structural model of several Exomer/Arf1 complexes on the constricted neck of a budding vesicle. One-half of a tangential cross-section of the budding vesicle neck is shown for clarity. The myristoylated N-terminal amphipathic helix of each Arf1 molecule is modeled based on a previous study [90].
Concluding remarks
The structures of cargo adaptors have provided deep insights into their function and regulation. Each cargo adaptor achieves its proper localization through a unique set of interactions, but several common themes have emerged from these recent studies.
It is clear that cargo adaptors are recruited to their site of action by more than one binding partner, often by a combination of a specific lipid and small GTPase, and cargo itself plays a role in recruitment of adaptors. Interestingly, several Arf and Rab family GTPases have been shown to recruit their effectors, which include other molecules important for trafficking as well as cargo adaptors through multivalent interactions [62, 63]. This reliance on more than one signal for recruitment is a prime example of coincidence detection, ensuring that cargo adaptors are recruited to the right place at the right time.
For several cargo adaptors, bivalent interactions with the same signal are utilized. The AP-1, COPI, and exomer complexes all bind to two molecules of the Arf1 GTPase [61, 71, 88], and AP-2 binds to at least two PI(4,5)P2 molecules [29]. Interestingly, the Arf1-dependent GGA clathrin adaptors have been reported to dimerize through their appendage domain [109], although the physiological significance of GGA dimerization remains unresolved. Nevertheless, most of the Golgi cargo adaptors form bivalent Arf1 complexes. Why are these bivalent interactions so common? One possibility is that cooperativity is a consequence of bivalency, and membrane recruitment of the cargo adaptor is therefore robust only once a critical threshold concentration of Arf1 has been reached (i.e., in the case of bivalent recruitment, there is a sharper transition between soluble and membrane-bound cargo adaptor, depending on the concentration of the recruiting GTPase or lipid). It remains to be determined whether this threshold hypothesis holds true in vivo.
Some cargo adaptors undergo dramatic conformational rearrangements in switching between open and closed conformations. This switching underlies allosteric regulation of cargo adaptor function, and further ensures that adaptors only engage and sort cargos at the correct membrane. The importance of this regulation becomes clear when one considers the itinerary of many cargos that cycle between different membranes. For example, after its biosynthesis the transferrin receptor (TfR) is delivered from the Golgi to the PM. After engaging with its ligand (transferrin), TfR is taken up into endocytic vesicles by interacting with AP-2. After releasing transferrin in endocytic compartments, TfR is then delivered back to the PM to repeat the cycle [110]. If the endocytic machinery (i.e. AP-2) were to mistakenly engage TfR at the Golgi and endosomes, TfR might never be delivered to the PM to carry out its function. Given the finding that AP-2 recruitment of clathrin is regulated by cargo and membrane binding, it is likely that additional mechanisms regulating the timing and activity of other cargo adaptors remain to be discovered.
Somewhat surprisingly, the exomer cargo adaptor plays a direct role in membrane remodeling [88]. This illustrates how a cargo adaptor can be directly involved in aspects of vesicle biogenesis beyond cargo selection. Exomer may be a unique example, in that there is no known structural scaffold in the exomer pathway that functions similarly to Sec13/31 or clathrin cages. Therefore, exomer may play a more direct role in membrane remodeling to compensate for the absence of such a structural scaffold. Alternatively, other cargo adaptors may also possess membrane-remodeling activity. Indeed, the structure of the COPII coat assembled on membrane tubules implies that the Sec23/24 cargo adaptor plays a direct role in shaping the membrane [48], and it has been proposed that COPI or AP-1 may drive membrane curvature through coordination of two Arf1 molecules [72].
Structural biology has proven to be a powerful approach for dissecting the regulatory mechanisms underlying cargo adaptor function. Of course, many outstanding questions remain (Box 1). We look forward to the results of future structural studies in this field.
Box 1. Outstanding Questions.
Does COPI exhibit closed and open states?
Is recruitment of clathrin by AP-1 and AP-3 regulated by a mechanism similar to that seen for AP-2?
Do cargo adaptors respond to “threshold” concentrations of their regulators in vivo?
Do cargo adaptors other than exomer directly participate in membrane remodeling?
Do cargos recruit adaptors or do adaptors recruit cargos?
How do specific GTPases control the cargo specificity of adaptors? (see Ref [111])
Are there other cargo adaptors that function in the TGN-to-apical PM exocytic pathway?
Highlights.
Recent crystal structures reveal new roles for cargo adaptors
The Golgi-localized AP-1, COPI, and exomer adaptors form bivalent Arf1 complexes
The AP-2 adaptor couples membrane and cargo binding to clathrin recruitment
The exomer cargo adaptor can remodel membranes when bound to Arf1
Acknowledgments
The authors are supported by NIH/NIGMS grant R01GM098621.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 3.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 4.Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325:1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 7.Miller EA, Barlowe C. Regulation of coat assembly--sorting things out at the ER. Curr Opin Cell Biol. 2010;22:447–453. doi: 10.1016/j.ceb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly BT, Owen DJ. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol. 2011;23:404–412. doi: 10.1016/j.ceb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Bonifacino JS. Adaptor proteins involved in polarized sorting. J Cell Biol. 2014;204:7–17. doi: 10.1083/jcb.201310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson LP. Structure and mechanism of COPI vesicle biogenesis. Curr Opin Cell Biol. 2014;29:67–73. doi: 10.1016/j.ceb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Hughson FM, Reinisch KM. Structure and mechanism in membrane trafficking. Curr Opin Cell Biol. 2010;22:454–460. doi: 10.1016/j.ceb.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson LP, Kummel D, Reinisch KM, Owen DJ. Structures and mechanisms of vesicle coat components and multisubunit tethering complexes. Curr Opin Cell Biol. 2012;24:475–483. doi: 10.1016/j.ceb.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato Y, Misra S, Puertollano R, Hurley JH, Bonifacino JS. Phosphoregulation of sorting signal-VHS domain interactions by a direct electrostatic mechanism. Nat Struct Biol. 2002;9:532–536. doi: 10.1038/nsb807. [DOI] [PubMed] [Google Scholar]
- 15.Misra S, Puertollano R, Kato Y, Bonifacino JS, Hurley JH. Structural basis for acidic-cluster-dileucine sorting-signal recognition by VHS domains. Nature. 2002;415:933–937. doi: 10.1038/415933a. [DOI] [PubMed] [Google Scholar]
- 16.Shiba T, Takatsu H, Nogi T, Matsugaki N, Kawasaki M, Igarashi N, Suzuki M, Kato R, Earnest T, Nakayama K, Wakatsuki S. Structural basis for recognition of acidic-cluster dileucine sequence by GGA1. Nature. 2002;415:937–941. doi: 10.1038/415937a. [DOI] [PubMed] [Google Scholar]
- 17.He X, Zhu G, Koelsch G, Rodgers KK, Zhang XC, Tang J. Biochemical and structural characterization of the interaction of memapsin 2 (beta-secretase) cytosolic domain with the VHS domain of GGA proteins. Biochemistry. 2003;42:12174–12180. doi: 10.1021/bi035199h. [DOI] [PubMed] [Google Scholar]
- 18.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 19.Shiba T, Kametaka S, Kawasaki M, Shibata M, Waguri S, Uchiyama Y, Wakatsuki S. Insights into the phosphoregulation of beta-secretase sorting signal by the VHS domain of GGA1. Traffic. 2004;5:437–448. doi: 10.1111/j.1600-0854.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki M, Shiba T, Shiba Y, Yamaguchi Y, Matsugaki N, Igarashi N, Suzuki M, Kato R, Kato K, Nakayama K, Wakatsuki S. Molecular mechanism of ubiquitin recognition by GGA3 GAT domain. Genes Cells. 2005;10:639–654. doi: 10.1111/j.1365-2443.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- 21.Prag G, Lee S, Mattera R, Arighi CN, Beach BM, Bonifacino JS, Hurley JH. Structural mechanism for ubiquitinated-cargo recognition by the Golgi-localized, gamma-ear-containing, ADP-ribosylation-factor-binding proteins. Proc Natl Acad Sci USA. 2005;102:2334–2339. doi: 10.1073/pnas.0500118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royle SJ, Qureshi OS, Bobanovic LK, Evans PR, Owen DJ, Murrell-Lagnado RD. Non-canonical YXXGPhi endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci. 2005;118:3073–3080. doi: 10.1242/jcs.02451. [DOI] [PubMed] [Google Scholar]
- 23.Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Mancias JD, Goldberg J. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol Cell. 2007;26:403–414. doi: 10.1016/j.molcel.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS. Sorting of the Alzheimer’s disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell. 2010;18:425–436. doi: 10.1016/j.devcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cramer JF, Gustafsen C, Behrens MA, Oliveira CL, Pedersen JS, Madsen P, Petersen CM, Thirup SS. GGA autoinhibition revisited. Traffic. 2010;11:259–273. doi: 10.1111/j.1600-0854.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 29.Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Honing S, Evans PR, Owen DJ. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010;141:1220–1229. doi: 10.1016/j.cell.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu A, Xing Y, Harrison SC, Kirchhausen T. Structural analysis of the interaction between Dishevelled2 and clathrin AP-2 adaptor, a critical step in noncanonical Wnt signaling. Structure. 2010;18:1311–1320. doi: 10.1016/j.str.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia X, Singh R, Homann S, Yang H, Guatelli J, Xiong Y. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol. 2012;19:701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson LP, Lewis M, Kent HM, Edeling MA, Evans PR, Duden R, Owen DJ. Molecular basis for recognition of dilysine trafficking motifs by COPI. Dev Cell. 2013;23:1255–1262. doi: 10.1016/j.devcel.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma W, Goldberg J. Rules for the recognition of dilysine retrieval motifs by coatomer. EMBO J. 2013;32:926–937. doi: 10.1038/emboj.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallon M, Clairfeuille T, Steinberg F, Mas C, Ghai R, Sessions RB, Teasdale RD, Collins BM, Cullen PJ. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc Natl Acad Sci USA. 2014;111:E3604–3613. doi: 10.1073/pnas.1410552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren X, Park SY, Bonifacino JS, Hurley JH. How HIV-1 Nef hijacks the AP-2 clathrin adaptor to downregulate CD4. Elife. 2014;3:e01754. doi: 10.7554/eLife.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross BH, Lin Y, Corales EA, Burgos PV, Mardones GA. Structural and functional characterization of cargo-binding sites on the mu4-subunit of adaptor protein complex 4. PLoS One. 2014;9:e88147. doi: 10.1371/journal.pone.0088147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiskoff AM, Fromme JC. Distinct N-terminal regions of the exomer secretory vesicle cargo Chs3 regulate its trafficking itinerary. Front Cell Dev Biol. 2014;2:47. doi: 10.3389/fcell.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fromme JC, Orci L, Schekman R. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 2008;18:330–336. doi: 10.1016/j.tcb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Jensen D, Schekman R. COPII-mediated vesicle formation at a glance. J Cell Sci. 2011;124:1–4. doi: 10.1242/jcs.069773. [DOI] [PubMed] [Google Scholar]
- 40.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 41.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23-Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- 43.Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 44.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/j.cell.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 45.Stagg SM, LaPointe P, Razvi A, Gurkan C, Potter CS, Carragher B, Balch WE. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharya N, JOD, Stagg SM. The structure of the Sec13/31 COPII cage bound to Sec23. J Mol Biol. 2012;420:324–334. doi: 10.1016/j.jmb.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noble AJ, Zhang Q, O’Donnell J, Hariri H, Bhattacharya N, Marshall AG, Stagg SM. A pseudoatomic model of the COPII cage obtained from cryo-electron microscopy and mass spectrometry. Nat Struct Mol Biol. 2013;20:167–173. doi: 10.1038/nsmb.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanetti G, Prinz S, Daum S, Meister A, Schekman R, Bacia K, Briggs JA. The structure of the COPII transport-vesicle coat assembled on membranes. Elife. 2013;2:e00951. doi: 10.7554/eLife.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Donnell J, Maddox K, Stagg S. The structure of a COPII tubule. J Struct Biol. 2010;173:358–364. doi: 10.1016/j.jsb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Edeling MA, Smith C, Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- 51.McNiven MA, Thompson HM. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/science.1118133. [DOI] [PubMed] [Google Scholar]
- 52.Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 53.ter Haar E, Harrison SC, Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc Natl Acad Sci USA. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- 55.Kelly BT, Graham SC, Liska N, Dannhauser PN, Honing S, Ungewickell EJ, Owen DJ. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 2014;345:459–463. doi: 10.1126/science.1254836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- 57.Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 58.Schledzewski K, Brinkmann H, Mendel RR. Phylogenetic analysis of components of the eukaryotic vesicle transport system reveals a common origin of adaptor protein complexes 1, 2, and 3 and the F subcomplex of the coatomer COPI. J Mol Evol. 1999;48:770–778. doi: 10.1007/pl00006521. [DOI] [PubMed] [Google Scholar]
- 59.Hara-Kuge S, Kuge O, Orci L, Amherdt M, Ravazzola M, Wieland FT, Rothman JE. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles. J Cell Biol. 1994;124:883–892. doi: 10.1083/jcb.124.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson LP. Structure and mechanism of COPI vesicle biogenesis. Curr Opin Cell Biol. 2014;29:67–73. doi: 10.1016/j.ceb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Yu X, Breitman M, Goldberg J. A structure-based mechanism for Arf1-dependent recruitment of coatomer to membranes. Cell. 2012;148:530–542. doi: 10.1016/j.cell.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan AR, Menetrey J. Structural biology of Arf and Rab GTPases’ effector recruitment and specificity. Structure. 2013;21:1284–1297. doi: 10.1016/j.str.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Cherfils J. Arf GTPases and their effectors: assembling multivalent membrane-binding platforms. Curr Opin Struct Biol. 2014;29C:67–76. doi: 10.1016/j.sbi.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Beck R, Prinz S, Diestelkotter-Bachert P, Rohling S, Adolf F, Hoehner K, Welsch S, Ronchi P, Brugger B, Briggs JA, Wieland F. Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J Cell Biol. 2011;194:765–777. doi: 10.1083/jcb.201011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldberg J. Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell. 1999;96:893–902. doi: 10.1016/s0092-8674(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 66.Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 67.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 69.Lee I, Doray B, Govero J, Kornfeld S. Binding of cargo sorting signals to AP-1 enhances its association with ADP ribosylation factor 1-GTP. J Cell Biol. 2008;180:467–472. doi: 10.1083/jcb.200709037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci USA. 2004;101:14108–14113. doi: 10.1073/pnas.0406102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren X, Farias GG, Canagarajah BJ, Bonifacino JS, Hurley JH. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell. 2013;152:755–767. doi: 10.1016/j.cell.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Canagarajah BJ, Ren X, Bonifacino JS, Hurley JH. The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci. 2013;22:517–529. doi: 10.1002/pro.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 74.Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ziman M, Chuang JS, Tsung M, Hamamoto S, Schekman R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1565–1576. doi: 10.1091/mbc.9.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchatjate S, Schekman R. Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface. Mol Biol Cell. 2006;17:4157–4166. doi: 10.1091/mbc.E06-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trautwein M, Schindler C, Gauss R, Dengjel J, Hartmann E, Spang A. Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi. EMBO J. 2006;25:943–954. doi: 10.1038/sj.emboj.7601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang CW, Hamamoto S, Orci L, Schekman R. Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barfield RM, Fromme JC, Schekman R. The exomer coat complex transports Fus1p to the plasma membrane via a novel plasma membrane sorting signal in yeast. Mol Biol Cell. 2009;20:4985–4996. doi: 10.1091/mbc.E09-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zanolari B, Rockenbauch U, Trautwein M, Clay L, Barral Y, Spang A. Transport to the plasma membrane is regulated differently early and late in the cell cycle in Saccharomyces cerevisiae. J Cell Sci. 2011;124:1055–1066. doi: 10.1242/jcs.072371. [DOI] [PubMed] [Google Scholar]
- 81.Rockenbauch U, Ritz AM, Sacristan C, Roncero C, Spang A. The complex interactions of Chs5p, the ChAPs, and the cargo Chs3p. Mol Biol Cell. 2012;23:4402–4415. doi: 10.1091/mbc.E11-12-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Starr TL, Pagant S, Wang CW, Schekman R. Sorting signals that mediate traffic of chitin synthase III between the TGN/endosomes and to the plasma membrane in yeast. PLoS One. 2012;7:e46386. doi: 10.1371/journal.pone.0046386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sacristan C, Manzano-Lopez J, Reyes A, Spang A, Muniz M, Roncero C. Oligomerization of the chitin synthase Chs3 is monitored at the Golgi and affects its endocytic recycling. Mol Microbiol. 2013;90:252–266. doi: 10.1111/mmi.12360. [DOI] [PubMed] [Google Scholar]
- 84.Ritz AM, Trautwein M, Grassinger F, Spang A. The prion-like domain in the exomer-dependent cargo Pin2 serves as a trans-Golgi retention motif. Cell reports. 2014;7:249–260. doi: 10.1016/j.celrep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Paczkowski JE, Richardson BC, Strassner AM, Fromme JC. The exomer cargo adaptor structure reveals a novel GTPase-binding domain. EMBO J. 2012;31:4191–4203. doi: 10.1038/emboj.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richardson BC, Fromme JC. The exomer cargo adaptor features a flexible hinge domain. Structure. 2013;21:486–492. doi: 10.1016/j.str.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Owen DJ, Vallis Y, Noble ME, Hunter JB, Dafforn TR, Evans PR, McMahon HT. A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- 88.Paczkowski JE, Fromme JC. Structural basis for membrane binding and remodeling by the exomer secretory vesicle cargo adaptor. Dev Cell. 2014;30:610–624. doi: 10.1016/j.devcel.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mourao A, Nager AR, Nachury MV, Lorentzen E. Structural basis for membrane targeting of the BBSome by ARL6. Nat Struct Mol Biol. 2014;21:1035–1041. doi: 10.1038/nsmb.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Kahn RA, Prestegard JH. Dynamic structure of membrane-anchored Arf*GTP. Nat Struct Mol Biol. 2010;17:876–881. doi: 10.1038/nsmb.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmid SL, Frolov VA. Dynamin: functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- 92.Boucrot E, Pick A, Camdere G, Liska N, Evergren E, McMahon HT, Kozlov MM. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell. 2012;149:124–136. doi: 10.1016/j.cell.2012.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 94.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 95.Chappie JS, Dyda F. Building a fission machine--structural insights into dynamin assembly and activation. J Cell Sci. 2013;126:2773–2784. doi: 10.1242/jcs.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 97.Daumke O, Roux A, Haucke V. BAR domain scaffolds in dynamin-mediated membrane fission. Cell. 2014;156:882–892. doi: 10.1016/j.cell.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 98.Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 100.Pinot M, Vanni S, Pagnotta S, Lacas-Gervais S, Payet LA, Ferreira T, Gautier R, Goud B, Antonny B, Barelli H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science. 2014;345:693–697. doi: 10.1126/science.1255288. [DOI] [PubMed] [Google Scholar]
- 101.Stachowiak JC, Brodsky FM, Miller EA. A cost-benefit analysis of the physical mechanisms of membrane curvature. Nat Cell Biol. 15:1019–1027. doi: 10.1038/ncb2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Campelo F, Malhotra V. Membrane fission: the biogenesis of transport carriers. Annu Rev Biochem. 2012;81:407–427. doi: 10.1146/annurev-biochem-051710-094912. [DOI] [PubMed] [Google Scholar]
- 104.Meinecke M, Boucrot E, Camdere G, Hon WC, Mittal R, McMahon HT. Cooperative recruitment of dynamin and BIN/amphiphysin/Rvs (BAR) domain-containing proteins leads to GTP-dependent membrane scission. J Biol Chem. 2013;288:6651–6661. doi: 10.1074/jbc.M112.444869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krauss M, Jia JY, Roux A, Beck R, Wieland FT, De Camilli P, Haucke V. Arf1-GTP-induced tubule formation suggests a function of Arf family proteins in curvature acquisition at sites of vesicle budding. J Biol Chem. 2008;283:27717–27723. doi: 10.1074/jbc.M804528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lundmark R, Doherty GJ, Vallis Y, Peter BJ, McMahon HT. Arf family GTP loading is activated by, and generates, positive membrane curvature. Biochem J. 2008;414:189–194. doi: 10.1042/BJ20081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 108.Hariri H, Bhattacharya N, Johnson K, Noble AJ, Stagg SM. Insights into the Mechanisms of Membrane Curvature and Vesicle Scission by the Small GTPase Sar1 in the Early Secretory Pathway. J Mol Biol. 2014;426:3811–3826. doi: 10.1016/j.jmb.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Inoue M, Shiba T, Ihara K, Yamada Y, Hirano S, Kamikubo H, Kataoka M, Kawasaki M, Kato R, Nakayama K, Wakatsuki S. Molecular basis for autoregulatory interaction between GAE domain and hinge region of GGA1. Traffic. 2007;8:904–913. doi: 10.1111/j.1600-0854.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 110.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo Y, Zanetti G, Schekman R. A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife. 2013;2:e00160. doi: 10.7554/eLife.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faini M, Prinz S, Beck R, Schorb M, Riches JD, Bacia K, Brugger B, Wieland FT, Briggs JA. The structures of COPI-coated vesicles reveal alternate coatomer conformations and interactions. Science. 2012;336:1451–1454. doi: 10.1126/science.1221443. [DOI] [PubMed] [Google Scholar]