Abstract
Background
The hypothalamus plays a key role in mediating the effects of estrogen on many physiological functions, including reproduction, metabolism, and thermoregulation. We have previously observed marked estrogen-dependent gene expression changes within the hypothalamus of rhesus macaques during aging, especially in the KNDy neurons of the arcuate-median eminence (ARC-ME) that produce kisspeptin, neurokinin B, and dynorphin A. Little is known, however, about the mechanisms involved in mediating the feedback from estrogen onto these neurons.
Methods
We used real-time PCR to profile age and estrogen-dependent gene expression changes in the rhesus macaque hypothalamus. Our focus was on genes that encode steroid receptors (ESR1, ESR2, PGR, and AR), and on enzymes that contribute to the local synthesis of 17β-estradiol (E2) (STS, HSD3B1/2, HSD17B5, and CYP19A). In addition, we used RT2 Profiler™ PCR Arrays to profile a larger set of genes that are integral to hypothalamic function.
Results
KISS1, KISS1R, TAC3, NPY2R mRNA levels increased in surgically menopausal (ovariectomized) old females, relative to age-matched ovariectomized animals that received E2 hormone therapy. In contrast, PGR, HSD17B, GNRH2, SLC6A3, KISS-1, TAC3, and NPY2R mRNA levels increased after E2 supplementation.
Conclusion
The rhesus macaque ARC-ME expresses many genes that are responsive to changes in circulating estrogen levels, even during old age, and these may contribute to the cause of normal and pathophysiological changes that occur during menopause.
Keywords: 17β-estradiol, Estrogen receptor-α, Estrogen receptor-β, Progestin receptor, Androgen receptor, Aging, Menopause, Hypothalamus
Introduction
Menopause in women and female rhesus macaques is associated with a marked decline in the production and secretion of 17β-estradiol (E2) from the ovaries [1–3]. This attenuation of circulating estrogen levels adversely impacts many physiological processes, and is thought to play a major role in the etiology of age-related pathologies, such as reproductive quiescence, hot flashes and disrupted sleep-wake cycles [4,5]. Little is known, however, about the mechanisms involved in mediating the feedback from E2 onto neurons in the hypothalamus.
Evidence from human and nonhuman primate studies suggests that loss of circulating estrogen can affect the cyto-architecture of the arcuate region of the hypothalamus and alter the pattern of gene expression [6–11]. For example, using in situ hybridization Rance et al. [6] demonstrated a significant increase in the size of estrogen receptor (ER) expressing neurons of post-menopausal compared to pre-menopausal women. ERα expressing neurons in the arcuate-median eminence (ARC-ME) colocalize with kisspeptin (KISS1), neurokinin B (NKB), and dynorphin A (DYN) (referred to as KNDy) and exert a major influence on the neuroendocrine reproductive axis by modulating the secretion of gonadotropin-releasing hormone (GnRH). Moreover, KNDy neurons show marked changes in their pattern of gene expression after menopause or ovariectomy, and these changes can be blocked by exposure to exogenous sex-steroids [12–17]. Consequently, it is plausible that the influence of sex-steroids on GnRH neuronal function is mediated by ERα, ERβ, and the progestin receptor (PR), associated with the KNDy-neural systems [18–25]. Furthermore, the expression of these receptors may change as animals show the characteristic menopausal decrease in circulating sex-steroid levels, and may represent a mechanism by which the hypothalamus undergoes a compensatory increase in its sensitivity to sex-steroids. Another possible menopause-associated compensatory mechanism could involve increased expression of enzymes involved in local intracrine synthesis of sex steroids from precursor steroids such as dehydroepiandrosterone (DHEA) [26–28]. The circulating levels of DHEA and DHEA sulfate (DHEAS) are especially pronounced in adult humans and nonhuman primates, and there is evidence that all of the key enzymes involved in DHEA to E2 conversion are expressed in the primate hypothalamus [29]. This suggests that local synthesis of sex steroids may also be contributing to the hormone milieu of the hypothalamus.
The aim of the present study was to help resolve these issues by examining the effect of age and E2 treatment on gene expression in the ARC-ME of female rhesus macaques. These nonhuman primates show similar age-related hormonal changes as women, though late in their lifespan [2, 30, 31], and so they represent a pragmatic translational animal model in which to study the neuroendocrine mechanisms that contribute to healthy human aging. Our primary focus was on genes that encode the main sex-steroid receptors [32–37]: ERα (encoded by ESR1), ERβ (encoded by ESR2), PR (encoded by PGR), as well as the androgen receptor (encoded by AR). In addition, we examined the expression of genes that play a key role in the intracrine conversion of DHEA to testosterone and E2: steroid sulfatase (encoded by STS); 17β-hydroxysteroid dehydrogenase type 5 (encoded by HSD17B5), 3β-hydroxysteroid dehydrogenase types 1 and 2 (encoded by HSD3B1/2), and aromatase (encoded by CYP19A1). Our second goal was to profile the expression of a larger set of genes that contribute to the control of hypothalamic-mediated functions, such as reproduction and metabolism. Our prediction was that the expression of hypothalamic genes would increase during aging, ensuring that the hypothalamus remains responsive to sex steroids even when the levels of these hormones in the circulation are attenuated.
Materials and Methods
Animals
Adult female rhesus macaques (Macaca mulatta) were cared for by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC), in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, and used in an Institutional Animal Care and Use Committee-approved study. The animals were housed indoors under controlled environmental conditions: 24 C temperature; 12-h light, 12-h dark photoperiods (lights on at 0700 h); regular meals at 0800 and 1500 h (Purina High Protein Monkey Chow; Purina Mills, Inc., St. Louis, MO) supplemented with fresh fruit and vegetables, and fresh drinking water available ad libitum.
Design of experiments
1. Effect of aging
Experiment 1 used real-time PCR to compare mRNA levels between young (~13 years) and old (~26 years) ovary-intact animals.
2. Effect of estradiol (E2)
Experiment 2 used real-time PCR to compare mRNA levels between young (~10 years) bilaterally ovariectomized (OVX) animals and young (~10 years) OVX animals that had been treated with E2 for ~1 month. This experiment also compared mRNA levels between old OVX controls and old OVX animals that were treated with E2 for ~4 years. The two older groups of animals were ~21 and ~22 years of age at the onset of the study, respectively.
3. Effect of menopause and hormone therapy (HT)
Experiment 3 used custom-made RT2 Profiler™ PCR Arrays to compare gene expression in the ARC-ME of young animals (~12 years), with that of old surgically menopausal animals (old OVX) from Experiment 2, and also with the long-term estradiol-treated animals (old OVX + E2).
Menstrual cycle status and serum hormone concentrations
Experiment 1
Serum levels of E2 were 36.5 ± 6.3 pg/ml in the young gonad-intact animals and 62 ± 16.5 pg/ml in the old gonad-intact animals. All but one of these animals had progesterone (P4) levels below the assay detection limit. One young animal showed elevated P4 concentrations (2.4 ng/ml) and was assumed to still be the luteal phase of the menstrual cycle. Based on individual animal menstruation records, all four young intact animals were normal cyclers and the old intact animals consisted of two cycling animals, an irregular cycler, and one post-menopausal animal.
Experiment 2
One month following OVX half of the animals in the young and old OVX groups were implanted subcutaneously with empty Silastic capsules (Dow Corning, Midland, MI), while the other half received capsules containing crystalline E2 (Steroloids, Wilton, NH), as previously described [38]; these were designed to last up to 1 year and to maintain circulating E2 levels between 100–200 pg/ml, which is similar to what is observed during the late follicular phase of the menstrual cycle. In the old OVX + E2 group the E2 capsules were replaced annually, to ensure sustained long-term delivery of the steroid for the entire duration of the study (~4 years); the untreated OVX animals maintained the same capsules throughout. Terminal serum E2 levels were undetectable in the young untreated OVX animals and had a mean level of 117 ± 7.0 pg/ml in the young OVX + E2 group. Serum E2 concentrations were measured at various times across the four-year experiment in the old OVX animals to confirm that the target hormone concentrations were being maintained. Serum E2 levels in the old untreated OVX group were < 30 pg/ml at all times, and often fell below the limit of assay sensitivity. In the old OVX + E2 animals, the mean E2 concentration during the final two years of the study was 118.8 ± 15.9 pg/ml and at the time of tissue collection was 94.3 ± 20.5 pg/ml.
Experiment 3
At the time of postmortem tissue collection, the young intact animals had mean serum E2 levels of 58.9 ± 24.5 pg/ml and very low serum P4 levels < 0.3 ng/ml. These hormone levels are indicative of the follicular phase of the menstrual cycle. In one of these animals, however, the E2 levels were considerably higher (128 pg/ml), suggesting that the animal was most likely in the late follicular phase. Note that the surgically menopausal animals used in Experiment 3, were the same old OVX and old OVX + E2 animals from Experiment 2.
The E2 and P4 assays were performed by the ONPRC Endocrine Technology and Support Core using a chemilluminescence-based automatic clinical platform (Immulite 2000; Siemens Healthcare Diagnostics, Deerfield, IL). The sensitivity limits of these assays were 20 pg/ml and 0.2 ng/ml for E2 and P4, respectively [39], and the intra-assay and inter-assay coefficients of variation were all less than 15%.
RNA isolation
All of the animals had previously been involved in various cross-sectional aging studies; their hypothalami and terminal blood serum became available for post-mortem analysis through the ONPRC Tissue Distribution Program. At the time of necropsy the mean (± sem) age of the gonad-intact young animals from Experiment 1 was 12.8 ± 2.3 yr (n = 4) and the age of the old animals was 25.8 ± 0.4 yr (n = 4). The animals from Experiment 2 were: young OVX (9.7 ± 0.3 yr; n = 4), young OVX ± E2 (9.6 ± 0.8 yr; n = 4), old OVX (25.0 ± 1.7 yr; n = 4), and old OVX + E2 (27.0 ± 0.9 yr; n = 4). The age of the young animals from Experiment 3 was 12.3 ± 0.9 yr (n = 4).
After sedation with ketamine (15–25 mg/kg i.m.) and pentobarbital sodium (25–30 mg/kg i.v.), a procedure consistent with the recommendations of the American Veterinary Medical Association’s Panel on Euthanasia, each brain was flushed with 1 L of 0.9% saline via a vascular catheter, and the hypothalamus was removed and preserved for ~2 weeks in RNAlater (Ambion, Austin, TX). A coronal slice encompassing the ARC-ME was dissected and stored frozen at −80 C. The boundaries for this tissue block included the exterior ventral edge of the ME, lateral cuts midway between the third ventricle and the optic nerve, an anterior cut along the posterior edge of the optic chiasma, a posterior cut just anterior to the mammillary bodies, and a cut 1 mm dorsal to the base of the third ventricle (i.e., based on stereotaxic coordinates, this represents the border between the ARC and the ventromedial hypothalamus).
Subsequently, each ARC-ME block was individually homogenized using a PowerGen rotor-stator homogenizer (Fisher Scientific, Pittsburgh, PA), and RNA was extracted using a QIAGEN RNeasy Mini Kit (Valencia, CA). An Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) was used to determine the quality of the RNA and a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA) was used to determine the concentration. For each sample, 1 μg of RNA was then converted to cDNA using random hexamers and the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen Life Technologies, Carlsbad, CA).
Gene expression profiling
The ARC-ME expression levels of genes encoding nuclear sex-steroid receptors ESR1, ESR2, PGR, and AR were determined using quantitative real-time PCR (qRT-PCR). Similarly, the ARC-ME expression levels of genes encoding the following steroidogenic enzymes were also examined: STS, HSD3B1/2, HSD17B5 and CYP19A1. Finally, RT2 Profiler™ PCR Arrays (SABiosciences) were used to profile a more extensive set of genes in the ARC-ME during aging and after treatment with E2.
Primers and probes
Table 1 depicts nucleotide sequences of the primers and probes that were used for detection of the steroid receptor genes (ESR1, ESR2, PGR, and AR), steroidogenic enzyme genes (STS, HSD3B1/2, HSD17B5, and CYP19A1), and also for two housekeeping genes (ALG9 and RPL13A) that we previously showed to be stably expressed in the rhesus ARC-ME across various sex-steroid environments [17, 40]. The nucleotide sequences for these genes were designed using Primer Express 2.0 software [Applied Biosystems (ABI), Foster City, CA] and were based on NCBI rhesus macaque reference sequences. The primers and probes were purchased from Invitrogen Life Technologies (Carlsbad, CA) and ABI (Foster City, CA), respectively. Reverse transcriptase-PCR (RT-PCR), using ARC-ME and ovarian RNA samples (as positive controls), was used to validate each of the primer sets by producing amplicons of a predicted size.
Table 1.
TaqMan, real-time polymerase chain reaction (PCR) primer and probe nucleotide sequences (5′ – 3′)
| Target Genes | Primer Sequences | Probe Sequences | GenBank Accession ID |
|---|---|---|---|
| ESR1 | F- ACAAGATCACAGACACTTTGATCCA R- CCTGATGTGGGAGAGGATGAG |
AGGCCTGACCCTGCAG | XM_002803858 |
| ESR2 | F- GATCGCTAGAACACACCTTACCTGTA R- GGTGCAACGGTTCCCACTAA |
AGAGACATTGAAAAGGAAG | NM_001265821 |
| PGR | F- CATGTCAGTGGGCAGATGCT R- TGCCACATGGTAAGGCATAATG |
CTGAATGAACAGCGGATG | NM_001278456 |
| AR | F- AAAATCCCACATCCTGCTCAA R- CAGGTCAAAAGTGAACTGATGCA |
TGCAGCCTATTGCGAGAG | NM_1032911 |
| STS | F- CCTTCCTCCGGCCTGTCT R- AGCTTTGCCACATGCATCTG |
CCAGTGCGACAGAGAAAAACAGGATAAGAGA | XM_001088752 |
| HSD3B1/2 | F- AGGACGTCTCGGTCGTCATC R- GAGCTGGGTACCTTTCACATTGA |
CATTGATGTCTTTGGTGTCACTCA | XM_001113873 |
| HSD17B5 | F- TGGAGGGCTTTGCTGAAGTCT R- GGTCCAGTCACCAGCATACAGA |
CAGAAGCCGTGCGTGTGGATGG | XM_001104543 |
| CYP19A1 | F- TAGCAGAAAAAAGACGCAGGATT R- CGTCAGGTCACCTCGTTTCTC |
AATGCATGGACTTTGCCACTGAGTTGATTTT | XM_001082665 |
| ALG9 | F- AACAGTGCCACAGAGCGAGAA R- CGATACCGCCTGGAGCACTA |
ACTGTCTTCCTGTTCGGG | XM_001106180 |
| RPL13A | F- TCACGAGGTTGGCTGGAAGT R- GATCTTGGCTTTCTCCTTCCTCTT |
CCAGGCAGTGACAGCCACCTTGG | XM_001115079 |
Reference sequences for each of the target genes can be accessed through GenBank accession ID.
TaqMan qRT-PCR
A 7900HT Fast Real-Time PCR thermal cycler and sequence detection system software (version 2.2.1; ABI, Foster City, CA) was used to obtain qRT-PCR data. Initially, pooled cDNA was used to create standard curves for each gene, and the experimental samples were subsequently diluted accordingly, so as to fall within the linear part of the curve. The PCR mixtures contained 5 μl TaqMan Universal PCR Master Mix, 0.3 μl of each specific forward and reverse primer (300 nm final concentration), 0.25 μl of specific probe (250 nm final concentration), and 2 μl of cDNA. The reaction sequence included 2 min at 50 C, 10 min at 95 C, and 50 cycles of 15 sec at 95 C and 1 min at 60 C. Automatic baseline and threshold levels were determined by ABI sequence detection system software (version 2.2.1.), and the final expression values are normalized to the arithmetic mean of two reference housekeeping genes, ALG9 and RPL13A. Individual genes from Experiments 1 and 2 were examined together on the same 384-well optical plate. A negative control included the omission of cDNA templates from the reaction mixture.
RT2 Profiler™ PCR Array
Total RNA samples (0.5 μg) were reverse transcribed using the RT2 First Strand Kit (Qiagen, Valencia, CA). Each RT- PCR reaction was performed in 25 μl of solution containing cDNA, 2 x RT2 SYBR Green Mastermix, and RNase-free water, using custom-made RT2 Profiler™ PCR Arrays Custom PCR Arrays (Qiagen, Valencia, CA) and a QuantStudio™ 12K Flex thermocycler (Life Technologies, Grand Island, NY). The RT-PCR reaction sequence included 10 min incubation at 95 C followed by 40 cycles of 15 sec at 95 C, 1 min at 60 C, 1 min at 60 C, and 15 sec at 95 C. The relative gene expression was calculated using the ΔΔCT method, and the results are expressed with reference to the arithmetic mean of three reference housekeeping genes (ALG9, GAPDH, and RPL13A).
Statistical analysis
Paired Student’s t-tests were used to compare difference in ESR1, ESR2, PGR, AR, STS, HSD3B1/2, HSD17B5, and CYP19A1 expression between young and old gonad-intact animals (Experiment 1), and between OVX and OVX+E2 animals (Experiment 2). ANOVA followed by the Dunnett multiple-range test was used to assess between-group difference in the expression for all other analyses (Experiment 3). Significance was considered at P < 0.05.
Result
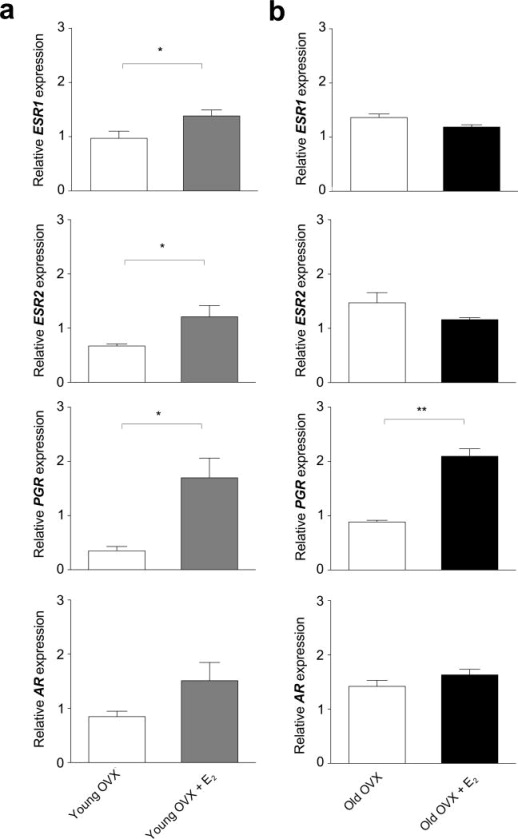
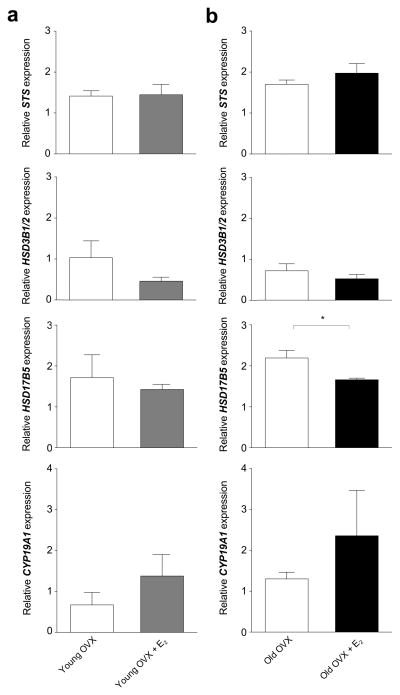
The ovary-intact animals showed no detectable effect of age on the expression of genes encoding steroid receptors or steroidogenic enzymes in the ARC-ME (Table 2). In both young and old OVX animals, however, PGR expression was significantly enhanced by E2 supplementation (Fig. 1). Similarly, ESR1 and ESR2 expression was enhanced by E2 supplementation in the young (Fig. 1a) but not in the old (Fig. 1b) animals. In the ovariectomized animals, there was no detectable effect of E2 supplementation on the expression of AR (Fig. 1), or on genes encoding steroidogenic enzymes (Fig. 2), except for a significant (P < 0.05) decrease in HSD17B5 expression in the old OVX + E2 animals relative to the untreated age-matched controls.
Table 2.
Effect of Age on Gene Expression in the ARC-ME of Female Rhesus Macaques
| Gene Symbol | Young Intact (n = 4) | Old Intact (n = 4) |
|---|---|---|
| ESR1 | 0.81 ± 0.25 | 1.32 ± 0.17 |
| ESR2 | 0.86 ± 0.10 | 1.32 ± 0.20 |
| PGR | 0.44 ± 0.12 | 1.25 ± 0.43 |
| AR | 0.89 ± 0.17 | 1.48 ± 0.12 |
| STS | 1.39 ± 0.27 | 1.96 ± 0.26 |
| HSD3B1/2 | 0.35 ± 0.15 | 0.53 ± 0.16 |
| HSD17B5 | 1.28 ± 0.26 | 1.87 ± 0.20 |
| CYP19A1 | 0.58 ± 0.36 | 1.57 ± 0.62 |
Values represent mean (± sem) mRNA levels, relative to the arithmetic mean of ALG9 and RPL13A.
No significant differences were detected between the two age groups (Student’s t -test, P >0.05).
Fig. 1.
Effect of estradiol (E2) treatment on steroid receptor gene expression in the ARC-ME of ovariectomized (OVX) rhesus macaques. The animals either served as controls or were treated with E2 for ~ 1 month (young animals) or for ~ 4 years (old animals). Each bar represents the mean (± sem) mRNA levels of four animals, normalized to the arithmetic mean of two housekeeping genes, ALG9 and RPL13A. In the young animals (a) ESR1, ESR2, and PGR mRNA levels were significantly higher in the OVX+E2 group than in the OVX group, whereas AR mRNA levels were not significantly different. In the old animals (b), only PGR, mRNA levels were significantly higher in the OVX+E2 relative to OVX group. * P < 0.05, ** P < 0.01 (Paired Student’s t-test).
Fig. 2.
Effect of estradiol (E2) treatment on on steroidogenic enzymes gene expression in the ARC-ME of ovariectomized (OVX) rhesus macaques. Each bar represents the mean (± sem) mRNA levels of four animals, normalized to the arithmetic mean of two housekeeping genes, ALG9 and RPL13A. In the young animals (a) there was no significant effect of E2 on any of the mRNA levels examined. In the old animals (b), HSD17B5 mRNA levels were significantly higher in the OVX + E2 relative to OVX group. * P < 0.05, (Paired Student’s t-test).
To corroborate and extended the findings from the real-time PCR experiments we also performed gene expression analysis on RNA extracts obtained from young gonad-intact, old OVX, and old OVX + E2 animals, using custom-made RT2 Profiler™ PCR arrays. These three groups were selected to provide insights into ARC-ME gene expression during sexually maturity, after menopause, and after subsequent hormone therapy (HT). Our focus was on genes that encode steroid receptors, as well as genes associated with the control of reproductive system, metabolism, and neurotransmission (Table 3). As expected, array analysis revealed a significant decrease (P < 0.01) in PGR expression in old OVX animals and showed that this decrease could be blocked by long-term HT. The expression of GNRH2 and SLC6A3 was also significantly enhanced by E2 (P < 0.05 and P < 0.01, respectively). In contrast, a significant increase in expression levels was observed for several genes associated with the KNDy neuronal system or metabolism (KISS1, P < 0.01; KISS1R, P < 0.05; TAC3, P < 0.01; NPY2R, P < 0.05), and in most cases the increase was reversed by E2.
Table 3.
Gene Expression in the Arcuate-Median Eminence of Rheus Macaques During Reproductive Aging
| Gene Symbol | Gene Name | Young Intact | Old OVX | Old OVX + E2 |
|---|---|---|---|---|
| Sex-steroid Receptors | ||||
| AR | androgen receptor | 1.00 ± 0.13 | 0.91 ± 0.10 | 1.02 ± 0.07 |
| ESR1 | estrogen receptor 1 | 1.00 ± 0.11 | 1.19 ± 0.06 | 0.90 ± 0.07 |
| ESR2 | estrogen receptor 2 | 1.00 ± 0.18 | 1.22 ± 0.13 | 1.08 ± 0.14 |
| PGR | progesterone receptor | 1.00 ± 0.10 ** | 0.33 ± 0.13 ** | 1.82 ± 0.14 |
| Reproduction | ||||
| GNRH1 | gonadotropin-releasing hormone 1 | 1.00 ± 0.17 | 1.09 ± 0.33 | 0.89 ± 0.20 |
| GNRH2 | gonadotropin-releasing hormone 2 | 1.00 ± 0.15 | 0.59 ± 0.07 * | 3.31 ± 1.01 |
| KISS1 | KiSS-1 metastasis-suppressor | 1.00 ± 0.32 ** | 11.72 ± 2.20 ** | 0.25 ± 0.08 |
| KISS1R | KISS1 receptor | 1.00 ± 0.11 * | 1.99 ± 0.15 | 2.18 ± 0.38 |
| PDYN | prodynorphin | 1.00 ± 0.15 | 0.89 ± 0.06 | 1.19 ± 0.07 |
| TAC3 | tachykinin 3 | 1.00 ± 0.09 ** | 3.21 ± 0.64 ** | 0.49 ± 0.05 |
| Metabolism | ||||
| AGRP | agouti-related protein homolog | 1.00 ± 0.25 | 1.48 ± 0.24 | 1.00 ± 0.07 |
| INSR | insulin receptor | 1.00 ± 0.16 | 1.27 ± 0.25 | 1.12 ± 0.07 |
| LEPR | leptin receptor | 1.00 ± 0.09 | 1.23 ± 0.17 | 1.12 ± 0.27 |
| MC2R | melanocortin 2 receptor (adrenocorticotropic hormone) | 1.00 ± 0.32 | 0.37 ± 0.11 | 0.69 ± 0.43 |
| MC4R | melanocortin 4 receptor | 1.00 ± 0.29 | 0.62 ± 0.07 | 0.71 ± 0.11 |
| MCHR1 | melanin-concentrating hormone receptor 1 | 1.00 ± 0.05 | 1.22 ± 0.17 | 1.71 ± 0.34 |
| NPY | neuropeptide Y | 1.00 ± 0.53 | 0.81 ± 0.40 | 0.40 ± 0.05 |
| NPY1R | neuropeptide Y receptor Y1 | 1.00 ± 0.19 | 1.08 ± 0.28 | 1.05 ± 0.16 |
| NPY2R | neuropeptide Y receptor Y2 | 1.00 ± 0.15 * | 1.53 ± 0.12 * | 1.04 ± 0.12 |
| NPY5R | neuropeptide Y receptor Y5 | 1.00 ± 0.12 | 1.05 ± 0.17 | 1.31 ± 0.17 |
| POMC | proopiomelanocortin | 1.00 ± 0.19 | 1.17 ± 0.27 | 0.83 ± 0.11 |
| Neurotransmission | ||||
| ACHE | acetylcholinesterase | 1.00 ± 0.10 | 1.17 ± 0.04 | 1.13 ± 0.10 |
| ADRA1A | adrenergic, alpha-1A, receptor | 1.00 ± 0.14 | 0.80 ± 0.03 | 0.73 ± 0.21 |
| DRD1 | dopamine receptor D1 | 1.00 ± 0.10 | 0.89 ± 0.08 | 0.82 ± 0.09 |
| DRD2 | dopamine receptor D2 | 1.00 ± 0.21 | 0.82 ± 0.11 | 1.02 ± 0.22 |
| SLC18A2 | solute carrier family 18, member 2 (vesicular monoamine) | 1.00 ± 0.22 | 0.88 ± 0.08 | 1.07 ± 0.26 |
| SLC6A3 | solute carrier family 6, member 3 (dopamine transporter) | 1.00 ± 0.34 | 0.62 ± 0.06 ** | 4.52 ± 0.74 |
| SLC6A4 | member 3, solute carrier family 6, member 4 (serotonin transporter) | 1.00 ± 0.57 | 0.67 ± 0.62 | 0.55 ± 0.25 |
| GABBR1 | gamma-aminobutyric acid B receptor, 1 | 1.00 ± 0.06 | 0.99 ± 0.02 | 1.08 ± 0.10 |
| GABBR2 | gamma-aminobutyric acid B receptor, 2 | 1.00 ± 0.27 | 0.69 ± 0.04 | 0.77 ± 0.05 |
| GABRA1 | gamma-aminobutyric acid A receptor, alpha 1 | 1.00 ± 0.23 | 0.75 ± 0.03 | 1.01 ± 0.07 |
| GABRA5 | gamma-aminobutyric acid A receptor, alpha 5 | 1.00 ± 0.12 | 0.82 ± 0.06 | 0.87 ± 0.16 |
| HTR1A | 5-hydroxytryptamine (serotonin) receptor 1A | 1.00 ± 0.14 | 0.71 ± 0.06 | 0.86 ± 0.15 |
| HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A | 1.00 ± 0.04 | 0.81 ± 0.12 | 0.63 ± 0.07 |
| HTR2C | 5-hydroxytryptamine (serotonin) receptor 2C | 1.00 ± 0.12 | 1.35 ± 0.15 | 1.20 ± 0.06 |
RT2 Profiler™ PCR Arrays were used to profile gene expression in young intact, old ovariectomized (OVX), and old OVX estrogen-treated (OVX+E2) females. The values (mean ± sem) are expressed relative to the arithmetic mean of ALG9, GAPDH, and RPL13A, and normalized against the Young Intact group.
P < 0.05,
P < 0.01 (relative to the Old OVX group; ANOVA followed by the Dunnett test).
Discussion
Previous studies of the rhesus macaque hypothalamus relied on RT-PCR and in situ hybridization to examine the expression of ESR1, ESR2 and PGR [41, 42]. Although, these studies were essentially qualitative or semi-quantitative, taken together their findings suggested that ESR1 and ESR2 expression is unaffected by circulating E2 levels, whereas PGR expression is highly stimulated by E2. In the present study we used qRT-PCR, a more quantitative molecular approach, to corroborate these previous observations and to examine if these genes show age-associated change in their expression. First of all, ESR1, ESR2, PGR were all expressed in the ARC-ME region of the hypothalamus, thus confirming the results from previous studies. Despite their old age, however, we failed to detect a significant change in the expression of these genes in our old ovary-intact animals. This most likely stems from the fact that most of these animals had not completely entered menopause, and their circulating E2 levels had not yet fallen to a sustained basal levels. Consequently, to more closely mimic the human postmenopausal condition, we also examined age-related differences in the ARC-ME of young and old OVX animals, and exposed some of them to supplementary E2 in order to gain insights into the influence of HT.
E2 HT had an age-related effect on ESR1 and ESR2 expression in OVX animals. While young OVX+E2 treated animals expressed more ESR1 and ESR2 mRNA than untreated age-matched controls, the old animals did not respond to E2 supplementation. Although the reason for this difference is unclear, one possibility is that the hypothalamus of older animals is itself less responsive to exogenous E2. Another possibility is that the stimulatory effect of E2 on ESR1 and ESR2 mRNA is transitory and that it gradually decreases during long-term HT. In contrast to ESR1 and ESR2, PGR expression was highly stimulated by E2 regardless of age. It should be emphasized that the two major isoforms of the progestin receptor (PGR-A and PGR-B) show different expression patterns [43, 44] and independent functions [45–47]. In the present study, however, we amplified both isoforms of PGR, and so cannot establish which form plays the dominant physiological role in the primate hypothalamus. Expression of the androgen receptor in the female rhesus macaque hypothalamus has not been described previously, but in the present study we found AR to be highly expressed in the female hypothalamus and to be unaffected by circulating E2 levels. This result is consistent with, and complements, the finding from a previous study in which ribonuclease protection assay showed no effect of testosterone on the hypothalamic expression of AR in male rhesus macaques [48]. Taken together, the quantitative data from the present study clearly establish that gonadal nuclear steroid receptors are highly expressed in the female rhesus macaque hypothalamus, and that circulating E2 levels can significantly modulate the progestin receptor gene expression even in old animals.
Recently, Naugle et al. [49], used immunohistochemistry to examine the effects of long-term E2 treatment on ERα and PGR expression in the rhesus macaque ARC-ME. Semi-quantitative analysis of the immunoreactive cells revealed no effect of E2 on the number and density of ERα-positive cells in old OVX females, which is in agreement with the mRNA findings from the present study. On the other hand, E2 also failed to significantly affect the number and density of PGR-positive cells, which does not agree with our mRNA findings or those previously reported by Bethea et al. [33]. It is unclear whether this discrepancy is a reflection of the relative insensitivity of the semi-quantitative immunohistochemistry methodology, or whether it is indicative that E2-induced PGR expression changes are not reflected at the translation level. Western blot analysis would help to resolve this issue in the future.
Given that the brain has an intrinsic capacity to synthesize sex steroids de novo [26–29], it is plausible that the hypothalamus can also compensate for the menopausal loss of sex-steroids by increasing its local production of these steroids. There are several key enzymes involved in the synthesis of testosterone and E2 in the brain, using DHEA as a precursor. In the present study we examined the hypothalamic expression of STS, HSD3B1/2, HSD17B5 and CYP19A1. These genes encode key enzymes in the conversion of DHEA to testosterone and E2, and are expressed in the rhesus macaque hypothalamus [29]. The findings from the current study corroborate these earlier observations. Moreover, they show that the expression of HSD17B5 becomes enhanced in old animals when circulating levels of E2 are low, which may result in a compensatory increase in hypothalamic E2 levels, due to enhanced intracrine synthesis from DHEA. On the other hand, circulating levels of DHEA (particularly in the sulfated form as DHEAS) show a significant age-related decline [29,50]. Consequently, it is unclear if the expression of STS, HSD3B1/2, HSD17B5 and CYP19A1 alone is sufficient to maintain high hypothalamic sex-steroid levels during aging. Nevertheless, the results suggest that local steroid synthesis in the hypothalamus is likely to be preserved in older animals, provided that there is sufficient DHEA precursor available.
Other significant age-related gene expression changes have previously been observed in the human and nonhuman primate ARC-ME, especially in the KNDy neurons [12–17]. We observed the same neuronal hypertrophy-related increase in KISS1 and TAC3 gene expression observed in postmenopausal women, and this increase could be blocked by the administration of exogenous E2 [17]. This suggests that the observed gene expression changes stemmed from an age-associated decline in circulating E2 levels, rather than from a sex-steroid independent aging mechanism. Although the physiological significance of the E2-dependent changes in KISS1, KISS1R, and TAC3, expression is unclear these changes may serve to maintain homeostatic control of the reproductive neuroendocrine axis after secretion of sex steroids from the ovaries has declined [2]. In addition, we found that the expression of NPY2R, a central mediator of food intake, was also suppressed following chronic E2 supplementation in old OVX animals [51]. In postmenopausal women, therefore, NPY2R of the ARC may help to mediate the beneficial effects of estrogen HT in the treatment of disorders such as diabetes, obesity, and metabolic syndrome [52].
Analysis of the RT2 Profiler™ PCR arrays also established that most of the selected genes are stably expressed during reproductive aging and after HT. On the other hand E2 supplementation was found to significantly stimulate the expression of GNRH2. We previously reported that E2 exerts a stimulatory action GNRH2 gene expression in the hypothalamus of young OVX animals [53–55]. The finding that GNRH2 expression in old OVX animals also responds to E2, suggests that this second form of primate GnRH may play an important role in mediating E2 positive feedback to the reproductive neuroendocrine axis, as previously suggested [54,55]. E2 supplementation was also found to stimulate the expression of SLC6A3, the gene that encodes the dopamine transporter (DAT). Voytko et al. have reported that chronic E2 therapy did not have an effect on dopaminergic activity in the ventral tegmentum and dorsolateral prefrontal cortex [56]. However, the present results show that chronic E2 was able to significantly increase the expression of the DAT in ARC neurons of aged OVX animals. The influence of estrogen on the dopaminergic system is less well understood, but our results suggest that chronic E2 treatment may facilitate reuptake of dopamine by presynaptic neurons in the ARC.
Menopause in women and female rhesus macaques is associated with attenuated circulating E2 concentrations [1–3], which is thought to contribute to the development of many age-related pathologies. On the other hand, the results from the present study suggest that the hypothalamus retains its capacity to respond to sex steroids, even in old age, and that the sensitivity of the hypothalamus to E2 shows some compensatory changes that may help to maintain local homeostasis.
Acknowledgments
This work was supported by NIH grants AG-019100, AG-023477, AG-029612, AG-036670 and OD-011092. We wish to thank Laurie Renner for managing the in vivo component of the study, and Dr. Steven Kohama for collecting and processing the postmortem tissues. We also wish to thank the ONPRC Endocrine Technology and Support Core for conducting the hormone assays, and the ONPRC Molecular and Cell Biology Core for assistance with the mRNA quantitation.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Rec Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 2.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 3.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 2008;61:4–16. doi: 10.1016/j.maturitas.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109:19846–19851. doi: 10.1073/pnas.1211517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarti CD, Chiantera A, Graziottin A, Ognisanti F, Sidoli C, Mincigrucci M, Parazzini F. Hormone therapy and sleep quality in women around menopause. Menopause (New York, NY) 2005;12:545–551. doi: 10.1097/01.gme.0000172270.70690.5e. [DOI] [PubMed] [Google Scholar]
- 6.Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., 3rd Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- 7.Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab. 1996;81:3540–3546. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- 8.Abel TW, Rance NE. Proopiomelanocortin gene expression is decreased in the infundibular nucleus of postmenopausal women. Brain Res Molec Brain Res. 1999;69:202–208. doi: 10.1016/s0169-328x(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 9.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 10.Krajewski SJ, Abel TW, Voytko ML, Rance NE. Ovarian steroids differentially modulate the gene expression of gonadotropin-releasing hormone neuronal subtypes in the ovariectomized cynomolgus monkey. J Clin Endocrinol Metab. 2003;88:655–662. doi: 10.1210/jc.2002-020887. [DOI] [PubMed] [Google Scholar]
- 11.Escobar CM, Krajewski SJ, Sandoval-Guzman T, Voytko ML, Rance NE. Neuropeptide Y gene expression is increased in the hypothalamus of older women. J Clin Endocrinol Metab. 2004;89:2338–2343. doi: 10.1210/jc.2003-031899. [DOI] [PubMed] [Google Scholar]
- 12.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-b and substance-p messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 13.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 14.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20:1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rance NE. Menopause and the human hypothalamus: Evidence for the role of kisspeptin/neurokinin b neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin b and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17beta-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol. 1977;7:13–31. doi: 10.1111/j.1365-2265.1977.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 19.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinol. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 20.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan KA, Witkin JW, Ferin M, Silverman AJ. Gonadotropin-releasing hormone neurons in the rhesus macaque are not immunoreactive for the estrogen receptor. Brain Res. 1995;685:198–200. doi: 10.1016/0006-8993(95)00352-q. [DOI] [PubMed] [Google Scholar]
- 22.Terasawa E. Control of luteinizing hormone-releasing hormone pulse generation in nonhuman primates. Cell Mol Neurobiol. 1995;15:141–164. doi: 10.1007/BF02069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- 24.Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci U S A. 1998;95:10978–10983. doi: 10.1073/pnas.95.18.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 26.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 27.Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dodr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487 e1481–1413. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- 31.Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod. 2008;79:398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J Comp Neurol. 1976;170:279–293. doi: 10.1002/cne.901700302. [DOI] [PubMed] [Google Scholar]
- 33.Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology. 1996;137:4372–4383. doi: 10.1210/endo.137.10.8828498. [DOI] [PubMed] [Google Scholar]
- 34.Blurton-Jones MM, Roberts JA, Tuszynski MH. Estrogen receptor immunoreactivity in the adult primate brain: Neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol. 1999;405:529–542. [PubMed] [Google Scholar]
- 35.Mills RH, Romeo HE, Lu JK, Micevych PE. Site-specific decrease of progesterone receptor mRNA expression in the hypothalamus of middle-aged persistently estrus rats. Brain Res. 2002;955:200–206. doi: 10.1016/s0006-8993(02)03440-6. [DOI] [PubMed] [Google Scholar]
- 36.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Kohama SG, Bethea CL. Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulations of monkey hypothalamus. Endocrinology. 1995;136:1790–1800. doi: 10.1210/endo.136.4.7895692. [DOI] [PubMed] [Google Scholar]
- 39.Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL. The phosphodiesterase 3 inhibitor org 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception. 2008;77:303–307. doi: 10.1016/j.contraception.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noriega NC, Kohama SG, Urbanski HF. Microarray analysis of relative gene expression stability for selection of internal reference genes in the rhesus macaque brain. BMC Mol Biol. 2010;11:47. doi: 10.1186/1471-2199-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pau CY, Pau KY, Spies HG. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol. 1998;146:59–68. doi: 10.1016/s0303-7207(98)00197-x. [DOI] [PubMed] [Google Scholar]
- 42.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 43.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor a form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 44.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 45.Duffy DM, Wells TR, Haluska GJ, Stouffer RL. The ratio of progesterone receptor isoforms changes in the monkey corpus luteum during the luteal phase of the menstrual cycle. Biol Reprod. 1997;57:693–699. doi: 10.1095/biolreprod57.4.693. [DOI] [PubMed] [Google Scholar]
- 46.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 47.Stouffer RL. Progesterone as a mediator of gonadotrophin action in the corpus luteum: Beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- 48.Abdelgadir SE, Roselli CE, Choate JV, Resko JA. Androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biol Reprod. 1999;60:1251–1256. doi: 10.1095/biolreprod60.5.1251. [DOI] [PubMed] [Google Scholar]
- 49.Naugle MM, Nguyen LT, Merceron TK, Filardo E, Janssen WG, Morrison JH, Rapp PR, Gore AC. G-protein coupled estrogen receptor, estrogen receptor α, and progesterone receptor immunohistochemistry in the hypothalamus of aging female rhesus macaques given long-term estradiol treatment. J Exp Zool A Ecol Genet Physiol. 2014;321:399–414. doi: 10.1002/jez.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorwell KG, Kohama SG, Urbanski HF. Testosterone increases circulating dehydroepiandrosterone sulfate levels in the male rhesus macaque. Front Endocrinol (Lausanne) 2014;5:101. doi: 10.3389/fendo.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koegler FH, Enriori PJ, Billes SK, Takahashi DL, Martin MS, Clark RL, Evans AE, Grove KL, Cameron JL, Cowley MA. Peptide yy(3-36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes. 2005;54:3198–3204. doi: 10.2337/diabetes.54.11.3198. [DOI] [PubMed] [Google Scholar]
- 52.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes, obesity & metabolism. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 53.Densmore VS, Urbanski HF. Effect of 17beta-estradiol on hypothalamic GnRH-II gene expression in the female rhesus macaque. J Molec Endocrinol. 2004;33:145–153. doi: 10.1677/jme.0.0330145. [DOI] [PubMed] [Google Scholar]
- 54.Urbanski HF. Differential roles of GnRH-I and GnRH-II neurons in the control of the primate reproductive axis. Front Gen Endocrinol. 2012;3:20. doi: 10.3389/fenod.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbanski HF. Selective targeting of GnRH-II neurons to block ovulation. Contraception. 2014 doi: 10.1016/j.contraception.2014.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voytko ML, Tinkler GP, Browne C, Tobin JR. Neuroprotective effects of estrogen therapy for cognitive and neurobiological profiles of monkey models of menopause. Am J Primatol. 2009;71:794–801. doi: 10.1002/ajp.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]