Abstract
Dysfunction of the retinal pigment epithelium (RPE) resulting from chronic inflammation is implicated in the pathogenesis of age-related macular degeneration (AMD). RPE cells adjacent to drusen deposits in the AMD eye are known to contain CXCL11, a chemokine involved in inflammatory cell recruitment. We investigated the CXCL11 production by the human RPE (ARPE-19) cells under inflammatory conditions and tested its response to resveratrol, a naturally occurring anti-inflammatory antioxidant. A proinflammatory cytokine mixture consisting of IFN-γ, IL-1β and TNF-α highly increased CXCL11 mRNA expression and CXCL11 protein secretion by ARPE-19 cells. Resveratrol substantially inhibited the proinflammatory cytokines-induced CXCL11 production while partially blocking nuclear factor-κB activation. This inhibitory action of resveratrol was also observed for the cytokines-induced expression of chemokines CXCL9, CCL2 and CCL5. Our results indicate that resveratrol could potentially attenuate RPE inflammatory response implicated in the pathogenesis of AMD.
Keywords: Resveratrol, Retinal pigment epithelium, Age-related macular degeneration, CXCL11, Nuclear factor-kappa B
1. Introduction
Retinal pigment epithelium (RPE) dysfunction resulting from abnormal inflammatory response is implicated in the pathogenesis of age related macular degeneration (AMD) [1]. CXCL11 (I-TAC), a chemokine involved in inflammatory cell recruitment, has been found to be present in RPE cells adjacent to drusen deposits in the AMD eye [2, 3]. Human RPE cells are known to highly increase the production of CXCL11 when co-cultured with activated T-cells [4]. Also, fetal RPE cells secrete large amounts of CXCL11 when treated with the proinflammatory cytokines IFN-γ, IL-1β, and TNF-α [5]. Resveratrol, a naturally occurring polyphenol, is a well characterized anti-inflammatory antioxidant and a popular nutraceutical [6]. Recently, we have shown that resveratrol could effectively suppress VEGF production by RPE cells stimulated with IFN-γ, IL-1β, and TNF-α [7]. Therefore, the possibility that this anti-inflammatory agent could inhibit the CXCL11 production by RPE cells responding to IFN-γ, IL-1β, and TNF-α was investigated in the present study.
2. Materials and methods
2.1. Cell culture
ARPE-19 human RPE cells obtained from ATCC (Manassas, VA) were grown to confluence as we reported earlier [8, 9]. The cells were treated with the proinflammatory cytokines IFN-γ (10 u/ml), IL-1β (1 ng/ml) and TNF-α (1 ng/ml) in the absence of serum for 16 h unless otherwise indicated. The cells were pre-incubated with resveratrol (50 μM) for 4 hours also in the absence of serum prior to the cytokine treatment when required. Resveratrol was obtained from Sigma-Aldrich, St. Louis, MO and dissolved in ethanol before adding to cell culture medium. Equal volume of ethanol was added to controls. Human IL-1β was purchased from R&D Systems, Minneapolis, MN while TNF-α and IFN-γ were from Roche Applied Science, Indianapolis, IN.
2.2. Real-time RT-PCR
Total RNA fraction isolated from ARPE-19 cells was reverse transcribed using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and used as the template for quantitative real-time PCR. Each PCR reaction (20 μl) was set up using validated TaqMan probes (labeled with reporter dye FAM at the 5′ end), primers specific for the gene of interest and TaqMan Universal PCR Master Mix (Applied Biosystems). Human 18S rRNA was used as the endogenous control. Gene amplification was analyzed with an Applied Biosystems ViiA 7 Real-Time PCR System and the results were expressed as n-fold induction in gene expression calculated using relative quantification (ΔΔCT) method.
2.3. NF-κB activation analysis
Western blot analysis of phospho-NF-κB p65 was performed using extracts of ARPE-19 cells prepared with cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA) containing protease and phosphatase inhibitors. The proteins in the cell extracts were separated by sodium dodecyl sulfate- polyacrylamide gel electrophoresis, and then blotted onto a nitrocellulose membrane. Immunoreactive bands on blots were detected with Amersham ECL prime Western blot detection reagents (GE Healthcare, Pittsburgh, PA) using anti-phospho-NF-kB p65 (Ser536) antibody (Cell Signaling Technology). The blot was then stripped and reprobed with mouse anti-actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO). Chemiluminescence films were scanned and the signal intensities of immunoreactive bands were estimated using Image Studio (Li-Cor Biosciences).
The active form of NF-κB p65 present in the ARPE-19 cell extracts was also analyzed using the TransAM NFκB p65 transcription factor assay kit (Active Motif, Carlsbad, CA). Briefly, the transcription factor is detected by ELISA after capturing it with an oligonucleotide containing NF-κB consensus sequence.
2.4. ELISA
CXCL11 secreted into cell culture media was analyzed using I-TAC/CXCL11 ELISA kit (RayBiotech, Norcross, GA). The amount of CXCL9, CXCL10, CXCL11, CCL2 and CCL5 secreted into the culture media was estimated by multiplex ELISA at Aushon Biosystems, Inc. (Billerica, MA).
3. Results and Discussion
3.1. Proinflammatory cytokines highly increase CXCL11 expression in ARPE-19 cells
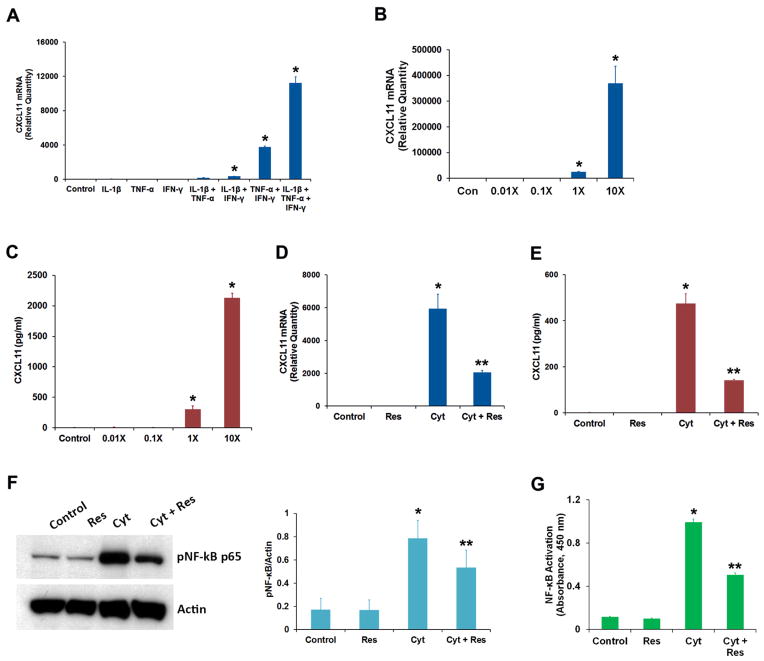
Human adult RPE-derived ARPE-19 cells are reported to produce large amounts of CXCL11 when co-cultured with activated T-cells [4]. Therefore, we employed this cell line to study the effect of proinflammatory cytokines on CXCL11 expression. We used IFN-γ, IL-1β, and TNF-α as proinflammatory cytokines since they are known to stimulate CXCL11 secretion from fetal RPE cells [5]. An increase in CXCL11 mRNA expression was observed when ARPE-19 cells were treated with a combination of IFN-γ either with TNF-α or IL-1β (Fig. 1A). A combination of all three cytokines was required for the maximum response. The increase in CXCL11 mRNA expression was dependent on the concentration of the cytokines (Fig. 1B). This treatment also resulted in a concentration-dependent secretion of CXCL11 protein into the cell culture medium (Fig. 1C). Thus, ARPE-19 cells respond to IFN-γ, IL-1β, and TNF-α by markedly increasing the secretion of CXCL11.
Fig. 1.
Effect of resveratrol on the production of chemokine CXCL11 by RPE cells exposed to IFN-γ, IL-1β and TNF-α. (A) ARPE-19 cells were treated for 16 h with different combinations of proinflammatory cytokines (IFN-γ, 10 u/ml; IL-1β, 1 ng/ml and TNF-α, 1 ng/ml) as indicated, and CXCL11 mRNA expression was analyzed by real-time PCR. (B) The cells were treated for 16 h with different concentrations of proinflammatory cytokines (1X = IFN-γ, 10 u/ml + IL-1β, 1 ng/ml + TNF-α, 1 ng/ml) and the CXCL11 mRNA expression was analyzed by real-time PCR. (C) The CXCL11 protein secretion into culture medium from the cells treated with increasing concentrations of proinflammatory cytokines was analyzed by ELISA. (D) The cells were treated with the proinflammatory cytokine mixture (Cyt) consisting of IFN-γ (10 u/ml), IL-1β (1 ng/ml) and TNF-α (1 ng/ml) for 16 h in the presence or absence of 50 μM resveratrol (Res) and the CXCL11 mRNA expression analyzed by real-time PCR. (E) The secretion of CXCL11 protein into culture medium under conditions described above was estimated by ELISA. (F) Cell extracts (20 μg protein) prepared from ARPE-19 cells treated for 16 h with proinflammatory cytokines in the presence or absence of resveratrol were analyzed by Western immunoblotting using anti-phospho-NF-κB p65 antibody. Actin was used as the gel loading control. The histogram shows the relative signal intensities of pNF-κB immunoreactive bands from three separate blots after normalization with actin. (G) NF-κB p65 transcription factor assay was performed using cell extracts (12 μg protein) prepared from ARPE-19 cells treated for 16 h with the proinflammatory cytokines in the presence or absence of resveratrol.
* = p < 0.05 when compared to control; ** = p < 0.05 when compared to Cyt; n = 4 for histograms A, B, C, D, E & G while n = 3 for histogram F.
3.2. Resveratrol attenuates CXCL11 production by ARPE-19 cells treated with proinflammatory cytokines
We investigated the effect of the anti-inflammatory agent resveratrol on the CXCL11 production by ARPE-19 cells responding to IFN-γ, IL-1β, and TNF-α. Increases in both CXCL11 mRNA expression and CXCL11 protein secretion resulting from exposure to proinflammatory cytokines were considerably reduced when ARPE-19 cells were treated with 50 μM resveratrol (Fig. 1D&E). This resveratrol concentration was found to be optimal for suppressing VEGF secretion by RPE cells exposed to IFN-γ, IL-1β, and TNF-α [7]. Resveratrol is known to block the activation of NF-κB signal transduction pathway by TNF-α and IL-1β [10, 11]. Therefore, we examined the effect of resveratrol on cytokines-mediated formation of phospho-NF-κB p65, an indicator of NF-κB acivation. Western blot analysis of cell extracts obtained from ARPE-19 cells treated for 16 h with the cytokines in the presence or absence of resveratrol showed that the phospho-NF-κB p65 formation was considerably decreased by resveratrol (Fig. 1F). This observation was further corroborated by performing the NF-κB p65 transcription factor assay with the same cell extracts (Fig. 1G). Resveratrol caused approximately 50% reduction in the activation of NF-κB p65 by the cytokines. Thus, resveratrol could effectively block NF-κB activation in ARPE-19 cells, and this effect could play a role in suppressing proinflammatory cytokines-induced CXCL11 expression.
3.3. Resveratrol suppresses the production of CXCL9, CCL2 and CCL5 by ARPE-19 cells exposed to proinflammatory cytokines
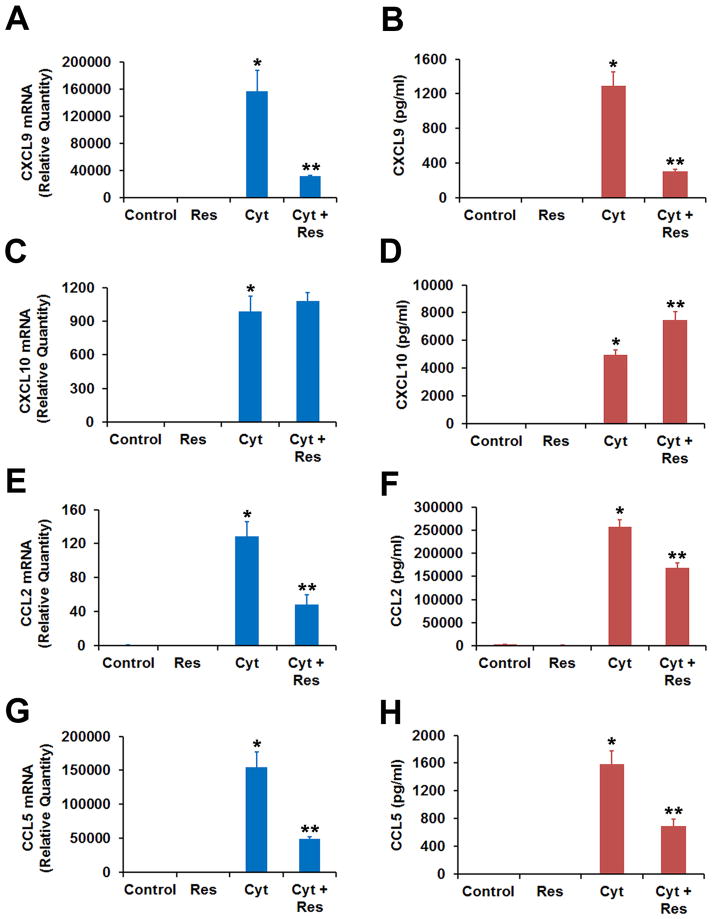
Proinflammatory cytokines IFN-γ, IL-1β, and TNF-α are known to greatly increase the expression of chemokines CXCL9, CXCL10, CCL2 and CCL5 in human RPE cells in culture [12, 13]. Therefore, we studied the induction of these chemokines in ARPE-19 cells by the proinflammatory cytokines and its potential inhibition by resveratrol (Fig. 2). The cytokines-induced increases in both mRNA expression and protein secretion of CXCL9, CCL2 and CCL5 were noticeably inhibited by resveratrol similar to what was observed for CXCL11 (Fig. 2). However, resveratrol failed to inhibit the induction of CXCL10 transcript by the cytokines. Interestingly, the cytokines-induced secretion of CXCL10 protein into the culture medium by the cells was significantly increased in the presence of resveratrol. The atypical response of CXCL10 to resveratrol needs to be investigated further.
Fig. 2.
Effect of resveratrol on the production of chemokines CXCL9, CXCL10, CCL2 and CCL5 by RPE cells exposed to IFN-γ, IL-1β and TNF-α. ARPE-19 cells pre-incubated with or without 50 μM resveratrol (Res) were treated with cytokine mixture (Cyt) consisting of IFN-γ (10 u/ml), IL-1β (1 ng/ml) and TNF-α (1 ng/ml) for 16 h. Chemokine mRNA expression was analyzed by real-time PCR while the chemokine protein secretion into the culture medium was estimated by ELISA. (A) CXCL9 mRNA expression. (B) CXCL9 protein secretion. (C) CXCL10 mRNA expression. (D) CXCL10 protein secretion. (E) CCL2 mRNA expression. (F) CCL2 protein secretion. (G) CCL5 mRNA expression. (H) CCL5 protein secretion.
* = p < 0.05 when compared to control, ** = p < 0.05 when compared to Cyt; n = 4.
Increased expression of CXCL11 by RPE cells potentially arising from inflammatory insult is indicated in AMD pathogenesis [3, 14]. We have shown that human adult RPE derived ARPE-19 cells can produce and secrete CXCL11 when stimulated with proinflammatory cytokines IFN-γ, IL-1β, and TNF-α. Our study also shows that resveratrol, a natural product and an anti-inflammatory agent, can inhibit this increase in CXCL11 production by the RPE cells responding to inflammatory stimuli. Resveratrol appears to elicit this effect by its known ability to negatively modulate NF-κB activation [10, 11]. The inhibitory effect of resveratrol was also observed for the cytokines-mediated increase in CCL2, CCL5 and CXCL9 production. Thus, resveratrol could possibly suppress the RPE inflammatory response and the resulting CXCL11 production implicated in the pathogenesis of AMD.
HIGHLIGHTS.
IFN-γ, IL-1β and TNF-α highly increased the production of CXCL11 by RPE cells.
Resveratrol suppressed the proinflammatory cytokines-induced CXCL11 production.
Resveratrol also blocked nuclear factor-κB activation by proinflammatory cytokines.
Resveratrol may attenuate RPE inflammatory response implicated in AMD.
Acknowledgments
This study was supported by the Intramural Research Program of the National Eye Institute, NIH.
ABBREVIATIONS
- RPE
Retinal pigment epithelium
- AMD
Age-related macular degeneration
- NF-κB
Nuclear factor-kappa B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438–51. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187(12):2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin T, Walker GB, Kurji K, Fang E, Law G, Prasad SS, Kojic L, Cao S, White V, Cui JZ, Matsubara JA. Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62(3):369–81. doi: 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juel HB, Faber C, Udsen MS, Folkersen L, Nissen MH. Chemokine expression in retinal pigment epithelial ARPE-19 cells in response to coculture with activated T cells. Invest Ophthalmol Vis Sci. 2012;53(13):8472–80. doi: 10.1167/iovs.12-9963. [DOI] [PubMed] [Google Scholar]
- 5.Shi G, Maminishkis A, Banzon T, Jalickee S, Li R, Hammer J, Miller SS. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49(10):4620–30. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svajger U, Jeras M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol. 2012;31(3):202–22. doi: 10.3109/08830185.2012.665108. [DOI] [PubMed] [Google Scholar]
- 7.Nagineni CN, Raju R, Nagineni KK, Kommineni VK, Cherukuri A, Kutty RK, Hooks JJ, Detrick B. Resveratrol Suppresses Expression of VEGF by Human Retinal Pigment Epithelial Cells: Potential Nutraceutical for Age-related Macular Degeneration. Aging Dis. 2014;5(2):88–100. doi: 10.14366/AD.2014.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62(2):155–69. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 9.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK, Hooks JJ, Redmond TM. Differential regulation of microRNA-146a and microRNA- 146b-5p in human retinal pigment epithelial cells by interleukin-1beta, tumor necrosis factor-alpha, and interferon-gamma. Mol Vis. 2013;19:737–50. [PMC free article] [PubMed] [Google Scholar]
- 10.Busch F, Mobasheri A, Shayan P, Lueders C, Stahlmann R, Shakibaei M. Resveratrol modulates interleukin-1beta-induced phosphatidylinositol 3-kinase and nuclear factor kappaB signaling pathways in human tenocytes. J Biol Chem. 2012;287(45):38050–63. doi: 10.1074/jbc.M112.377028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509–19. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 12.Hooks JJ, Nagineni CN, Hooper LC, Hayashi K, Detrick B. IFN-beta provides immuno-protection in the retina by inhibiting ICAM-1 and CXCL9 in retinal pigment epithelial cells. J Immunol. 2008;180(6):3789–96. doi: 10.4049/jimmunol.180.6.3789. [DOI] [PubMed] [Google Scholar]
- 13.Wang XC, Jobin C, Allen JB, Roberts WL, Jaffe GJ. Suppression of NF-kappaB-dependent proinflammatory gene expression in human RPE cells by a proteasome inhibitor. Invest Ophthalmol Vis Sci. 1999;40(2):477–86. [PubMed] [Google Scholar]
- 14.Newman AM, Gallo NB, Hancox LS, Miller NJ, Radeke CM, Maloney MA, Cooper JB, Hageman GS, Anderson DH, Johnson LV, Radeke MJ. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012;4(2):16. doi: 10.1186/gm315. [DOI] [PMC free article] [PubMed] [Google Scholar]