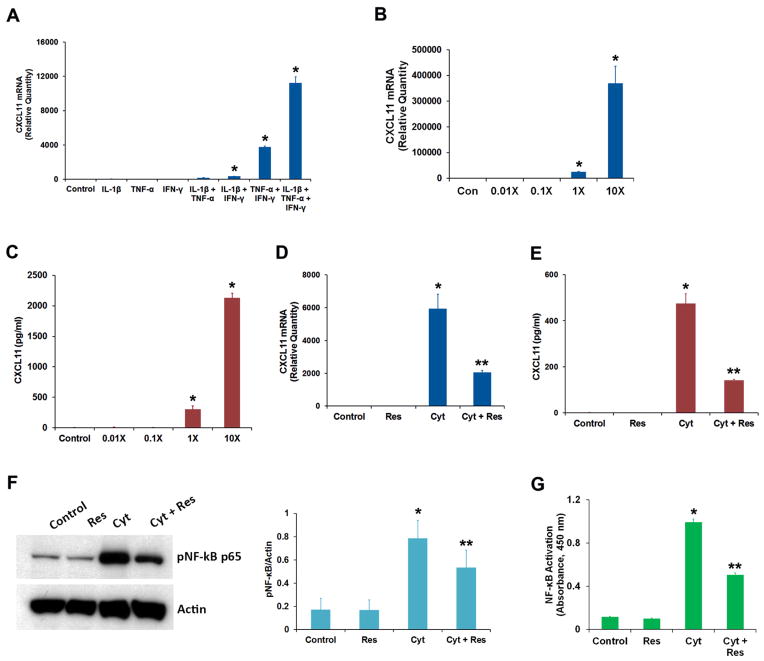
Fig. 1.
Effect of resveratrol on the production of chemokine CXCL11 by RPE cells exposed to IFN-γ, IL-1β and TNF-α. (A) ARPE-19 cells were treated for 16 h with different combinations of proinflammatory cytokines (IFN-γ, 10 u/ml; IL-1β, 1 ng/ml and TNF-α, 1 ng/ml) as indicated, and CXCL11 mRNA expression was analyzed by real-time PCR. (B) The cells were treated for 16 h with different concentrations of proinflammatory cytokines (1X = IFN-γ, 10 u/ml + IL-1β, 1 ng/ml + TNF-α, 1 ng/ml) and the CXCL11 mRNA expression was analyzed by real-time PCR. (C) The CXCL11 protein secretion into culture medium from the cells treated with increasing concentrations of proinflammatory cytokines was analyzed by ELISA. (D) The cells were treated with the proinflammatory cytokine mixture (Cyt) consisting of IFN-γ (10 u/ml), IL-1β (1 ng/ml) and TNF-α (1 ng/ml) for 16 h in the presence or absence of 50 μM resveratrol (Res) and the CXCL11 mRNA expression analyzed by real-time PCR. (E) The secretion of CXCL11 protein into culture medium under conditions described above was estimated by ELISA. (F) Cell extracts (20 μg protein) prepared from ARPE-19 cells treated for 16 h with proinflammatory cytokines in the presence or absence of resveratrol were analyzed by Western immunoblotting using anti-phospho-NF-κB p65 antibody. Actin was used as the gel loading control. The histogram shows the relative signal intensities of pNF-κB immunoreactive bands from three separate blots after normalization with actin. (G) NF-κB p65 transcription factor assay was performed using cell extracts (12 μg protein) prepared from ARPE-19 cells treated for 16 h with the proinflammatory cytokines in the presence or absence of resveratrol.
* = p < 0.05 when compared to control; ** = p < 0.05 when compared to Cyt; n = 4 for histograms A, B, C, D, E & G while n = 3 for histogram F.