Abstract
Efficient wound healing requires the coordinated responses of various cell types within an injured tissue. To react to the presence of a wound, cells have to first detect it. Judging from their initial biochemical and morphological responses, many cells including leukocytes, epithelial and endothelial cells, detect wounds from over hundreds of micrometers within seconds-to-minutes. Wound detection involves the conversion of an injury-induced homeostatic perturbation, such as cell lysis, an unconstrained epithelial edge, or permeability barrier breakdown, into a chemical or physical signal. The signal is spatially propagated through the tissue to synchronize protective responses of cells near the wound site and at a distance. This review summarizes the triggers and mechanisms of wound detection in animals.
The physiological triggers of wound detection
Epithelial barriers allow organisms to maintain internal homeostasis amid changes of external environment and protect against infection. Some epithelia, for example those covered by liquid, close breaches extremely fast [1–4], effectively limiting the entry of pathogens. Yet, closure is never instantaneous. To protect the exposed tissue during healing, organisms mount provisional defenses around the injury site. Those include local secretion of antimicrobials and/or recruitment of phagocytes. If these cytotoxic responses are erroneously activated or improperly scaled or timed, they damage the host itself [5]. Aberrant wound responses are a hallmark of many epithelial diseases, such as asthma, cystic fibrosis and Crohn’s.
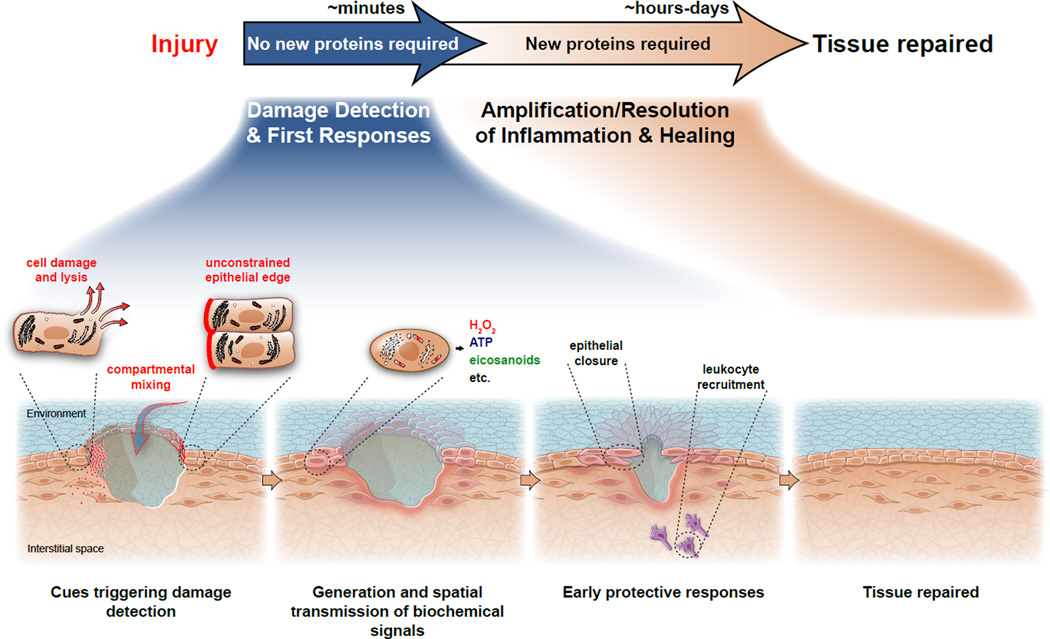
Epithelial injury causes (i) cell damage and lysis, (ii) an unconstrained (“free”) epithelial edge, and (iii) barrier breaching that allows compartmental mixing (Figure 1). Cell lysis releases cytoplasmic molecules into the extracellular space that directly trigger chemotaxis or the production of chemokines in target cells. Unconstrained epithelial edges and compartmental mixing displace cells near the injury site from their normal chemical and mechanical homeostasis. All these cues are thought to establish tissue-scale signaling patterns in the extracellular space, such as chemotactic or haptotactic concentration gradients, which alert distant cells to the presence of a wound, and spatially coordinate their responses.
Figure 1. Phases of wound detection and repair in the larval zebrafish.
Wound detection is triggered by tissue intrinsic cues, such as factors released from lysing cells and signaling initiated in cells at unconstrained wound margins. Injury also disrupts the epithelial barrier, which leads to compartmental mixing and induces stress signaling. Minutes following injury, these signals provide spatial and temporal guidance cues for the earliest protective responses, such as epithelial closure and leukocyte recruitment. The initial ‘detection phase’ is followed by the amplification and resolution of the inflammatory response, cell proliferation, and regenerative events. These later processes are thought to be coordinated by complex transcriptional chemokine, cytokine, and growth factor cascades. Completion of tissue healing can take anywhere from hours to days.
Most research on wound healing has focused on the transcriptional growth factor and chemokine cascades that govern proper execution of tissue repair and inflammation [6], but how these responses are initiated remains little understood [7,8]. Here, we summarize current concepts of epithelial wound detection in animals. Intriguing mechanistic analogies between wound responses in animals and plants exist as reviewed elsewhere [9]. We concentrate on mechanisms triggered solely by epithelial damage; that is, injury in the absence of blood vessel trauma. In higher animals, non-bleeding epithelial wounds result from particulate/allergen exposure, mechanical stress (for example, bowel movements, coughing, etc.), chemical injury, or lytic infection of mucosal linings. Such lesions are abundant in diseases that increase the fragility of epithelial barriers, such as asthma [10], and can permeabilize large surface areas to microbes, allergens and irritants. Although many different cell types participate in the wound response, we focus on wound detection by epithelial cells and leukocytes. To this end, we preferentially refer to data derived from animal model systems (mice, zebrafish, fruit fly and worms) where available.
Epithelial wound detection on the cellular level
Any type of tissue damage, including epithelial injury, is ultimately detected on the cellular level either as cell lysis or sub-lytic cell stress (Figure 2). Cell lysis and stress can occur as a direct, momentary consequence of the injury method itself. Some injury types, e.g., burn injury, cause more cell lysis than others, such as epithelial tearing. Thus, the amount of direct cell lysis is often a poor indicator of actual wound/breach size. Cell stress, and in extreme cases, lysis can be a secondary consequence of tissue-level perturbations, such as loss of epithelial sheet structure or barrier function. Below, we summarize signals that mediate epithelial wound detection on the cellular level.
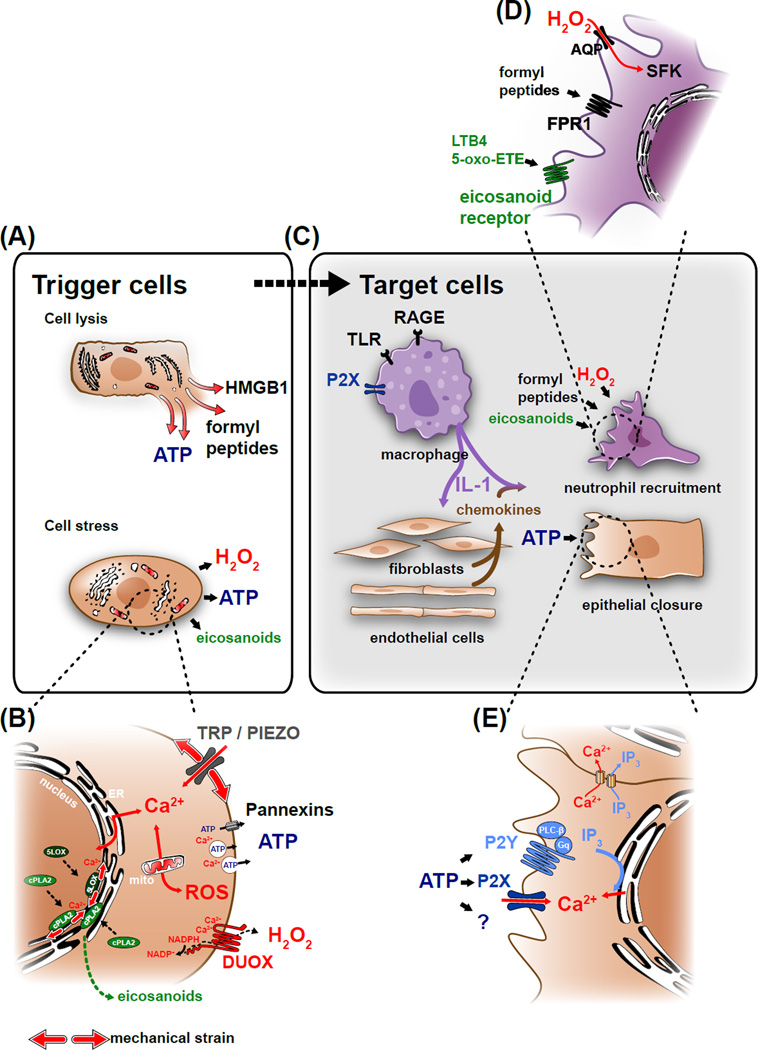
Figure 2. Molecular mechanism of cell lysis- and stress-mediated damage detection.
(A) Wound detection is triggered when damaged cells release cytoplasmic factors into the extracellular space. These “damage associated molecular patterns” (DAMPs) include molecules, such as HMGB1, formyl peptides or ATP. Epithelial injury also perturbs normal tissue homeostasis and results in cell stress, which induces the production of wound signals. (B) Homeostatic perturbations resulting in cell stress induce cytoplasmic Ca2+ signals by opening mechanosensitive, cation permeable channels (TRPs, Piezo) or by releasing Ca2+ from intracellular stores. Calcium signals initiate ROS production, either by activating the epithelial NADPH-oxidase DUOX (dual oxidase) through binding to the enzyme’s EF-hand domains, or by triggering mitochondrial permeability transition. Calcium signals also initiate the production and enzymatic oxidation of arachidonic acid into eicosanoids by activating cytosolic phospholipase A2 (cPLA2), and 5-lipoxygenase (5LOX). Ca2+ binding is required for these proteins to efficiently associate with nuclear membranes, giving them access to their lipid substrates. This leads to the production of proinflammatory eicosanoids, such as 5-oxo-ETE or leukotriene B4 (LTB4). Ca2+ also governs the secretion of paracrine mediators into the extracellular space (e.g., ATP) through regulating exocytosis and/or plasma membrane channel opening. (C) Most DAMPs act through transcriptional relay mechanisms in “sentinel” cells such as tissue-resident macrophages dispersed throughout the tissue. DAMPs are recognized by pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), or receptors for advanced glycation end-products (RAGE). PRR and inflammasome signaling lead to processing and release of IL-1, which in turn stimulates chemokine secretion in target cells. Signals from lysed and stressed cells along with these chemokines trigger neutrophil recruitment and epithelial migration. (D) Rapid leukocyte migration to injury sites is mediated by G-protein coupled receptors, such as the N-formyl peptide receptor FPR1 and the eicosanoid receptors OXER and LTB4R. Leukocyte recruitment also depends on the H2O2-mediated oxidation of Src family kinases (SFK), such as LYN. (E) Extracellular ATP regulates wound closure by acting either as a DAMP or a stress signal through purinergic P2X channels or G-protein coupled P2Y receptors, which cause cytosolic [Ca2+]-elevation and IP3 production. Alternatively, ATP may initiate wound responses through yet unknown mechanisms.
Cell lysis detection
Epithelial wounds may be detected through factors that leak out of lysing cells (termed “Damage Associated Molecular Patterns,” or DAMPs). DAMPs include various cytoplasmic metabolites, peptides, and proteins (e.g., uric acid, ATP, nucleic acids, HMGB1, lactoferrin, S100, mitochondrial components, 4-hydroxyphenyllactic acid in C. elegans, etc.) [11,12]. Some DAMPs, such as formylated peptides and ATP, can act as migratory signals themselves (Box 1). However, most DAMPs are thought to act indirectly by stimulating transcriptional cytokine and chemokine cascades in responding cells. Interleukin 1 (IL-1) is one of the first transcriptionally induced cytokines after tissue damage, and crucial for cell lysis detection. Neutrophil recruitment to injected necrotic cells, liver burn damage in mice, or tail fin wounds in zebrafish larvae is severely inhibited in animals deficient in IL-1 signaling [13–15]. DAMPs enhance IL-1 transcription through binding to pattern recognition receptors and by promoting proteolytic maturation of IL-1 precursor peptide through inflammasome-mediated activation of caspase-1 [16,17]. IL-1 synthesis can be also independent of caspase-1 [13,18–20]. Experiments in mice suggest that bone marrow derived cells (e.g., tissue resident-macrophages) are essential for DAMP dependent IL-1 production. Accordingly, neutrophils of macrophage-depleted mice are severely impaired in their ability to detect necrotic cells [14,21]. By contrast, neutrophil recruitment to tail fin wounds in macrophage-depleted zebrafish larvae is normal [22], which points to developmental-stage or species-dependent differences in the cell types required to transduce DAMP/IL-1 signals. Unlike commonly assumed, IL-1 signaling (or ATP release for that purpose) may not always depend on cell death and cytoplasmic leakage. Cell swelling has been recently described as an alternative, conserved inflammasome activator [23]. IL-1 is thought to regulate leukocyte recruitment by stimulating the production of chemotactic chemokines, such as the CXCR2 ligand interleukin 8 (IL-8) [15,24]. The cells that produce IL-8 and other leukocyte chemoattractants in response to IL-1 remain poorly characterized in vivo, but do not seem to originate from bone marrow [11,16]. Where and when chemokine gradients act in an injured organism remains a largely open question. In mice, the CXCR2 ligand MIP-2 (mice lack IL-8) was shown to form a haptotactic concentration gradient within the vasculature attracting neutrophils to necrotic liver damage sites [14]. Gradient formation required the immune signaling adaptor MYD88, which mediates both IL-1 and Toll-like receptor (TLR) signaling, but not IL-1 receptor activation [14]. This indicates that detection of cell lysis by endothelial cells may involve IL-1 independent pathways. Initial migration (“swarming”) of CXCR2 deficient neutrophils to dermal injury sites in mice is normal [25], arguing against the idea that interstitial IL-8 gradients mediate initial damage detection by leukocytes in this model. Zebrafish, like humans (and unlike mice) possess an IL-8 ortholog, which mediates leukocyte recruitment to tail fin injury [26]. Whether this depends on vascular or interstitial chemokine gradients remains unclear.
Box 1. Chemotactic/kinetic DAMPs.
Rapid induction of cell motility in epithelial cells and leukocytes is crucial for efficient epithelial repair and defense. DAMPs, such as ATP and formylated peptides directly mediate cell movements and directional sensing through G-protein coupled receptor pathways. ATP is present at millimolar concentration in the cytoplasm. It is passively released (as a DAMP) from lysing cells, or in response to physical or chemical stresses from intact cells to trigger purinergic signaling [85]. Several non-lytic ATP secretion routes have been characterized in cell culture [43]. Less is known about the relative physiological relevance of these mechanisms for wound healing in vivo. Immune-modulatory functions of extracellular ATP include inflammasome activation through P2X7 channels [17], enhancement of neutrophil and microglia chemotaxis [39,86], and recruitment of eosinophils and dendritic cells after allergic challenge [87–89]. Extracellular ATP stimulates epithelial cell motility during wound healing in vitro [71,72,74,75] and in vivo [4]. The healing-promoting effect of extracellular ATP in cell culture is mediated by the P2Y2 receptor [72,90]. P2Y2 activation is thought to stimulate epithelial cell migration through transactivation of the EGF receptor [71,74,76,91], possibly through activation of phospholipase D [91] and NADPH oxidases [49,76,92]. Secreted nucleotides are rapidly removed from the extracellular space by ecto- ATPases [93], which negatively regulate the spatial range of nucleotide-induced wound closure movements after zebrafish tail fin injury [4]. Owing to ATP’s second-to-millisecond extracellular half-life [94,95], physiological ATP reaction-diffusion gradients around lysing cells are probably highly transient and short-ranged (~single cell diameter), raising the question of how they can mediate sustained wound responses at a distance.
Bacterial and mitochondrial proteins and peptides contain N-formylmethionyl groups [96–98]. Cell lysis allows mitochondrial formylated peptides to enter the interstitial space and act as chemoattractants by binding to G-protein coupled receptors (N-formyl peptide receptors, FPRs) on target cells [99]. The relative contribution of formylated peptide signaling to damage detection may strongly depend on injury- and tissue-type. FPRs guide neutrophil migration to liver burn injuries [14], but not to dermal laser injury sites in the mouse ear [25]. Formylated peptide signaling has also been reported to mediate epithelial wound healing in the gut [100]. However, after gut injury, the bulk of formylated peptides probably derives from commensal bacteria.
Cell stress detection
Ideally, wound responses should correct injury-induced homeostatic perturbations before they inflict irreversible damage upon the organism. To mount “preventive” wound responses, tissues need to sense reversible, pre-lytic stresses in addition to terminal cell lysis. However, wound relevant stress signals, such as calcium (Ca2+), reactive oxygen species (ROS) and mechanical stress are multipurpose cues that control a plethora of processes unrelated to tissue injury (e.g., muscle contraction, nerve transmission, etc.). How these promiscuous signals allow tissues to specifically distinguish between injury-induced and harmless homeostatic perturbations remains fundamentally unclear. Principally, pathway specificity could be encrypted by signal intensity, spatiotemporal pattern or context dependent signal interpretation. Physiological “AND-gates,” i.e., signaling circuits, which only trigger responses upon the coincidence of two concomitant inputs, are frequently utilized by immune-regulatory circuits [27]. During the zebrafish wound response the activation of pro-inflammatory cytosolic phospholipase A2 (cPLA2) requires the coincidence of a Ca2+ signal and a hypotonic stimulus to initiate eicosanoid production that mediates rapid leukocyte recruitment [28].
Stress signals: Calcium
Ca2+ is a ubiquitous second messenger [29] and an essential regulator of cellular [30] and multicellular wound responses (Box 2). Epithelial wounds trigger intercellular Ca2+ waves that propagate several hundreds of micrometers into the tissue within seconds-to-minutes after injury in vitro and in vivo [28,31–38]. Wave propagation principally allows Ca2+ signals to transport information on tissue damage to distant cells in the tissue, which appears to be important for regulating diverse wound responses, such as early immune cell recruitment, wound margin contraction and regeneration in vivo [28,36,37,39–42]. Wound-induced elevation of cytoplasmic [Ca2+] can result from plasma membrane damage, mechanical/chemical/electrical activation of Ca2+ channels, or ligand-mediated receptor activation (e.g., growth factor receptors, G-protein coupled receptors). Self-regenerative, intercellular Ca2+ wave propagation occurs when a diffusible, [Ca2+]-elevating ligand triggers its own secretion. For example, ATP released into the extracellular space upon cell lysis or mechanical stress activates G-protein coupled purinergic signaling in neighboring cells [29], leading to inositole trisphosphate (IP3) production and IP3-mediated Ca2+ release from intracellular stores. To complete the propagation cycle, Ca2+ stimulates ATP secretion through exocytosis or plasma membrane channels [43]. In this way, Ca2+ signals can spread through a wounded epithelial sheet in a domino-like fashion. IP3 diffusion through gap junctions also contributes to intercellular wave propagation [38,44]. Epithelial Ca2+ waves typically subside with increasing distance from the wound, indicating that signal propagation is not completely self-regenerative. In addition to chemical ligands, propagated physical cues, such as tissue tension or electrical fields, can contribute to wave-propagation through opening mechano- or voltage-sensitive Ca2+ channels. In the nematode C. elegans and the fruit fly D. melanogaster, injury-induced epidermal Ca2+ waves depend on transient receptor potential ion channels (TRPM) [37,40,42]. Members of the TRP superfamily sense a wide variety of chemical and physical stresses [45], and are possible mediators of physically stimulated Ca2+ wave propagation after injury.
Box 2. Calcium-dependent wound signaling.
Ca2+-binding to specific protein domains (e.g., C2- or EF-hand domains) induces activation and translocation of important signaling proteins [29]. In the context of epithelial wound responses, Ca2+ signals initiate ROS production by activating the epithelial NADPH-oxidase DUOX through binding to the enzyme’s EF-hand domains [37,101], or by triggering mitochondrial permeability transition through an unknown mechanism [41,102]. In addition, Ca2+ stimulates production and enzymatic oxidation of arachidonic acid into powerful paracrine mediators, collectively called eicosanoids. To this end, Ca2+ binds to the C2-domains of cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase (5-LOX). Ca2+ binding allows the enzymes to associate with nuclear membranes giving them access to their lipid substrates [103–105]. Oxidized arachidonic acid metabolites, such as 5-oxo-ETE or leukotriene B4, are powerful inflammatory signals that mediate rapid leukocyte recruitment to zebrafish tail fin wounds and dermal injury sites in the mouse ear, respectively [25,28]. In addition, Ca2+ governs the secretion of paracrine mediators via exocytosis and/or plasma membrane channels [106,107]. Aside from paracrine wound signaling, Ca2+ is also required for epithelial sheet motility and integrity. Ca2+ triggers actin-myosin contraction through calmodulin-mediated activation of myosin light chain kinase. Myosin contractility is required for mesenchymal and amoeboid modes of cell migration as well as for epithelial wound constriction by a contractile actin cable (“purse-string” mechanism) [108–110].
Stress signals: Reactive oxygen species
ROS have typically been regarded as unwanted, cytotoxic byproducts of aerobic metabolism. More recent studies have highlighted beneficial signaling functions of ROS during the wound response. Regardless of its particular source (Box 3), ROS production generally indicates the presence of homeostatic tissue perturbations that require the organisms’ attention. Wound-induced ROS production mediates early leukocyte recruitment in the zebrafish and fly, healing in mammalian cell culture models, and regeneration of tail fins and sensory neurons in zebrafish and frog tadpoles [36,37,41,46–51]. ROS have also been implicated in wound responses of plants [9]. Altogether, ROS appear to be integral wound regulators throughout phyla.
Box 3. Physiological sources of ROS.
ROS comprise a group of chemically highly reactive, oxygen-containing metabolites that are produced by cells upon exposure to extracellular signals as well as mechanical, chemical or metabolic stress. A major source o f ROS during pathological situations is NADPH-oxidases located on the plasma membrane or on intracellular vesicles. These enzymes generate extracellular/luminal superoxide or hydrogen peroxide (H2O2) in response to stimulation by extracellular ligands or elevated cytoplasmic Ca2+, respectively [111]. H2O2, a particularly stable ROS, can enter the cytoplasm by passive diffusion through the lipid bilayer or aquaporin water channels (AQP) [112]. ROS are also constitutively produced by the mitochondrial electron transport chain (ETC) at low, sub-micromolar concentrations. Mitochondrial ROS production is stimulated by ETC inhibition downstream of complex I or III (e.g. by hypoxia or metabolic toxins) [113], possibly owing to cosubstrate compensation within the ETC [114]. Ca2+ triggers mitochondrial ROS production through a molecularly poorly defined process termed mitochondrial permeability transition [41,102]. Unlike NADPH oxidases, mitochondria can produce ROS directly into the cytoplasm.
Given their high chemical reactivity and accordingly short half-life, ionic ROS or ROS-radicals (e.g., OH·, O2−, etc.) do not diffuse far within biological systems. Superoxide dismutase converts superoxide (O2−) into hydrogen peroxide (H2O2), which is less reactive and diffuses over larger distances, particularly within the highly oxidizing environment of the extracellular space. Owing to its extended half-life, H2O2 is capable of forming tissue-scale gradients in the interstitial space, principally allowing it to act as a paracrine signal [52]. The existence of ROS gradients around epithelial wounds has been confirmed in live zebrafish larvae by intravital imaging of a fluorescent H2O2 reporter [46]. Likewise, ROS production at epithelial wounds has been observed in D. melanogaster, C. elegans, and in mammalian cell culture [37,46–49]. In wounded zebrafish tail fin, fly epidermis, and scratched monolayers of lung epithelial cells, H2O2 is produced by the epithelial NADPH oxidase DUOX. In C. elegans, mitochondria are the major source of injury-induced ROS [41]. Ca2+ is a conserved upstream trigger for ROS production by DUOX and mitochondria [37,40,41]. ROS are thought to modulate wound-induced signaling by transient oxidation of redox sensitive cysteine residues in growth factor receptors, kinases, phosphatases, and other cytoplasmic signaling proteins [53]. This can directly modulate cell motility or chemotactic signaling in wound-responding cells [47,54,55], or enhance the enzymatic [56], or transcriptional production of wound chemoattractants, respectively [57]. Redox sensitive wound response regulators are beginning to be identified: H2O2 mediated oxidation of the leukocyte specific non-receptor tyrosine kinase LYN mediates rapid leukocyte recruitment to injured zebrafish tail fins [47]. In C. elegans, oxidation of two redox sensitive cysteines within the small GTPase RHO-1 stimulates wound closure by enhancing actin polymerization [41].
Stress signals: Mechanical stress
Cells near the wound site experience drastic changes in mechanical forces, caused either by the injury procedure itself or by disruption of epithelial sheet force balance [58]. In addition, compartmental mixing after barrier breakage perturbs extracellular homeostasis leading to secondary mechanical stress. For example, injuring the mucosal epithelium of the upper digestive tract of mammals (oral cavity and esophagus) exposes cells inside the tissue to hypotonic saliva. A similar situation is encountered upon epidermal injury of freshwater fish or amphibians [4,28,59]. A decrease in extracellular osmotic pressure leads to cell swelling and membrane stretch around the wound site. In the absence of a hypotonic stimulus, cell swelling can, for example, result from necrotic energy depletion (preceding cell lysis), or from chemotactic ligand exposure [60,61]. Cell swelling stimulates NLRP3 inflammasome activation, eicosanoid production and extracellular ATP secretion [4,28,62]. Altogether, mechanotransduction involving cell swelling or other stimuli is emerging as an important regulator of epithelial wound detection (Box 4).
Box 4. Wound-relevant mechanotransduction.
Mechanotransduction denotes a process in which force-induced conformational changes in biomolecules or macromolecular assemblies induce chemical signaling [58,115–117]. Mechanosensitive proteins (e.g., plasma membrane channels, cytoskeletal linkers) are typically associated with the force-bearing structures of a cell or the extracellular matrix. Force-induced changes in protein conformation lead to opening of channel pores, unmasking of hidden protein-binding or post-translational modification sites. Piezo- and transient receptor potential ion channels (TRP) allow influx of extracellular Ca2+ in response to plasma membrane stretch [118,119]. TRPM, a member of the TRP channel family, has been shown to be important for the injury-induced Ca2+ wave that stimulates wound closure, and ROS production in C. elegans and fruit fly [37,40–42]. Also, vesicle/membrane trafficking is subject to mechanical regulation [120]. Increased plasma membrane tension, often in conjunction with Ca2+ signals, stimulates the secretion of wound mediators, such as nucleotides through exocytotic or channel (e.g., pannexin) mechanisms [43]. In the zebrafish tail fin model, hypotonic cell swelling leads to local release of nucleotides at the wound site, which stimulates epidermal breach closure through basal epithelial cell migration [4]. Alternatively, mechanical signaling is induced by force-induced changes in membrane tension and lipid packing, for example during cell swelling. Interestingly, activation of various membrane associated proteins (curvature sensing proteins, phospholipases and pore-forming peptides, etc.) requires insertion of hydrophobic protein residues into the hydrophobic core of the lipid bilayer. Lateral membrane tension increases the spacing between the negatively charged phospholipid head groups thereby facilitating molecular access to the hydrophobic membrane core. Concordantly, purified phospholipases, amphiphilic peptides, and a variety of other membrane associated signalling proteins preferentially insert into pre-tensed membranes in vitro [121,122]. The activity of purified venom phospholipase A2 on artificial lipid vesicles increases when vesicles are osmotically swollen [123]. Similarly, phospholipase C and protein kinase C activity depends on lipid packing density in vitro [124–126]. Hypotonic exposure of cultured cells activates phospholipase A2 to generate arachidonic acid [127]. Arachidonic acid released in response to injury-induced hypotonic shock of zebrafish tail fins is further metabolized into inflammatory eicosanoids that mediate rapid wound detection by leukocytes [28].
Wound detection on the tissue level
Injury disrupts epithelial sheet continuity and barrier function. By detecting the resultant tissue perturbations (i.e., lack of epithelial constraint, or drop of osmotic pressure, etc.), an organism can continuously measure wound/breach size and adjust protective responses accordingly in an auto-regulatory fashion.
Epithelial edge detection
Unconstrained epithelial edges are associated with epithelial sheet motility after injury and during development [63,64]. Cells at an epithelial edge lack neighbors on one side, and thus experience less mechanical constraint, and receive less adhesion and junctional signaling input than cells that are entirely integrated within the sheet. Various cell culture studies have explored the individual contributions of cell damage, availability of free space, or lack of constraint to injury-induced epithelial sheet motility. Free edges stimulate epidermal growth factor receptor (EGFR) and MAP kinase signaling [64–70], indicating that lack of constraint is biochemically sensed. Interestingly, EGFR activity, which is important for sheet motility and healing, is suppressed when epithelial edges are physically constrained by a biologically inert, solid barrier [70]. This suggests that detection of epithelial edges does not likely involve surveillance of specific cell-cell interactions, but rather direct sensing of cellular constraint [64,70]. How constraint, or lack thereof, is sensed remains unknown. Propagated MAP kinase waves are involved in the regulation of long-distance wound responses in injured epithelial monolayers [67,68]. Cell damage may additionally increase migration speed and directionality near the wound border [66,67], possibly by further stimulating MAP kinase activation through ROS [67]. Extracellular ATP-induced purinergic (P2Y) signaling [65,71–75] has been described as a primary trigger for wound-induced ROS production and EGFR activation in cultured mammalian epithelia [49,76]. Although wound-induced ATP release is generally assumed to result from cytoplasmic leakage of damaged cells, cells also secrete ATP in response to non-lytic stimulation [43]. Significant amounts of ATP are released from epithelial monolayers when experimental wounds are introduced by gentle removal of agarose growth barriers, which causes minimal direct cell damage [65]. Thus, extracellular nucleotides may present a universal signal by which lysing cells, tissue edges and epithelial breaches (below) initiate epithelial motility after injury. In lung epithelial cells [49,76,77], injured zebrafish [46] and fly epidermis [37], injury-induced ROS production is mediated by DUOX. The Ca2+ required for DUOX activation was reported to derive from ATP mediated P2Y receptor activation in fish and mammalian cell culture [49,78]. By contrast, TRPM channels stimulate ROS production in flies and worms through extracellular Ca2+ influx [37,40,42]. The physiological or evolutionary rationale for the species-dependent, mechanistic differences in wound-induced Ca2+ elevation remains to be investigated.
Epithelial barrier-breach detection
Intact epithelia prevent compartmental mixing between the environment and the interstitial space of tissues. Breakdown of intercompartmental differences in charge, or concentration of molecular species may be interpreted by cells inside the tissue as a sign that the epithelium has been injured.
Electric cues may contribute to surveillance of epithelial barrier integrity. Active ion transport leads to charge separation between the inside and the outside of an epithelium, thereby generating a transepithelial potential difference (TEPD). When the electrical resistance of the epithelium is breached, the TEPD rapidly dissipates, which gives rise to a laterally oriented electric field that extends up to a millimeter away from the wound, and persists over hours [79–81]. Artificial electric fields of similar magnitude as those that occur around wounds stimulate directed migration (“electrotaxis” or “galvanotaxis”) of various cell types, including immune cells, fibroblasts and epithelial cells. A correlation has been noted between the magnitude of endogenous electric wound fields and the rate of wound healing, which holds upon TEPD perturbation with various ion channel modulators [80]. However, partial reversal of the direction of wound-induced endogenous currents by heterozygous Pax6 deletion (Pax6+/−) does not impair corneal wound healing in mice [82]. This is inconsistent with a guidance function of endogenously occurring electric fields after epithelial injury. Further work is necessary to clarify the physiological role of electric cues for wound detection.
Barrier surveillance can be also mediated by chemical cues. Intact epithelial barriers maintain chemical concentration differences between the apical and basal compartment. In human airways, the epithelial growth factor receptor erb2 is enriched on the basolateral epithelial surface, while its ligand, heregulin, is apically enriched in alveolar lung fluid. Upon injury, apical heregulin can access its basolaterally localized cognate receptor to initiate wound healing [83]. Likewise, freshwater organisms, such as Xenopus laevis and zebrafish, appear to utilize naturally occurring differences in ion and osmolyte concentration between their aquatic habitat and their interstitial fluid to survey skin integrity. Indirect observations consistent with the notion of an “osmotic surveillance” mechanism came from experiments comparing the protrusion speeds of epithelial cells in wounded Xenopus tadpoles in situ, with the protrusion speeds measured after cells were taken into culture. Namely, migration was considerably faster in situ than in cell culture, and could be reduced to cell culture levels by increasing the tonicity of the tissue immersion medium with sorbitol. It was speculated that slow cell protrusion in vitro may be due to the higher tonicity of the culture medium [59]. Other researchers found wound closure in early Xenopus embryos to be strongly inhibited by isotonic sodium chloride. However, the possibility of an osmotic effect was rejected, because isotonic glucose did not similarly inhibit wound closure [84]. Whether these findings point to developmental stage-dependent differences in wound closure mechanisms such as between early frog embryos and tadpoles, or differences in cell-specific permeability of the applied osmolytes is unclear. In support of an osmotic surveillance mechanism, recent studies have found that initial leukocyte recruitment and rapid epithelial wound closure in zebrafish is inhibited by injuring the larvae in isotonic salt or sucrose solutions [4,28]. The entry of hypotonic bathing medium into fish after epithelial breaching, triggers basal epithelial cell motility and leukocyte recruitment through (probably cell swelling-induced) nucleotide release and cPLA2 activation near the wound site. These data suggest that osmotic surveillance is a master regulator of epithelial wound detection in larval zebrafish, and raise the possibility that wet epithelia utilize environmental cues to enhance wound detection more generally, but whether this is the case stands to be seen. Regardless, it is of interest to recall that modern land-living mammals descend from freshwater fish. Curiously, and for unknown reasons, land-living mammals invest energy to desalt their saliva by ductal passage, and maintain a freshwater-like environment in the proximal part of their upper digestive tract. Through food and liquid uptake, these internal skins are directly exposed to the outside world, and likely face similar challenges as the epidermis of freshwater organisms. It is intriguing to speculate that homeostatic adjustment of their internal liquid environment to an ancestral habitat allows land-living animals to take advantage of optimized epithelial defenses that evolved millions of years ago in lakes and rivers.
Concluding remarks
Epithelial integrity is guarded by several surveillance systems that induce rapid wound closure and alert host defenses upon injury. Wound healing and inflammation function robustly in organisms that are exposed to very different physicochemical environments (air, salt/fresh water, etc.). To achieve robustness, these processes are thought to be instructed by tissue-intrinsic cues that are independent of the environment. Intrinsic cues include DAMPs that alert leukocytes to irreversible cell damage. Unconstrained epithelial edges present another intrinsic cue, which is associated with epithelial sheet motility after injury and during development. Surprisingly, recent experiments have pointed to the existence of additional, tissue extrinsic detection mechanisms. Through osmotic surveillance, wounds are detected by changes in epithelial permeability: namely, breach-induced osmotic stress on cells near wounds in larval zebrafish epidermis triggers wound signal release. Monitoring epithelial structure and barrier function by epithelial edge- or breach surveillance allows tissues to accurately measure wound size and adjust protective responses accordingly through feedback control. On the cellular level, these mechanisms operate through stress signals instead of, or in addition to DAMPs, and can function independent of cell lysis. Although the molecular identity of several stress-induced wound signals is known, numerous questions regarding their physiological function remain. Ca2+ regulates a plethora of biological processes, raising the fundamental question of how it specifically instructs wound responses. Although an increasing number of molecular ROS targets are being identified, the mechanisms by which ROS instruct directional migration remain little understood. On this note, it is worthwhile to mention that NOX/DUOX-mediated ROS generation is associated with cytoplasmic NADPH consumption, and low [NADPH] may mediate tissue damage detection by promoting the production of chemotactic oxoeicosanoids. Finally, it remains to be elucidated how chemical and mechanical stress signals are integrated in live tissues to mediate specific wound detection. Deciphering the combinatorial code of epithelial injury signaling provides a compelling challenge for future research.
Highlights.
Cell lysis, unconstrained epithelial edges, and barrier permeability function as principal cues for wound detection.
On the cellular level, wounds are detected by cell lysis, but also by reversible, sub-lytic cell stress.
Multipurpose “stress” signals, such as reactive oxygen species, calcium and mechanical strain, transducer wound cues into cellular responses.
To efficiently detect wounds, wet epithelia integrate cues from their liquid environment, in addition to tissue-intrinsic damage cues.
Acknowledgements
We thank Michelina Stoddard and Mark Jelcic for their valuable thoughts and suggestions on the manuscript. We apologize to our colleagues whose work we did not cite due to space limitations.
B.E. was supported by a Lucille Castori Center for Microbes, Inflammation and Cancer Fellowship. This work was supported by NIH grant GM099970, American Asthma Foundation Scholar grant, and a Louis V. Gerstner, Jr. Young Investigator award to P.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szpaderska AM, et al. Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 2.Seifert AW, et al. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One. 2012;7:e32875. doi: 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson R, et al. Adult zebrafish as a model system for cutaneous wound-healing research. J. Invest. Dermatol. 2013;133:1655–1665. doi: 10.1038/jid.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gault WJ, et al. Osmotic surveillance mediates rapid wound closure through nucleotide release. J. Cell Biol. 2014;207:767–782. doi: 10.1083/jcb.201408049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock KL, et al. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 7.Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat. Rev. Mol. Cell Biol. 2013;14:249–262. doi: 10.1038/nrm3541. [DOI] [PubMed] [Google Scholar]
- 8.LeBert DC, Huttenlocher A. Inflammation and wound repair. Semin. Immunol. 2014;26:315–320. doi: 10.1016/j.smim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki N, Mittler R. Reactive oxy gen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012;53:2269–2276. doi: 10.1016/j.freeradbiomed.2012.10.538. [DOI] [PubMed] [Google Scholar]
- 10.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 11.Kono H, et al. Molecular determinants of sterile inflammation. Curr. Opin. Immunol. 2014;26:147–156. doi: 10.1016/j.coi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Zugasti O, et al. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat. Immunol. 2014;15:833–838. doi: 10.1038/ni.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C-J, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 14.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 15.Yan B, et al. IL-1β and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. J. Immunol. 2014;192:5998–6008. doi: 10.4049/jimmunol.1301645. [DOI] [PubMed] [Google Scholar]
- 16.Rock KL, et al. Innate and adaptive immune responses to cell death. Immunol. Rev. 2011;243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross O, et al. The inflammasome: an integrated view. Immunol. Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 18.Guma M, et al. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joosten LAB, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miwa K, et al. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat. Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 21.Kono H, et al. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J. Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray C, et al. Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 2011;105:811–819. doi: 10.1160/TH10-08-0525. [DOI] [PubMed] [Google Scholar]
- 23.Compan V, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Kuppner MC, et al. TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8 mRNA levels and IL-6 production by human retinal pigment epithelial cells. Immunology. 1995;84:265–271. [PMC free article] [PubMed] [Google Scholar]
- 25.Lämmermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Oliveira S, et al. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. 2013;190:4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 28.Enyedi B, et al. Tissue damage detection by osmotic surveillance. Nat. Cell Biol. 2013;15:1123–1130. doi: 10.1038/ncb2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge MJ. Elementary and global aspects of calcium signalling. J. Physiol. 1997;499(Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bement WM, et al. Rehabilitation and the single cell. Curr. Opin. Cell Biol. 2007;19:95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen M, et al. Intercellular calcium signaling induced by extracellular adenosine 5’-triphosphate and mechanical stimulation in airway epithelial cells. J. Cell Sci. 1993;106(Pt 4):995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- 32.Enomoto K, et al. The increase in the intracellular Ca2+ concentration induced by mechanical stimulation is propagated via release of pyrophosphorylated nucleotides in mammary epithelial cells. Pflugers Arch. 1994;427:533–542. doi: 10.1007/BF00374271. [DOI] [PubMed] [Google Scholar]
- 33.Churchill GC, et al. Mechanical stimulation initiates cell-to-cell calcium signaling in ovine lens epithelial cells. J. Cell Sci. 1996;109(Pt 2):355–365. doi: 10.1242/jcs.109.2.355. [DOI] [PubMed] [Google Scholar]
- 34.Frame MK, de Feijter AW. Propagation of mechanically induced intercellular calcium waves via gap junctions and ATP receptors in rat liver epithelial cells. Exp. Cell Res. 1997;230:197–207. doi: 10.1006/excr.1996.3409. [DOI] [PubMed] [Google Scholar]
- 35.Klepeis VE, et al. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J. Cell Sci. 2001;114:4185–4195. doi: 10.1242/jcs.114.23.4185. [DOI] [PubMed] [Google Scholar]
- 36.Yoo SK, et al. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J. Cell Biol. 2012;199:225–234. doi: 10.1083/jcb.201203154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razzell W, et al. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boitano S, et al. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 39.Sieger D, et al. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev. Cell. 2012;22:1138–1148. doi: 10.1016/j.devcel.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Xu S, Chisholm AD. A Gαq-Ca2+ signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr. Biol. 2011;21:1960–1967. doi: 10.1016/j.cub.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antunes M, et al. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J. Cell Biol. 2013;202:365–379. doi: 10.1083/jcb.201211039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sneyd J, et al. A model for the propagation of intercellular calcium waves. Am. J. Physiol. 1994;266:C293–C302. doi: 10.1152/ajpcell.1994.266.1.C293. [DOI] [PubMed] [Google Scholar]
- 45.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat. Rev. Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 46.Niethammer P, et al. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo SK, et al. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreira S, et al. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 49.Wesley UV, et al. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J. Biol. Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 50.Rieger S, Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011;9:e1000621. doi: 10.1371/journal.pbio.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Love NR, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013;15:222–228. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enyedi B, Niethammer P. H2O2: A Chemoattractant? Methods Enzymol. 2013;528:237–255. doi: 10.1016/B978-0-12-405881-1.00014-8. [DOI] [PubMed] [Google Scholar]
- 53.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klyubin IV, et al. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur. J. Cell Biol. 1996;70:347–351. [PubMed] [Google Scholar]
- 55.Kuiper JWP, et al. Rac regulates PtdInsP3 signaling and the chemotactic compass through a redox-mediated feedback loop. Blood. 2011;118:6164–6171. doi: 10.1182/blood-2010-09-310383. [DOI] [PubMed] [Google Scholar]
- 56.Erlemann K-R, et al. Airway epithelial cells synthesize the lipid mediator 5-oxo-ETE in response to oxidative stress. Free Radic. Biol. Med. 2007;42:654–664. doi: 10.1016/j.freeradbiomed.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juarez MT, et al. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet. 2011;7:e1002424. doi: 10.1371/journal.pgen.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez GA, et al. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Radice GP. Locomotion and cell-substratum contacts of Xenopus epidermal cells in vitro and in situ. J. Cell Sci. 1980;44:201–223. doi: 10.1242/jcs.44.1.201. [DOI] [PubMed] [Google Scholar]
- 60.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grinstein S, et al. Volume changes in activated human neutrophils: the role of Na+/H+ exchange. J. Cell. Physiol. 1986;128:33–40. doi: 10.1002/jcp.1041280107. [DOI] [PubMed] [Google Scholar]
- 62.Muñoz-Planillo R, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rand H. Wound closure in actinian tentacles with reference to the problem of organization. Arch. für Entwicklungsmechanik der Org. 1915;41:159–214. [Google Scholar]
- 64.Klarlund JK, Block ER. Free edges in epithelia as cues for motility. Cell Adh. Migr. 5:106–110. doi: 10.4161/cam.5.2.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Block ER, et al. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J. Biol. Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 66.Murrell M, et al. Tension, free space, and cell damage in a microfluidic wound healing assay. PLoS One. 2011;6:e24283. doi: 10.1371/journal.pone.0024283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boettiger AN, et al. Role of boundary conditions in an experimental model of epithelial wound healing. 2006;08544:68–75. doi: 10.1152/ajpcell.00411.2005. [DOI] [PubMed] [Google Scholar]
- 68.Matsubayashi Y, et al. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr. Biol. 2004;14:731–735. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 69.Poujade M, et al. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Block ER, et al. Free edges in epithelial cell sheets stimulate epidermal growth factor receptor signaling. Mol. Biol. Cell. 2010;21:2172–2181. doi: 10.1091/mbc.E09-12-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boucher I, et al. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp. Eye Res. 2007;85:130–141. doi: 10.1016/j.exer.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boucher I, et al. The P2Y2 receptor mediates the epithelial injury response and cell migration. Am. J. Physiol. Cell Physiol. 2010;299:C411–C421. doi: 10.1152/ajpcell.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinger I, et al. Tri-nucleotide receptors play a critical role in epithelial cell wound repair. Purinergic Signal. 2005;1:281–292. doi: 10.1007/s11302-005-8132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin J, et al. Wound-induced ATP release and EGF receptor activation in epithelial cells. J. Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klepeis VE, et al. P2Y receptors play a critical role in epithelial cell communication and migration. J. Cell. Biochem. 2004;93:1115–1133. doi: 10.1002/jcb.20258. [DOI] [PubMed] [Google Scholar]
- 76.Sham D, et al. ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17. PLoS One. 2013;8:e54391. doi: 10.1371/journal.pone.0054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gieger C, et al. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480:201–208. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Oliveira S, et al. ATP modulates acute inflammation in vivo through dual oxidase 1-derived H2O2 production and NF-κB activation. J. Immunol. 2014;192:5710–5719. doi: 10.4049/jimmunol.1302902. [DOI] [PubMed] [Google Scholar]
- 79.Zhao M, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 80.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin. Cell Dev. Biol. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kucerova R, et al. The role of electrical signals in murine corneal wound reepithelialization. J. Cell. Physiol. 2011;226:1544–1553. doi: 10.1002/jcp.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vermeer PD, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 84.Fuchigami T, et al. Exposure to external environment of low ion concentrations is the trigger for rapid wound closure in Xenopus laevis embryos. Zoolog. Sci. 2011;28:633–641. doi: 10.2108/zsj.28.633. [DOI] [PubMed] [Google Scholar]
- 85.Burnstock G. Introductory overview of purinergic signalling. Front. Biosci. (Elite Ed) 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 87.Müller T, et al. The purinergic receptor P2Y2 r eceptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 88.Vanderstocken G, et al. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. J. Immunol. 2010;185:3702–3707. doi: 10.4049/jimmunol.0903908. [DOI] [PubMed] [Google Scholar]
- 89.Idzko M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 90.Lu D, et al. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 2012;26:2580–2591. doi: 10.1096/fj.12-204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Block ER, Klarlund JK. Wounding sheets of epithelial cells activates the epidermal growth factor receptor through distinct short- and long-range mechanisms. Mol. Biol. Cell. 2008;19:4909–4917. doi: 10.1091/mbc.E08-01-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gorissen SH, et al. Dual oxidase-1 is required for airway epithelial cell migration and bronchiolar reepithelialization after injury. Am. J. Respir. Cell Mol. Biol. 2013;48:337–345. doi: 10.1165/rcmb.2012-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu D, Insel PA. Hydrolysis of extracellular ATP by ectonucleoside triphosphate diphosphohydrolase (ENTPD) establishes the set point for fibrotic activity of cardiac fibroblasts. J. Biol. Chem. 2013;288:19040–19049. doi: 10.1074/jbc.M113.466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orriss IR, et al. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell. Physiol. 2009;220:155–162. doi: 10.1002/jcp.21745. [DOI] [PubMed] [Google Scholar]
- 95.Orriss IR, et al. Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralisation. PLoS One. 2013;8:e69057. doi: 10.1371/journal.pone.0069057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta. 1999;1410:103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 97.Marcker K, Sanger F. N-FORMYL-METHIONYL-S-RNA. J. Mol. Biol. 1964;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- 98.Sagan L. On the origin of mitosing cells. J. Theor. Biol. 1967;14:255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 99.Le Y, et al. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 100.Babbin BA, et al. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 2007;179:8112–8121. doi: 10.4049/jimmunol.179.12.8112. [DOI] [PubMed] [Google Scholar]
- 101.Donkó A, et al. Dual oxidases. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:2301–2308. doi: 10.1098/rstb.2005.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium. 2014;56:437–445. doi: 10.1016/j.ceca.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009;50(Suppl):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rådmark O, et al. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 105.Brock TG. Regulating leukotriene synthesis: the role of nuclear 5-lipoxygenase. J. Cell. Biochem. 2005;96:1203–1211. doi: 10.1002/jcb.20662. [DOI] [PubMed] [Google Scholar]
- 106.Gustavsson N, et al. Calcium sensing in exocytosis. Adv. Exp. Med. Biol. 2012;740:731–757. doi: 10.1007/978-94-007-2888-2_32. [DOI] [PubMed] [Google Scholar]
- 107.Penuela S, et al. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta. 2013;1828:15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 108.Vicente-Manzanares M, Horwitz AR. Cell migration: an overview. Methods Mol. Biol. 2011;769:1–24. doi: 10.1007/978-1-61779-207-6_1. [DOI] [PubMed] [Google Scholar]
- 109.Bement WM, et al. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J. Cell Biol. 1993;121:565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 111.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 112.Bienert GP, et al. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 113.Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kueh HY, et al. Maintenance of mitochondrial oxygen homeostasis by cosubstrate compensation. Biophys. J. 2013;104:1338–1348. doi: 10.1016/j.bpj.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 116.Chicurel ME, et al. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- 117.DuFort CC, et al. Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eijkelkamp N, et al. Transient receptor potential channels and mechanosensation. Annu. Rev. Neurosci. 2013;36:519–546. doi: 10.1146/annurev-neuro-062012-170412. [DOI] [PubMed] [Google Scholar]
- 119.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gauthier NC, et al. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22:527–535. doi: 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 121.Janmey PA, Kinnunen PKJ. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Polozov IV, et al. Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophys. J. 2001;81:949–959. doi: 10.1016/S0006-3495(01)75753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys. J. 1995;68:1888–1894. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slater SJ, et al. The modulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 1994;269:4866–4871. [PubMed] [Google Scholar]
- 125.Souvignet C, et al. Activation of protein kinase C in lipid monolayers. J. Biol. Chem. 1991;266:40–44. [PubMed] [Google Scholar]
- 126.Boguslavsky V, et al. Effect of monolayer surface pressure on the activities of phosphoinositide-specific phospholipase C-beta 1, -gamma 1, and -delta 1. Biochemistry. 1994;33:3032–3037. doi: 10.1021/bi00176a036. [DOI] [PubMed] [Google Scholar]
- 127.Hoffmann EK, et al. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]