Abstract
Tourette Syndrome is a disorder characterized by tics. It typically begins in childhood and often improves in adult life. Tics are best described as voluntary movements made automatically so that volition is not ordinarily appreciated. There is frequently an urge, sometimes in the form of a specific sensory feeling (sensory tic), that precedes the tic. Patients say that they make the tic in order to reduce the urge, although shortly after the tic, the urge recurs. The sensory feeling may arise due to defective sensory habituation. Since tics relieve the urge, this can be considered rewarding, and repetition of this behavior may perpetuate the tic as a habit. Tourette Syndrome affects boys more than girls and is associated with attention deficit hyperactivity disorder and obsessive compulsive disorder. Although Tourette Syndrome often appears to be autosomal recessive in inheritance, it has been difficult to find any abnormal genes. There is a loss of inhibition in these patients and recent studies show abnormalities in brain GABA. Certainly there is also an abnormality in dopamine function and dopamine blocking agents are effective therapy. In severe drug-refractory patients, deep brain stimulation can be effective.
Keywords: Tic, Urge, Sensory habituation, Habit, Inhibition, Dopamine
Tourette Syndrome (TS) is a disorder, mainly of childhood, with a prominent manifestation of tics. In DSM-5, an alternate term for TS is Tourette’s Disorder. Tics, as formally defined in DSM-5, are “sudden, rapid, recurrent, nonrhythmic motor movements or vocalizations, generally preceded by urge”. It should be noted that young children, less than ten years of age, most often do not report urge, and that could be either because there is no urge or it is difficult to describe. There are a number of tic syndromes, and perhaps the most common is provisional tic disorder (previously called transient tic of childhood) where the tics last about one year. In order to qualify for TS, again by DSM-5, there must be multiple motor and vocal tics present at some time with the disorder beginning before age 18 and lasting more than one year, and where the tics are not secondary to a physiological substance or other neurological disorder. There are a number of patients who do not have vocal tics, but are in other ways similar, and, while that disorder would be called persistent tic disorder (previously, chronic motor tic), the disorder is likely the same.
The Tourette International Consortium reported on the characteristics of 6805 patients with TS. [1] The male to female ratio is 4.4 to 1; the mean age of onset is 6.4 years. A family history is present in 51.7% of patients. Attention deficit/Hyperactivity Disorder (ADHD) is seen in 55.6%, and obsessive compulsive disorder (OCD) is seen in 54.9%. In terms of clinical course, tics disappear in adult life in approximately half of patients, are improved in 40 to 45% of cases, and remain in only 5 to 10% of patients. [2, 3]
Tics can affect any part of the body, but seem particularly prominent in the face, such as eye blinking. Tics can be either simple or complex movements, but there is no evidence that they are different in type. Vocalizations can be a variety of sounds and words, including coprolalia. The urge, or premonitory phenomenon, can be just an inner tension of wanting to move or can be a specific feeling in a specific part of the body. If in a specific body part, it can be called a sensory tic, and many patients will say that the sensory tic is the main aspect of the disorder, since the movement is made voluntarily to make the sensation go away. Unfortunately, the benefit is only short lasting and the urge or sensory tic builds up shortly again. In a series of 50 patients, sensory tics were localized in face and head in 73%, neck in 66%, shoulders 56%, arms 39%, hands 34%, throat 34%, and other sites less than 30% each. [4] In the same series, character of the urge most frequently, between 80 and 90%, was “urge to move” or “had to do it”, whereas more sensory feeling such as “ache”, “itching” and “tingling/numbness” were only a little more than 20% each.
While the family history is often positive and a genetic etiology is assumed, finding relevant mutations has been very difficult. It appears that the disorder is complex and multifactorial with some rare Mendelian genes and many risk genes. In the risk gene category, many seem to involve the dopaminergic and serotonergic systems. A recent large genome-wide association study identified the largest signal from rs7868992 on chromosome 9q32 within the gene COL27A1, but the meaning of this is unclear. [5] There is evidence for a mutation in the gene leading to histidine decarboxylase deficiency as a rare stronger influence in causing TS. [6]
Pathophysiology
In order to determine where the abnormalities are in the brain in patients with TS, there have been many neuroimaging studies. Studies examining brain structure have used a variety of methods, and while they seem reasonable many studies are not compatible with each other. This might be due to small numbers of subjects, difficulties with the methods, or other factors not determined. As examples, a few of these studies will be noted here. In a study of caudate volume in 43 patients studied once before age 14 and again about 7.5 years later, showed that caudate volume correlates significantly and inversely with severity of tics (and OCD) in early adulthood. [7] Using voxel based morphometry (VBM), one study showed increased gray matter in the midbrain, [8] while another showed increased gray matter in the ventral putamen and left hippocampus. [9] The white matter tracts have also been examined using diffusion tensor imaging (DTI). [10] Analyzing the data with probabilistic fiber tractography, there were reductions in connectivity between supplementary motor area (SMA) and basal ganglia, as well as in frontal cortico-cortical circuits. [11]
Physiological studies have revealed some valuable details about the nature of the disorder. Starting on the sensory side, not only are there frequency sensory tics in the patients, but often they report that sensory stimuli seem particularly bothersome. In a questionnaire study, patients reported that there were very sensitive to stimuli in every modality, sound, light, smell and taste as well as touch. [12] Moreover, the patients noted that faint stimuli might well be more troublesome than more intense stimuli. Following the questionnaire, the investigators did some quantitative sensory testing. They found that sensory thresholds were the same as normal for tactile stimuli on the leg or site of sensory trick and the same for olfactory stimuli. Moreover, the subjective intensity near threshold was similar to normal, despite noting that faint stimuli were generally more bothersome. The interpretation of these data suggests that the problem is not that of simple sensory perception, but might be a deficiency of habituation.
Evaluating the brain activity associated with tic has been done with EEG and neuroimaging techniques. Starting with the EEG, a number of groups have looked specifically for the EEG activity preceding the tic. EEG activity preceding voluntary movement is characterized by a slowly rising negativity for about 1 second, called the Bereitschaftspotential (BP). [13] The BP has two components, called early and late. The early component arises from both medial and lateral area 6, the SMA and premotor cortex, respectively. The later component, which is more lateralized over the motor cortex contralateral to the moving limb, includes activity of the primary motor cortex. Since many of the patients say the tic is actually voluntary, one might expect a BP preceding the tic. However, the BP may well be fully absent [14] or just consisting of the late component. [15] In a study of 14 patients, 6 had a BP and only 4 of these included the early component. [16] This finding suggests diminished involvement of area 6. Certainly some patients will say that the movement is involuntary, and some will be unsure of whether the tics are voluntary or involuntary. This confusion might arise if the tics are highly automatic and the sense of volition might well be weak.
Functional neuroimaging studies have been particularly revealing. Since tics are discrete events, it is possible to do event-related design functional MRI (fMRI). The BOLD signal is aligned with the tic, similar to how the BP is identified with EEG. In the critical study, the investigators looked at brain events at tic onset and two seconds before (Figure 1). [17] Two seconds before is presumed to be at the peak of the urge to tic. At tic onset, the sensorimotor area and a region in the superior parietal lobule were active, not surprising since they are related to movement production. Two second prior to tic, activation was prominent in the mesial and lateral premotor areas, the anterior cingulate cortex and insula bilaterally. The latter two regions are closely related to each other and the limbic system. In another study using functional positron emission tomography (PET) with blood flow methods, subjects were evaluated in several states. [18] For patients, it was presumed that their waking state is constantly dominated by urge and tics, but that this would be dampened by sleep. Thus, the change of waking compared with sleep should indicate tic activated areas. This activity could be compared with the difference in normal subjects awake and asleep. Remarkably, this study showed prominent activity in anterior cingulate and insula (as well as the cerebellum) similar to what was seen in the urge state in the fMRI study.
Figure 1.
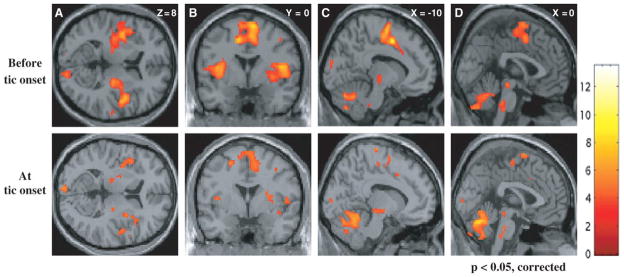
Event related functional MRI of tics. Statistical parametric maps superimposed on axial (A), coronal (B) and sagittal (C and D) views are shown. The upper row shows significant activations (P < 0.05, corrected for multiple comparisons) of paralimbic areas (ACC and insular region bilaterally) before tic onset; these activations were much less prominent at tic onset (lower row). From Bohlhalter S, Goldfine A, Matteson S et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain 2006;129:2029–2037, with permission.
Importantly, there are now a number of studies showing that the insula and anterior cingulate are critical structures for urge in normal subjects. A good model for this is blink suppression. Persons can suppress blinking for a period of time, but only at the expense of increasing urge to blink. Using blood flow PET, comparing blink suppression and rest, there was prominent activation in both insula and anterior cingulate cortex. [19] Also using event-related design fMRI, the activity related to blink after attempted suppression shows the same regions (among some others). [20]
Moreover, there has now been a demonstration of increased resting state connectivity in Tourette patients between the insula and the sensorimotor cortex. [21] Hence it is possible to speculate that urge activity in the insula directly influences the motor cortex to produce tics, bypassing the premotor cortex (which then explains the reduced amplitude of the BP).
There is evidence for loss of inhibition in the brains of patients with TS. This loss of inhibition might well contribute to triggering of movement more readily. One bit of evidence is decreased short intracortical inhibition (SICI) assessed with paired-pulse transcranial magnetic stimulation (TMS). [22] SICI assesses one network of intracortical inhibitory circuitry. Another physiological measure of inhibition is prepulse inhibition, where an initial relatively small sensory stimulus inhibits a reflex response to a second stronger sensory stimulus. This is also reduced in TS patients. [23, 24] Underlying the loss of inhibition might well be an abnormal pattern of GABA-ergic neurons. In postmortem examination of the basal ganglia of TS patients, an abnormal distribution of parvalbumin positive neurons in the different nuclei was found. [25] Flumazenil, radioactively labeled, can bind to the GABA receptor complex. Using this as a PET ligand, a maldistribution of flumazenil binding was identified. [26] The regions of most significant decrease were in the bilateral ventral striatum, thalamus, amygdala and the right insula (Figure 2).
Figure 2.
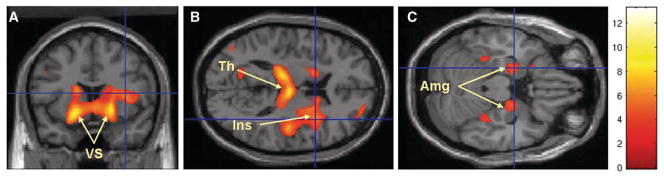
Brain areas with decreased binding of [11C]flumazenil in Tourette syndrome patients versus control subjects: the most significant decreases were seen in the bilateral ventral striatum (VS), bilateral thalamus (Th), right insula (Ins) and bilateral amygdala (Amg). From Lerner A, Bagic A, Simmons JM, et al. Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain 2012;135:1926–1936, with permission.
Another theme in the physiological understanding of tic relates to the dopamine system. There is strong evidence for overactivity of dopaminergic systems. A valuable theory synthesizing considerable information suggests that there is a developmental hypofunction of dopamine neurons leading to dopamine receptor hypersensitivity. [27–29] One important function of the dopamine system is to signal reward. If performing a tic relieves urge and makes a patient feel better, this is essentially a reward. Rewarded behavior tends to be repeated. Repeated behaviors can become habits. [30] Hence, tics could well be a form of habit, arising from a dopamine signal in response to making a movement to relieve an urge. Objection to this idea for young children would be that the description of urge is not common.
Treatment
There are a variety of methods that can be used to help patients with bothersome tics. [3, 31, 32] The first consideration of course is whether to treat or not. Treatment is only symptomatic and some patients have only mild tics; hence, treatment might be worse than the disease. Moreover, in most patients, tics are self-limited and will eventually go away. However, if symptomatic treatment is needed, there can be effective therapy.
A popular approach these days that avoids drugs is behavior therapy, most commonly habit reversal therapy. [33, 34] This procedure follows from the idea that tics are habits. Patients are taught to recognize the urge and to try to deal with it by doing something more socially acceptable than making a tic.
In terms of drug therapy, [35–37] should that be needed, most experts recommend the use of alpha-adrenergic agonists as the first line. These include clonidine and guanfacine. The next step would be the second-generation, or atypical antipsychotics, of which the best evidence is for risperidone and weaker evidence is for aripiprazole. Another consideration, perhaps preceding the atypicals, would be tetrabenazine. [38] Step three would be the first generation, or typical, antipsychotics which are more potent, but may have more side effects. These would include pimozide, perhaps as first choice since it has regulatory approval in the USA, haloperidol and fluphenazine.
Another consideration is a focal injection of botulinum neurotoxin. [31, 32] Interestingly, these injections not only reduce motor tics, but also sensory tics.
It should be recognized that cannabinoids appear to have efficacy also for tics in Tourette syndrome, [39] and while this therapy has been mostly done outside the medical arena, the use of these agents is coming into the main stream.
Should all else fail, if a patient has very severe symptoms, particularly if already an adult, then deep brain stimulation (DBS) can be considered. [40, 41] While the evidence is largely anecdotal, and the optimal target is yet to be determined, DBS does seem to work. Surgeons have targeted the medial thalamus, the globus pallidus, the nucleus accumbens, the anterior limb of the internal capsule and the subthalamic nucleus.
Acknowledgments
This work was supported by the NINDS Intramural Program.
Footnotes
Parts of this paper was presented as an invited lecture at the 56th annual meeting of the Japanese Society of Child Neurology, Hamamatsu, Japan, May 30, 2014.
Conflicts of interest
None relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freeman RD Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16 (Suppl 1):15–23. doi: 10.1007/s00787-007-1003-7. [DOI] [PubMed] [Google Scholar]
- 2.Bagheri MM, Kerbeshian J, Burd L. Recognition and management of Tourette’s syndrome and tic disorders. Am Fam Physician. 1999;59:2263–72. 74. [PubMed] [Google Scholar]
- 3.Robertson MM. The Gilles de la Tourette syndrome: the current status. Arc Dis Child Educ Pract Ed. 2012;97:166–75. doi: 10.1136/archdischild-2011-300585. [DOI] [PubMed] [Google Scholar]
- 4.Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette’s syndrome. Mov Disord. 2003;18:1530–3. doi: 10.1002/mds.10618. [DOI] [PubMed] [Google Scholar]
- 5.Scharf JM, Yu D, Mathews CA, Neale BM, Stewart SE, Fagerness JA, et al. Genome-wide association study of Tourette’s syndrome. Mol Psychiatry. 2013;18:721–8. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellan Baldan L, Williams KA, Gallezot JD, Pogorelov V, Rapanelli M, Crowley M, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81:77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–8. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette’s syndrome. Ann Neurol. 2006;59:381–5. doi: 10.1002/ana.20765. [DOI] [PubMed] [Google Scholar]
- 9.Ludolph AG, Juengling FD, Libal G, Ludolph AC, Fegert JM, Kassubek J. Grey-matter abnormalities in boys with Tourette syndrome: magnetic resonance imaging study using optimised voxel-based morphometry. Br J Psychiatry. 2006;188:484–5. doi: 10.1192/bjp.bp.105.008813. [DOI] [PubMed] [Google Scholar]
- 10.Muller-Vahl KR, Grosskreutz J, Prell T, Kaufmann J, Bodammer N, Peschel T. Tics are caused by alterations in prefrontal areas, thalamus and putamen, while changes in the cingulate gyrus reflect secondary compensatory mechanisms. BMC Neurosci. 2014;15:6. doi: 10.1186/1471-2202-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng B, Braass H, Ganos C, Treszl A, Biermann-Ruben K, Hummel FC, et al. Altered intrahemispheric structural connectivity in Gilles de la Tourette syndrome. Neuroimage Clin. 2014;4:174–81. doi: 10.1016/j.nicl.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belluscio BA, Jin L, Watters V, Lee TH, Hallett M. Sensory sensitivity to external stimuli in Tourette syndrome patients. Mov Disord. 2011;26:2538–43. doi: 10.1002/mds.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–56. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Obeso JA, Rothwell JC, Marsden CD. Simple tics in Gilles de la Tourette’s syndrome are not prefaced by a normal premovement EEG potential. J Neurol Neurosurg Psychiatry. 1981;44:735–8. doi: 10.1136/jnnp.44.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karp BI, Porter S, Toro C, Hallett M. Simple motor tics may be preceded by a premotor potential. J Neurol Neurosurg Psychiatry. 1996;61:103–6. doi: 10.1136/jnnp.61.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Salm SM, Tijssen MA, Koelman JH, van Rootselaar AF. The bereitschaftspotential in jerky movement disorders. J Neurol Neurosurg Psychiatry. 2012;83:1162–7. doi: 10.1136/jnnp-2012-303081. [DOI] [PubMed] [Google Scholar]
- 17.Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–37. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 18.Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68:1979–87. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- 19.Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, et al. Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex. 2009;19:218–23. doi: 10.1093/cercor/bhn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman BD, Horovitz SG, Morel B, Hallett M. Neural correlates of blink suppression and the buildup of a natural bodily urge. Neuroimage. 2012;59:1441–50. doi: 10.1016/j.neuroimage.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tinaz S, Belluscio BA, Malone P, van der Veen JW, Hallett M, Horovitz SG. Role of the sensorimotor cortex in tourette syndrome using multimodal imaging. Hum Brain Mapp. 2014;35:5834–46. doi: 10.1002/hbm.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–84. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- 23.Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: Preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 24.Zebardast N, Crowley MJ, Bloch MH, Mayes LC, Wyk BV, Leckman JF, et al. Brain mechanisms for prepulse inhibition in adults with Tourette syndrome: initial findings. Psychiatry Res. 2013;214:33–41. doi: 10.1016/j.pscychresns.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102:13307–12. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner A, Bagic A, Simmons JM, Mari Z, Bonne O, Xu B, et al. Widespread abnormality of the γ-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135:1926–36. doi: 10.1093/brain/aws104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segawa M. Neurophysiology of Tourette’s syndrome: pathophysiological considerations. Brain Dev. 2003;25:S62–9. doi: 10.1016/s0387-7604(03)90011-8. [DOI] [PubMed] [Google Scholar]
- 28.Nomura Y, Segawa M. Neurology of Tourette’s syndrome (TS) TS as a developmental dopamine disorder: a hypothesis. Brain Dev. 2003;25:S37–42. doi: 10.1016/s0387-7604(03)90007-6. [DOI] [PubMed] [Google Scholar]
- 29.Nomura Y, Fukuda H, Terao Y, Hikosaka O, Segawa M. Abnormalities of voluntary saccades in Gilles de la Tourette’s syndrome: pathophysiological consideration. Brain Dev. 2003;25:S48–54. doi: 10.1016/s0387-7604(03)90009-x. [DOI] [PubMed] [Google Scholar]
- 30.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 31.Termine C, Selvini C, Rossi G, Balottin U. Emerging treatment strategies in Tourette syndrome: what’s in the pipeline? Int Rev Neurobiol. 2013;112:445–80. doi: 10.1016/B978-0-12-411546-0.00015-9. [DOI] [PubMed] [Google Scholar]
- 32.Kurlan RM. Treatment of Tourette syndrome. Neurotherapeutics. 2014;11:161–5. doi: 10.1007/s13311-013-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta N, Cavanna AE. The effectiveness of habit reversal therapy in the treatment of Tourette syndrome and other chronic tic disorders: a systematic review. Funct Neurol. 2013;28:7–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Wile DJ, Pringsheim TM. Behavior Therapy for Tourette Syndrome: A Systematic Review and Meta-analysis. Curr Treat Options Neurol. 2013;15:385–95. doi: 10.1007/s11940-013-0238-5. [DOI] [PubMed] [Google Scholar]
- 35.Shaw ZA, Coffey BJ. Tics and Tourette Syndrome. Psychiatr Clin North Am. 2014;37:269–86. doi: 10.1016/j.psc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann A, Worbe Y. Pharmacological treatment of Gilles de la Tourette syndrome. Neurosci Biobehav Rev. 2013;37:1157–61. doi: 10.1016/j.neubiorev.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Egolf A, Coffey BJ. Current pharmacotherapeutic approaches for the treatment of Tourette syndrome. Drugs Today. 2014;50:159–79. doi: 10.1358/dot.2014.50.2.2097801. [DOI] [PubMed] [Google Scholar]
- 38.Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012;34:1487–504. doi: 10.1016/j.clinthera.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Muller-Vahl KR. Treatment of Tourette syndrome with cannabinoids. Behav Neurol. 2013;27:119–24. doi: 10.3233/BEN-120276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller-Vahl KR. Surgical treatment of Tourette syndrome. Neurosci Biobehav Rev. 2013;37:1178–85. doi: 10.1016/j.neubiorev.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Pouratian N. Deep brain stimulation for Tourette syndrome. Neurosurg Clin N Am. 2014;25:117–35. doi: 10.1016/j.nec.2013.08.009. [DOI] [PubMed] [Google Scholar]


