Abstract
Background & Aims
Alcoholic liver disease (ALD) is characterized by the development of fatty liver, alcoholic hepatitis, fibrosis and cirrhosis. However, the underlying mechanism(s) associated with progression remains elusive. Pro-inflammatory cytokines have been implicated in ALD progression due to pro-apoptotic effects on hepatocytes. Wnt/β-catenin signaling recently has been shown to promote inflammation and apoptosis, suggesting that activation of this signaling pathway may modulate ALD progression. The current study was designed to test whether pharmacological activation of Wnt/β-catenin signaling altered ALD development and progression in a rat model.
Methods
Adult male Long Evans rats were fed with isocaloric liquid diets containing 0% or 37% ethanol for 8 weeks, and also treated with Wnt agonist during the last 3 weeks of the feeding regimen. Liver and blood samples were subjected to histology, TUNEL assay, immunoblot analysis, real-time quantitative PCR, and alanine transaminase (ALT) assay.
Results
Wnt/β-catenin signaling was negatively correlated with Foxo3A expression and reduced steatosis, cellular injury and apoptosis in ALD rats. Mutation experiments demonstrated that Foxo3A was critical for modulating these effects. Activation of Wnt/β-catenin signaling suppressed Foxo3Ainduced apoptosis through up-regulation of serum/glucocorticoid regulated kinase 1 (SGK1). Moreover, pharmacological restoration of Wnt/β-catenin signaling reduced ALD progression in vivo.
Conclusion
Wnt/β-catenin signaling plays a protective role in ALD progression via antagonizing Foxo3A-induced apoptosis and activation of the Wnt/β-catenin signaling cascade attenuates ALD progression.
Keywords: Wnt, alcoholic liver disease, apoptosis, Foxo3A, SGK1
Introduction
Alcoholic liver disease (ALD) is associated with heavy consumption and often presents after 20–30 years chronic abuse. ALD progression features three histologic stages, i.e.fatty liver, alcoholic hepatitis, and hepatic fibrosis or cirrhosis. Fatty liver is often reversible with cessation of drinking. On the other hand, chronic alcoholic hepatitis is a more severe form of ALD and 20% of patients develop hepatic cirrhosis, which is the 12th leading cause of death in the United States in 2008[1, 2]. Although the early stages of ALD are usually reversible with abstinence, chronic alcoholic hepatitis is a potentially progressive and difficult disease to control due to inflammation-induced hepatic damage and hepatocyte apoptosis. These pro-inflammatory cytokines that trigger inflammatory responses and apoptosis have been observed in the liver of animal models of ALD as well as in chronic alcoholics[3, 4]. Moreover, targeting elevated levels of inflammatory cytokines, such as TNFα and IL1β has been investigated as potential therapeutic approaches to alter ALD progression[5, 6]. However, inhibition of proinflammatory cytokine signaling by anti-TNFα agents has been shown to increase the risk of bacterial or viral infection[7–14]. Currently, other potential targets which modulate inflammatory responses are being explored in a therapeutic context to arrest ALD progression via suppressing inflammation[4].
Wnt/β-catenin signaling has been evaluated for its role in liver development and regeneration. Conditional depletion of β-catenin in hepatocytes with hepatocyte nuclear factor 3 gamma (HNF3γ) driven by Cre recombinase produced an undeveloped liver at embryonic day 12 and lethality at day 17 in mice[15]. In addition, loss of β-catenin expression in the liver delayed proliferation of hepatocytes in mice following partial hepatectomy[16, 17]. However, β-catenin recently has been investigated for its function in modulating inflammatory responses. Specific knockout of β-catenin in dendritic cells has been shown to inhibit the immune response against bacterial infection[18]. Wnt/β-catenin signaling has also been shown to be involved in Foxo3A-mediated apoptosis. Overexpression of β-catenin antagonizes Foxo3A-induced apoptosis in colon cancer cells[19]. Interestingly, liver-specific β-catenin knockout in mice show more severe ALD phenotypes than wild-type mice after chronic ethanol consumption and the mechanisms appear to involve induction of oxidative stress[20, 21].
Although evidence is presented to indicate that Wnt/β-catenin signaling is very important in liver development, regeneration or ALD progression, there is no information on whether activation of Wnt/β-catenin signaling could reduce ALD progression. Herein, the current study was designed to test whether pharmacological activation of Wnt/β-catenin signaling altered ALD development and progression in a rat model.
Materials and methods
Animal experiments
Long Evans (LE) rats were purchased from Harlan Laboratories (South Easton, MA). This ALD model was developed as previously described[22]. Briefly, LE rats were pair-fed with a liquid diet containing either 37% ethanol or an isocaloric liquid diet for 8 weeks. During the last three weeks of feeding, a Wnt agonist (Sigma; 5mg/kg) was introduced by intra-peritoneal injection twice weekly. Rats were sacrificed with isoflurane and the liver and blood collected for biochemical analysis.
Cells and reagents
Immortalized human hepatocytes, Huh7, and Hep3B cells were grown in DMEM Dulbecco's Modified Eagle Medium with 10% fetal bovine serum, L -glutamine (Life Technologies, Gaithersburg, MD), and MEM nonessential amino acids (Sigma Chemical Co, St Louis, MO). Alanine aminotransferase enzymatic activity assay kit was purchased from Cayman Chemical. TUNEL staining kit (In Situ Cell Death Detection Kit) was purchased from Roche. CID 11210285 hydrochloride (2-Amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3-methoxyphenyl)pyrimidine hydrochloride, AMBMP, N4-(1,3-benzodioxol-5-ylmethyl)-6-(3-methoxyphenyl)-2,4-pyrimidinediamine hydrochloride, Wnt Agonist) and LiCl were purchased from Sigma. SGK1 inhibitor was purchased from Santa Cruz Biotechnology. The plasmids of Foxo3A (FLAG-Foxo3A WT, #8360) and Foxo3A without DNA binding domain (FLAG-Foxo3A TM, #8361)[23] were from Addgene.
Immunoblot analysis
Immunoblot analysis (IB) was performed as previously described[24]. In brief, 50 µg of total protein was used for all IB data. Antibodies of pAkt, Akt, pGSK3β, GSK3β, β-catenin, β-actin, pFoxo3A, Foxo3A, pro-caspase 3, cleaved-caspase 3, and Bcl-XL were purchased from Cell Signaling Technology and the dilution factor was 1:1000. α-tubulin antibody was purchased from Sigma and the dilution factor was 1:3000. Antibodies of SGK1, cyclin D1, Sirt1, lamin A/C, and GADPH were purchased from Santa Cruz Biotechnology and the dilution factor was 1:500. Relative expression density was quantified using Quantity One software (Bio-Rad).
Real Time quantitative PCR (RT-qPCR)
RT-qPCR was performed as previously described[25]. Briefly, 1 µg of total mRNA was used to reverse transcribe cDNA with iScript kit (Bio-Rad). cDNA was diluted 10 folds before performing qPCR. mRNA expression was relative to internal control and assayed in triplicate.
Immunohistochemical staining
Rat liver were obtained, fixed in 10% neutral buffered formalin, and processed to a paraffin embedded block as previously described[26]. Basically, 5 µm tissue slides were processed for antigen retrieval with 10 mM Sodium Citrate (pH value of 6). The tissue slides were blocked with 5% BSA for 1 hr and incubated with indicated 1st antibodies prepared with 5% BSA in PBS overnight at 4°C. Then slides were washed 5 times with 0.5% PBST and incubated with 1:1000 diluted biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for another hour. Slides were washed 5 times with 0.5% PBST and incubated with ABC solution (Vector Laboratories). Tissues were visualized by AEC (DAKO, Carpenteria, CA), followed by Mayor's hematoxylin counterstaining. Stained tissue slides were dehydrated and covered with cover slides. Images were obtained with the microscopic camera and quantified with the image pro plus software.
TUNEL staining
TUNEL staining was performed following instruction manual. Basically, liver slides were processed and stained with TUNEL kit. Images were taken and analyzed as previously described[27].
Statistical analysis
All statistical analysis was performed using student t test. p-value was considered significant when p-values is smaller than 0.05. The results were reported as mean ± SD or SE (n = 3–10).
Results
Chronic ethanol consumption produces injury, apoptosis, and reduced β-catenin signaling
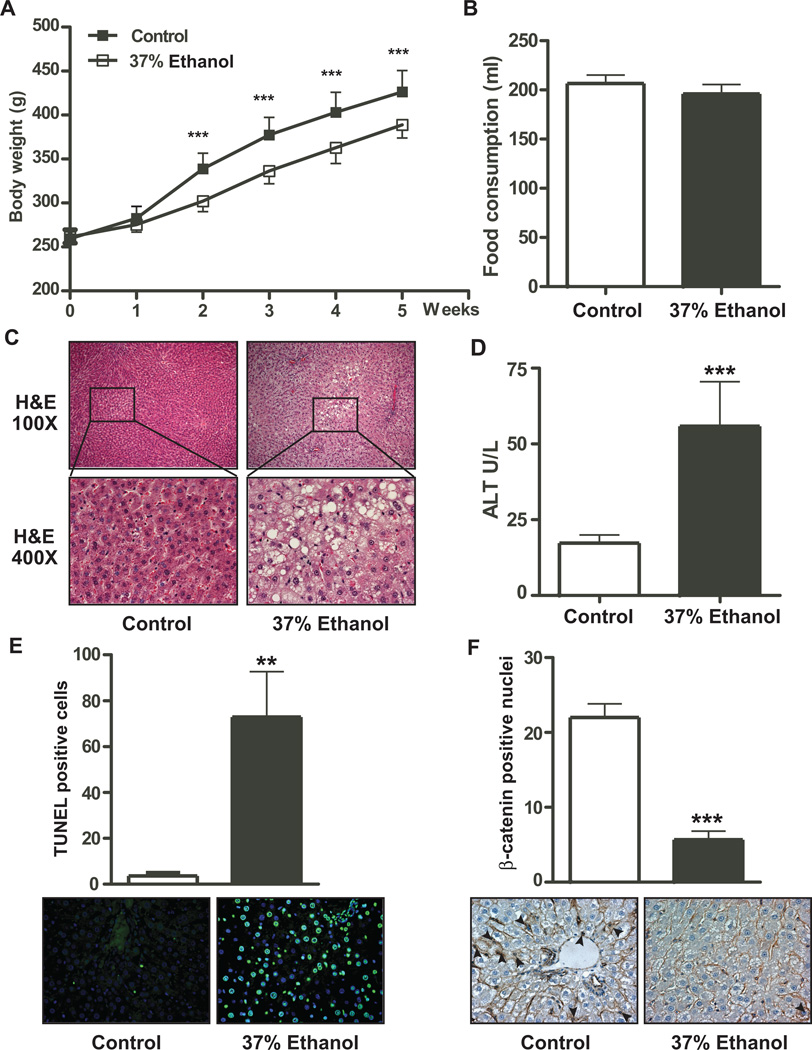
Eight weeks of 37% ethanol consumption reduced body weight in ALD rats starting as early as week 2 (Fig. 1A and sFig. 1A). Although body weight gain was reduced in ALD rats, no significant differences in food consumption was measured between control and 37% ethanol fed animals (Fig. 1 B). There was no difference in liver to body weight ratio as well (sFig. 1B). However, fat to body weight ratio and blood glucose level were decreased (non-fasting) in ethanol fed rats (sFigs. 1C and 1D). Significantly elevated ethanol levels were found in ethanol fed rats (sFig. 1E). To determine the degree of liver injury produced by chronic ethanol consumption, we performed H&E staining and measured alanine transaminase (ALT) levels to assess lipid accumulation and hepatic injury, respectively. The H&E staining revealed extensive steatosis in ethanol fed rats (Fig. 1C). An ALT enzymatic assay revealed significantly higher ALT activity compared to isocaloric pair fed controls. Further studies with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay also exhibited robust positive signals in the liver of ethanol fed rats but it was barely detectable in isocaloric pair fed controls which suggest extensive hepatic DNA damage produced by chronic ethanol. Interestingly, nuclear and cytoplasmic expression of β-catenin was decreased in the livers of ALD rats as well (Fig. 1F), suggesting that Wnt/β-catenin signaling may be disrupted and could place the liver at risk for enhanced apoptosis.
Fig. 1. Chronic ethanol feeding of rats promotes steatosis, apoptosis, and reduced nuclear β-catenin accumulation.
(A) Body weights were measured weekly in isocaloric pair fed control and 37% ethanol fed rats. (B) Food consumption was determined and measured 6 weeks after chronic ethanol feeding. (C) H&E staining demonstrating liver injury and steatosis in control and 37% ethanol fed rats. (D) ALT enzymatic activity was measured in the blood of control and 37% ethanol fed rat. (E) TUNEL staining was used to detect apoptotic cells in the liver. Green fluorescence represents an apoptosis positive signal. (F) IHC staining was employed to detect nuclear localization of β-catenin in control and ethanol fed rats. p-value was calculated by student t test. **, p < 0.01; ***, p < 0.001 vs. control (n = 10 per group).
Down-regulation of β-catenin hepatic levels negatively correlates with Foxo3A expression and apoptosis in ALD rats
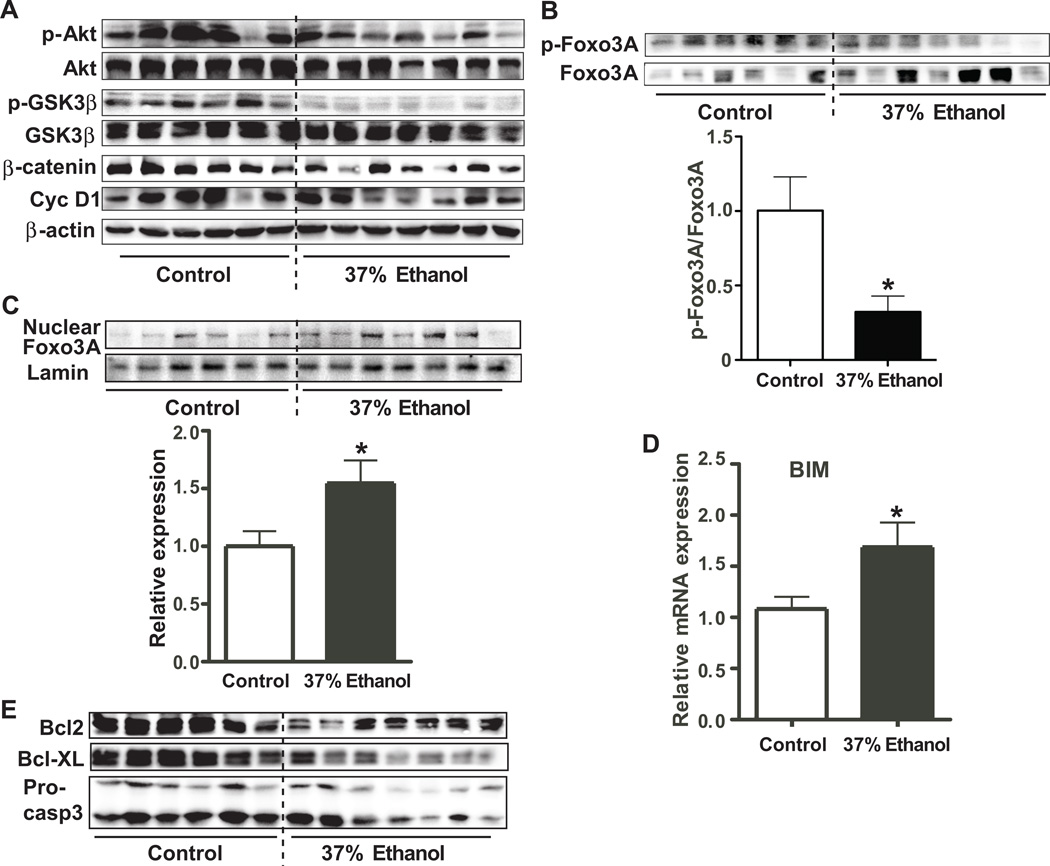
The Wnt/β-catenin signaling pathway has been shown to be regulated by Akt/GSK3β signaling[28]. Accordingly, we tested whether the observed down-regulation of hepatocyte nuclear/cytoplasmic β-catenin levels was influenced by the Akt/GSK3β pathway, we measured expression levels of p-Akt, Akt, β-catenin, p-GSK3β, and GSK3β. As shown in Fig. 2A and sFig. 2A, levels of p-Akt, p-GSK3β, and β-catenin were significantly reduced in the livers of ethanol fed rats, implying that decreased generation of p-Akt could reduce phosphorylation of GSK3β which led to activation of GSK3β and resulted in the degradation of β-catenin. Furthermore, inhibition of cyclin D1 expression (Fig. 2A), which is one of Wnt/β-catenin target genes, supported that impaired β-catenin signaling is associated with Akt/GSK3β pathway in ALD rats.
Fig. 2. β-Catenin cellular accumulation is inversely associated with Foxo3A expression and apoptosis in ALD rats.
(A) Expression levels of of pAkt, Akt, pGSK3β, GSK3β, β-catenin, cyclin D1 (Cyc D1), and β-actin in the liver of control and 37% ethanol fed rats as assessed by immunoblot analysis. (B) Expression levels of pFoxo3A and Foxo3A in the liver of control and 37% ethanol fed rats. The graph below depicts the ratio of pFoxo3A/Foxo3A. (C) Levels of nuclear Foxo3A in the liver of control and 37% ethanol fed rats. Graph below shows relative expression of nuclear Foxo3A normalized by lamin. (D) Level of BIM mRNA expression in the liver of control and 37% ethanol fed rats. (E) Bcl2, Bcl-XL, and pro-caspase3 (Pro-casp3) expression levels were determined in the liver of control and ethanol fed rats. p-value was calculated using student t test. *, p < 0.05 vs. control (n = 6–7).
Foxo3A is one of the p-Akt regulated target genes and is highly associated with apoptosis[29, 30]. To determine whether p-Akt regulates Foxo3A, levels of p-Foxo3A and Foxo3A (Figs. 2B and C) were examined. We observed that the ratio of p-Foxo3A/Foxo3A was lower in the liver of 37% ethanol fed rats compared to isocaloric fed controls. Interestingly, the nuclear fraction of Foxo3A was significantly higher in the ethanol fed rats. Moreover, ethanol treatment reduced phosphorylation of Foxo3A in human hepatocyte and increased nuclear localization of Foxo3A in hepatocytes, suggesting that it may promote hepatocyte apoptosis (Fig. 2C, sFig. 3A and B). The pro-apoptotic protein BIM, which is one of Foxo3A downstream target genes, was upregulated in the livers of ethanol fed rats (Fig. 2D). Moreover, anti-apoptotic signals mediated by Bcl2, Bcl-XL, and pro-caspase 3 were down-regulated in the 37% ethanol fed rats (Fig. 2E and sFig. 2B). Taken together, these results suggest that ALD-associated apoptosis may be related to Foxo3A overexpression.
Foxo3A has a pivotal role in promoting apoptosis of hepatocytes
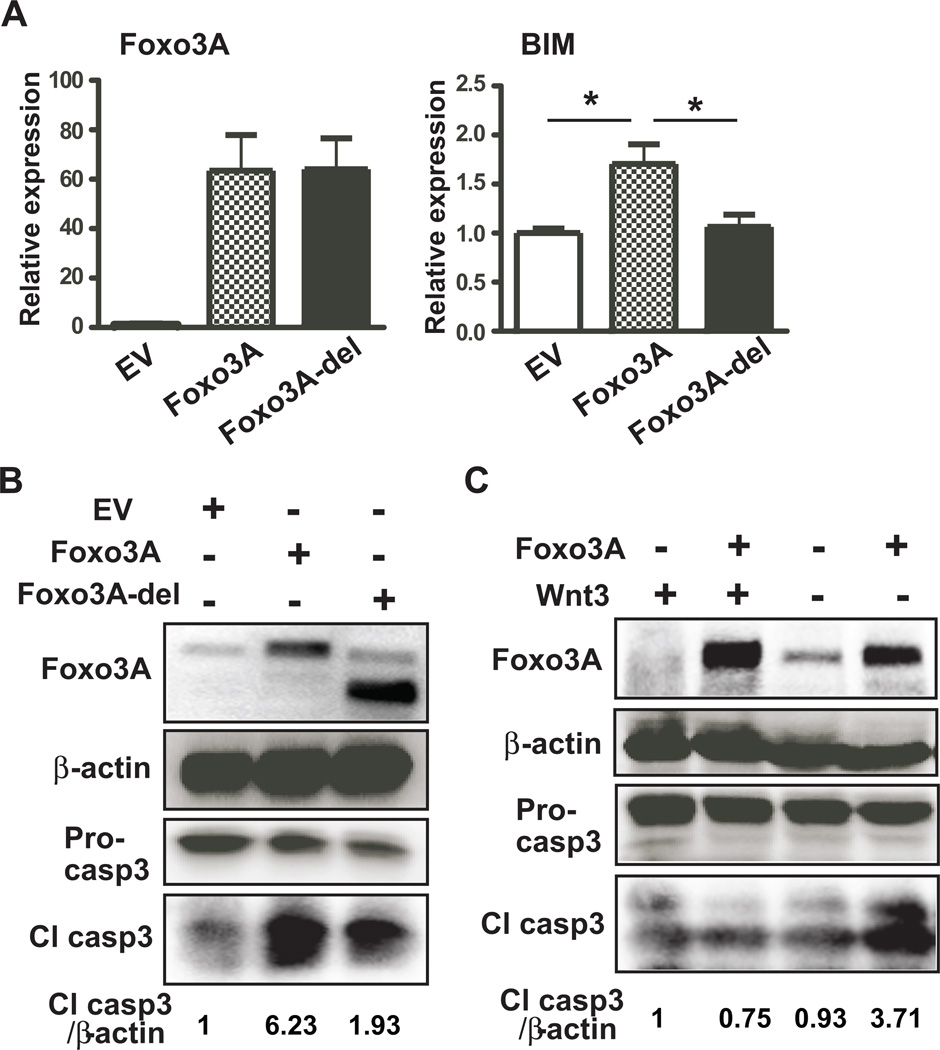
Foxo3A expression correlates with enhanced apoptosis in the liver of chronic ethanol fed rats, but the direct consequences of increased Foxo3A activity has not been clarified. To test whether the function of Foxo3A was essential to promoting apoptosis of hepatocytes in ALD, we transfected empty vector, Foxo3A, and Foxo3A-del (Foxo3A without DNA binding domain) expression plasmids into human hepatocytes and Hep3B cells and subsequently assessed apoptotic signals. Expression of full length Foxo3A and Foxo3A-del were verified by real-time RT-PCR as well as immunoblot analysis of human hepatocytes and Hep3B cells (Figs. 3A, B, sFigs. 4A, and 4B). Interestingly, the apoptotic signals produced by BIM and cleaved-caspase 3 increased only in Foxo3A transfected human hepatocytes and Hep3B cells but not in Foxo3A-del transfected cells, indicating that the transcriptional activity of Foxo3A was crucial for biologic activity and mediating hepatic apoptosis (Figs. 3B and sFig. 4B). In addition, TUNEL positive cells were increased in Foxo3A transfected hepatocytes (sFig. 5). Since Wnt/β-catenin signaling has been shown to antagonize Foxo3A-mediated apoptosis, we assessed whether activation of the Wnt/β-catenin signal could antagonize Foxo3A-induced hepatic apoptosis. As anticipated, expression of Foxo3A alone promoted apoptosis of human hepatocytes and Hep3B cells as evidenced by enhanced caspase 3 cleavage. However, co-expression of Wnt3 and Foxo3A in both cell lines blunted Foxo3A-induced caspase 3 cleavage (Fig. 3C and sFig. 4C). Therefore, exogenous expression of Wnt3 significantly reversed Foxo3A-induced apoptosis, which implies that activation of Wnt/β-catenin signaling may play a regulatory role.
Fig. 3. Foxo3A modulates apoptosis of hepatocytes.
(A) Expression levels of Foxo3A and BIM in human hepatocytes transfected with empty vector (EV), Foxo3A, or Foxo3A without DNA binding domain (Foxo3A-del). (B) Immunoblot analysis of Foxo3A, pro-caspase 3 (Pro-casp3), cleaved-caspase 3 (Cl casp3), and β-actin in human hepatocyte transfected with EV, Foxo3A, or Foxo3A-del. Cl casp3/β-actin represents a fold expression of Cl casp3 compared to control (EV) after normalized by β-actin. (C) Expression levels of Foxo3A, pro-caspase 3, cleaved-caspase 3, and β-actin in human hepatocyte transfected with EV or Foxo3A in the presence or absence of Wnt3. p-value was calculated using student t test. *, p < 0.05 vs. control (EV) (n = 3 independent experiments, assayed in triplicate).
Foxo3A-induced apoptosis is down-regulated by pharmacologic activation of Wnt/β-catenin signaling
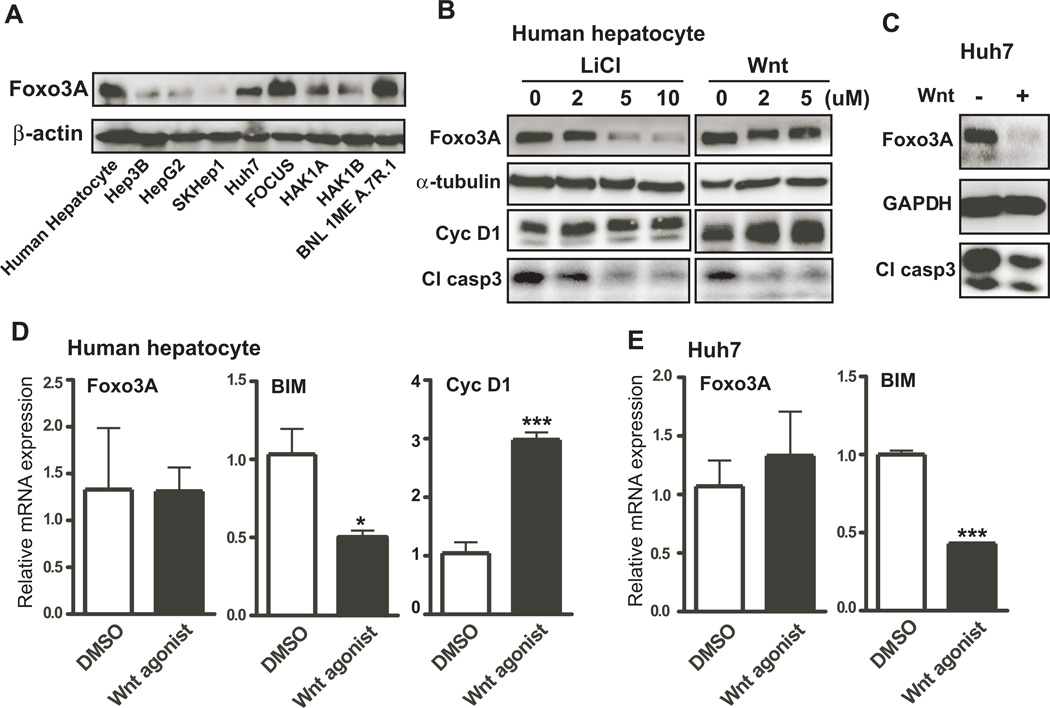
To determine if activation of Wnt/β-catenin signaling would suppress Foxo3A-induced apoptosis, endogenous levels of Foxo3A expression were measured in various liver cell lines (Fig. 4A and sFig. 6A). Among the four cell lines that exhibited high levels of Foxo3A expression; human hepatocytes and Huh7 cells were selected for further studies. To activate Wnt/β-catenin signaling, we used LiCl and a Wnt agonist. LiCl is widely used to repress the activity of GSK3β, which phosphorylates and subsequently degrades cytoplasmic β-catenin leading to activation of β-catenin signaling. In human hepatocytes, LiCl treatment decreased Foxo3A expression and cleaved caspase 3 at concentration as low as 2 µM LiCl (Fig. 4B and sFig. 6B). Elevated cyclin D1, which is one of the Wnt/β-catenin responsive target genes, was measured to validate activation of β-catenin signaling. To further demonstrate that Wnt/β-catenin signaling was critical to Foxo3A-mediated apoptosis, the Wnt agonist was used to treat human hepatocytes and Huh7 cell lines. As shown in Fig. 4B, C, sFig. 6B and C, Foxo3A and cleaved-caspase 3 were reduced upon Wnt agonist treatment in both cells. However, the results of mRNA expression only revealed decrease in apoptotic signal of Foxo3A downstream target, BIM, but no change in Foxo3A itself, suggesting that Wnt/β-catenin signaling may modulate Foxo3A protein expression by non-transcriptional mechanisms (Figs. 4D and E).
Fig. 4. Pharmacological activation of Wnt/β-catenin signaling suppresses Foxo3A-associated apoptosis.
(A) Endogenous expression level of Foxo3A in several liver derived HCC cell lines. (B) Immunoblot analysis of Foxo3A, α-tubulin, cyclin D1 (Cyc D1), and cleaved-caspase 3 (Cl casp3) are shown in human hepatocytes treated with 0, 2, 5, and 10 µM LiCl, or 0, 2, and 5 µM Wnt agonist (Wnt). (C) Levels of Foxo3A, GAPDH, and cleaved-caspase 3 in Huh7 cells treated with or without Wnt agonist. (D) Expression of Foxo3A, BIM, and cyclin D1 mRNA in human hepatocytes treated with or without Wnt agonist. (E) Levels of Foxo3A and BIM mRNA were determined in Huh7 cells treated with or without Wnt agonist. p-value was calculated using student t test. *, p < 0.05; ***, p < 0.001 vs. control (DMSO) (n = 3 independent experiments, assayed in triplicate).
Wnt/β-catenin signaling modulates Foxo3A-associated apoptosis through SGK1
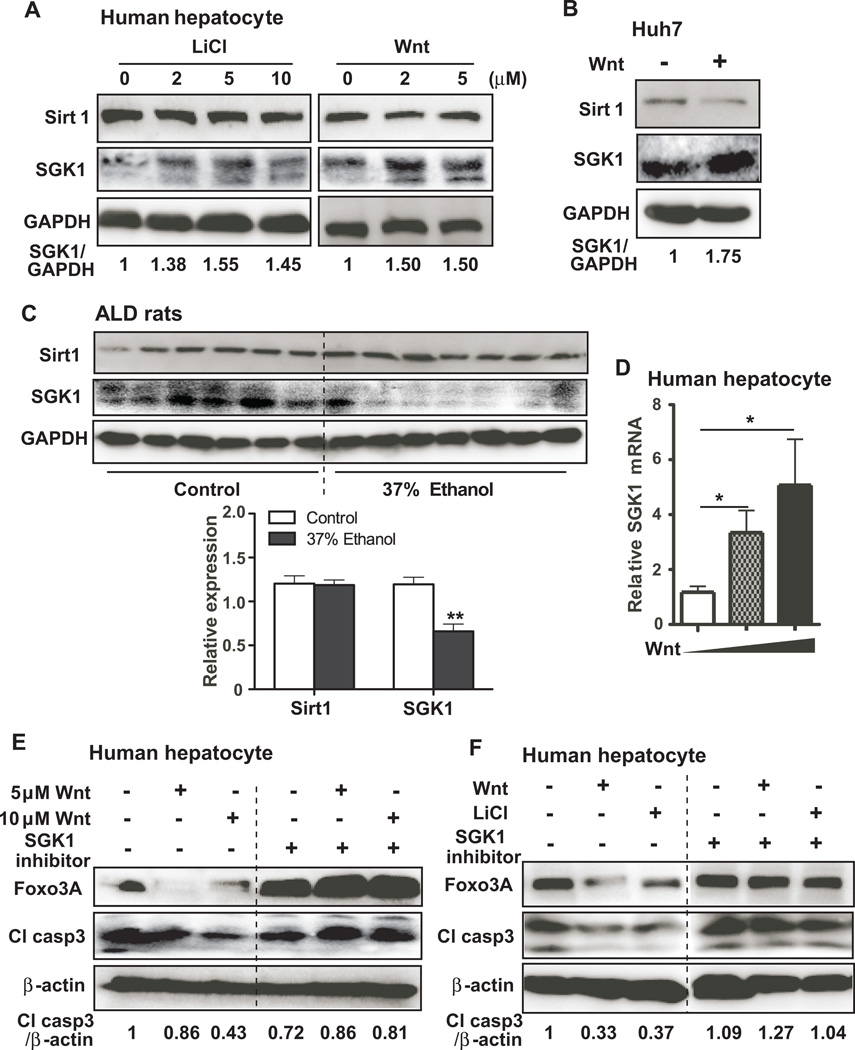
It has been shown that Foxo3A function may be regulated through de-acetylation by Sirt1 or phosphorylation by SGK1[23, 31]. To evaluate whether activation of Wnt/β-cateinin signaling would promote down-regulation of Foxo3A through Sirt1 or SGK1, expression levels were measured following treatment with LiCl or a Wnt agonist in human hepatocytes or Huh7 cells. As shown in Figs. 5A and B, activation of Wnt/β-catenin signaling promoted SGK1 expression but did not alter the expression of Sirt1 in both cell lines. This observation was further verified in vivo with liver samples taken from ethanol fed rats. Levels of SGK1 expression were significantly reduced in the livers from ethanol fed compared to isocaloric pair fed control rats, but no difference of Sirt1 expression was observed between the livers of control and ethanol fed rats (Fig. 5C). Furthermore, these findings were consistent with previous reports that Wnt/β-catenin signaling was transcriptionally regulated by SGK1[31]; the Wnt agonist treatment was found to enhance SGK1 mRNA expression in human hepatocytes (Fig. 5D).
Fig. 5. Wnt/β-catenin signaling modulates Foxo3A-mediated apoptosis through SGK1.
(A) The protein expression of Sirt1, SGK1, and GAPDH in human hepatocyptes treated with 0, 2, 5, and 10 µM LiCl, or 0, 2, and 5 µM Wnt agonist. GAPDH served as a loading control. SGK1/GAPDH represents a fold expression of SGK1 compared to control after normalized by GAPDH. (B) Representative immunoblot of Sirt1, SGK1, and GAPDH in Huh7 cells treated with or without Wnt agonist. (C) The protein expression of Sirt1, SGK1, and GAPDH in the liver of control and 37% ethanol fed rats. (D) Expression of SGK1 mRNA in human hepatocytes treated with 0, 10, and 20 µM Wnt agonist. (E) Levels of Foxo3A, cleaved-caspase 3 (Cl casp3), and β-actin are shown in human hepatocytes treated with 0, 5, and 10 µM Wnt agonist in the presence or absence of 10 µM SGK1 inhibitor. (F) Foxo3A, Cl casp3, and β-actin were determined in human hepatocytes treated with LiCl or Wnt agonist in the presence or absence of SGK1 inhibitor, plus etoposide. Cl casp3/β-catein represents a fold expression of Cl casp3 compared to control after normalized by β-actin. p-value was calculated using student t test. *, p < 0.05; **, p < 0.01 vs. control.
Although Wnt/β-catenin signaling induces SGK1 mRNA and protein expression, it is not clear if the functional kinase activity of SGK1 was critical for Wnt/β-catenin mediated Foxo3A-associated apoptosis. In this regard, a SGK1 inhibitor which inhibits SGK1 kinase activity was used to pre-treat human hepatocytes before Wnt agonist exposure. Pre-treatment with the SGK1 inhibitor reversed the effects of Wnt/β-catenin signaling on the suppressed expression of Foxo3A and caspase 3 cleavage (Fig. 5E). Moreover, the SGK1 inhibitor reversed the effects of LiCl and Wnt agonist on expression of Foxo3A and cleaved-caspase 3 in the presence of etoposide, an apoptosis inducer (Fig. 5F), suggesting that SGK1 functional kinase activity was required for Wnt/β-catenin mediated Foxo3A-associated apoptosis.
Pharmacologic activation of Wnt/β-catenin signaling attenuates ALD progression
Based on the results obtained from human hepatocytes, it is possible that activation of Wnt/β-catenin signaling could play a role in the pathogenesis ALD. Thus, a Wnt agonist was evaluated with respect to activation of Wnt/β-catenin signaling and could this treatment attenuate ALD development.
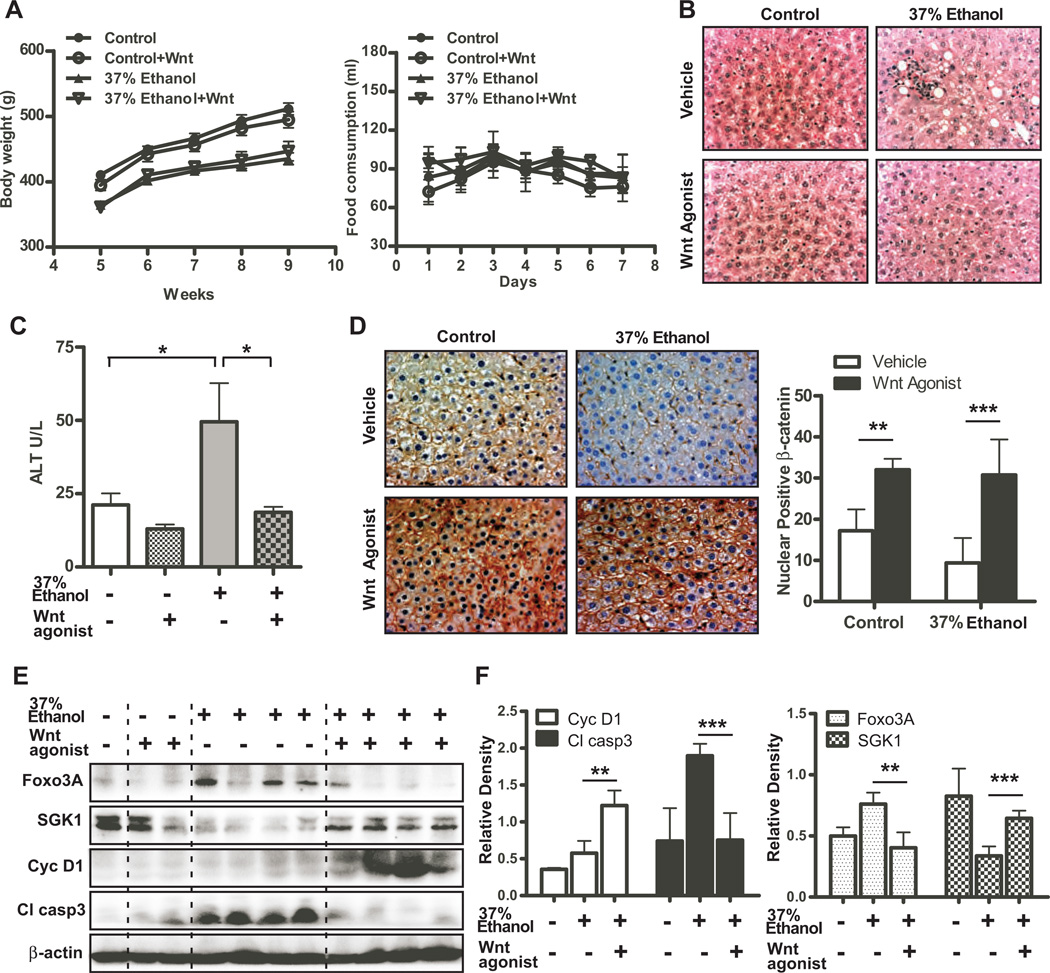
Wnt agonist administration had a significant impact on improving steatosis in the liver, but did not affect body weight (Fig. 6A and B). In addition, Wnt agonist treatment significantly reduced ALT blood levels in ethanol fed rats (Fig. 6C), suggesting that Wnt agonist not only improves steatosis but also blunts liver injury. To further determine if the therapeutic effects were mediated by activation of Wnt/β-catenin signaling, nuclear and/or cytoplasmic expression of β-catenin was examined by immunohistochemical staining. As shown in Fig. 6D, Wnt agonist treatment significantly increased nuclear and cytoplasmic β-catenin expression in the liver of both isocaloric pair fed control and chronic ethanol fed rats. As expected, SGK1 and cyclin D1 were increased upon Wnt agonist treatment (Figs. 6E and F). On the other hand, the expression of Foxo3A and cleaved-caspase 3, and TUNEL positive cells were significantly suppressed (Figs. 6E, F, and sFig. 7) suggesting that activation of Wnt/β-catenin signaling improves Foxo3Ainduced apoptosis in the ALD rat model.
Fig. 6. Wnt agonist treatment suppresses ALD progression.
(A) Body weight and food consumption measured in control and 37% ethanol fed rats treated with or without Wnt agonist (n = 10 per group). (B) H&E staining was performed to visualize microstructure of the livers in control and 37% ethanol fed rats treated with or without Wnt agonist. Note that Wnt agonist reduced steatosis in ethanol fed rats. (C) ALT enzymatic activity was measured in the same samples as (B). (D) Representative pictures of β-catenin staining in the liver of control and 37% ethanol fed rats treated with or without Wnt agonist. Right graph depicts quantitation of results and indicates enhanced nuclear accumulation with Wnt agonist. (E) Expression levels of Foxo3A, SGK1, cyclin D1 (Cyc D1), cleaved-caspase 3 (Cl casp3), and β-actin in the liver of control and 37% ethanol fed rats treated with or without Wnt agonist. (F) The densitometric quantitation of these results of (E). p-value was calculated using student t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
Although Wnt/β-catenin signaling has been investigated for its role in liver organogenesis, including prenatal and postnatal development, little information is available on its function in liver disease and especially ALD. It is known that following birth and during postnatal development from days 0 to 90, total and active β-catenin levels are increased. Also, specific knockout of β-catenin in hepatocytes using HNF3γ driven Cre recombinase led to an undeveloped liver at embryonic day 12 and lethality at embryonic day 17 due to strikingly enhanced oxidative stress and apoptosis in the liver [15, 16]. Moreover, delayed liver regeneration has been observed in the liver-specific β-catenin knockout mice following partial hepatectomy, although there was no difference regarding total regenerated liver weight between wild type and liver-specific β-catenin knockout mice and these finding suggest that this signaling cascade may be pivotal for hepatic growth[32]. In accordance with previous studies, Tan et al. also used albumin driven liver-specific β-catenin knockout mice to demonstrate that the lack of β-catenin delayed liver regeneration but did not alter the eventual accumulation of a normal liver weight following regeneration. Notably, specific knockout of β-catenin in the liver enhanced apoptosis during the liver regeneration process and this phenotype was also observed in HNF3γ driven liver-specific β-catenin knockout mice, suggesting that β-catenin signaling was involved in regulation of hepatocyte apoptosis.
We investigated a molecular mechanism of how ALD development and progression might occur and surprisingly found that β-catenin signaling was down-regulated in ALD compared to isocaloric pair fed control rats. It has been previously shown that knockout of β-catenin enhanced oxidative stress and altered mitochondrial function and was associated with severe liver damage[20, 21]. Moreover, ethanol-induced hepatic apoptosis was inversely related to activation of β-catenin signaling, which complements the finding of a previous report that liver-specific knockout of β-catenin induced hepatocyte apoptosis. Collectively, evidence is accumulating that β-catenin not only is involved in liver growth but also participates in regulation of hepatocyte apoptosis. Indeed, there is one other report in colon cancer cell lines revealing that overexpression of Foxo3A, a known pro-apoptotic gene, enhances cell death but co-expression with β-catenin antagonized this Foxo3A-induced effect. This finding suggests that Foxo3A may be one of the mediators of β-catenin-modulated apoptosis[19]. We found that activated Foxo3A was increased in ALD compared to controls and was negatively correlated with reduced β-catenin signaling in the liver. Indeed, Foxo3A has been shown to be involved in ethanol-induced liver damage in a mouse model. For example, using Foxo3A−/− mice, Ni et al. demonstrated that ethanol exposure stimulated Foxo3A expression accompanied by elevated autophagy and concluded that Foxo3A may be critical for ethanol-induced autophagy and hepatotoxicity in this animal model[33]. In addition, Liu et al. revealed that Foxo3A was important in ethanol-induced oxidative stress in the murine liver[20]. We demonstrated that Foxo3A has a pivotal role in promoting human hepatocyte apoptosis and its transcriptional activity was essential for Foxo3A-induced programmed cell death of human hepatocytes. Furthermore, we observed that activation of Wnt/β-catenin signaling rescued Foxo3A-induced apoptosis, which is a finding consistent with a previous report on colon cancer cells[19]. Taken together, ethanol consumption induces Foxo3A expression to generate hepatocyte apoptosis and does so by inhibiting Wnt/β-catenin signaling.
Although activation of β-catenin signaling antagonizes Foxo3A-induced apoptosis in hepatocytes, it was unclear how β-catenin signaling promotes these effects. In this respect, Sirt1 and SGK1 have been previously shown to suppress Foxo3A-induced apoptosis through de-acetylation and phosphorylation of Foxo3A, respectively[23, 31]. We found that activation of β-catenin had no effects on Sirt1 levels but significantly increased SGK1 expression through transcriptional up-regulation. We also determined that enhanced β-catenin signaling was associated with SGK1 upregulation in liver of chronic ethanol fed rats. In this context, a previous report has demonstrated that β-catenin promotes SGK1 transcription to suppress Foxo3A activity in tumor cell lines[31]. Our finding also demonstrated that this regulation was operative in human hepatocyte and the intact rat liver. The regulation was partially dependent on SGK1 expression, because pre-treating human hepatocytes with SGK1 inhibitor reversed β-catenin-inhibited Foxo3A expression and the consequent downstream effects on apoptosis as evident by caspase 3 cleavage.
In addition to its role in apoptosis, Foxo3A has also been linked to promotion of insulin resistance through Akt-mediated binding between Foxo3A and 14-3-3 protein[34, 35]. Insulin resistance and down-regulated pAkt expression are common features in ALD animal models[36, 37]. In the current study, down-regulated pAkt and Foxo3A were both observed in the ALD rat model, which is known to have severe insulin resistance in the liver[38]. Recently, activated Wnt/β-catenin signaling has been suggested to regulate energy homeostasis and glucose metabolism. Using large-scale RNAi screen, Yoon et al. identified that Wnt/β-catenin signals modulate mitochondrial biogenesis through insulin receptor substrate-1[39], and Abiola et al. found that activation of Wnt/β-catenin signaling increased insulin sensitivity through the Sterol regulatory elementbinding transcription factor 1[40], suggesting that loss of Wnt/β-catenin signaling may reduce insulin signaling and result in hepatic insulin resistance associated with ALD in these experimental animal models. More important, we also observed that Wnt/β-catenin signaling modulates Foxo3A level through SGK1.
The finding of β-catenin signaling may inhibit apoptosis and possibly modulate insulin resistance in hepatocytes leads to a hypothesis that targeting β-catenin to increase its signaling activity may attenuate ALD injury and progression. In this regard, two compounds, LiCl and a Wnt agonist, were used to test this hypothesis that activation of β-catenin signaling suppressed generation of cleaved caspase 3 in human hepatocytes and Huh7 cells; these findings suggest that pharmacological activation of β-catenin signaling will inhibit apoptosis of hepatocytes. The Wnt agonist was beneficial for reducing liver injury in the ALD model, as demonstrated by reduced ALT activity and hepatic steatosis. Ethanol feeding suppressed β-catenin signaling and led to less SGK1 expression which in turn promoted Foxo3A upregulation to induce hepatocyte apoptosis, whereas Wnt agonist administration reversed this process. Therefore, pharmacologic activation of β-catenin signaling reduces liver injury produced by chronic ethanol consumption.
Supplementary Material
Acknowledgements
We thank Dr. Hironori Koga for the help of human hepatocyte, OUMS29 (Kurume University). We thank Drs. Ming Tong, Teresa Ramirez, and Chetram Deochand for the technical supports (Liver research Center, Brown University). Foxo3A related plasmids were kindly provided by Dr. Michael Greenberg (Harvard Medical School).
Financial support: This work was supported by the grant AA20587 (to MK) from the National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations
- Foxo3A
Forkhead box O3
- Wnt
Wingless-type MMTV integration site family
- ALD
alcoholic liver disease
- SGK1
serum/glucocorticoid regulated kinase 1
- Sirt1
Sirtuin 1
- LiCl
Lithium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Author’s contributions:
Study concept and design: C.H., M.K.
Acquisition of data: C.H., T.Y.
Analysis and interpretation of data: C.H., S.M., and M.K.
Drafting of the manuscript: C.H. M.K.
Revision of manuscript: J.R.W., M.K.
Technical support: T.Y., S.M.
Material support: S.M., Z.D.
Obtained funding: M.K.
Study supervision: M.K.
References
- 1.Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Sys. 2011;59:1–126. [PubMed] [Google Scholar]
- 2.Bird GL, Williams R. Factors determining cirrhosis in alcoholic liver disease. Mol Asp Med. 1988;10:97–105. doi: 10.1016/0098-2997(88)90017-9. [DOI] [PubMed] [Google Scholar]
- 3.Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, et al. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54:846–856. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Ann Rev Nutri. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naveau S, Emilie D, Balian A, Grangeot-Keros L, Borotto E, Portier A, et al. Plasma levels of soluble tumor necrosis factor receptors p55 and p75 in patients with alcoholic liver disease of increasing severity. J Hepatol. 1998;28:778–784. doi: 10.1016/s0168-8278(98)80227-4. [DOI] [PubMed] [Google Scholar]
- 6.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 8.Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology (Oxford) 2003;42:617–621. doi: 10.1093/rheumatology/keg263. [DOI] [PubMed] [Google Scholar]
- 9.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. J Am Med Assoc. 2011;306:2331–2339. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J Am Med Assoc. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 11.Wendling D, Auge B, Bettinger D, Lohse A, Le Huede G, Bresson-Hadni S, et al. Reactivation of a latent precore mutant hepatitis B virus related chronic hepatitis during infliximab treatment for severe spondyloarthropathy. Ann Rheu Dis. 2005;64:788–789. doi: 10.1136/ard.2004.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteve M, Saro C, Gonzalez-Huix F, Suarez F, Forne M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut. 2004;53:1363–1365. doi: 10.1136/gut.2004.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winthrop KL, Baddley JW, Chen L, Liu L, Grijalva CG, Delzell E, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. J Am Med Assoc. 2013;309:887–895. doi: 10.1001/jama.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. New Eng J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 15.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, et al. beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastroand Liver Physiol. 2007;292:G1578–G1585. doi: 10.1152/ajpgi.00359.2006. [DOI] [PubMed] [Google Scholar]
- 17.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, et al. beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Yeh TH, Singh VP, Shiva S, Krauland L, Li H, et al. beta-catenin is essential for ethanol metabolism and protection against alcohol-mediated liver steatosis in mice. Hepatology. 2012;55:931–940. doi: 10.1002/hep.24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehwald N, Tao GZ, Jang KY, Papandreou I, Liu B, Pysz MA, et al. beta-Catenin regulates hepatic mitochondrial function and energy balance in mice. Gastroenterology. 2012;143:754–764. doi: 10.1053/j.gastro.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Pang M, de la Monte SM, Longato L, Tong M, He J, Chaudhry R, et al. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol. 2009;50:1192–1201. doi: 10.1016/j.jhep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 24.Koga H, Tsedensodnom O, Tomimaru Y, Walker EJ, Lee HC, Kim KM, et al. Loss of the SxxSS motif in a human T-cell factor-4 isoform confers hypoxia resistance to liver cancer: an oncogenic switch in Wnt signaling. PloS one. 2012;7:e39981. doi: 10.1371/journal.pone.0039981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyama T, Lee HC, Koga H, Wands JR, Kim M. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol Can Res. 2010;8:254–265. doi: 10.1158/1541-7786.MCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CK, Lee SO, Lai KP, Ma WL, Lin TH, Tsai MY, et al. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology. 2013;57:1550–1563. doi: 10.1002/hep.26135. [DOI] [PubMed] [Google Scholar]
- 27.Lai KP, Yamashita S, Huang CK, Yeh S, Chang C. Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of proinflammatory cytokines/chemokines. EMBO Mol Med. 2012;4:791–807. doi: 10.1002/emmm.201101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verheyen EM, Gottardi CJ. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev Dyn. 2010;239:34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 30.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 31.Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283:19201–19210. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- 32.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 33.Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol. 2013;183:1815–1825. doi: 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endo Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, et al. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Tonks KT, Ng Y, Miller S, Coster AC, Samocha-Bonet D, Iseli TJ, et al. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia. 2013;56:875–885. doi: 10.1007/s00125-012-2811-y. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez T, Longato L, Dostalek M, Tong M, Wands JR, de la Monte SM. Insulin resistance, ceramide accumulation and endoplasmic reticulum stress in experimental chronic alcohol-induced steatohepatitis. Alc Alc. 2013;48:39–52. doi: 10.1093/alcalc/ags106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derdak Z, Lang CH, Villegas KA, Tong M, Mark NM, de la Monte SM, et al. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. J Hepatol. 2011;54:164–172. doi: 10.1016/j.jhep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Gene Dev. 2010;24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abiola M, Favier M, Christodoulou-Vafeiadou E, Pichard AL, Martelly I, Guillet-Deniau I. Activation of Wnt/beta-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PloS one. 2009;4:e8509. doi: 10.1371/journal.pone.0008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.