Abstract
Rationale
Apolipoprotein E (apoE) exerts anti-inflammatory properties that protect against atherosclerosis and other inflammatory diseases. However, mechanisms by which apoE suppresses the cellular activation of leukocytes commonly associated with atherosclerosis remain incompletely understood.
Objective
To test the hypothesis that apoE suppresses inflammation and atherosclerosis by regulating cellular microRNA levels in these leukocytes.
Methods and Results
An assessment of apoE expression among such leukocyte subsets in wild-type mice revealed that only macrophages and monocytes express apoE abundantly. An absence of apoE expression in macrophages and monocytes resulted in enhanced nuclear factor-κB (NF-κB) signaling and an exaggerated inflammatory response upon stimulation with lipopolysaccharide. This correlated with reduced levels of microRNA-146a, a critical negative regulator of NF-κB signaling. Ectopic apoE expression in Apoe−/− macrophages and monocytes raised miR-146a levels, while its silencing in wild-type cells had an opposite effect. Mechanistically, apoE increased the expression of transcription factor PU.1, which raised levels of pri-miR-146 transcripts, demonstrating that apoE exerts transcriptional control over miR-146a. In vivo, even a small amount of apoE expression in macrophages and monocytes of hypomorphic apoE mice led to increased miR-146a levels, and inhibited macrophage pro-inflammatory responses, Ly-6Chigh monocytosis, and atherosclerosis in the settings of hyperlipidemia. Accordingly, cellular enrichment of miR-146a through the systemic delivery of miR-146a mimetics in Apoe−/−Ldlr−/− and Ldlr−/− mice attenuated monocyte/macrophage activation and atherosclerosis in the absence of plasma lipid reduction.
Conclusions
Our data demonstrate that cellular apoE expression suppresses NF-κB–mediated inflammation and atherosclerosis by enhancing miR-146a levels in monocytes and macrophages.
Keywords: Atherosclerosis, miR-146a, apoE, macrophages, NF-Kb, microRNA
INTRODUCTION
Apolipoprotein E (apoE), a 34 kDa secreted protein, is well recognized for its ability to suppress atherosclerosis.1 Beyond its pivotal role in lipoprotein cholesterol transport and in regulating cellular lipid levels, apoE has long been known to exert anti-inflammatory properties. ApoE deficient (Apoe−/−) mice display enhanced chronic inflammation in response to spontaneous and diet-induced hypercholesterolemia as well as an exaggerated acute immune response when challenged with bacterial lipopolysaccharide (LPS), which predisposes them to death from sepsis.2, 3 Macrophages are an important source of inflammatory cytokine production in the settings of both acute and chronic inflammation and apoE is known to be highly expressed by macrophages.1 Although the addition of exogenous apoE and its mimetic peptides has been reported to suppress macrophage inflammatory responses4 and induce anti-inflammatory macrophage phenotypes,5 far less is known regarding how endogenous apoE expression mediates anti-inflammatory functions in macrophages and in other leukocytes.
Macrophages and circulating monocytes are important effector cells in many inflammatory diseases, including atherosclerosis.6 Induction of inflammatory mediators in these cells is primarily mediated by the activation of the transcription factor nuclear factor-κB (NF-κB) pathway. Several lines of evidence suggest that NF-κB is activated in peripheral blood monocytes and lesional macrophages in patients with acute coronary syndrome and unstable angina.7 Experimental data using animal models have suggested that aberrant NF-κB activation in myeloid cells can contribute to the initiation and progression of atherosclerosis.8, 9 Thus, understanding how NF-κB activity in monocytes and macrophages is controlled in the context of hyperlipidemia is of great interest and may provide new therapeutic avenues for the treatment of atherosclerosis.
Multiple regulatory mechanisms have evolved to limit NF-κB activation in macrophages and monocytes. Of these, microRNA-146a (miR-146a) has emerged as a key negative regulator of NF-κB.10 In this study, we tested the hypothesis that apoE expression can suppress NF-κB–mediated inflammation by regulating cellular microRNA levels in macrophages and monocytes. Our findings reveal that cellular apoE expression impacts on the levels of several microRNAs, including miR-146a, and thereby provide new insight to understand how apoE suppresses inflammation and atherosclerosis beyond reducing plasma lipid levels.
METHODS
In vivo administration of microRNA mimetics
The in vivo delivery of microRNA mimetics was performed as previously described.11 The uptake of miR-146a mimetics by target cells in vivo was validated by using miR-146a−/− mice obtained from the Jackson Laboratories. Briefly, miR-146a−/− mice were injected intravenously via the tail vein with a mixture of Lipofectamine 2000 and mimetics (miRNA negative control, or miR-146a; 1 nmol/mouse) suspended in 200 μL PBS twice a week for two weeks. Ly-6Chigh monocytes sorted from spleens and macrophages purified from peritoneal lavage were analyzed for miR-146a expression. To study the effects of microRNA mimetics on atherosclerosis, Apoe−/−Ldlr−/− mice (11 ± 1-week-old) were continuously fed a normal chow diet and injected with microRNA mimetics as above for a period of 6 weeks. To control for endogenous apoE expression on miR-146a effects in atherosclerosis, the study was repeated with 10 week-old Ldlr−/− mice fed a high fat diet. After 2 weeks of diet initiation, the mice were treated with miR-146a mimetics or controls as above for a period of 6 weeks. MiRNA and mRNA in resident peritoneal macrophages were examined by quantitative RT-PCR. Blood leukocytes were collected and analyzed by flow cytometry. The heart and aorta were harvested for histological analyses.
An expanded Materials and Methods section is available in Online Data Supplement.
RESULTS
ApoE is primarily expressed by monocytes and macrophages among the major leukocyte subsets known to participate in atherosclerosis
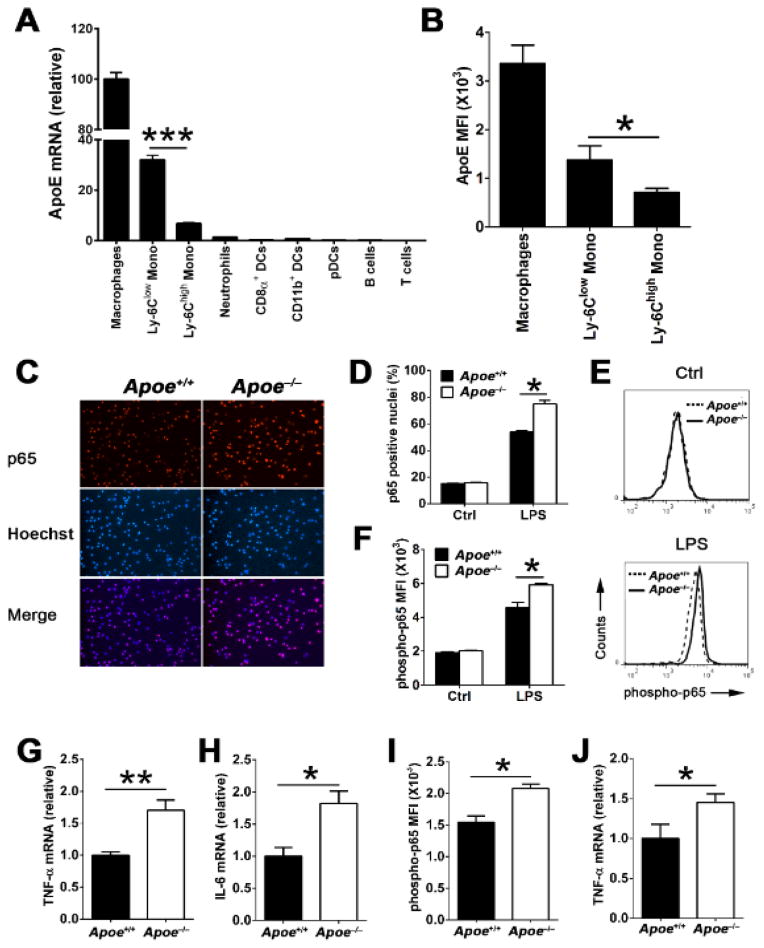
Although apoE is well known to be expressed by tissue macrophages,1 its expression pattern among other leukocyte subsets known to participate in atherosclerosis is not well defined. We explored this question by measuring apoE mRNA levels in various subsets of mature myeloid and lymphoid cells isolated from wild-type (WT) C57BL/6 mice by quantitative real-time PCR. The mRNA levels of apoE in these cells were then compared to those in resident peritoneal macrophage set arbitrarily to 100%. As shown in Figure 1A, apoE expression was found to be restricted to myeloid cells, primarily among monocytes. Interestingly, Ly-6Clow monocytes expressed robust amounts (32.0 ± 5.8) that were on average fivefold more apoE mRNA than Ly-6Chigh monocytes (6.7 ± 1.4, P<0.001). Consistent with mRNA expression data, quantitation of cellular apoE protein content by flow cytometry showed that Ly-6Clow monocytes had twofold more apoE than Ly-6Chigh monocytes (Figure 1B). In contrast, apoE was expressed far less in neutrophils (1.2 ± 0.2), DC subsets (<1.0), pDCs (<0.5), and B cells (<1.0), and it was undetectable in T lymphocytes (Figure 1A). The strikingly different apoE expression levels observed between the two major monocyte subsets suggests that it may play a role in regulating their functional heterogeneity.
Figure 1. ApoE is primarily expressed in macrophages and monocytes, and suppresses NF-κB inflammatory signaling in these cells.
(A) ApoE mRNA expression levels among purified bone marrow Ly-6Chigh monocytes, Ly-6Clow monocytes, and neutrophils (n=9), and splenic CD8α+ DCs, CD11b+ DCs, pDCs, B cells, and T cells (n=6) from WT mice were detected by quantitative real-time PCR (qRT-PCR). Mono, monocytes; DCs, dendritic cells; pDCs, plasmacytoid dendritic cells; The results were normalized by β-2-microglobulin (β2m) level and the mRNA expression level of apoE in peritoneal macrophages (n=9) was set arbitrarily to 100%. (B) ApoE expression in peritoneal macrophages, bone marrow Ly-6Chigh monocytes and Ly-6Clow monocytes from WT mice (n=8) were examined by flow cytometry. MFI, mean fluorescence intensity; Data are presented as the means ± SEM. ***, P<0.001; *, P<0.05. Representative images (C) and quantitative analysis (D) of immunofluorescence of p65 in peritoneal macrophages derived from 4-week-old WT (Apoe+/+) and Apoe−/− mice before (Ctrl) or after LPS stimulation (100 ng/mL) for 15 min. Representative histograms (E) and quantitation (F) of p65 phosphorylation in 4-week-old WT and Apoe−/− macrophages before (Ctrl) or after (LPS) 15 min of stimulation with LPS (100 ng/mL) analyzed by flow cytometry. TNF-α (G) and IL-6 (H) mRNA expression in WT and Apoe−/− macrophages stimulated with LPS (100 ng/mL) for 6 hrs were analyzed by qRT-PCR. Phosphorylated p65 (I) and TNF-α mRNA (J) levels were examined in Ly-6Chigh monocytes of WT and Apoe−/− mice after LPS stimulation for 30 min and 18 hrs, respectively. The results of WT cells were set arbitrarily to 1. n=4~5 for C, D, E, F, G, and H; n=3~4 for I and J. Data are shown as the means ± SEM and are representative of three independent experiments. *, P<0.05; **, P<0.01
ApoE expression reduces NF-κB inflammatory signaling in macrophages and monocytes
Numerous studies have shown that NF-κB signaling driven by toll-like receptors (TLRs) in monocytes and macrophages is an important contributor to acute and chronic inflammation including atherosclerosis.6, 12 However, little is known about the impact that cellular apoE expression can exert on NF-κB signaling in response to TLR signaling. We addressed this question with cultured macrophages and monocytes exposed to lipopolysaccharide (LPS), a well-established TLR4 ligand and activator of NF-κB signaling in these cells. Peritoneal macrophages from 4-week-old WT and Apoe−/− mice were stimulated with LPS and subsequently analyzed for the subcellular distribution and phosphorylation of the major NF-κB subunit p65 by immunofluorescence and flow cytometry. At a basal unstimulated state, resident peritoneal macrophages cultured from either WT or Apoe−/− mice displayed a similarly low level of nuclear p65 (Figure 1D). In contrast, TLR4 activation by LPS stimulation caused a pronounced nuclear translocation of p65 in both WT and Apoe−/− macrophages (Figure 1C and 1D). However, macrophages derived from Apoe−/− mice displayed almost 20% more nuclei containing p65 than those of WT mice (Figure 1C and 1D). Moreover, we also observed that the absolute cellular levels of p65 phosphorylation were significantly greater among macrophages derived from Apoe−/− mice than those of WT mice (Figure 1E and 1F). We next explored the consequence of increased p65 nuclear localization and phosphorylation on the cellular expression of NF-κB target genes in Apoe−/− macrophages. As shown in Figure 1G and 1H, Apoe−/− macrophages consistently displayed higher mRNA expression levels of TNF-α and IL-6 compared to WT macrophages. A similar increase in LPS-induced p65 phosphorylation (Figure 1I) and TNF-α mRNA expression (Figure 1J) was also observed among Ly-6Chigh monocytes isolated from Apoe−/− mice compared to those isolated from WT mice. Collectively, our findings show that apoE expression in macrophages and monocytes restrains NF-κB signaling and downstream inflammatory cytokine expression in response to LPS stimulation.
ApoE deficiency results in reduced miR-146a expression in macrophages and monocytes
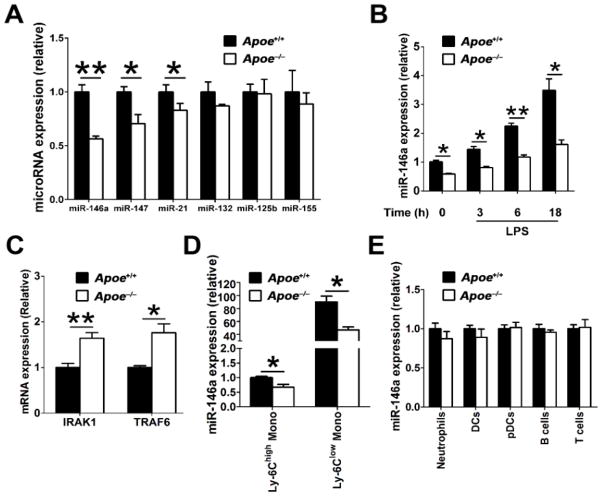
We sought to identify the underlying mechanisms by which apoE controls NF-κB activity in macrophages. Several microRNAs are known to differentially regulate NF-κB signaling in macrophages, including miR-146a, miR-147, miR-21, miR-132, miR-125b and miR-155.10, 13, 14 We explored whether a loss of apoE expression in macrophages could impact on levels of these microRNAs to alter their cellular responsiveness to LPS-induced NF-κB activation. Our results show that compared to WT macrophages, Apoe−/− macrophages displayed lower levels of miR-146a, miR-147 and miR-21. However, miR-132, miR-125b and miR-155 were present at similar levels in these two types of macrophages (Figure 2A).
Figure 2. MiR-146a levels are reduced in apoE-deficient macrophages and monocytes, but not other myeloid and lymphoid cells.
A, Select microRNAs in 4-week-old WT and Apoe−/− (n=4) peritoneal macrophages 3 hrs after LPS stimulation were analyzed by qRT-PCR. B, three-week-old WT and Apoe−/− BMDM (bone marrow-derived macrophages) were unstimulated or stimulated with LPS for indicated time and miR-146a expression were analyzed by qRT-PCR (n=4). C, qRT-PCR analysis of IRAK1 and TRAF6 mRNA expression in unstimulated WT and Apoe−/− macrophages (n=4). D, qRT-PCR analysis of miR-146a expression in Ly-6Chigh and Ly-6Clow monocytes purified from WT and Apoe−/− mice (n=3). The results of WT Ly-6Chigh were set arbitrarily to 1. E, qRT-PCR analysis of miR-146a expression in various immune cells purified from WT and Apoe−/− mice (n=3). The results of WT cells were set arbitrarily to 1. Data are shown as the means ± SEM and are representative of three independent experiments. *, P<0.05; **, P<0.01.
In beginning to explore the relationship between cellular apoE expression and microRNA levels, we focused on microRNA-146a because of its well established role in suppressing NF-κB–driven inflammatory signaling and cytokine expression in macrophages.10, 15 Bone marrow-derived macrophages (BMDM) prepared from 3-week-old Apoe−/− and WT mice were exposed to LPS and levels of microRNA-146a were determined by qRT-PCR at various time points after stimulation. A loss of apoE expression in Apoe−/− BMDM led to a 2-fold decrease in miR-146a expression even prior to LPS stimulation, indicating the importance of apoE expression in the maintenance of baseline miR-146a levels in macrophages (Figure 2B). Although miR-146a levels increased in Apoe−/− BMDM following LPS stimulation, this occurred at a much lower magnitude than in WT BMDM (Figure 2B). Interestingly, the difference in miR-146a levels were even more pronounced at the 6 hr and 18 hr time points, demonstrating that a loss of apoE impairs the cell’s ability to produce and maintain a normal pool of miR-146a in response to an inflammatory stimulus (Figure 2B). We explored the functional consequences of miR-146a paucity in Apoe−/− macrophages on the expression levels of its target genes including TRAF6 and IRAK1.10, 15 Not surprisingly, we noted that the loss of apoE expression in macrophages consistently led to a 2-fold increase in the mRNA levels of these two key molecular activators of NF-κB signaling (Figure 2C). These findings therefore explain in part our observations of increased NF-κB activation and pro-inflammatory cytokine expression in Apoe−/− macrophages (Figure 1).
To test the possibility that hyperlipidemia contributed to the observed reduced miR-146a levels in cells derived from Apoe−/− mice, we repeated our experiments with resident peritoneal macrophages isolated from Ldlr−/− mice that displayed a similar level of hyperlipidemia (Online Figure IA). As shown in Online Figure IB-ID, macrophages isolated from Ldlr−/− mice exhibited similar levels of miR-146a (P=0.18), as wells as mRNA levels of TRAF6 (P=0.71) and IRAK1 (P=0.49) as those isolated from WT mice. These data demonstrate that a loss of apoE expression but not the presence of hyperlipidemia is responsible for reduced miR-146a expression and activity in macrophages derived from Apoe−/− mice.
Lastly, we tested if other leukocytes derived from Apoe−/− mice were also susceptible to reduced miR-146a levels. Interestingly, we detected significantly reduced amounts of miR-146a in both Ly-6Chigh and Ly-6Clow monocytes derived from Apoe−/− mice compared to those of WT mice at 3–4 weeks of age (Figure 2D). However, this effect did not extend to other myeloid cells or lymphocytes examined including neutrophils, DCs, pDCs, B cells and T cells (Figure 2E), which do not express appreciable amounts of apoE (Figure 1A). Collectively, our findings demonstrate that cellular apoE expression enables the production and maintenance of miR-146a in monocytes and macrophages to effectively suppress NF-κB inflammatory signaling that is required for the fine-tuning and resolution of inflammation.
ApoE expression enhances miR-146a in macrophages and monocytes
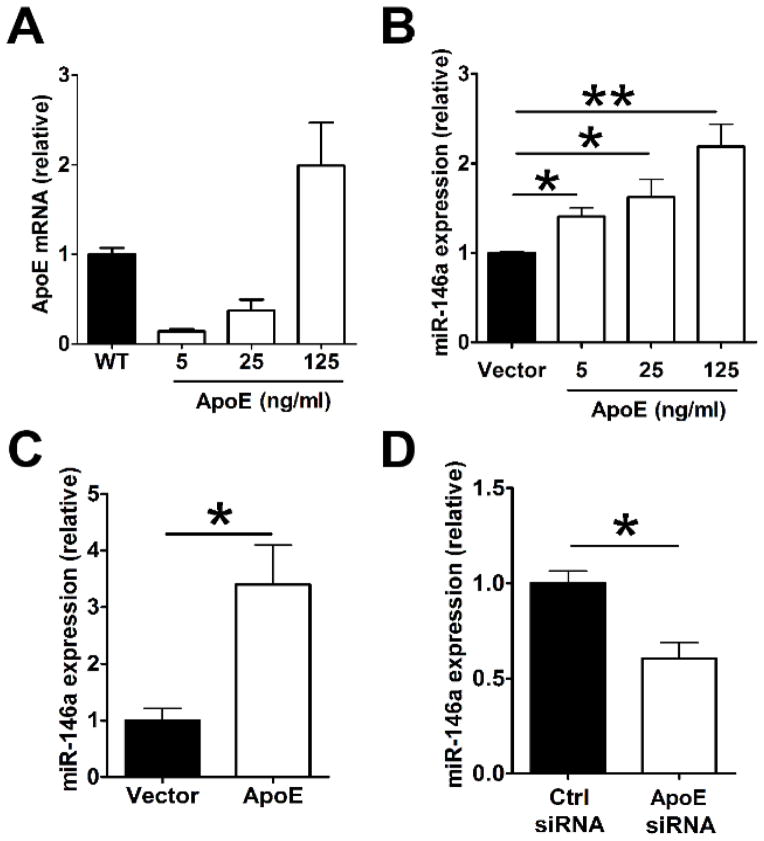
We next tested the causality between cellular apoE expression and enhanced miR-146a levels in macrophages and monocytes. This was accomplished by detecting cellular miR-146a levels following a transduced upregulation of apoE expression or its suppression. Restoring apoE expression in Apoe−/− BMDM using a mouse apoE gene expression vector (Figure 3A) increased miR-146a levels in a concentration-dependent manner (Figure 3B). Remarkably, restoring only 14% of WT apoE mRNA expression levels in Apoe−/− BMDM transduced with a low amount of apoE expression plasmid delivered at 5 ng/ml (Figure 3A) led to a pronounced 40% increase in miR-146a levels (Figure 3B). Interestingly, expressing 14-fold more apoE mRNA expression in cells transduced with 125 ng/ml apoE expression plasmid (Figure 3A) resulted in only a 50% increase in miR-146a levels (Figure 3B). These results suggest that only a small amount of cell-derived apoE expression is required to positively drive an accumulation of miR-146a in macrophages. The induction of miR-146a by apoE expression was even more effective in Ly-6Chigh monocytes which displayed a three-fold increase in miR-146a levels when transduced with the apoE expression plasmid at 125 ng/ml (Figure 3C). We also tested whether suppressing apoE expression could have an opposite effect on miR-146a levels in these cells. As shown in Figure 3D, transducing WT mouse monocyte-derived macrophages with an apoE siRNA led to a 40% reduction of miR-146a levels 48 hrs after transfection. Together, our in vitro data demonstrate a causative role for cellular apoE expression in the enhancement of miR-146a levels in macrophages and monocytes and that low amounts of cellular apoE expression are sufficient to drive a meaningful biological effect.
Figure 3. Ectopic apoE expression enhances miR-146a expression in macrophages and monocytes.
A, BMDM prepared from Apoe−/− mice were transfected with an empty vector (Vector) or increasing amounts ranging from 5 to 125 ng/ml of an apoE expression vector using Lipofectamine 2000. After 48 hrs, cells were analyzed for apoE mRNA levels. The results were compared to WT apoE mRNA levels set arbitrarily to 1. B, Apoe−/− BMDMs were transfected as described in (A) and were examined for miR-146a levels. MiR-146a expression in Apoe−/− BMDM receiving control vector were set arbitrarily to 1. C, miR-146a expression in Apoe−/− Ly-6Chigh monocytes transfected with control empty vector or apoE expression vector (125 ng/ml). D, WT monocytes were transfected with a control siRNA (siCtrl) (Ctrl siRNA) or an apoE siRNA (ApoE siRNA) using Lipofectamine RNAiMAX. Cells were analyzed for miR-146a expression 48 hrs after transfection. The results of control groups were set arbitrarily to 1. Similar results were obtained in 3 separate experiments using 4 mice per group. Data are shown as the means ± SEM. *, P<0.05; **, P<0.01.
ApoE increases miR-146a by enhancing the transcription factor PU.1
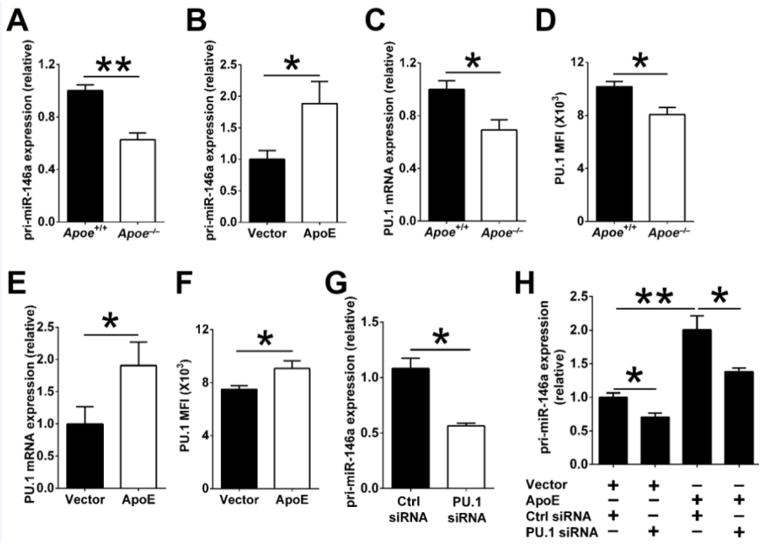
We next explored the mechanisms by which cellular apoE expression increases miR-146a levels in macrophages. At first, levels of miR-146a primary transcript (pri-miR146a) were detected to examine whether apoE affects miR-146a transcription. As shown in Figure 4A, peritoneal macrophages from WT mice expressed two fold more pri-miR146a than those from Apoe−/− mice. Moreover, transducing apoE expression in Apoe−/− BMDM raised cellular pri-miR-146a levels (Figure 4B). These results suggest that apoE increases miR-146a in macrophages by enhancing its transcription.
Figure 4. ApoE increases miR-146a transcription by enhancing transcription factor PU.1.
A, Quantitative PCR analysis of primary miR-146a transcripts (pri-miR-146a) in peritoneal macrophages derived from 4-week-old Apoe−/− and WT mice (n=12). The results of WT cells were set arbitrarily to 1. B, Apoe−/− BMDM (n=4) were transfected with an empty vector (Vector) or an apoE expression vector (ApoE) using Lipofectamine 2000. After 48 hrs, cells were analyzed for pri-miR-146a levels. Results of the control group were set arbitrarily to 1. (C) qPCR and (D) FACS analysis of PU.1 expression in peritoneal macrophages derived from Apoe−/− and WT mice (n=8); the results of WT cells were set arbitrarily to 1. (E) qPCR and (F) FACS analysis of PU.1 expression in Apoe−/− BMDM (n=4) transfected with a control empty vector (Vector) or an apoE expression vector (ApoE). G, Quantitative PCR analysis of pri-miR-146a expression in WT BMDM (n=4) transfected with a control siRNA or a PU.1-specific siRNA (100 μM) for 24 hrs using Lipofectamine RNAiMAX; the results of control siRNA-treated cells were set arbitrarily to 1. H, Apoe−/− BMDM were first transfected with empty or apoE expression vectors for 24 hrs, and then transfected with either control siRNA or PU.1 siRNAs for 24 hrs. The cells were examined for pri-miR-146a expression by real-time PCR. The results of Apoe−/− BMDM receiving the empty vector and control siRNA were set arbitrarily to 1. Data are shown as the means ± SEM. The data in B, E, F, G and H are representative of two or three independent experiments *, P<0.05; **, P<0.01.
MiR-146a expression in hematopoietic cells is known to be transcriptionally induced by NF-κB and PU.1.10, 16 Because NF-κB activity is similarly low in freshly isolated resident peritoneal macrophages derived from young, 3–4 week-old Apoe−/− and WT mice (Figure 1D and 1F), we wondered whether apoE enhances miR-146a transcription by regulating basal PU.1 levels. Indeed, PU.1 mRNA and protein levels were significantly greater in WT macrophages than in Apoe−/− macrophages (Figure 4C and 4D). Furthermore, transduced expression of apoE in Apoe−/− macrophages increased PU.1 mRNA and protein expression (Figure 4E and 4F).
We then investigated the functional role of PU.1 in apoE-induced miR-146a transcription by down-regulating its expression. As shown in Figure 4G, the knockdown of PU.1 in WT BMDM by siRNA led to a 50% reduction in pri-miR-146a expression levels. Importantly, the induction of pri-miR-146a by ectopic apoE expression in Apoe−/− BMDM was significantly reduced but not completely abrogated by PU.1 siRNA (Figure 4H). Together, these data demonstrate that apoE drives the transcription of miR-146a in part by enhancing cellular expression of PU.1.
ApoE raises cellular miR-146a levels and restrains the activation of macrophages and monocytes in the settings of hyperlipidemia
The chronic activation of monocytes and macrophages has long been established as a key underlying mechanism for atherosclerosis initiation and progress.6, 14, 17 We therefore examined whether apoE expression could suppress monocyte and macrophage inflammation, and thereby atherosclerosis, by increasing miR-146a in the settings of hyperlipidemia.
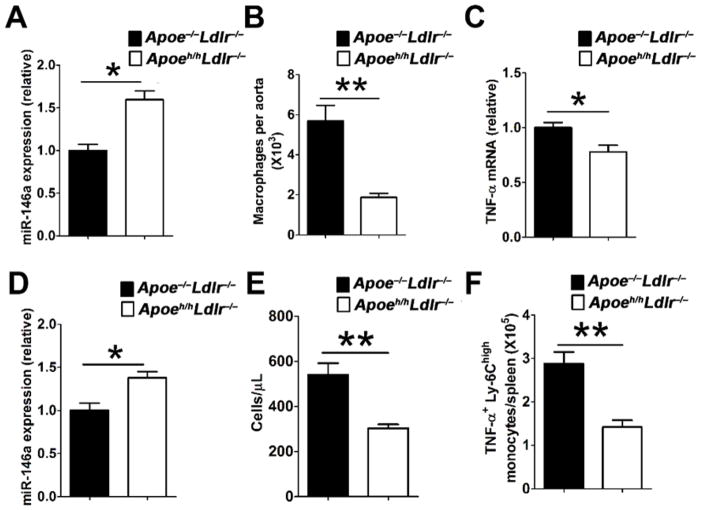
To address this question we made use of the hypomorphic apoE (Apoeh/h) mouse model of reduced apoE gene expression in all tissues, which we previously designated HypoE mice.18 We recently reported that HypoE mice deficient in low density lipoprotein receptor expression (Apoeh/hLdlr−/− mice) spontaneously display similarly elevated plasma cholesterol and triglycerides as Apoe−/−Ldlr−/− mice, but develop 4-fold less atherosclerotic lesion burden.19 In this study, we examined if apoE expression, albeit at reduced levels in monocytes and macrophages of hyperlipidemic Apoeh/hLdlr−/− mice, could raise cellular miR-146a levels to suppress cellular activation and atherosclerosis caused by chronic hyperlipidemia. Both macrophages (Figure 5A) and Ly-6Chigh monocytes (Figure 5D) isolated from Apoeh/hLdlr−/− mice displayed a 50% increase in cellular miR-146a levels as compared to those isolated from Apoe−/−Ldlr−/− mice. We also observed a three-fold reduction in the number of aortic macrophages in Apoeh/hLdlr−/− mice as compared to Apoe−/−Ldlr−/− mice (Figure 5B). Moreover, resident peritoneal macrophages isolated from Apoeh/hLdlr−/− mice displayed lower TNF-α mRNA expression compared to those from Apoe−/−Ldlr−/− mice (Figure 5C). In peripheral blood, Apoeh/hLdlr−/− mice had two-fold less Ly-6Chigh monocytes compared to Apoe−/−Ldlr−/− mice (Figure 5E). Intracellular cytokine analysis revealed a two-fold reduction in the numbers of Ly-6Chigh monocytes producing TNF-α in the spleen of Apoeh/hLdlr−/− mice as compared to those of Apoe−/−Ldlr−/− mice (Figure 5F). Collectively, these results demonstrate that cellular apoE expression, even at low levels seen in HypoE mice, substantially raise miR-146a levels in macrophages and monocytes to reduce chronic inflammation in the setting of hyperlipidemia and thereby suppress atherosclerosis.
Figure 5. Hyperlipidemic HypoE mice expressing reduced apoE levels display enhanced miR-146a in macrophages and monocytes that suppress their cellular activation.
MiR-146a expression in (A) peritoneal macrophages and (D) bone marrow Ly-6Chigh monocytes isolated from Apoe−/−Ldlr−/− and Apoeh/hLdlr−/− (HypoE) mice (n=6). (B) The number of macrophages in the aortas of Apoe−/−Ldlr−/− and Apoeh/hLdlr−/− mice (n=4). (C) TNF-α expression in peritoneal macrophages isolated from Apoe−/−Ldlr−/− and Apoeh/hLdlr−/− mice (n=6). The number of (E) blood Ly-6Chigh monocytes and (F) splenic TNF-α-producing Ly-6Chigh monocytes in Apoe−/−Ldlr−/− and Apoeh/hLdlr−/− mice (n=9). The results of Apoe−/−Ldlr−/− mice were set arbitrarily to 1 for A, C and D. Data are shown as the means ± SEM. *, P<0.05; **, P<0.01.
Systemic delivery of a miR-146a mimetic reduces macrophage activation, Ly-6Chigh monocytosis and atherosclerosis in the setting of hyperlipidemia and apoE deficiency
Our in vitro and in vivo findings collectively point to a deficit in miR-146a levels in monocytes and macrophages as a source of increased inflammation and atherosclerosis in mice deficient in apoE expression. We investigated whether correcting such a miR-146a deficit could suppress monocytosis and macrophage activation and thereby atherosclerosis in apoE-deficient mice.
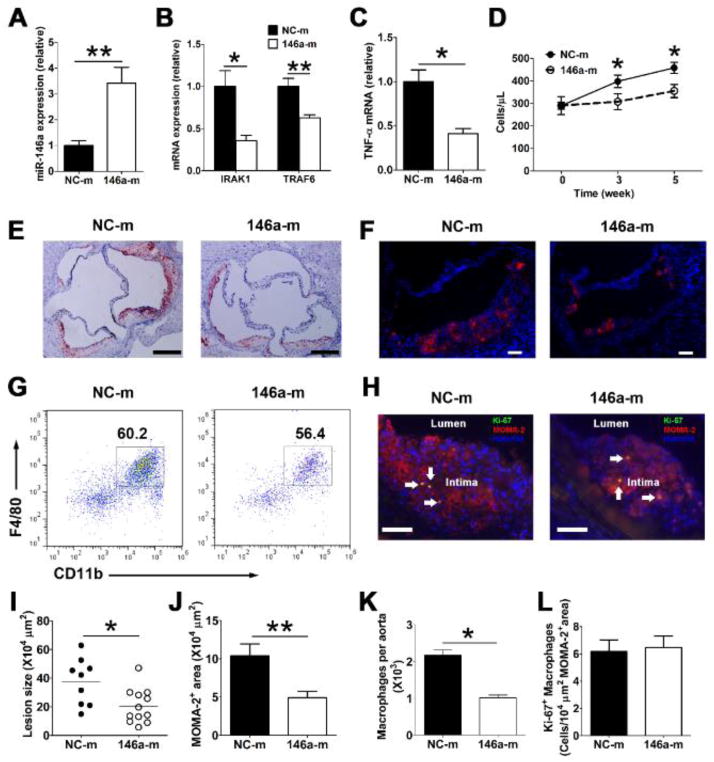
To explore the feasibility of restoring miR-146a levels in monocytes and macrophages in vivo, we systemically delivered a miR-146a mimetic (146a-m) or negative control mimetic (NC-m) encapsulated in liposomes (1 nmol/mouse) via tail vein injection twice a week for two weeks in miR-146a deficient (miR-146a−/−) mice. This method effectively restored miR-146a expression in Ly-6Chigh monocytes and macrophages (Online Figure IIA and IIB), confirming an efficient uptake of miR-146a mimetics by these cells in vivo. We then explored the benefit of delivering such miR-146a mimetics for a period of 6 weeks into Apoe−/−Ldlr−/− mice fed a normal chow diet. At the time of euthanasia, there was a three-fold increase in miR-146a expression in peritoneal macrophages isolated from mice injected with the 146a-m than those from mice receiving the NC-m (Figure 6A). Consistent with this finding, the mRNA expression of IRAK1 and TRAF6 were substantially reduced (Figure 6B). Moreover, there was a 50% reduction in TNF-α mRNA levels in resident peritoneal macrophage (Figure 6C), indicating a substantial reduction of NF-κB signaling and inflammation in Apoe−/−Ldlr−/− mice that received infusions of 146a-m. In addition, as soon as three weeks after the 146a-m treatment, Apoe−/−Ldlr−/− mice also displayed a 30% reduction in the number of blood Ly-6Chigh monocytes that persisted at the 5 week time point (Figure 6D).
Figure 6. In vivo delivery of miR-146a mimetics inhibits macrophage inflammatory responses, Ly-6Chigh monocytosis and atherosclerosis in hyperlipidemic Apoe−/−Ldlr−/− mice.
Apoe−/−Ldlr−/− mice (~10 week old, n=9~12/group) receiving a chow diet were intravenously administered miRNA negative control mimetics (NC-m) or miR-146a mimetics (146a-m) twice a week for 6 weeks. MiR-146a levels (A), IRAK1 and TRAF6 (B), as well as TNF-α mRNA (C) levels in resident peritoneal macrophages were analyzed by qRT-PCR. The absolute numbers of Ly-6Chigh monocytes (D) in peripheral blood were determined by flow cytometry before, 3 weeks and 5 weeks after initiation of the treatment. Representative oil red O staining (E) and MOMA-2 staining (F) of aortic root cross-sections from 146a-m or NC-m treated Apoe−/−Ldlr−/− mice; Scale bar, 200 μm in E, 50 μm in F. Quantification of aortic root lesion area (I) and MOMA-2 macrophage area (J). Dots represent values for individual mice; bars are group means. Representative FACS plots (G) and absolute cell number (K) of macrophages in aortas of Apoe−/−Ldlr−/− mice receiving either 146a-m or NC-m. Live CD45+ cells were gated. H, Representative images of Ki-67 and MOMA-2 co-staining of aortic sinus lesions, arrows denote double positive cells, scale bar, 50 μm; L, Quantification of Ki-67+ MOMA-2+ cells in atherosclerotic plaque; data were normalized by MOMA-2 positive area. n=9~12 per group in A–D, I, J and L, n=4~6 per group in K. The results of control mice were set arbitrarily to 1 (A–C). Data are shown as the means ± SEM. *, P<0.05; **, P<0.01.
We next examined how such a beneficial reduction in systemic inflammation impacted on the progression of atherosclerosis in these hyperlipidemic mice. Despite similarly elevated plasma cholesterol levels (Online Figure IIIA), a 6-week period of systemic miR-146a mimetic delivery led to a 2-fold reduction in oil-red O positive neutral-lipid surface area (Figure 6E and 6I), as well as MOMA-2 positive macrophage surface area (Figure 6F and 6J) in the aortic root. In addition, there was a two-fold reduction in the absolute number of CD11b+ F4/80+ macrophages in the aorta of Apoe−/−Ldlr−/− mice (Figure 6G and 6K). Double immunostaining for MOMA-2 and Ki-67 (a marker for cell proliferation) in sections of aortic sinus showed that lesional macrophages in 146a-m–treated mice proliferated at a rate similar to those of control mice (Figure 6H and 6L). Collectively, our findings demonstrate that systemic delivery of a miR-146a mimetic results in an attenuation of atherosclerosis by suppressing Ly-6Chigh monocytosis, macrophage activation but not macrophage proliferation in atheroma.
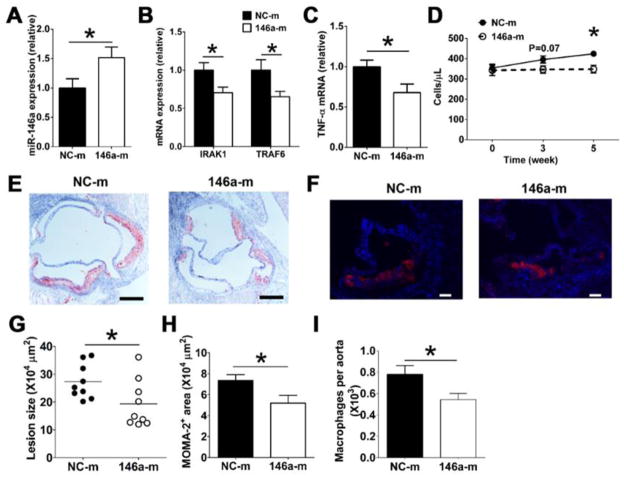
Finally, we assessed the capacity for systemic delivery of a miR-146a mimetic to suppress atherosclerosis in hyperlipidemic mice with an intact apoE gene. This was accomplished by using Ldlr−/− mice fed a high fat diet that develop twice the amount of plasma cholesterol as chow fed Apoe−/−Ldlr−/− mice (Online Figure IIIB). Bi-weekly infusions of 146a-m for six weeks led to a 50% increase of miR-146a in resident peritoneal macrophages of Ldlr−/− mice (Figure 7A). This contrasts the 3-fold increase detected in macrophages of 146a-m–treated Apoe−/−Ldlr−/− mice (Figure 6A). Such modest increase in miR-146a nonetheless reduced the expression of IRAK1, TRAF6, and TNF-α expression in these cells (Figure 7B and 7C). It also led to a 20% reduction in Ly-6Chigh monocytosis (Figure 7D) and a significant suppression of atherosclerotic plaque formation (Figure 7E and 7G) and macrophage accumulation in the aorta (7F, 7H and 7I).
Figure 7. In vivo delivery of miR-146a mimetics reduces macrophage inflammatory responses, Ly-6Chigh monocytosis and atherosclerosis in Ldlr−/− mice fed a high fat diet.
Ldlr−/− mice (~10 week old, n=9/group) were fed a high fat diet for 2 weeks, and then were injected with either 146a-m or NC-m via tail vein twice weekly for 6 weeks. MiR-146a levels (A), IRAK1, TRAF6 (B), and TNF-α mRNA in macrophages (C) as well as absolute numbers of blood Ly-6Chigh monocytes (D) were examined as detailed in Figure 6. Representative oil red O staining (E) and MOMA-2 staining (F) of aortic root cross-sections from 146a-m or NC-m treated Ldlr−/− mice; Scale bar, 200 μm in E, 50 μm in F. Aortic root lesion area (G), MOMA-2 macrophage area (H) and absolute cell number of aortic macrophages (I) in Ldlr−/− mice receiving either 146a-m or NC-m were quantified as in Figure 6. *, P<0.05
DISCUSSION
ApoE is recognized for its unparalleled ability to suppress atherosclerosis.20 Beyond its participation in the removal of atherogenic remnant lipoproteins from plasma,20 apoE exerts a direct influence on myeloid cell activity.21 The expression of apoE in the macrophage has long been recognized to suppress atherosclerosis by enhancing the efflux of cellular cholesterol, thereby preventing foam cell formation in the vessel wall.1, 22 More recent findings have added to the list of apoE’s anti-atherogenic properties that include an ability to suppress myelopoeisis23 and the cellular activation of circulating monocytes in hyperlipidemic mice.19 The underlying mechanisms of these protective properties have largely been ascribed to the lipid efflux capacity of apoE both through its cellular expression23 and by its ability to enhance the cholesterol efflux capacity of plasma HDL.19 As illustrated in Figure 8, results of our study uncovered a new anti-inflammatory property of apoE. Our findings demonstrate that cellular apoE expression participates to enhance and sustain miR-146a levels in macrophages to suppress NF-κB–mediated cellular activation in response to acute and chronic inflammatory signaling via TLR receptors, including in a hyperlipidemic environment. We also found that among other leukocytes commonly involved in atherosclerosis, monocytes are the only ones to express appreciable amounts of apoE. Moreover, we noted that apoE is expressed at much higher levels in anti-inflammatory Ly-6Clow monocytes that also express far more miR-146a than Ly-6Chigh monocytes.
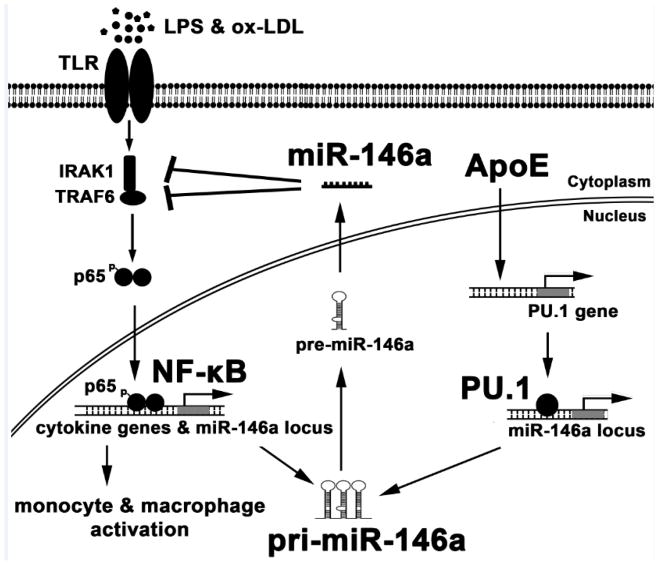
Figure 8. ApoE enhances PU.1–dependent miR-146a transcription to suppress NF-κB–driven monocyte and macrophage activation and thereby inflammation and atherosclerosis.
Environmental ligands of Toll like receptors (TLR) including LPS and oxLDL caused by hyperlipidemia provoke inflammatory signaling in monocytes and macrophages resulting in NF-κB activation. Gene transcription from NF-κB activity results in the production of inflammatory mediators including pro-atherogenic cytokines. It also results in the production of pri-miR-146a that is subsequently processed into mature miR-146a that silences the expression of key TLR-adaptor molecules IRAK1 and TRAF6. The production of miR-146a thereby serves as a regulatory feedback loop to suppress NF-κB activity and resolve inflammation. Findings from our study identified that cellular apoE expression contributes to amplify this regulatory feedback loop by increasing PU.1-dependent transcription of pri-miR-146a and thereby mature miR-146a production.
MicroRNAs have emerged as key regulators of chronic inflammation and atherosclerosis in hyperlipidemic mice.11, 14 In fact, miRNA-146a has been identified as a critical regulator of myeloid cell activation and expansion.10, 15 It has been shown to control the balance between pro- and anti-inflammatory monocytes by down-regulating the expression of the transcription factor RelB that controls the proliferation of Ly-6Chigh monocytes, which are recognized for their inflammatory24 and atherogenic properties.17 MiR-146a is also recognized for its ability to potently suppress inflammatory challenges by reducing TLR-driven NF-κB signaling in macrophages and in other hematopoietic cells. 10 This function is crucial to prevent an immunological overload and fatal inflammation following a bout of sepsis or LPS injection.15 But the relevance of miR-146a in controlling immunity during prolonged periods of chronic inflammation such as hyperlipidemia has not been explored. Therefore, our findings demonstrating reduced miR-146a levels in macrophages and monocytes that lack apoE expression provide new insight to explain the susceptibility to atherosclerosis and sepsis 2, 3 reported in Apoe−/− mice.
Our findings are unexpected, as miR-146a is known to emerge from NF-κB transcription,10 and Apoe−/− mice have been reported to express increased NF-κB activity,2 which we confirmed in our study. As such, one would legitimately expect to detect increased levels of miR-146a in macrophages isolated from Apoe−/− mice. Contrary to this expectation, we uncovered that cellular apoE expression enables both macrophages and monocytes to upregulate and maintain elevated levels of miR-146a in response to acute and chronic inflammatory challenges. Our findings, therefore, provide provocative new insight to appreciate the influence that apoE can exert on the plasticity of monocytes and macrophages to suppress inflammation and atherosclerosis beyond contributing to enhance cellular lipid efflux. This new mode of cellular regulation could explain results of earlier reports that documented a capacity for apoE to suppress type I inflammation2 and promote atherosclerosis regression beyond reducing plasma lipid levels. 25
The ability of apoE to regulate cellular miRNA levels is not limited to myeloid cells. A study has recently reported that apoE can control miR-145 to suppress smooth muscle cell activation and aortic stiffness.26 Because apoE is known to suppress endothelial activation, it is possible that it could be doing so in part by raising cellular miR-146a levels. Our demonstration of apoE’s capacity to increase miR-146a expression in monocytes and macrophages may constitute a key regulatory mechanism to suppress NF-κB–driven inflammation and atherosclerosis. In addition, because apoE is also expressed by hematopoietic progenitors,23 ongoing studies are aimed at exploring whether it can increase miR-146a to suppress NF-κB signaling and thereby add another level of control over myeloproliferation that has so far been ascribed to its ability to suppress cytokine-driven proliferative signaling by enhancing cellular lipid efflux.23
The physiological relevance of our findings with respect to suppression of chronic inflammation comes from observations of our hypomorphic apoE mouse model of reduced apoE expression that we termed HypoE mice.18 We recently reported that HypoE mice deficient in LDL receptor expression (Apoeh/hLdlr−/− mice) develop a similar hyperlipidemia as Apoe−/−Ldlr−/− mice, despite significantly lower levels of apoE expression in all tissues including myeloid cells and hepatocytes.19 A key observation for reduced atherosclerosis in Apoeh/hLdlr−/− mice was a reduced number of activated circulating Ly-6Chigh monocytes that we ascribed to reduced cellular lipid levels due to enhanced cholesterol efflux capacity of plasma HDL. 19 In this study, we uncovered another explanation as Ly-6Chigh monocytes isolated from these mice were found to express more miR-146a and less TNF-α than those from hyperlipidemic Apoe−/−Ldlr−/− mice. In parallel, peritoneal macrophages isolated from both mouse models demonstrated that a low amount of apoE expression in the HypoE mice was sufficient to raise miR-146a levels and suppress NF-κB–driven cellular activation. An ability for low apoE expression levels to induce an appreciable amount of miR-146a as seen in the HypoE mice was confirmed by our in vitro studies showing that a 25-fold difference in the ectopic expression of apoE in macrophages deficient in apoE results only in a marginal increase in cellular miR-146a levels. Our findings, therefore, underscore the importance of apoE’s capacity to raise cellular miR-146a levels in monocytes and macrophages to suppress NF-κB inflammatory signaling and thereby atherosclerosis. A similar enhancement of miR-146a by apoE in brain astrocytes could play an important role in the protection from NF-κB–driven neurodegenerative disorders.
Cellular miRNA levels are influenced by transcriptional and post-transcriptional events.27 Data from our study demonstrate that cellular apoE expression raises miR-146a levels in monocytes and macrophages by enhancing its transcription. MiR-146a expression is transcriptionally induced by NF-κB during inflammatory challenges and by PU.1 during hematopoietic development. 10, 16 Our data show that a source of added transcriptional activity at the miR-146a locus by apoE derives in part from increased levels of PU.1. Further research will be required to understand how cellular apoE expression enhances miR-146a levels via PU.1 or other yet to be determined mechanisms and whether plasma lipoprotein-associated apoE can regulate these effects through cell-surface receptor interactions.
To our knowledge, our study is the first in vivo demonstration of microRNA delivery to macrophages and monocytes that reduces inflammation and atherosclerosis in the absence of plasma lipid reduction. An appealing aspect of miR-146a delivery is the ability to silence multiple inflammatory targets including IRAK1 and TRAF6 to suppress NF-κB–driven TNF-α expression in macrophages. Also, the observed decrease in Ly-6Chigh monocytosis could be due to reduced expression of the M-CSF receptor15 or the RelB transcription factor24, both targets of miR-146a in these cells. Importantly, our findings reveal that the level of macrophage miR-146a enrichment and intensity of its anti-inflammatory effects produced by an administration of miR-146a mimetics are less pronounced when given to Ldlr−/− mice than to Apoe−/−Ldlr−/− mice. The naturally higher expression levels of miR-146a caused by endogenous ApoE expression in monocytes and macrophages of Ldlr−/− mice could make them less responsive to infusions of microRNA mimetics. Nonetheless, even a modest 50% increase in macrophage miR-146a levels effectively reduced chronic inflammation and atherosclerosis in hyperlipidemia Ldlr−/− mice. Moreover, a similarly mild elevation in miR-146a levels in macrophages was observed to substantially reduce atherosclerosis in hyperlipidemic HypoE mice (Figure 5). Collectively, our findings support a mild enhancement of miR-146a levels in monocytes and macrophages as a potential therapeutic avenue for chronic inflammatory disorders including atherosclerosis while minimizing a compromise of host defense.
In conclusion, our data demonstrate that apoE expression in macrophages and monocytes controls inflammation by enhancing PU.1-dependent miR-146a expression that suppresses inflammatory NF-κB signaling (Figure 8). Our findings provide a new mechanistic link to explain how apoE exerts its anti-inflammatory properties to suppress atherosclerosis beyond reducing plasma lipid levels or enhancing cellular lipid efflux. Our findings also highlight the potential utility of targeting the cellular regulation of miR-146a by apoE to promote the resolution of inflammation and atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Apolipoprotein (apo) E can protect against atherosclerosis beyond reducing blood lipid levels.
Among its pleiotropic properties, apoE has long been recognized to exert regulatory control over cells of the innate and adaptive immune system.
ApoE is known to protect monocytes and macrophages from lipid-driven activation and contribution to atherosclerosis mainly through its ability to promote cellular lipid efflux.
What New Information Does This Article Contribute?
Cellular apoE expression in monocytes and macrophages increases PU.1–dependent expression of miR-146a that suppresses NF-κB activation in response to Toll-like receptor (TLR) signaling.
Sub-physiological apoE expression is sufficient to positively drive monocyte and macrophage heterogeneity by increasing miR-146a levels that suppress inflammation and atherosclerosis in hyperlipidemic mice.
In vivo delivery of miR-146a mimetics in hyperlipidemic mice raises miR-146a levels in monocytes and macrophages and effectively suppresses NF-κB–driven inflammation and atherosclerosis in the absence of plasma lipid lowering.
Our understanding of how apoE suppresses atherosclerosis beyond reducing plasma and cellular lipid levels remains incomplete. We found that apoE expression in monocytes and macrophages enhances the production of microRNAs that suppress NF-κB inflammatory signaling, including miR-146a. Although NF-κB activation normally drives miR-146a expression as a natural feedback mechanism, this process is impaired in monocytes and macrophages of Apoe−/− mice. The capacity for apoE to increase miR-146a levels was independent of NF-κB activation, but rather was dependent on PU.1, a transcription factor that enhances the expression of miR-146a. Even very low levels of cellular apoE expression enhanced miR-146a expression, and this process contributed to suppressed monocyte and macrophage activation and atherosclerosis in hypomorphic apoE mice with hyperlipidemia similar to apoE–deficient mice. Intravenous infusions of miR-146a mimetics rescued the miR-146a paucity in monocytes and macrophages of Apoe−/− mice and suppressed NF-κB inflammatory responses and atherosclerosis in the absence of plasma lipid reduction. Such delivery of miR-146a mimetics to hyperlipidemic mice with an intact apoE gene led to a more modest increase in cellular miR-146a levels that nonetheless suppressed atherosclerosis, demonstrating its potential therapeutic utility for human atherosclerosis.
Acknowledgments
We thank Drs. Lewis L. Lanier and K. Mark Ansel for critical review of the manuscript and David Wong and King Yeung Hong for excellent technical assistance.
SOURCES OF FUNDING
This work was supported by HL089871 (RLR) from the National Institutes of Health, which was administered by the Northern California Institute for Research and Education; and a Merit Review award, 5I01BX000532, from the Department of Veterans Affairs to RLR. The work was performed at the Veterans Affairs Medical Center, San Francisco, California.
Nonstandard Abbreviations and Acronyms
- apoE
apolipoprotein E
- NF-κB
nuclear factor-κB
- IRAK1
interleukin-1 receptor-associated kinase 1
- TRAF6
TNF receptor-associated factor 6
- BMDM
bone marrow-derived macrophages
- Mono
monocytes
- DC
dendritic cells
- pDC
plasmacytoid dendritic cells
- LPS
lipopolysaccharide
- TLR
toll like receptor
- qRT-PCR
quantitative real-time PCR
- MOMA-2
Macrophage/monocyte monoclonal antibody
- 146a-m
miR-146a mimetic
- NC-m
negative control mimetic
- LDLR
low density lipoprotein receptor
- M-CSF
macrophage colony stimulating factor
- PU.1
purine-rich PU-box binding protein 1
Footnotes
DISCLOSURES
None.
References
- 1.Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr Opin Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ali K, Middleton M, Pure E, Rader DJ. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 3.de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- 4.Christensen DJ, Ohkubo N, Oddo J, Van Kanegan MJ, Neil J, Li F, Colton CA, Vitek MP. Apolipoprotein E and peptide mimetics modulate inflammation by binding the SET protein and activating protein phosphatase 2A. J Immunol. 2011;186:2535–2542. doi: 10.4049/jimmunol.1002847. [DOI] [PubMed] [Google Scholar]
- 5.Baitsch D, Bock HH, Engel T, Telgmann R, Muller-Tidow C, Varga G, Bot M, Herz J, Robenek H, von Eckardstein A, Nofer JR. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–2661. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 8.Wolfrum S, Teupser D, Tan M, Chen KY, Breslow JL. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-kappaB target genes. Proc Natl Acad Sci U S A. 2007;104:18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res. 2009;50(Suppl):S340–345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 14.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghani S, Riemke P, Schonheit J, Lenze D, Stumm J, Hoogenkamp M, Lagendijk A, Heinz S, Bonifer C, Bakkers J, Abdelilah-Seyfried S, Hummel M, Rosenbauer F. Macrophage development from HSCs requires PU.1-coordinated microRNA expression. Blood. 2011;118:2275–2284. doi: 10.1182/blood-2011-02-335141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raffai RL, Weisgraber KH. Hypomorphic apolipoprotein E mice: a new model of conditional gene repair to examine apolipoprotein E-mediated metabolism. J Biol Chem. 2002;277:11064–11068. doi: 10.1074/jbc.M111222200. [DOI] [PubMed] [Google Scholar]
- 19.Gaudreault N, Kumar N, Posada JM, Stephens KB, Reyes de Mochel NS, Eberle D, Olivas VR, Kim RY, Harms MJ, Johnson S, Messina LM, Rapp JH, Raffai RL. ApoE suppresses atherosclerosis by reducing lipid accumulation in circulating monocytes and the expression of inflammatory molecules on monocytes and vascular endothelium. Arterioscler Thromb Vasc Biol. 2012;32:264–272. doi: 10.1161/ATVBAHA.111.238964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davignon J. Apolipoprotein E and atherosclerosis: beyond lipid effect. Arterioscler Thromb Vasc Biol. 2005;25:267–269. doi: 10.1161/01.ATV.0000154570.50696.2c. [DOI] [PubMed] [Google Scholar]
- 21.Raffai RL. Apolipoprotein E regulation of myeloid cell plasticity in atherosclerosis. Curr Opin Lipidol. 2012;23:471–478. doi: 10.1097/MOL.0b013e328356f967. [DOI] [PubMed] [Google Scholar]
- 22.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci U S A. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etzrodt M, Cortez-Retamozo V, Newton A, Zhao J, Ng A, Wildgruber M, Romero P, Wurdinger T, Xavier R, Geissmann F, Meylan E, Nahrendorf M, Swirski FK, Baltimore D, Weissleder R, Pittet MJ. Regulation of monocyte functional heterogeneity by miR-146a and Relb. Cell Rep. 2012;1:317–324. doi: 10.1016/j.celrep.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raffai RL, Loeb SM, Weisgraber KH. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2005;25:436–441. doi: 10.1161/01.ATV.0000152613.83243.12. [DOI] [PubMed] [Google Scholar]
- 26.Kothapalli D, Liu SL, Bae YH, Monslow J, Xu T, Hawthorne EA, Byfield FJ, Castagnino P, Rao S, Rader DJ, Pure E, Phillips MC, Lund-Katz S, Janmey PA, Assoian RK. Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep. 2012;2:1259–1271. doi: 10.1016/j.celrep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.