Abstract
The adaptive immune system consists of two types of lymphocytes: T and B cells. These two lymphocytes originate from a common precursor, yet are fundamentally different with B cells mediating humoral immunity while T cells mediate cell mediated immunity. In cytokine production, naïve T cells produce multiple cytokines upon activation while naïve activated B cells do not. B cells are capable of producing cytokines, but their cytokine production depends on their differentiation state and activation conditions. Hence, unlike T cells that can produce a large amount of cytokines upon activation, B cells require specific differentiation and activation conditions to produce cytokines. Many cytokines act on B cells as well. Here, we discuss several cytokines and their effects on B cells including: Interleukins, IL-7, IL-4, IL-6, IL-10, and Interferons, IFN-α, IFN-β, IFN-γ. These cytokines play important roles in the development, survival, differentiation and/or proliferation of B cells. Certain chemokines also play important roles in B cell function, namely antibody production. As an example, we discuss CCL28, a chemokine that directs the migration of plasma cells to mucosal sites. We conclude with a brief overview of B cells as cytokine producers and their likely functional consequences on the immune response.
Keywords: B cells, cytokines, interleukins, interferon, chemokines, autoimmunity
1. Introduction
The immune system is a highly evolved mechanism designed to protect us from pathogens present in our environment. If a pathogen breaches our primary defense mechanisms, represented by barrier tissues such as the skin and mucosal epithelia, we are equipped with an arsenal of molecular and cellular weaponry that has adapted over millions of years of host-pathogen interactions. In its earliest stages, the immune system consisted of a group of generic receptors capable of recognizing conserved pathogen patterns that could elicit a host response [1–3]. The ability to recognize conserved pathogen associated molecular patterns or “PAMP’s” is a fundamental characteristic of the innate immune system. Despite the capacity to recognize conserved patterns present on pathogens, the innate system lacks the ability to remember a previous assailant and respond with a larger and more rapid response against that insult.
The adaptive immune system includes of two main types of lymphocytes: T and B cells. Each of these originate from different lymphoid organs: the thymus and bone marrow, respectively. The ability to generate diverse antigen receptors, a key feature associated with the adaptive immune system, is driven by the gene AID, which encodes an activation-induced deaminase. This gene plays a crucial role in the recombination process that generates a variable T or B cell receptor (TCR/BCR) [4, 5]. The two main types of lymphocytes work in concert to produce an adaptive immune response.
We begin this review with an overview of B cell development and differentiation. Given the large number of cytokines that act on B cells we have chosen to focus on several that play significant roles in the development, survival, differentiation and proliferation of B cells. Interleukins IL-7, IL-4, IL-6, and IL-10 are discussed because of their role in B cell development, B cell proliferation and isotype secretion, and the ability of B cells to regulate the immune response, respectively. The interferons: IFN-α, IFN-β, IFN-γ, also play important roles in the development of B cell responses. Next, we discuss CCL28, a chemotactic cytokine (chemokine) that recruits IgA+ plasma cells to the mucosal tissues. For a list of the cytokines discussed and their functions see Table 1. Finally, we conclude with a brief overview of B cells as cytokine producers and their effects on the immune system
Table 1.
Effects of cytokines on B cells.
| Cytokine | Function | Reference |
|---|---|---|
| IL-7 | B cell development, Ig gene rearrangement | 31–36, 38, 41–43, 47 |
| IL-4 | B cell proliferation, isotype switching | 10, 48, 50–54, 56, 57, 59 |
| IL-6 | B cell proliferation, isotype switching | 62–66, 70 |
| IL-10 | Regulate response | 73, 77, 81–85 |
| IFN-α | B cell development, increased BCR sensitivity | 95–97 |
| IFN-β | B cell development, increased BCR sensitivity | 95, 96 |
| IFN-γ | Inhibit/stimulate B cell proliferation, isotype switching | 99–102 |
| CCL28 | Recruitment of IgA+ plasma cells to mucosa | 107–109, 112 |
2. B cell development, differentiation, and their role in adaptive immunity
B cells undergo a molecular process to rearrange the heavy and light chains of their immunoglobulin genes. This is known as V-D-J and V-J recombination [6] and it applies to the heavy and light chains, respectively. It occurs in the fetal liver and bone marrow and is supported by stromal cell-derived IL-7 [7]. Upon completion of this rearrangement, B cells express a unique BCR [8]. The BCR is required for further B cell development and survival [9]. Upon exiting the bone marrow a B cell is considered ‘immature’ or “transitional”. This name is based on cell surface markers expressed at this particular stage in the differentiation program of the B cell, which includes membrane-bound IgM and IgD. Although technically immature, a B cell can respond to type-I antigens including lipopolysaccharide (LPS) which can induce a rapid antibody response.
Upon migration to secondary lymphoid organs (spleen or lymph nodes) B cells may encounter antigen through interactions with other immune cells such as dendritic cells or macrophages. The B cell can either differentiate into a short lived plasma cell or enter a germinal center (GC). Within the GC, B cells undergo clonal expansion, class switch recombination (CSR), and somatic hypermutation [10]. This process results in the production of high affinity antibody-producing plasma and memory B cells [11].
B cells, like T cells, can also be divided into subsets based on location and function. Some subsets include: Marginal Zone (MZ) B cells, Follicular (FO) B cells, and B-1 cells. Like their name implies, MZ B cells are sessile cells found in the marginal zone of the spleen. This location allows them to capture blood borne pathogens and respond with a rapid antibody response [12]. However, most of the data available on MZ B cells comes from murine models, likely a result of anatomical differences in the marginal zone of the spleen between humans and mice. FO B cells circulate throughout the periphery, but upon encountering their cognate antigen they enter a GC [13]. Memory B cells, generated during the GC reaction, persist and differentiate into plasma cells in a secondary immune response to provide rapid antibody production [14]. B-1 B cells are different from conventional B-2 cells in their location, phenotype, and self-renewing capacity. B-1 cells can be further subdivided into B-1a and B-1b cells based on the expression of CD5 [15]. B-la are fetal B cell progenitors and are known as “innate B cells” because of their ability to produce natural antibodies without T cells help [16]. While B-1b cells are involved with clearance of specific pathogens such as Borrelia hermsii and are therefore considered to be involved in adaptive immune responses [16–18]. B-1 cells can respond to T-independent antigens by secreting natural IgM antibodies which they produce without T cell help [19, 20]. Unfortunately, most information on B-1 cells has been obtained in the mouse, and little information is available on human B-1 cells. This is probably because B-1 cells reside in the peritoneal cavity. Their peritoneal location makes it challenging to study them in humans. Interestingly, B-1-like cells have been implicated in human diseases, for example, endometriosis [21].
Since their discovery in the mid-1960’s, B cells were recognized for their ability to produce antibodies [8, 22]. More recently, it has been recognized that B cells are more than antibody factories. For example, B cells are required for optimal T cell activation to certain antigens including low dose foreign proteins, pathogen challenge, and auto-antigens [23]. Furthermore, their presence facilitates the genesis of the immune system, and maintains its integrity. Mice that develop without B cells exhibit a dramatic decrease in thymocyte numbers and diversity, and also show defects in the spleen, dendritic cells (DC), [24] and T cell compartments, lack of Peyer’s Patches (PP), organogenesis and follicular DC networks, have a paucity of MZ macrophages, and reduced chemokine expression [8, 25, 26]. The importance of B cells in immune system homeostasis is apparent in the function of T and DC functions, regulation of lymphoid tissue organization, wound healing, tissue rejection, and tumor immunity [8, 27]. This information indicates that B cells are linked to the development and maintenance of the immune system.
3. Cytokines that act on B cells
Cytokines are proteins produced and secreted by a variety of cells including stromal cells, fibroblasts, and endothelial cells. In the immune system they are produced by leukocytes and exert their function on other leukocytes or tissues that express the cytokine receptor [28]. Some of them are called interleukins (between leukocytes). The term interleukin (IL) was first used in 1979 to describe two different molecules secreted by leukocytes with a similar molecular weight. These two early interleukins are now known as IL-1 and IL-2 [29]. Since the introduction of the term, and concurrent identification of the first two interleukins, 37 more interleukins have been described [30, 31]. Our laboratory has contributed to the discovery and characterization of interleukins and recently described IL-39 (meteorin-like) [32]. Many of the new additions are members of the IL-1 superfamily [30, 33]. Here, we review IL-7, IL-4, IL-6, and IL-10. These interleukins play important roles in B cell development (IL-7), survival/proliferation of B cells, and isotype switching (IL-4 and IL-6), and regulation of the immune response (IL-10).
3.1. IL-7
IL-7 is essential to B cell development in mice [34–36]. Mice deficient in IL-7, IL-7R or treated with anti-IL-7 antibodies exhibit the same phenotype: B cell development arrest [37–39]. The developmental arrest occurs at different stages: pro-B to pre-B cell transition and the earlier stage of pre-pro B cells for IL-7 deficient mice and IL-7Rα deficient mice, respectively.
In developing B cells, IL-7 acts as a survival factor. This effect may be due to its ability to regulate Bcl-2 family members [40]. Other extrinsic signaling can synergize with IL-7 signaling. IL-7 drives expansion of developing B cells [41]; this activity originally established IL-7 as a pro-B cell growth factor. IL-7 and IL-7Rα are critical for the development of B cells in mice, but this may not apply to humans. In humans, mutations to the IL-7Rα gene result in SCID (Severe Combined Immune Deficiency), making IL-7 indispensable for T cell development; yet SCID patients have normal B cell populations [42]. Therefore, while IL-7 is not strictly required for the development of normal human B cells. However, numerous reports have documented that IL-7 can influence B cell development in humans [43]. We conclude that the exact effects of IL-7 in human B cell development remain to be defined.
IL-7-mediated induction of BCR rearrangement in animal models has been difficult to study. An IL-7Rα−/− mouse exhibits impaired immunoglobulin gene rearrangements [44]; while the IL-7 deficient mouse does not show impaired V-D-J or V-J gene rearrangements [45]. These discrepancies were recently resolved using mutated constructs of the IL-7Rα that were transferred into IL-7Rα−/− mice. This experimental approach demonstrated that the IL-7Rα has a direct role in promoting immunoglobulin gene rearrangement [46]. The previously reported data may not necessarily be contradictory; instead, the discrepancy may be explained by a second ligand of IL-7Rα called thymic stromal lymphopoietin (TSLP). TSLP is a cytokine that has stimulatory effects on B cells under in vitro culture conditions and plays a role in early B cell development [47].
Generally, only developing B cells respond to IL-7 signaling [48]. However, FO B cells that enter GC’s undergo various reactions that result in the production of higher affinity antibodies. The events that lead to the production of higher affinity antibodies require the reactivation of certain genes including: RAG (recombination activating genes), responsible for the GC’s reactions [49, 50] and IL-7Rα [51], which, as discussed above, plays a direct role in immunoglobulin gene rearrangement.
3.2. IL-4
IL-4 was discovered over thirty years ago, but remains a topic that deserves further research. It is mainly produced by activated CD4+ Th2 cells and can act on a wide array of cells of hematopoietic origin [52]. The IL-4 receptor (IL-4R) is expressed on diverse cells including hematopoetic, endothelial, epithelial, muscle, fibroblast, hepatocyte, and brain cells [53]; the expression of IL-4R on these widely diverse cells reflects the pleiotropic effects of IL-4.
IL-4 was initially described as a B cell growth factor, due to its ability to co-stimulate B cell proliferation [54]. It acts on both resting B cells (by increasing their volume and homotypic aggregation) [55] and activated B cells by acting synergistically with CD40 ligation to enable division, survival, and differentiation [56]. Hence, it is a potent survival factor for B cells [10, 57, 58]. IL-4 stimulates the preferential secretion of certain immunoglobulin isotypes, such as IgG1 and IgE [52]; thereby driving the immune response towards a Type 2 reaction. IL-4 also induces the expression of class II MHC (major histocompatibility complex), in B cells [59].
STAT6, a latent component of IL-4 signaling, is essential for the IL-4 response [60]. STAT6 deficient mice have B cells that are unresponsive to IL-4 stimulation, reduced T cell proliferative ability (likely a result of B cell unresponsiveness), a severe reduction in Th2 cytokines, and lack of IgE and IgG1 production during parasitic infection [61, 62]. IL-4 is produced by activated CD4+ Th2 cells [63] and it promotes Th2 responses by inducing the differentiation of naïve T cells to the Th2 phenotype. This leads to the production of other anti-inflammatory cytokines and inhibits pro-inflammatory conditions thereby serving as a cross-regulator of the immune response [64].
3.3. IL-6
IL-6 is produced by a variety of cells including lymphocytes, fibroblasts, and peripheral blood mononuclear cells (PBMC) [65]. Although there is low homology between human and mouse IL-6 (65% and 42% at the DNA and protein level, respectively) there are four conserved cysteine residues, reflecting an evolutionary relationship between these molecules [66]. Moreover, the genes encoding mouse and human IL-6 are located in syntenic regions. This indicates that the IL-6 gene is conserved and that its function is similar in both species.
IL-6 is expressed under inflammatory conditions including viral infections [67, 68] and LPS stimulation [69, 70]. Initially, IL-6 was named BSF-2, or B cell stimulation factor 2, due to its ability to increase immunoglobulin secretion, IgM and IgG, in either freshly stimulated B cells or EBV, Epstein-Barr virus, immortalized cells [71].
IL-6-producing B cells exacerbate inflammatory conditions and autoimmune pathologies. Conversely, lack of IL-6 can lead to immune defects. For example, an IL-6 deficient mouse is resistant to myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE). This phenotype is a consequence of poor lymphocyte proliferation that leads to reduced inflammation and demyelination in the central nervous system [72–75]. Furthermore, mouse studies have demonstrated that IL-6-deficient B cells do not support the polarization of T cells to Th17, a cell type linked to various inflammatory conditions [75]. In humans, IL-6 is elevated in both Systemic lupus erythematosus (SLE) and Castleman’s disease patients [75–77]. However, the exact origin of IL-6 producing B cells remains unknown. Overall, IL-6 over-production by B cells promotes inflammation through the generation of pathogenic antibodies and increased proliferation of pathogenic T cells.
3.4. IL-10
IL-10 is best known for its anti-inflammatory properties. It was identified as an inhibitor of type 1 (pro-inflammatory) cytokines including: IL-1, TNF-α, and IL-12 [78–81]. It can also down-regulate MHC class II expression on immune cells [78].
An IL-10−/− mouse exhibits an exaggerated inflammatory response to microbial challenges; however, other pathogens are better cleared in absence of IL-10 [82]. This dichotomy is illustrated by the spontaneous development of irritable bowel disease (IBD) observed in IL-10−/− mice [83] as a consequence of their gut being colonized with enteric bacteria [84]. This in vivo phenotype mirrors in vitro data and indicates that IL-10 is a non-redundant cytokine, that is, there is no alternative regulatory mechanism to compensate for its ablation [85].
Despite the fact that an early study documented IL-10 production by a B cell lymphoma [86], the ability of B cells to produce IL-10 remained unrecognized. Recently, a unique B cell subpopulation, identified by its ability to secrete IL-10, has been characterized in mice. These B cells are phenotypically CD1dhiCD5+ and represent only 1–2% of splenic B220+ cells in wild-type (WT) mice. Furthermore, adoptive transfer these IL-10-producing B cells can reverse inflammation in mice lacking CD20+ B cells and CD19−/− mice. The negative regulation of inflammation is mediated by IL-10, a cytokine strongly produced by this B cell subpopulation. These cells are now known as B regulatory or B10 cells [87].
In the mouse, B10 cells are also found in the peritoneal cavity and are similar to their spleen counterparts: same surface marker phenotype and both secrete IL-10. However, can peritoneal B10 cells modulate inflammation? To investigate this, a RAG2−/− mouse (a mouse that lacks both B and T cells), received CD25−CD45RBhiCD4+ T cells, and either IL-10−/− CD19+ B cells or WT CD19+ B cells [24]. The RAG2−/− mice that received the IL-10−/− CD19+ B cells developed higher colitis scores [88]. Taken together, these results suggest that peritoneal B10 cells are important in gut homeostasis and can modulate T cell function during inflammatory conditions such as colitis.
In 2010, a human B cell subset with the ability to produce IL-10 and capacity to suppress the differentiation of Th1 cells was described [89]. This regulatory capacity was IL-10 dependent and ablated by the addition of either anti-CD80 or anti-CD86 antibodies suggesting that T cell help is required for Breg function. This regulatory B cell subset is phenotypically defined as CD19+CD24hiCD38hi and was found in both normal and SLE patients; however, the suppressive capacity of these cells was diminished in cells from SLE patients [89]. Taken together, this indicates that a breach in the interplay of between T and B cells may lead to autoimmunity.
In humans, the inflamed gut leads to the differentiation of Breg cells that negatively regulate inflammation via the release of IL-10 [90]. Although the mechanism behind the differentiation of the Breg cells has not been completely elucidated, IL-1β and IL-6 are important cytokines in the induction of these regulatory B cells. Interestingly, both IL-1β and IL-6 are pro-inflammatory cytokines; hence overt immune responses would be regulated through the production of IL-10. These data are reminiscent of animal studies where Breg cells have been shown to lead to the resolution of colitis in mice [88].
While there are cell surface markers that define the B10 population, (for example, CD1dhiCD5+), a specific transcription factor has yet to be associated to this cell subset. However, Blimp1 and IRF4 are upregulated while pax5 and Bcl6 are down-regulated [91]. Given that these transcription factors are linked to plasma cells, these observations suggest that B10 cells have the ability to differentiate into plasma cells capable of secreting polyreactive IgM and IgG antibodies. This may reflect their ability to dampen inflammation through the clearance of potentially threatening antigens. The B10 cell lineage has yet to be well defined. B10 cells share some phenotypic characteristics with B1a cells of the peritoneal cavity, T2-MZ, transitional-2, precursors and MZ B cells [92–94].
4. Interferons that act on B cells
Several interferons, including IFN-α, IFN-β, IFN-γ have interesting effects on B cells [95]. Both type I and type II IFN’s are involved in generating an antiviral state. They accomplish this by regulating both branches of the immune system. However, sustained antiviral responses are dependent on IFN-γ [96–98]. Beyond their ability to interfere with the replication of viruses they also have a role in the type of response produced. For example, both types of interferons up-regulate peptides associated with class I MHC, but only IFN-γ can induce the expression of class II MHC molecules in macrophages [99]
4.1. Type I IFN’s: IFN-α and IFN-β
Type I IFN’s (IFN-α/β) are constitutively produced in the bone marrow and promote the generation and selection of normal B cell populations [100]. Treatment of mature B cells with IFN-α/β results in partial activation that is associated with increased sensitivity to BCR engagement [101]. This increased sensitivity has been proposed to be a link between innate and acquired immune responses because IFN-α/β (associated with innate responses) leads to the amplification of B cell responses (associated with acquired immune responses) [101]. Similar results were obtained using plasmacytoid DC-derived IFN-α [24] or recombinant IFN-α [102]. Moreover, IFN-α, regardless of the source (endogenous or exogenous), results in increased B cell activation. This renders B cells more receptive to T cells; thus, IFN-α promotes B cell proliferation and their differentiation to antibody secreting cells [102].
4.2. Type II IFN: IFN-γ
IFN-γ is produced by Th1, natural killer T cells (NKT), and natural killer (NK) cells. Its production is induced by IL-12 and IL-18, and inhibited by IL-4, IL-10, and TGF-β [95]. This regulation reflects its strong association with type 1 responses driven by Th1 polarized cells, which produce of large amounts of IFN-γ [103].
IFN-γ was initially reported as an inhibitor of B cell responses [104] due to inhibition of IgM production as a result of a reduction in IgM precursor cells. However, its inhibitory effects are limited to pre-activated B cells and do not affect resting B cells. This suggests that IFN-γ may play a role in the control of polyclonal B cell responses [104].
IFN-γ can also induce B cell proliferation when used with anti-CD40 antibody [105]. This suggests that the manner in which B cells are activated, i.e. LPS, anti-IgM or CD40 ligation, is a critical factor in the fate of B cells treated with IFN-γ. Moreover, it reveals a finely-tuned adaptation of the humoral immune response to IFN-γ
Immunoglobulin secretion by B cells is a direct consequence of the cytokines shaping the response (Table 2). IFN-γ, a Th1 cytokine, is involved in the induction/repression of various immunoglobulin classes. In humans, IFN-γ reduces total IgG production, and it specifically inhibits IgG1, a major component of total IgG, while increasing IgG2, with no notable effects on either IgG3 or IgG4 production levels [106]. This phenotype is also observed in the mouse. LPS-activated B cells treated with IFN-γ produced increased levels of IgG2a and IgG3 while IgG1, IgM, and IgE were inhibited [107]. These data indicate that IFN-γ has similar functions in both humans and mice and that it is involved in the humoral response by directly controlling the immunoglobulin isotypes produced by B cells.
Table 2.
Role of cytokines in murine isotype class switching.
| IgM | IgG1 | IgG2a | IgG2b | IgG3 | IgA | IgE | |
|---|---|---|---|---|---|---|---|
| IL-4 | Inhibits | Induces | Inhibits | Induces | |||
| IL-6 | Induces | ||||||
| IFN-γ | Inhibits | Inhibits | Induces | Induces | Inhibits | ||
| TGF-β | Inhibits | Induces | Inhibits | Induces |
5. Chemokines and B cells
Chemokines are small secreted chemotactic cytokines that control both the innate and adaptive branches of the immune system. In the immune system, their primary function is to direct the migration of cells of the immune system in the body; hence they are often considered the ‘traffic directors’ of the immune system because they guide different leukocyte subsets to a given destination. Here we will discuss CCL28 as an example, because of its important role in directing B cells, specifically IgA+ plasma cells, to mucosal sites.
5.1. CCL28
CCL28, a β-chemokine, is expressed by epithelial cells that line the mucosa, and has a high level of homology with another chemokine (CCL27) [108]. This homology explains their shared receptor, CCR10, and suggests that these two genes arose through a gene duplication event. CCL27 is expressed in the skin and directs T cell homing to cutaneous sites; therefore the gene duplication event that occurred led to selective chemokine differentiation and specialized tissue/cell type expression [109]. We should note that both the mucosa and skin are barrier tissues, an observation that may account for the specialization of their functions and may explain their expression patterns.
The highest site of human CCL28 expression is the salivary gland. Other mucosal sites including small intestine and colon [110] also express CCL28. Deregulated levels of CCL28 have been correlated to various pathologies including salivary gland tumors and [110] Hodgkin’s disease (HD) [111], and Sjögren’s syndrome [112]
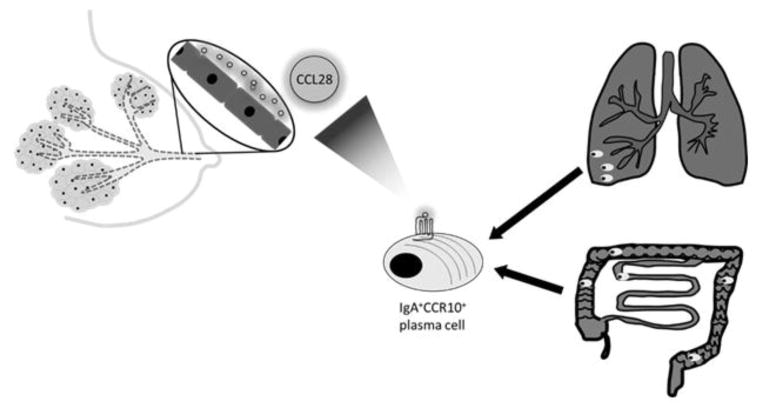
The CCL28/CCR10 axis strongly correlates with CCL28 function, namely the recruitment of IgA+ plasma cells to mucosal sites [113–115]. The mammary gland, an exocrine gland responsible for the production and secretion of milk, is unlike other mucosal immune organs because it develops in stages [116]. Importantly, the ductal epithelia in mammary gland express CCL28 upon the onset of lactation. Expression of CCL28 in the mammary gland parallels the accumulation of IgA antibody secreting cells in the mammary gland, a process that can be inhibited by anti-CCL28 antibodies [113]. Taken together, these results indicate that the CCL28/CCR10 axis regulates the recruitment of IgA secreting plasma cells to the mammary gland (Figure 1).
Figure 1. CCL28 expression is induced upon lactation.
This results in the recruitment of IgA+ CCR10+ plasma cells from the respiratory and gastrointestinal tract. Hence, neonates receive passive immunity against respiratory and gut pathogens via breast milk.
CCL28 has also been reported to bind CCR3 [109]. However, CCR3 is not the primary physiological receptor under healthy conditions [109, 117]. During pathological conditions, the ability of CCL28 to bind CCR3 may become relevant. For example, levels CCL28 levels increase in patients with atopic asthma and this leads to the accumulation of IgE-producing plasma cells. This effect is likely due to the CCL28/CCR3 axis [118].
6. B cells as cytokine producers
Naïve B cells do not secrete many cytokines upon activation. In contrast, naïve T cells initiate cytokine production almost immediately after activation. This inherent difference between T and B cells reflects the fact that B cells require additional signaling beyond activation to become cytokine producers. The additional signaling can be provided by the immune microenvironment and specific differentiation stages of the B cell. For example, the main cytokines produced by naïve B cells upon activation are the chemokines CCL22 and CCLl7 [119–121]. These two chemokines share the same receptor (CCR4), which is strongly expressed in CD4+ Th2 type T cells. Therefore, the production of CCL17 and CCL22 by naïve B cells reflects the ability of activated B cells to recruit Th2 cells. In turn, the recruited Th2 cells produce cytokines, such as IL-4, that shape the B cell response and induce the differentiation of B cells towards cytokine producing B cells (Figure 2). Thus, cytokine secretion by B cells is regulated by extrinsic signaling provided by other immune cell types. Once B cells acquire the capacity to produce cytokines, they become capable of cross-regulating responses via polarization/inhibition and can even negatively regulate the entire immune system.
Figure 2. B cells recruit Th2 cells to receive stimuli.
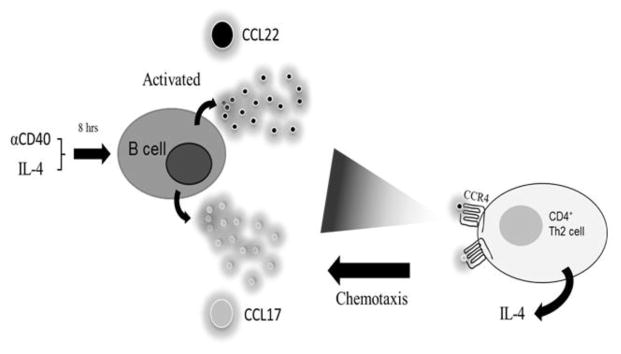
Upon activation, naïve B cells express CCL17 and CCL22. These chemokines recruit CD4+CCR4+ Th2 T cells that provide B cells with appropriate cues to differentiate and produce cytokines.
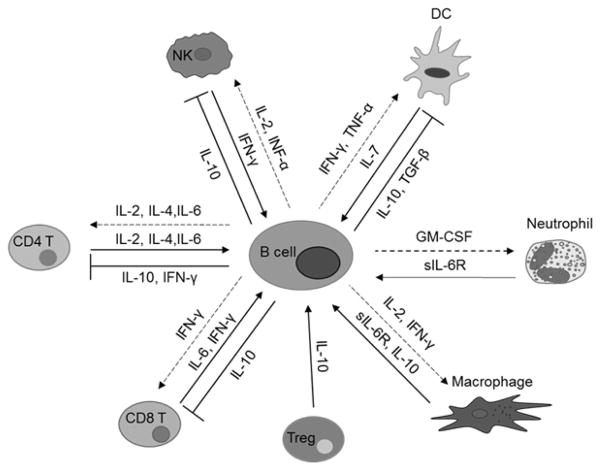
The first report of B cells as cytokine producers described ROHA-9, an EBV transformed human B cell line that constitutively produces IL-1 and leads to enhanced T cell proliferation [122, 123]. These findings have been replicated using mouse B cells. For example, EBV transformed mouse B cells secrete IL-5, which promotes proliferation of eosinophil precursors, B cell proliferation, and antibody production [124]. This set the stage for B cells as effectors of the immune response and suggested that B cells are able to modulate the magnitude of the immune response by both antigen-presentation and cytokine production (Figure 3).
Figure 3. Cytokine production by B cells.
Arrows are cytokines produced by an immune cells that act on B cells. Inhibition lines are cytokines that B cells produce that have a regulatory effect on immune cells. Dashed arrows are cytokines produced by B cells that propagate/magnify effects on immune cells
B cells are classified into effector subtypes depending on the cytokines they secrete. There are two main B effector (Be) populations: Be1 and Be2, which either drive Th1/Th2 responses and cross-regulate the other [125, 126]. In a recent study, it was demonstrated that cytokine secretion by human B cells depends on the stimuli they encounter [127]. B cells stimulated with CD40L and BCR signaling proliferated and produced pro-inflammatory cytokines including TNF-α, lymphotoxin, and IL-6; however, stimulation through CD40 alone led to a significant production of IL-10 which down-regulates ‘unnecessary’ responses [127]. In the presence of autoimmunity this “cytokine network” becomes deregulated. For example, cells collected from multiple sclerosis (MS) patients have a reduced capacity to secrete IL-10 [128]. Naïve B cells from patients with MS retain the ability to be polarized in vitro to Be1 cells which produce pro-inflammatory cytokines, or Be2 cells which produce anti-inflammatory cytokines. Treatment with Mitoxantrone, a therapeutic agent used for MS, recapitulated these results in vivo suggesting an in vivo “cytokine network” switch [128]. Taken together, these studies demonstrate that in addition to receiving activation signaling, B cells require further cues from their immune microenvironment to produce cytokines.
As the list of cytokines produced by B cells expands, we must reconsider the function of B cells. B cells ultimately become plasma cells; yet throughout their journey to their final differentiated state, they are active immune response modulators with the ability to either augment, suppress or skew a given immune response depending on the cytokines they secrete. Furthermore, the identified effector subtypes, Be1 and Be2, are of critical importance to the initiation and propagation of either type I or type II responses because their products not only stimulate either response, but also participate in cross regulation mechanisms that inhibit opposite responses [129]. Overall, we conclude that B cells should be considered an integral component of the adaptive immune system. Their ability to produce cytokines likely reflects their increasingly important role as regulatory cells of the immune system. Given the therapeutic success of B cell ablation (using anti-CD20 antibodies) in the treatment of human autoimmune diseases [130], we predict that the potential role of cytokine-producing B cells in human disease is a field that will yield many future surprises.
Highlights.
Cytokines drive the differentiation of B cells
Differentiation and immune microenvironment determine B cell cytokine production
Cytokines produced by B cells can modulate the adaptive immune response
Acknowledgments
We would like to thank Dr. Amanda M. Burkhardt for her critical review of the manuscript. This work was supported by NIAID NIH grants R01 AI93548 and R21 AI096278 (to AZ). MIV was supported by NSF-GK-12 grant DGE-0638751 and NIH MBRS-IMSD grant GM055246. JCD was supported by CONACYT/SEP #329416/BC-1455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–8. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–22. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Molecular biology and evolution. 2005;22:367–77. doi: 10.1093/molbev/msi026. [DOI] [PubMed] [Google Scholar]
- 6.Brack C, Hirama M, Lenhard-Schuller R, Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978;15:1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- 7.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–62. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 8.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–83. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 10.Illera VA, Perandones CE, Stunz LL, Mower DA, Jr, Ashman RF. Apoptosis in splenic B lymphocytes. Regulation by protein kinase C and IL-4. Journal of immunology. 1993;151:2965–73. [PubMed] [Google Scholar]
- 11.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 12.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nature reviews Immunology. 2013;13:118–32. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nature reviews Immunology. 2009;9:767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 14.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature reviews Immunology. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 15.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nature reviews Immunology. 2007;7:213–9. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 16.Hardy RR. B-1 B cell development. Journal of immunology. 2006;177:2749–54. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 17.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–90. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. The Journal of experimental medicine. 1983;157:202–18. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy RR, Hayakawa K. B cell development pathways. Annual review of immunology. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 21.Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12451–6. doi: 10.1073/pnas.0703451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper MD, Peterson RD, South MA, Good RA. The functions of the thymus system and the bursa system in the chicken. 1966. Journal of immunology. 2006;176:6370–404. [PubMed] [Google Scholar]
- 23.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20878–83. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. The Journal of experimental medicine. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgarth N, Jager GC, Herman OC, Herzenberg LA. CD4+ T cells derived from B cell-deficient mice inhibit the establishment of peripheral B cell pools. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4766–71. doi: 10.1073/pnas.97.9.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonnella PA, Waldner HP, Weiner HL. B cell-deficient (mu MT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. Journal of immunology. 2001;166:4456–64. doi: 10.4049/jimmunol.166.7.4456. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. Journal of immunology. 1998;161:1439–46. [PubMed] [Google Scholar]
- 28.Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Human genomics. 2010;5:30–55. doi: 10.1186/1479-7364-5-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello CA, Mier JW. Interleukins. Annual review of medicine. 1986;37:173–8. doi: 10.1146/annurev.me.37.020186.001133. [DOI] [PubMed] [Google Scholar]
- 30.Clavel G, Thiolat A, Boissier MC. Interleukin newcomers creating new numbers in rheumatology: IL-34 to IL-38. Joint, bone, spine: revue du rhumatisme. 2013;80:449–53. doi: 10.1016/j.jbspin.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 31.van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3001–5. doi: 10.1073/pnas.1121534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ushach I, Burkhardt AM, Martinez C, Hevezi PA, Gerber PA, Buhren BA, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clinical immunology. 2014 doi: 10.1016/j.clim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. European cytokine network. 2011;22:127–47. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 34.Namen AE, Schmierer AE, March CJ, Overell RW, Park LS, Urdal DL, et al. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. The Journal of experimental medicine. 1988;167:988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin RG, Lupton S, Schmierer A, Hjerrild KJ, Jerzy R, Clevenger W, et al. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:302–6. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–3. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 37.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. The Journal of experimental medicine. 1995;181:1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. The Journal of experimental medicine. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, et al. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. The Journal of experimental medicine. 1993;178:257–64. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annual review of immunology. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 41.Namikawa R, Muench MO, de Vries JE, Roncarolo MG. The FLK2/FLT3 ligand synergizes with interleukin-7 in promoting stromal-cell-independent expansion and differentiation of human fetal pro-B cells in vitro. Blood. 1996;87:1881–90. [PubMed] [Google Scholar]
- 42.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nature genetics. 1998;20:394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 43.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nature reviews Immunology. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 44.Lange K, Gold MM, et al. Autoantibodies in human glomerulonephritis. The Journal of clinical investigation. 1949;28:50–5. [PubMed] [Google Scholar]
- 45.Wei C, Zeff R, Goldschneider I. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. Journal of immunology. 2000;164:1961–70. doi: 10.4049/jimmunol.164.4.1961. [DOI] [PubMed] [Google Scholar]
- 46.Corcoran AE, Smart FM, Cowling RJ, Crompton T, Owen MJ, Venkitaraman AR. The interleukin-7 receptor alpha chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. The EMBO journal. 1996;15:1924–32. [PMC free article] [PubMed] [Google Scholar]
- 47.Scheeren FA, van Lent AU, Nagasawa M, Weijer K, Spits H, Legrand N, et al. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. European journal of immunology. 2010;40:955–65. doi: 10.1002/eji.200939419. [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto Y, Montecino-Rodriguez E, Leathers H, Stephan RP, Dorshkind K. B-cell development in the thymus is limited by inhibitory signals from the thymic microenvironment. Blood. 2002;100:3504–11. doi: 10.1182/blood-2002-03-0733. [DOI] [PubMed] [Google Scholar]
- 49.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–5. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 50.Papavasiliou F, Casellas R, Suh H, Qin XF, Besmer E, Pelanda R, et al. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 51.Hikida M, Nakayama Y, Yamashita Y, Kumazawa Y, Nishikawa SI, Ohmori H. Expression of recombination activating genes in germinal center B cells: involvement of interleukin 7 (IL-7) and the IL-7 receptor. The Journal of experimental medicine. 1998;188:365–72. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul WE. Interleukin 4/B cell stimulatory factor 1: one lymphokine, many functions. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1987;1:456–61. doi: 10.1096/fasebj.1.6.3315808. [DOI] [PubMed] [Google Scholar]
- 53.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annual review of immunology. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 54.Idzerda RL, March CJ, Mosley B, Lyman SD, Vanden Bos T, Gimpel SD, et al. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. The Journal of experimental medicine. 1990;171:861–73. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chomarat P, Banchereau J. An update on interleukin-4 and its receptor. European cytokine network. 1997;8:333–44. [PubMed] [Google Scholar]
- 56.Rush JS, Hodgkin PD. B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. European journal of immunology. 2001;31:1150–9. doi: 10.1002/1521-4141(200104)31:4<1150::aid-immu1150>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 57.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. The Journal of experimental medicine. 1997;186:325–30. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paul WE. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Foundation symposium. 1997;204:208–16. doi: 10.1002/9780470515280.ch14. discussion 16–9. [DOI] [PubMed] [Google Scholar]
- 59.Thieu VT, Nguyen ET, McCarthy BP, Bruns HA, Kapur R, Chang CH, et al. IL-4-stimulated NF-kappaB activity is required for Stat6 DNA binding. Journal of leukocyte biology. 2007;82:370–9. doi: 10.1189/jlb.1106707. [DOI] [PubMed] [Google Scholar]
- 60.Wurster AL, Rodgers VL, White MF, Rothstein TL, Grusby MJ. Interleukin-4-mediated protection of primary B cells from apoptosis through Stat6-dependent up-regulation of Bcl-xL. The Journal of biological chemistry. 2002;277:27169–75. doi: 10.1074/jbc.M201207200. [DOI] [PubMed] [Google Scholar]
- 61.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 63.Howard M, Farrar J, Hilfiker M, Johnson B, Takatsu K, Hamaoka T, et al. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. The Journal of experimental medicine. 1982;155:914–23. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respiratory research. 2001;2:66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Snick J. Interleukin-6: an overview. Annual review of immunology. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 66.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 67.Paludan SR. Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes. Journal of virology. 2001;75:8008–15. doi: 10.1128/JVI.75.17.8008-8015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. The Journal of allergy and clinical immunology. 1996;98:1080–7. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 69.Eggesbo JB, Hjermann I, Hostmark AT, Kierulf P. LPS induced release of IL-1 beta, IL-6, IL-8 and TNF-alpha in EDTA or heparin anticoagulated whole blood from persons with high or low levels of serum HDL. Cytokine. 1996;8:152–60. doi: 10.1006/cyto.1996.0022. [DOI] [PubMed] [Google Scholar]
- 70.Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. Journal of neuroinflammation. 2009;6:9. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, et al. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2) Proceedings of the National Academy of Sciences of the United States of America. 1985;82:5490–4. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okuda Y, Sakoda S, Bernard CC, Fujimura H, Saeki Y, Kishimoto T, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. International immunology. 1998;10:703–8. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 73.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. Journal of immunology. 1998;161:6480–6. [PubMed] [Google Scholar]
- 74.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. European journal of immunology. 1998;28:2178–87. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 75.Bao Y, Cao X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. Journal of autoimmunity. 2014;55C:10–23. doi: 10.1016/j.jaut.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka Y, Saito K, Shirakawa F, Ota T, Suzuki H, Eto S, et al. Production of B cell-stimulating factors by B cells in patients with systemic lupus erythematosus. Journal of immunology. 1988;141:3043–9. [PubMed] [Google Scholar]
- 77.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74:1360–7. [PubMed] [Google Scholar]
- 78.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. The Journal of experimental medicine. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. Journal of immunology. 1991;147:3815–22. [PubMed] [Google Scholar]
- 80.Isler P, de Rochemonteix BG, Songeon F, Boehringer N, Nicod LP. Interleukin-12 production by human alveolar macrophages is controlled by the autocrine production of interleukin-10. American journal of respiratory cell and molecular biology. 1999;20:270–8. doi: 10.1165/ajrcmb.20.2.3313. [DOI] [PubMed] [Google Scholar]
- 81.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. Journal of immunology. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 82.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Critical reviews in immunology. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 84.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and immunity. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature reviews Immunology. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 86.Suda T, O’Garra A, MacNeil I, Fischer M, Bond MW, Zlotnik A. Identification of a novel thymocyte growth-promoting factor derived from B cell lymphomas. Cellular immunology. 1990;129:228–40. doi: 10.1016/0008-8749(90)90200-b. [DOI] [PubMed] [Google Scholar]
- 87.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 88.Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, et al. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. Journal of immunology. 2013;191:2780–95. doi: 10.4049/jimmunol.1300649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 90.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nature medicine. 2014;20:1334–9. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- 91.Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. Journal of immunology. 2012;188:1036–48. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. Journal of immunology. 2007;178:7868–78. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 93.Spencer NF, Daynes RA. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5- B cells: possible involvement in age-associated cytokine dysregulation. International immunology. 1997;9:745–54. doi: 10.1093/intimm/9.5.745. [DOI] [PubMed] [Google Scholar]
- 94.Brummel R, Lenert P. Activation of marginal zone B cells from lupus mice with type A(D) CpG-oligodeoxynucleotides. Journal of immunology. 2005;174:2429–34. doi: 10.4049/jimmunol.174.4.2429. [DOI] [PubMed] [Google Scholar]
- 95.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of leukocyte biology. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 96.Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Current opinion in immunology. 2002;14:111–6. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 97.Lu B, Ebensperger C, Dembic Z, Wang Y, Kvatyuk M, Lu T, et al. Targeted disruption of the interferon-gamma receptor 2 gene results in severe immune defects in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8233–8. doi: 10.1073/pnas.95.14.8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. Journal of virology. 1999;73:3418–23. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giroux M, Schmidt M, Descoteaux A. IFN-gamma-induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor-1 is regulated by protein kinase C-alpha. Journal of immunology. 2003;171:4187–94. doi: 10.4049/jimmunol.171.8.4187. [DOI] [PubMed] [Google Scholar]
- 100.Demengeot J, Vasconcellos R, Modigliani Y, Grandien A, Coutinho A. B lymphocyte sensitivity to IgM receptor ligation is independent of maturation stage and locally determined by macrophage-derived IFN-beta. International immunology. 1997;9:1677–85. doi: 10.1093/intimm/9.11.1677. [DOI] [PubMed] [Google Scholar]
- 101.Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. International immunology. 2002;14:411–9. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- 102.Gujer C, Sandgren KJ, Douagi I, Adams WC, Sundling C, Smed-Sorensen A, et al. IFN-alpha produced by human plasmacytoid dendritic cells enhances T cell-dependent naive B cell differentiation. Journal of leukocyte biology. 2011;89:811–21. doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bradley LM, Dalton DK, Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. Journal of immunology. 1996;157:1350–8. [PubMed] [Google Scholar]
- 104.Abed NS, Chace JH, Fleming AL, Cowdery JS. Interferon-gamma regulation of B lymphocyte differentiation: activation of B cells is a prerequisite for IFN-gamma-mediated inhibition of B cell differentiation. Cellular immunology. 1994;153:356–66. doi: 10.1006/cimm.1994.1034. [DOI] [PubMed] [Google Scholar]
- 105.Johnson-Leger C, Hasbold J, Holman M, Klaus GG. The effects of IFN-gamma on CD40-mediated activation of B cells from X-linked immunodeficient or normal mice. Journal of immunology. 1997;159:1150–9. [PubMed] [Google Scholar]
- 106.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. Journal of immunology. 1994;153:4948–58. [PubMed] [Google Scholar]
- 107.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. Journal of immunology. 1988;140:1022–7. [PubMed] [Google Scholar]
- 108.Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2) The Journal of biological chemistry. 2000;275:22313–23. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- 109.Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. Journal of immunology. 2000;165:2943–9. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 110.Liu GX, Lan J, Sun Y, Hu YJ, Jiang GS. Expression of the chemokine CCL28 in pleomorphic adenoma and adenolymphoma of the human salivary glands. Experimental and therapeutic medicine. 2012;4:65–9. doi: 10.3892/etm.2012.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanamoto H, Nakayama T, Miyazato H, Takegawa S, Hieshima K, Tatsumi Y, et al. Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin’s disease with frequent infiltration of eosinophils and/or plasma cells. The American journal of pathology. 2004;164:997–1006. doi: 10.1016/S0002-9440(10)63187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hernandez-Molina G, Burkhardt AM, Lima G, Zlotnik A, Betanzos JL, Bahena S, et al. Absence of salivary CCL28 in primary Sjogren’s syndrome. Rheumatology international. 2015 doi: 10.1007/s00296-014-3210-0. [DOI] [PubMed] [Google Scholar]
- 113.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. The Journal of experimental medicine. 2004;200:805–9. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, et al. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. Journal of immunology. 2008;181:6309–15. doi: 10.4049/jimmunol.181.9.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. The Journal of clinical investigation. 2003;111:1001–10. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nature reviews Molecular cell biology. 2005;6:715–25. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 117.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. Journal of immunology. 2005;175:5341–50. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 118.Scanlon KM, Hawksworth RJ, Lane SJ, Mahon BP. IL-17A induces CCL28, supporting the chemotaxis of IgE-secreting B cells. International archives of allergy and immunology. 2011;156:51–61. doi: 10.1159/000322178. [DOI] [PubMed] [Google Scholar]
- 119.Ghadially H, Ross XL, Kerst C, Dong J, Reske-Kunz AB, Ross R. Differential regulation of CCL22 gene expression in murine dendritic cells and B cells. Journal of immunology. 2005;174:5620–9. doi: 10.4049/jimmunol.174.9.5620. [DOI] [PubMed] [Google Scholar]
- 120.Nakayama T, Hieshima K, Nagakubo D, Sato E, Nakayama M, Kawa K, et al. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein-Barr virus. Journal of virology. 2004;78:1665–74. doi: 10.1128/JVI.78.4.1665-1674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takegawa S, Jin Z, Nakayama T, Oyama T, Hieshima K, Nagakubo D, et al. Expression of CCL17 and CCL22 by latent membrane protein 1-positive tumor cells in age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorder. Cancer science. 2008;99:296–302. doi: 10.1111/j.1349-7006.2007.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scala G, Kuang YD, Hall RE, Muchmore AV, Oppenheim JJ. Accessory cell function of human B cells. I. Production of both interleukin 1-like activity and an interleukin 1 inhibitory factor by an EBV-transformed human B cell line. The Journal of experimental medicine. 1984;159:1637–52. doi: 10.1084/jem.159.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsushima K, Procopio A, Abe H, Scala G, Ortaldo JR, Oppenheim JJ. Production of interleukin 1 activity by normal human peripheral blood B lymphocytes. Journal of immunology. 1985;135:1132–6. [PubMed] [Google Scholar]
- 124.Paul CC, Keller JR, Armpriester JM, Baumann MA. Epstein-Barr virus transformed B lymphocytes produce interleukin-5. Blood. 1990;75:1400–3. [PubMed] [Google Scholar]
- 125.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Current opinion in immunology. 2008;20:332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature immunology. 2000;1:475–82. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 127.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? Journal of immunology. 2004;172:3422–7. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 128.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. Journal of immunology. 2007;178:6092–9. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 129.Luu VP, Vazquez MI, Zlotnik A. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity. 2014;47:1–12. doi: 10.3109/08916934.2013.856006. [DOI] [PubMed] [Google Scholar]
- 130.Kao D, Lux A, Schwab I, Nimmerjahn F. Targeting B cells and autoantibodies in the therapy of autoimmune diseases. Seminars in immunopathology. 2014;36:289–99. doi: 10.1007/s00281-014-0427-7. [DOI] [PubMed] [Google Scholar]