Abstract
Background and aims
The mechanisms of hypoxia-induced tumor growth remain unclear. Hypoxia induces intracellular translocation and release of a variety of damage associated molecular patterns (DAMPs) such as nuclear HMGB1 and mitochondrial DNA (mtDNA). In inflammation, Toll-like receptor (TLR)-9 activation by DNA-containing immune complexes has been shown to be mediated by HMGB1. We thus hypothesize that HMGB1 binds mtDNA in the cytoplasm of hypoxic tumor cells and promotes tumor growth through activating TLR9 signaling pathways.
Methods
C57BL6 mice were injected with Hepa1-6 cancer cells. TLR9 and HMGB1 were inhibited using shRNA or direct antagonists. Huh7 and Hepa1-6 cancer cells were investigated in vitro to investigate how the interaction of HMGB1 and mtDNA activates TLR9 signaling pathways.
Results
During hypoxia, HMGB1 translocates from the nucleus to the cytosol and binds to mtDNA released from damaged mitochondria. This complex subsequently activates TLR9 signaling pathways to promote tumor cell proliferation. Loss of HMGB1 or mtDNA leads to a defect in TLR9 signaling pathways in response to hypoxia, resulting in decreased tumor cell proliferation. Also, the addition of HMGB1 and mtDNA leads to the activation of TLR-9 and subsequent tumor cell proliferation. Moreover, TLR9 is overexpressed in both hypoxic tumor cells in vitro and in human hepatocellular cancer (HCC) specimens; and, knockdown of either HMGB1 or TLR9 from HCC cells suppressed tumor growth in vivo after injection in mice.
Conclusions
Our data reveals a novel mechanism by which the interactions of HMGB1 and mtDNA activate TLR9 signaling during hypoxia to induce tumor growth.
Keywords: mitochondrial DNA, HMGB1, Hepatocellular carcinoma, DAMPs, Hypoxia, Liver
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, ranking as the third most common cause of cancer-related deaths in the world [1]. One of the main reasons for the poor prognosis of many patients with HCC is due to the aggressive and high proliferative activity of HCC cells, which results from the up-regulation of multiple signaling pathways [2]. Significant evidence links cancer development and progression to the continuous oxidative stress and chronic inflammation found in the tumor microenvironment [3]. The hypoxic environment generated in the center of the HCC tumor can produce cell necrosis, which in turn releases damage associated molecular pattern (DAMP) proteins, such as High Mobility Group Box 1 (HMGB1) and mitochondrial DNA (mtDNA). These DAMP molecules interact with their receptors, triggering a pro-inflammatory signaling cascade that contributes to tumor progression. Though the underlying mechanisms linking hypoxia, inflammation, and cancer are far from clear, the imbalance of the microenvironment likely plays a key role.
HMGB1 is a chromatin-binding nuclear DAMP that has been implicated in both infectious and sterile inflammatory disease states, including cancer [4, 5]. Several studies have shown that HMGB1 is overexpressed in various solid tumors cancers and that elevated levels of HMGB1 may foster a chronic inflammatory state through the production of multiple inflammatory mediators [4]. We have previously reported that during hypoxia, HMGB1 translocates to the cytoplasm and is then actively secreted by HCC cells; and, extracellular HMGB1 can promote cancer invasion and metastasis through Toll-like receptor (TLR)-4 signaling [6]. HMGB1 within the cytosolic compartment can also serve as a sensor for nucleic acids and is required for a robust activation of innate immune response to nucleic acids in autoimmune diseases [7, 8]. Multiple receptors have evolved to recognize nucleic acids, in particular, TLR9, a pattern-recognition endosomal receptor, is involved in immune signaling and plays a crucial role in cell survival through recognition of various bacterial and viral nucleic components [9, 10]. HMGB1 has been shown to enhance the activation of this nucleic-acid-sensing TLR by binding to its respective ligands [11].
The mitochondria is another cellular source of DAMPs. Examples of mitochondrial DAMPs that are released into the extracellular space during different types of cell death and tissue injury include mitochondrial DNA (mtDNA), cytochrome c, and mitochondria derived reactive oxygen species (ROS) [12, 13]. Human mtDNA is a 16.6 kb circular double-stranded DNA molecule, which encodes 13 polypeptides involved in respiration and oxidative phosphorylation. Of interest, both mtDNA mutations and mtDNA copies have been associated with cancer development and progression [14]. In addition, mtDNA can stimulate inflammatory responses through TLR9 when released into plasma as a consequence of acute trauma and injury [12].
A more thorough understanding of the complex interactions between cancer cells, immune cells, DAMPs, and TLRs in the hypoxic setting is needed for the development of new strategies for cancer therapy. Therefore, in this study we demonstrate that hypoxic HCC cells quickly translocate HMGB1 and mtDNA to the cytosol. HMGB1 then binds to mtDNA to activate TLR9 signaling pathways. The activation of TLR-9 results in triggering of inflammatory and pro-tumorigenic signaling pathways, with subsequent increased cancer proliferation in vitro and heightened tumor growth in mice.
Materials and methods
Patient Samples, Cell lines, and Reagents
All materials used in this study were obtained under an approved Institutional Review Board protocol. Tumorous and adjacent nontumorous liver tissues (n=24) were collected from patients who underwent surgery for HCC at the University of Pittsburgh. Hepa1-6 and Huh7 cell lines were purchased from ATCC (Manassas, VA). Cells were transferred to hypoxia chamber with 1% O2 if necessary.
Animals
Male wild-type (C57BL/6) mice (8-weeks-old) were purchased from Jackson Laboratories (West Grove, PA). Animal protocols were approved by the Animal Care and Use Committee of the University of Pittsburgh and the experiments were performed in adherence to the National Institutes of Health Guidelines.
Establishment of TLR9 Stable Knockdown Cells
One day before transfection, 2×105 cells were seeded onto 6-well plates. TLR9 shRNA was transfected into cells with Lipofectin 2000. After 24 h, transfected cells were spread onto 100-mm culture dish at 1:100 dilution. To select for stable transfectants, cells were cultured in DMEM with 10 μg/ml puromycin (Sigma-Aldrich) for 4 weeks. Clones with puromycin resistance were selected and expanded.
Flow cytometry analysis
Mitochondrial ROS was measured in cells by MitoSOX (Invitrogen) staining (5 μM for 15 min at 37°C) [15]. To measure mitochondrial mass, cells were stained with 25nM of MitoTracker Green FM and MitoTracker Deep Red FM (Invitrogen) (15 min at 37°C) [16].
Determination of mtDNA copies
mtDNA in cytosol was measured as described previously [17]. In summary, 1×107 cells were homogenized with a Dounce homogenizer in 100 mM Tricine-NaOH solution (pH 7.4) containing 0.25 M sucrose, 1 mM EDTA and protease inhibitor, then were centrifuged at 700 g for 1min at 4°C. Protein concentration and volume of the supernatant were normalized, followed by centrifugation at 10,000 g for 30 min at 4°C for the production of a supernatant corresponding to the cytosolic fraction. DNA was isolated from 200 μl of the cytosolic fraction. The copy number of DNA encoding cytochrome c oxidase I (COX I) was measured by quantitative real-time PCR with same volume of the DNA solution. The following primers were used: mouse COXI forward, 5′-GCCCCAGATATAGCATTCCC-3′; mouse COXI reverse, 5′-GTTCATCCTGTTCCTGCTCC-3′.
Chromatin immunoprecipitation (ChIP)
Hepa1-6 cells suffered from hypoxia for 24 h were collected (cell lysate group). The culture medium were also collected after Hepa1-6 cells treated with exogenous rhHMGB1 (1 μg/ml) plus mtDNA (medium containing 10 μg/ml mtDNA), and served as control (rhHMGB1-mtDNA group). ChIP was performed as described previously [18]. Purified DNA samples were normalized and subjected to PCR analysis. Mouse COXI gene were amplified using the same primer as listed above. PCR products were gel purified and sequenced using the same primers as were used for PCR.
Immunoprecipitation (IP)
Following treatment, whole lysate protein diluted in immunoprecipitation buffer [50mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 0.5% Nonidet P-40, 150mM NaCl, 10% glycerol, 1mM EDTA]. Normal rabbit IgG was used as a negative control. Whole-cell lysates (400 μg) were incubated with 1μg anti-HMGB1 antibodies and 20μL protein A/G-beads at 4°C overnight. Samples were washed with PBS and subjected to Western blot analysis.
Tumor models
Hepa1-6 cells (2×106) were subcutaneously implanted into the flanks of each mouse. The left flank was implanted with control tumor cells, whereas the right side was injected with the tested tumor cells. Tumor growth was monitored by the measurement of the length and width of the tumor with a caliper. Tumor sizes were calculated with the following formula: Size =Length×Width2×(π/6). After the mice were sacrificed at 4 weeks, tumors were collected, photographed.
Statistical analysis
Data are shown as means±SEM. Data were pooled from at least three independent experiments to avoid possible variation of the cell cultures. For statistical analyses, Student’s t test or one way analysis of variance test were employed and p < 0.05 was considered to be statistically significant.
Results
TLR9 is overexpressed in hypoxic HCC and promotes tumor growth
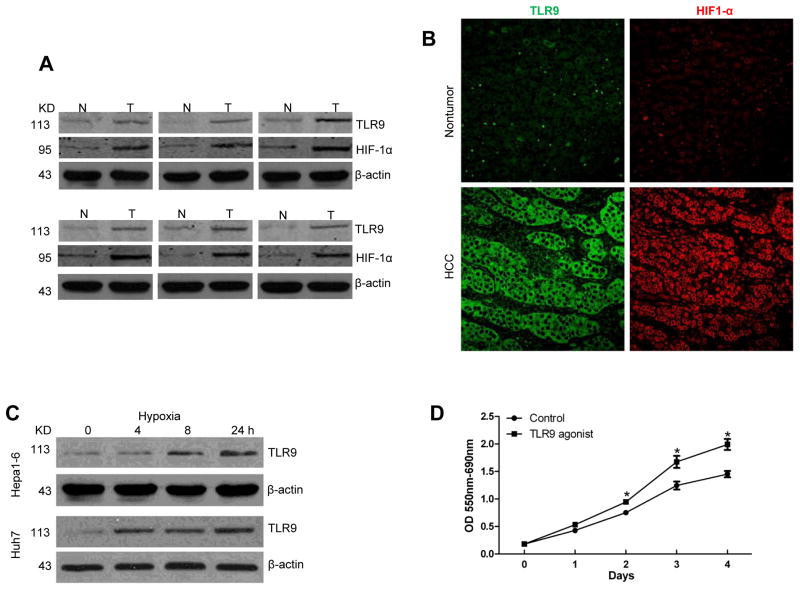
We have previously reported that TLR9 signaling pathways are involved in hepatic ischemia/reperfusion injury by promoting the expression of pro-inflammatory cytokines to induce immune responses and organ damage [19]. Accumulating data suggests that TLR9 is also associated with tumor progression [20, 21]; however, the role of TLR-9 in tumor associated hypoxia and the resulting tumor growth remains unstudied. To explore the role of TLR9 in human HCC, we examined the expression of TLR9 and Hypoxia-induced Factor-1 alpha (HIF-1α) in 24 paired human HCC tissue samples and their corresponding non-tumor liver tissue by Western blot analysis and immunoflorescent staining. We found that expression of TLR9 and HIF-1α was higher in HCC tissues (Fig. 1A, B). Since hypoxia is present in solid tumors including HCC, we examined whether hypoxia plays a role in inducing expression of TLR9 in HCC. Hepa1-6 cells and Huh7 cells, a murine and human HCC cell line, respectively, were cultured under normoxic or hypoxic conditions. After exposure to hypoxia, the expression of TLR9 was increased in a time dependent manner in both HCC cell lines (Fig. 1C). To determine the role TLR9 signaling on tumor growth in vitro, an MTT assay was used. Hepa1-6 cells treated with a TLR9 agonist (ODN 1668, 1μM) displayed a significant increase in cell proliferation in a time-dependent manner compared with the vehicle control (Fig. 1D). These results show that TLR9 is overexpressed in hypoxic cancer cells and stimulation of TLR9 enhances tumor growth.
Fig. 1. TLR9 is overexpressed in hypoxic HCC and promotes tumor growth.
(A) TLR9 and HIF-1α protein tissue levels were measured using Western blot analysis in paired HCC samples and their non-tumor liver counterparts. (N, nontumor liver; T, tumor). Selected samples are representative of 24 unique paired samples. (B) Representative images of the nontumor and HCC tissues stained for TLR9 and HIF-1α (Green, TLR9; red, HIF-1α). (C) TLR9 protein expression in Hepa1-6 and Huh7 cells subjected to a time course of hypoxia (1% O2). (D) MTT assay at different time points of hep1-6 cells after TLR9 agonist treatment. Data is presented as mean±SE and is representative of 3 independent experiments. *p< 0.05.
Hypoxia induces mitochondrial damage and DAMP release to the cytosol
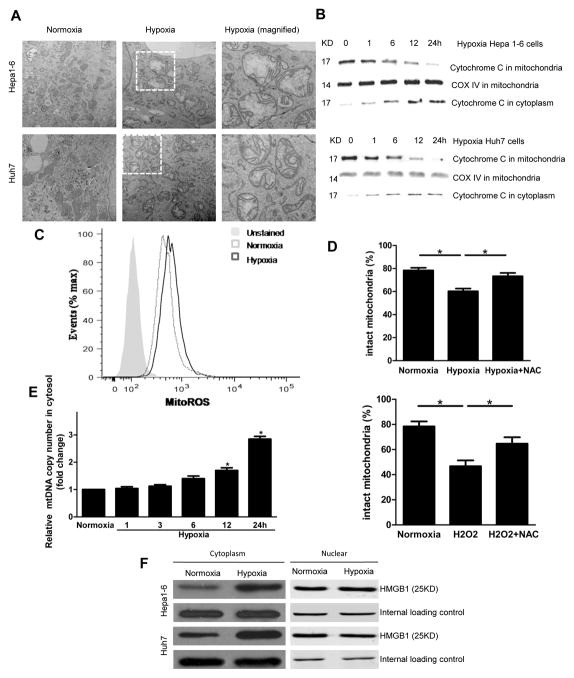
To elucidate the mechanisms behind hypoxia-driven tumor growth, we next investigated the intracellular DAMPs released during hypoxic stress that may interact with TLR9. Previous studies have shown that both cytoplasmic HMGB1 and mtDNA can activate TLR9 to elicit inflammatory responses [7, 13]. Mitochondrial DNA has been recently described as a DAMP; however, its role in cancer cells’ response to stress and tumor progression remains unexplored. Subjecting tumor cells to hypoxia produced a greater abundance of swollen mitochondria with severely disrupted cristae in murine Hepa1-6 and human Huh7 HCC cells when compared to cells in normoxia (Fig. 2A). Further, we observed the release of cytochrome c from the mitochondria, as the levels of mitochondrial cytochrome c was decreased in the mitochondrial fraction of hypoxic Hepa1-6 and Huh7 cells (Fig. 2B). There was also a simultaneous increase in the cytoplasmic portion of cytochrome c confirming that hypoxia induces not only structural damage but also functional changes in mitochondria. Mitochondria are particularly susceptible to damage induced by mitochondrial ROS, which are generated largely by the rate of electron flow through respiratory chain complexes [22]. We found that in hypoxia, cancer cells generated more mitochondrial superoxide anion radical (O2−) than in normoxia, as detected by the fluorescence of MitoSOX (a mitochondrial superoxide indicator, Fig. 2C), indicative of more mitochondrial ROS production and mitochondrial dysfunction [16, 23].
Fig. 2. Hypoxia induces mitochondrial damage and DAMP release to the cytosol.
(A) Transmission electron microscopy images showing swollen mitochondria and disrupted cristae in hypoxic Hepa1-6 and Huh7 cells. (B) Release of cytochrome c from mitochondria to cytoplasm in cancer cells subjected to a time course of hypoxia. (C) Cancer cells labeled with MitoSOX subjected to flow cytometric analysis to evaluate ROS production. (D) H2O2 treatment and hypoxia induced a significant decrease in the percentage of intact mitochondria. NAC decreases hypoxia induced mitochondrial damage. (E) Cytosolic levels of mtDNA significantly increase after 12 and 24 h of hypoxia. (F) HMGB1 translocates to cytosol in cancer cells subjected to hypoxia. *p<0.05.
We further assessed the functional mitochondria pool in HCC cells in normoxia and hypoxia through the use of MitoTracker DeepRed, a fluorescent probe sensitive to the mitochondrial inner transmembrane potential. To measure the total mitochondrial pool, we counterstained cells with MitoTracker Green, a probe that stains mitochondrial membrane lipids independently of membrane potential. Hypoxia induced a decrease in the percentage of intact mitochondria (positive for MitoTracker DeepRed and MitoTracker Green), from 78.6% to 60.4% of the total population (Fig. 2D). H2O2 treatment (500 μM) resulted in similar findings (Fig. 2D); and, as expected, ROS inhibitor N-acetyl cysteine (NAC) reversed this loss of intact mitochondria (Fig. 2D). In addition, we found that hypoxia not only induced an increase in mitochondrial ROS but also promoted mitochondrial DNA translocation in a time dependent manner from the mitochondria to cytosol in HCC cells (Fig. 2E). These data indicate that hypoxic HCC cells accumulated structurally and functionally abnormal mitochondria leading to translocation of mtDNA to the cytosol. Similar to the mitochondrial changes, hypoxia can also induce the translocation of HMGB1 from the nucleus to the cytoplasm in Hepa1-6 and Huh7 cells (Fig. 2F).
Hypoxia induces HMGB1 and mtDNA translocation, binding, and activation of TLR9
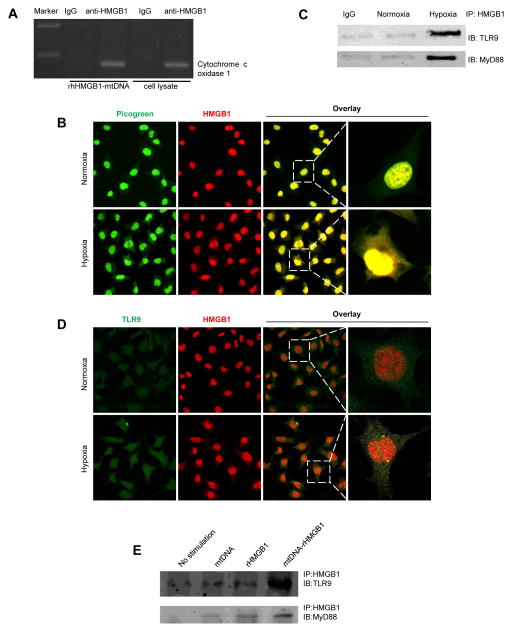
Since hypoxia induces both HMGB1 and mtDNA translocation to the cytosol and HMGB1 has been shown to form a complex with nucleic acids [8], we thus hypothesized that cytosolic HMGB1 can sense and bind to mtDNA to activate downstream signaling pathways. Because HMGB1 belongs to the HMGB proteins, it possesses a unique DNA-binding domain, the HMG-box, which can bind DNA structures with high affinity in a non-sequence specific manner. Thus, we believe that HMGB1 also binds to mtDNA in a non-sequence specific binding; and, in our experiments, we have decided to study the binding of HMGB1 to the specific mtDNA COXI gene to examine their interaction. Through chromatin immunoprecipitation (ChIP) assays that measure protein and DNA interactions and the sequence analysis of the 116 bp PCR product, we found that HMGB1 can bind to mtDNA COXI gene (Fig. 3A, Supplementary Fig. 1). To further confirm the co-localization of HMGB1 and mtDNA, we also performed immunofluorescent staining for HMGB1 and mtDNA. We used Picogreen stain, a dsDNA stain, for mtDNA as it colocalized with MitoTracker Deepred staining, a mitochondrial stain, under normoxic condition (Supplementary Fig. 2A). As expected, HMGB1 translocates from the nucleus to the cytoplasm under hypoxic conditions. In addition, HMGB1 and mtDNA colocalized in HCC cells subjected to hypoxia (Fig. 3B). Together these findings suggest that cytosolic HMGB1 localizes with and binds to mtDNA in HCC cells during hypoxia.
Fig. 3. Hypoxia induces HMGB1 and mtDNA translocation, binding, and activation of TLR9.
(A) HMGB1 can bind mtDNA as evident by ChIP assay. (B) Co-localization between HMGB1 and mtDNA in Hepa1-6 cells subjected to 24 h of hypoxia by confocal microscopy. (C) IP assays demonstrate that hypoxia induced the association of TLR9 with its downstream effector MyD88 and HMGB1. (D) Co-localization between HMGB1 and TLR9 in Hepa1-6 cells subjected to 24 h of hypoxia. (E) Combined stimulation of HMGB1 and mtDNA resulted in considerable association between TLR9 and MyD88 as shown by IP, which did not occur after stimulation with either HMGB1 or mtDNA alone.*p < 0.05.
Immunoprecipitation assays showed that hypoxia induced the association of TLR9 with its downstream effector MyD88 and HMGB1 (Fig. 3C), which indicates that hypoxia can initiate TLR9 activation in HCC cells through HMGB1. Since hypoxia can trigger both HMGB1 translocation and TLR9 activation, we examined the possible physical interaction between HMGB1 and TLR9. Hepa1-6 cells were treated with hypoxia, and the association of TLR9 with HMGB1 was assessed by immunofluorescent staining. We found that during normoxia most HMGB1 remained in the nucleus (Fig. 3D). After exposure to hypoxia, the majority of HMGB1 co-localized with TLR9 in the cytosol (Fig. 3D). Furthermore, since the redistribution of TLR9 from the ER to early endosomes is essential for TLR9 activation by DNA [24], we investigated the relationship between TLR9 and the early endosome marker EEA1. We found that the colocalization of TLR9 and EEA1 was increased during hypoxia (Supplementary Fig. 2B). Interestingly, only the combined stimulation of HMGB1 and mtDNA resulted in considerable association between TLR9 and MyD88 as shown by IP, which did not occur after stimulation with either HMGB1 or mtDNA alone (Fig. 3E). This suggests that HMGB1-mtDNA interactions are required for complete activation of the TLR9 signaling cascade.
HMGB1 and mtDNA activate TLR9 signaling pathways and promote tumor growth in vitro
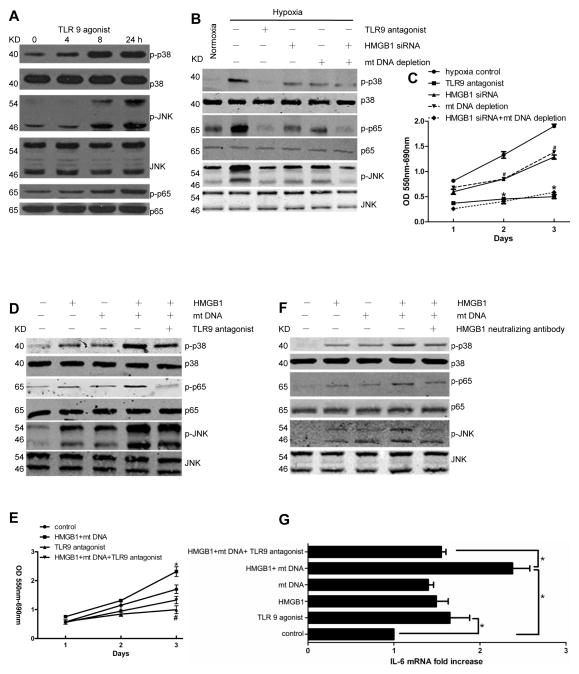
We next sought to determine how the interaction of mtDNA-HMGB1 and TLR9 might activate tumor growth signaling pathways during hypoxia. Mitogen-activated protein (MAP) kinase pathways are evolutionary conserved pathways that link extracellular or intracellular signals to the machinery that controls fundamental cellular processes such as growth, proliferation, differentiation, and migration, which are all fundamental aspects of tumor development. The JNK family and p38 isoforms are strongly activated by environmental stresses and inflammatory cytokines and contribute to cancer cell growth [25]. In addition, NF-κB signaling can lead to suppression of apoptosis in response to stress and continue to proliferate [26]. We first evaluated the role of TLR9 in the activation of mitogen-activated protein (MAP) kinases. Phosphorylation of p38, JNK, and marked phosphorylation at serine 536 of the p65 subunit of NF-κB was amplified with TLR9 agonist treatment in Hepa1-6 cells (Fig. 4A). When we subjected Hepa1-6 cells to hypoxia, MAP kinases were activated when compared to normoxic cancer cells (Fig. 4B). The hypoxia driven activation was similar to the activation seen when adding a TLR9 agonist in Fig. 4A. Depletion of HMGB1 and mtDNA was carried out as per the Methods section and confirmed prior to experiments (Supplementary Fig. 3A, B and 4A). Knocking down HMGB1 using shRNA or depleting mtDNA from the cancer cells, significantly decreased the activation of p38, p65 and JNK. We found similar findings above using Huh7 cells treated with the same conditions (data not shown). In addition, depleting both HMGB1 and mtDNA in the same cell or adding TLR9 antagonist (ODN 2088, 3μM) resulted in a more pronounced decreased in MAP kinase activation when compared to hypoxia or depleting either HMGB1 or mtDNA. These results paralleled the responses for tumor cell proliferation as evidenced by the MTT assay (Fig. 4C). In contrast, when we treated Hepa1-6 cells with HMGB1 (1 μg/ml) or mtDNA (10 μg/ml) for 24 h, there was a significant increase in the activation of MAP kinases. This effect was more pronounced when both HMGB1 and mtDNA were present and significantly decreased with the addition of a TLR9 antagonist (Fig. 4D). Again, the results of the MAP kinase activation in the different groups paralleled the results of the tumor cell proliferation as evidenced by the MTT assay (Fig. 4E). We also found that adding an HMGB1 neutralizing antibody (10 μg/ml), to prevent HMGB1-mtDNA complex formation, attenuated the activation of MAP kinases (Fig. 5F). Studies have shown that the tumorigenic effects of TLR9 depend on NF-κB mediated up regulation of IL-6 expression [27, 28]. Since we found that HMGB1 and mtDNA increased the activation of NF-κB via TLR9, we examined the downstream effect of NF-κB in increasing IL-6 expression. Indeed, we found that IL-6 was significantly elevated in the TLR9 agonist, HMGB1, and mtDNA treated groups and decreased in the groups treated with TLR9 antagonist or depleted from mtDNA or HMGB1 (Fig. 4G).
Fig. 4. HMGB1 and mtDNA activate TLR9 signaling pathways and promote tumor growth in vitro.
MAP kinases activation was determined in (A) Hepa1-6 cells treated with TLR9 agonist for different time points and (B) in different grouped Hepa1-6 cells for 24 h. (C) MTT assay showing tumor cell proliferation. #HMGB1 siRNA group, mtDNA depletion group compared with control. *TLR9 antagonist group, HMGB1 siRNA+mtDNA depletion group compared with control. (D) (E) MAP kinases activation and cell proliferation were determined during normoxia in Hepa1-6 cells. *HMGB1 + mtDNA group compared with control. #TLR9 antagonist group compared with control. (F) HMGB1 neutralizing antibody attenuated the activation of MAP kinases. (G) IL-6 mRNA expression in different groups.*and #p< 0.05.
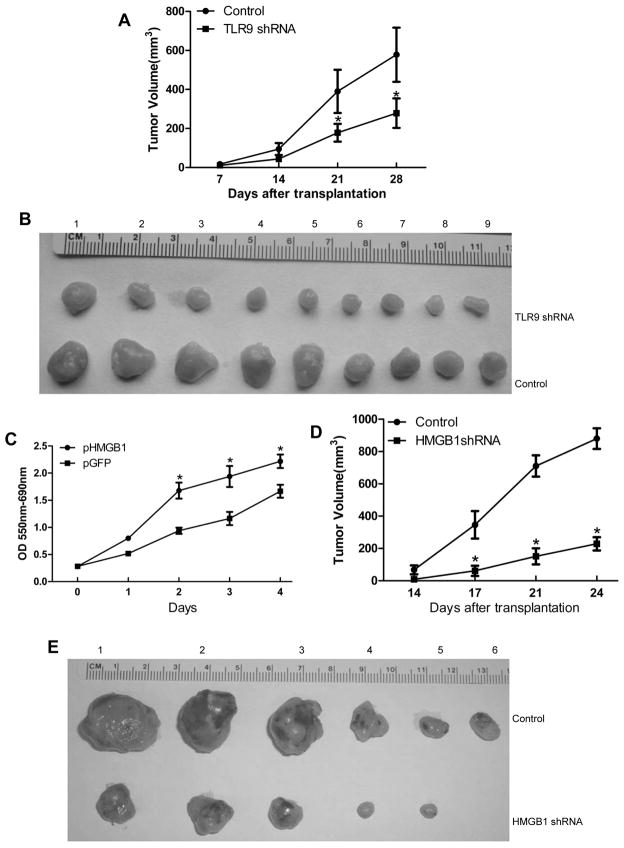
Fig. 5. TLR9 and HMGB1 promote tumor growth in vivo.
(A) Tumor volumes after Hepa1-6 cells with stable knockdown of TLR9 were engrafted in flanks of C57BL/9 mice with (B) representative tumor nodules shown. (C) Cancer cell proliferation was assessed by MTT in cells with stable HMGB1 overexpressing cells at different time points. Data is presented as mean±SE and is representative of 3 independent experiments. (D) Tumor volumes after Hepa1-6 cells with stable knockdown of HMGB1 were engrafted in flanks of C57BL/6 mice with (E) representative tumor nodules shown. Data is presented as mean±SE. * p < 0.05.
TLR9 and HMGB1 promote tumor growth in vivo
Our above findings demonstrate that TLR9 stimulation by both mtDNA and HMGB1 increases cancer cell proliferation. To confirm that TLR9 regulates the growth of HCC tumors in vivo, we engrafted Hepa1-6 cells transfected with either empty vector or TLR9 shRNA plasmid into the flanks of C57BL/6 mice and monitored the mice weekly for tumor size changes (n=9 mice/group). Four weeks following injection, the mice were sacrificed and their xenografted tumors were examined. Tumor nodule volumes were greater in the control group compared with the TLR9 shRNA group (Fig. 5A, B).
In addition, we studied the role of HMGB1 in promoting HCC growth. Constitutively active HMGB1 was stably transfected into the murine Hepa1-6 HCC cell line, and HMGB1 expression was confirmed via Western blot (Supplementary Fig. 4A). Stable HMGB1 expressing Hepa1-6 cells displayed a significant increase in cell growth in a time-dependent manner compared with the vector control transfected cells (Fig. 5C). In contrast to HMGB1 overexpression, stable knockdown of HMGB1 using siRNA in Hepa1-6 cells considerably decreased cell growth, as evidenced by the MTT assay (Supplementary Fig. 4B). To determine whether HMGB1 also regulates the growth of HCC tumors in vivo, we engrafted the Hepa1-6 cells transfected with either empty vector or HMGB1 shRNA plasmid into C57BL/6 mice and monitored the mice weekly for tumor size changes (n=6 mice/group, three independent experiments). Four weeks following injection, the mice were sacrificed and their xenografted tumors were examined. Tumor volumes were greater in the control group compared with the HMGB1 shRNA group (Fig. 5D, E).
Discussion
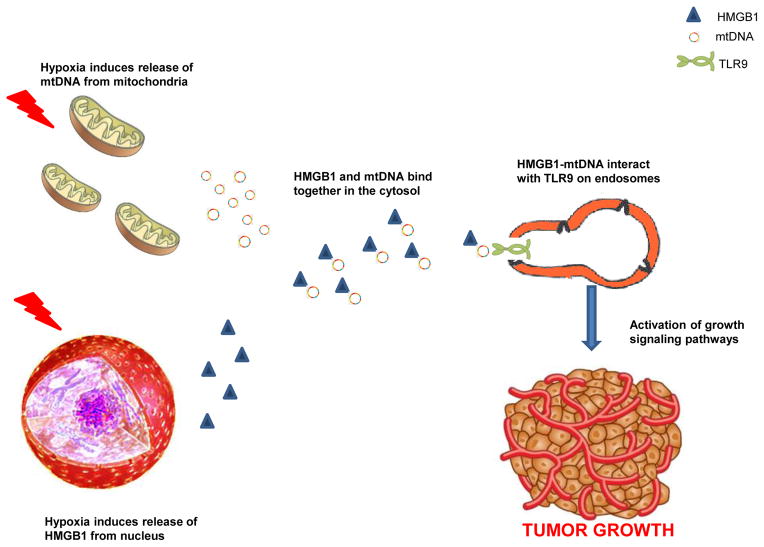
Overcoming hypoxic conditions is crucial for the progression and survival of solid tumors. The link between hypoxia and tumor progression has been recognized for decades; and, the extent of hypoxia in a solid tumor may represent an independent predictor of poor outcome [29]. In HCC, regions of hypoxia are commonly present throughout the tissue because of areas of necrosis, irregular blood flow, and poor oxygen diffusion across an ever growing tumor [30]. The mechanisms by which tumors activate responses to survive and progress despite the hypoxic microenvironment remain unclear. TLR9 is an innate immunity receptor that recognizes self and microbe-derived DNA [9, 10]. Although widely overexpressed in many tumors, the contribution of TLR9 to cancer pathophysiology remains incompletely understood [20, 21]. We have found that TLR9 is overexpressed in human HCC tissue where hypoxia is common and also in HCC cancer cell lines subjected to hypoxia. In addition we demonstrate that TLR9 is essential for HCC cells to proliferate under hypoxic conditions. The regulation of TLR9 signaling and the physiological ligands which may induce TLR9 mediated tumor growth remain poorly characterized. Here we have provided novel evidence that HMGB1 can interact with and bind to mtDNA in the cytoplasm and mediate the growth of tumor cells under hypoxic conditions by the activation of TLR9 signaling pathways (Fig. 6).
Fig. 6.
Schematic diagram of the proposed role of mtDNA and HMGB1 in promoting tumor growth during hypoxia via activation of TLR9 growth signaling pathways.
DAMP molecules are normally released by damaged or stressed tissues to ‘notify’ the immune system of tissue injury or impending danger. These molecules can originate from several different tissue compartments and essentially any cellular compartment. HMGB1 is a prototypical DAMP that intensifies the inflammatory response to injury. However, in cancer, HMGB1 has been reported to play paradoxical roles in both cancer cell survival and death [31]. In the nucleus, HMGB1 has antitumor roles in tumorigenesis. HMGB1 impacts chromosomal stability by maintaining telomere length through regulating telomerase activity and by directing DNA repair mechanisms [32]. In addition, HMGB1 functions as a tumor suppressor in breast cancer by directly binding to well-known tumor suppressor protein Rb and regulating its activity [33]. As an extracellular protein, HMGB1 has a predominant role of stimulating tumor growth and survival by sustaining a proinflammatory microenvironment, increase ATP production in cancer cells, and promotion of invasion, metastasis and angiogenesis [31]. The actions of HMGB1 in the cytoplasm of cancer cells remains less studied with regards to tumor pathophysiology compared to its well delineated nuclear and extracellular roles. Our results show that in HCC, HMGB1’s dominant role is protumorigenic as cancer cells treated with HMGB1 shRNA have significantly lower proliferation rate and less tumor growth when subjected to hypoxia or when injected in mice. In addition, we describe a novel mechanism through which HMGB1 contributes to HCC growth, i.e. by activation of TLR9 pro-tumorigenic signalling pathways. This body of work enhances our previous published findings that shows that HMGB1 levels are significantly elevated in patients with HCC and that HMGB1 can interact with its receptors RAGE and TLR4 to promote HCC metastasis [34].
Nucleic acids have been shown to be a potent trigger of innate inflammatory responses [35]. One of the more recent functions attributed to HMGB1 is that it can sense nucleic acids and is involved in the activation of all nucleic sensing TLRs (TLR 3, 7 and 9) [8, 36]. Both Tian et al. and Ivanov et al. demonstrated the role of HMGB1 in sensing circulating CpG-DNA immune complexes resulting in TLR-9 dependent augmentation of the inflammatory response [7, 36]. Even though the role of HMGB1 as a nucleic acid sensor has been well studied in autoimmune disease pathophysiology, this is the first report relating it to tumor pathophysiology. Similar to CpG ODN, mtDNA has CpG rich motifs, and thus we hypothesized that HMGB1 can sense mtDNA in a similar way as CpG ODN and other nucleic acids. In this study, our data support an innovative role of cytoplasmic HMGB1 as a sensor of mtDNA where it senses and binds to mtDNA in the cytoplasm. This complex later activates TLR9 to assist the cancer cells in surviving hypoxia. Although mtDNA by itself can stimulate tumor growth as shown for the first time in our study, HMGB1 is needed for the full effect of mtDNA activation of TLR9 signaling pathways. This solidifies the function of HMGB1 as a co-ligand for nucleic-acid receptors, such as TLR9. The binding of mtDNA to HMGB1 is required for more effective recognition and subsequent activation of TLR9 to enhance tumor growth under hypoxic conditions.
The mechanisms by which hypoxia is associated with tumor progression remain unclear and understanding the pathways involved is critical to develop new therapies. Our study proposes a novel mechanism by which HCC cells can turn hypoxic insults to their advantage to proliferate. Hypoxia is common in HCC due to the intrinsic fact of the high cancer proliferative rate. In addition, treatments aimed to cure HCC can actually accentuate tumor hypoxia. Surgery remains the cornerstone for the treatment and possible cure of HCC, but because portal triad clamping is a common maneuver during hepatectomy to control bleeding, the resultant hypoxia and release of DAMPs from this procedure may contribute to the growth of micro-metastatic disease and explain the high rates of recurrence post-operatively [37]. In addition, transarterial chemoembolization and radioembolization rely on causing increased tumor hypoxia as one of the mechanisms to kill tumor cells [38]. The targeted hypoxic tumor cells can eventually release HMGB1 and mtDNA that may act on distant non-targeted liver tumors in which TLR9 is overexpressed. Therefore, targeting TLR9 may represent an adjunct treatment in the battle against HCC.
In conclusion, our study shows that TLR9 is overexpressed in HCC and contributes to tumor growth in response to hypoxia. HMGB1 plays a pivotal role in TLR9 activation during hypoxia by way of mediating the binding of mtDNA and TLR9 with the subsequent activation of protumorigenic signaling pathways. These findings support the notion that TLR9, HMGB1 and mtDNA are suitable targets for the development of new anticancer drugs against hypoxia-driven tumor growth.
Supplementary Material
Acknowledgments
Financial support: This work was supported by Howard Hughes Medical Institute Physician-Scientist Award (A.T.), U.S. National Institutes of Health grants R01-GM95566 (A.T.), National Natural Science Foundation of China (No.81160257).
List of abbreviations
- HCC
hepatocellular carcinoma
- DAMPs
damage associated molecular patterns
- HMGB1
High mobility group box-1
- mtDNA
Mitochondrial Deoxyribonucleic acid
- TLR
toll-like receptor
- DMEM
Dulbecco’s Modified Eagle’s Medium
- shRNA
short hairpin ribonucleic acid
- siRNA
small interfering ribonucleic acid
- ROS
reactive oxygen species
- PBS
phosphate buffered saline
- EDTA
ethylene-diamineteraacetic acid
- COX
cytochrome c oxidase
- ChIP
chromatin immunoprecipitation
- IP
immunoprecipitation
- PCR
polymerase chain reaction
- Ig
immunoglobulin
- NAC
N-acetyl cysteine
- MyD88
myeloid differentiation factor 88
- MAP
mitogen activated protein
- NF-κB
Nuclear Factor Kappa Beta
- IL-6
interleukin-6
- RAGE
receptor for advanced glycation endproducts
- CpG ODN
CpG oligodeoxynucleotides
Footnotes
Conflict of interest: none to disclose
Authors’ contributions:
Conception and design: Yao Liu, Wei Yan, Dean Tian, Michael Lotze, Daolin Tang, Allan Tsung
Acquisition and analysis of data: Yao Liu, Wei Yan, Samer Tohme, Man Chen, Yu Fu, Allan Tsung
Drafting and revision of article: Yao Liu, Wei Yan, Samer Tohme, Dean Tian, Michael Lotze, Daolin Tang, Allan Tsung
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annual review of immunology. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 5.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, et al. High mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012 doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature immunology. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 8.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends in immunology. 2012;33:633–640. doi: 10.1016/j.it.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends in immunology. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Yu M. Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci. 2011;89:65–71. doi: 10.1016/j.lfs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, et al. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. American journal of respiratory cell and molecular biology. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Chen Y, Wu KC, Liu J, Zhao YQ, Pan YL, et al. RUNX3 directly interacts with intracellular domain of Notch1 and suppresses Notch signaling in hepatocellular carcinoma cells. Experimental cell research. 2010;316:149–157. doi: 10.1016/j.yexcr.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren T, Xu L, Jiao S, Wang Y, Cai Y, Liang Y, et al. TLR9 signaling promotes tumor progression of human lung cancer cell in vivo. Pathol Oncol Res. 2009;15:623–630. doi: 10.1007/s12253-009-9162-0. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka J, Sugimoto K, Shiraki K, Tameda M, Kusagawa S, Nojiri K, et al. Functional cell surface expression of toll-like receptor 9 promotes cell proliferation and survival in human hepatocellular carcinomas. Int J Oncol. 37:805–814. [PubMed] [Google Scholar]
- 22.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 24.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 25.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 26.Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. NF-kappaB and JNK: an intricate affair. Cell cycle. 2004;3:1524–1529. doi: 10.4161/cc.3.12.1321. [DOI] [PubMed] [Google Scholar]
- 27.Voorzanger N, Touitou R, Garcia E, Delecluse HJ, Rousset F, Joab I, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–5505. [PubMed] [Google Scholar]
- 28.Gao C, Kozlowska A, Nechaev S, Li H, Zhang Q, Hossain DM, et al. TLR9 signaling in the tumor microenvironment initiates cancer recurrence after radiotherapy. Cancer research. 2013;73:7211–7221. doi: 10.1158/0008-5472.CAN-13-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer metastasis reviews. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. Journal of cellular biochemistry. 2009;107:1053–1062. doi: 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- 31.Kang R, Zhang Q, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Molecular carcinogenesis. 2009;48:571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao Y, Wang HC, Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta pharmacologica Sinica. 2007;28:1957–1967. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 34.Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–1875. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tohme S, Geller DA, Cardinal JS, Chen HW, Packiam V, Reddy S, et al. Radiofrequency ablation compared to resection in early-stage hepatocellular carcinoma. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2013;15:210–217. doi: 10.1111/j.1477-2574.2012.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarnagin W, Chapman WC, Curley S, D’Angelica M, Rosen C, Dixon E, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2010;12:302–310. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.