Abstract
Transcatheter aortic valve replacement (TAVR) is an effective treatment for severe symptomatic aortic stenosis (AS) in patients who are inoperable or at high risk for surgery. However, the intermediate to long-term mortality is high, emphasizing the importance of patient selection. We therefore sought to evaluate the prognostic value of frailty among older TAVR recipients, hypothesizing that frail patients would experience a higher mortality rate and a higher likelihood of poor outcome 1 year after TAVR. This substudy of the PARTNER (Placement of AoRtic TraNscathetER Valves) Trial was conducted at 3 high-enrolling sites where frailty was assessed systematically prior to TAVR. In total, 244 patients received TAVR at the participating sites. Frailty was assessed using a composite of four markers (serum albumin, dominant hand grip strength, gait speed, and Katz activity of daily living (ADLs) survey), which were combined into a frailty score. The cohort was dichotomized at median frailty score. Outcomes measures were the time to death from any cause over 1 year of follow up and poor outcome at one year. Poor outcome was defined as: (1) death, (2) Kansas City Cardiomyopathy Questionnaire – Overall Summary score (KCCQ-OS) <60, or (3) decrease of ≥10 points in the KCCQ-OS score from baseline to 1 year. At 1 year, the Kaplan-Meier estimated all-cause mortality rate was 32.7% in the frail group and 15.9% in the non-frail group (log-rank p=0.004). At 1 year, poor outcome occurred in 50.0% of the frail group and 31.5% of the non-frail group (p=0.02). In conclusion, Frailty was associated with increased mortality and a higher rate of poor outcome 1 year after TAVR.
Clinical Trial Registration: ClinicalTrials.gov, Unique Identifier # NCT00530894
Keywords: Frailty, Transcatheter aortic valve replacement
Transcatheter aortic valve implantation (TAVR) can be an effective treatment option for older adults with severe symptomatic aortic stenosis (AS) who are inoperable or at high risk for traditional surgery, However, the intermediate to long-term mortality of patients undergoing TAVR is high,1,2 which is largely reflective of the underlying age and comorbidities of the treated population. Accordingly, there have been numerous efforts to understand which patients are unlikely to benefit from TAVR – either from a survival or quality of life standpoint. In these analyses, several clinical and physiological factors have been associated with a higher risk of poor outcomes.3 In elderly populations, frailty – a syndrome of impaired physiologic reserve and decreased resistance to stressors4 – has emerged as an important risk factor for morbidity and mortality in multiple clinical situations, including: the general population,5 older adults with coronary artery disease,6,7 and recovery after general 8 and cardiac surgery9,10. In addition, small, single center studies have shown that frailty is associated with increased morbidity and mortality after TAVR. 11–14 However, the relationship between frailty and quality of life after TAVR is unknown. Accordingly, we sought to evaluate the prognostic value of frailty among older adults who received TAVR in the Placement of AoRTic TraNscathetER Valve (PARTNER) Trial. We hypothesized that frail patients who undergo TAVR would experience increased mortality and a higher likelihood of a poor quality of life after TAVR, even after accounting for traditionally measured clinical risk factors.
Methods
The design and initial results of the PARTNER trial have been published previously.15,16 The PARTNER trial enrolled patients with severe symptomatic AS into 2 cohorts: those at high surgical risk (cohort A) and those considered inoperable due to severe coexisting conditions (cohort B). Patients in cohort B with a suitable iliofemoral vessel were randomized to transfemoral TAVR with the Edwards-Sapien heart valve system (Edwards Lifesciences, Irvine, California) or to standard medical care. Patients in cohort A were randomized to TAVR (transfemoral if iliofemoral vessels were suitable or transapical if not) or to conventional surgical aortic valve replacement. The current analyses pooled patients from both Cohort A and Cohort B (n=244) who were randomized to and received TAVR at 1 of 3 high-enrolling PARTNER trial sites where frailty was assessed systematically prior to TAVR (Medical City Dallas Hospital, Dallas, Texas[n=35]; Mayo Clinic, Rochester, Minnesota [n=83]; Columbia University Medical Center, New York, New York [n=126]). Among the 1057 participants enrolled in the PARTNER trial, 519 patients were randomized to and received TAVR (344 in cohort A, 175 in cohort B). Among these, 244 patients (47%; 215 in cohort A, 29 in cohort B) also completed a baseline frailty assessment at 3 high-volume centers and were included in this analysis. The study was approved by the institutional review board at each participating site, and all patients provided written informed consent.
Frailty is defined as a syndrome of impaired physiologic reserve and decreased resistance to stressors4 and is captured by the core domains of wasting and malnutrition, exhaustion and inactivity, weakness, and slowness.5 For this study, we operationalized frailty using a composite of four markers, which were chosen to parallel those operationalized by Fried.5,12 Malnutrition and wasting was assessed using serum albumin measured on the day before TAVR. Weakness was assessed by dominant hand grip strength measured using the average of 3 trials of maximal isometric grip measured in kilograms with a Jamar dynamometer (Sammons Preston, Chicago, Illinois). Slowness was assessed using gait speed on a 15 ft (4.57 m) walk. Participants were instructed to “walk at your comfortable pace” until a few steps past the 15-ft line. The timer was started with the first footfall after the 0-ft line and was stopped at the first footfall after the 15-ft line. The usual assist devices of subjects (e.g., walkers, canes) were permitted.17 If able, each subject completed one 15-ft walk. Gait speed was calculated by dividing 4.57 m by time to walk this distance in seconds and reported in meters/second, as has been previously recommended.18 Those subjects unable to walk 15 ft were considered to have a gait speed of 0 m/s. Instead of self-reported physical activity, independence in activities in daily living (ADLs) was assessed by the Katz ADL survey.19
The four assessments were then combined into a frailty score, as previously described.12 Briefly, gait speed and serum albumin were divided into quartiles. Grip strength was divided into quartiles stratified by sex. As nearly 75% of subjects were independent in all 6 Katz ADLs, ADL status was dichotomized into a group with dependence in any ADL versus those with no ADL dependence. With these quartiles, a frailty score was calculated in the following manner: 1) quartiles of albumin, gait speed, and grip strength were assigned values of 0 to 3 in descending order; and 2) a score of 0 for ADLs was assigned for ADL independence and 3 for any ADL dependence. These components were then summed to derive a frailty score for each subject (possible range 0 to 12), with the highest score representing the most frail, and the lowest score being the least frail. Frail, for the purposes of this study, was then defined as a frailty score above the median in this population. A patient with a frailty score of ≥ 6 was considered frail while those < 6 were considered non-frail.
The primary clinical outcome measure was the time to death from any cause over 1 year of follow-up. Other clinical outcomes of interest included 30-day cardiac death, repeat hospitalization due to AS or complications of the valve procedure, stroke, major bleeding, major vascular complications, permanent pacemaker, and renal failure requiring dialysis. Cardiac death, stroke, and major vascular complications were defined according to a modified version of the Valve Academic Research Consortium criteria20 as described in the PARTNER trial protocol.15,16 All events were adjudicated by an independent clinical events committee.
In addition, because TAVR recipients are often elderly with multiple comorbidities, it is likely that prolonged survival alone (without improved quality of life) would not be viewed as an acceptable outcome. To account for this need, a definition of poor outcome after TAVR that considers both survival and quality of life has been operationalized,3,21 which is defined as any of the following at 6 months after TAVR (definition #1): (1) death, (2) Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OS) score <45, or (3) decrease of ≥10 points in the KCCQ-OS score from baseline to 6 months. 3,21,22 In addition, we employed an alternative, expanded definition of poor outcome (definition #2) that included any of the following at 1 year after TAVR: (1) death, (2) KCCQ-OS score <60, or (3) decrease of ≥10 points in the KCCQ-OS score from baseline to 1 year. 3,21
All statistical analyses were based on the population of patients who actually received TAVR and underwent a baseline frailty assessment at participating sites. Continuous variables are summarized as medians (interquartile ranges) and were compared between frail and non-frail TAVR patients using the Wilcoxon rank-sum test. Categorical variables are presented as proportions and were compared by the chi-square test. Thirty-day event rates were compared between frail and non-frail patients using univariable Cox proportional hazards models. Time to event variables were summarized by means of Kaplan-Meier estimates and compared with the log-rank test. Cox proportional hazards models were then used to evaluate the independent association between baseline frailty status and all-cause mortality over 1 year after TAVR. Logistic regression was used to evaluate the association between frailty status and poor outcome after TAVR using definitions 1 and 2, as described above.
The following models were used to evaluate the relationship between frailty and 1 year mortality or poor outcome after TAVR. Using separate unadjusted Cox proportional hazards models, the relation between each component of the frailty score and mortality was evaluated. Gait speed, grip strength, and albumin were modeled as continuous variables and in quartiles. Because the results did not differ, only the results of the continuous analyses are shown. ADL was modeled as a dichotomous variable (independent in all ADLs versus dependent in any ADL). The unadjusted relationship between frailty score, both modeled as a continuous variable as a categorical variable (dichotomized at the median), was evaluated. Unadjusted logistic regression was used to evaluate the relationship between frailty status and poor outcome after TAVR using definitions 1 and 2, as described above.
Multivariable modeling was used to evaluate the independent relationship between frailty status (frailty score dichotomized at the median) and 1 year mortality after TAVR. Frailty status was forced into the multivariable models. Covariates for the multivariable models were selected using stepwise Cox regression with entry/stay criteria of 0.1/0.1, and a maximal ratio of 1 covariate for every 10 events in order to avoid over-fitting. Candidate variables included age, male gender, body mass index, transfemoral TAVR, Society of Thoracic Surgery score, diabetes mellitus, hypertension, angina pectoris, heart failure, NYHA Class IV, coronary artery disease, previous coronary angioplasty, previous coronary bypass, cerebrovascular disease, peripheral vascular disease, previous balloon aortic valvuloplasty, permanent pacemaker, renal disease, liver disease, chronic pulmonary disease, aortic valve mean gradient, ejection fraction, moderate or severe mitral regurgitation. Using multivariable logistic regression, a similar modeling strategy was used to evaluate the independent relationship between frailty and poor outcome after TAVR. A 2-sided alpha level of 0.05 was used for all significance testing. All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Results
Among the 244 patients included in this analysis, overall, median gait speed was 0.38 m/s (interquartile range 0.23–0.64 m/s), median serum albumin was 3.9 g/dL (interquartile range 3.6–4.2 g/dL), and 172 (71%) performed all ADLs independently. Among men, median grip strength was 23.6 kg (interquartile range 17.0–28.3) and among women, median grip strength was 12.2 kg (interquartile range 10.0–15.7). (Table 1) The median frailty score was 5 (interquartile range 3–7). Accordingly, for the purposes of this study 134 (120 cohort A; 14 cohort B) participants with a frailty score <6 were considered not frail and 110 (95 cohort A; 15 cohort B) participants with a frailty score ≥6 were considered frail. Baseline demographic, clinical, and echocardiographic characteristics stratified by baseline frailty status are summarized in table 2. Notably, frail participants walked shorter distances when performing the six-minute walk test and were less likely to be able to perform the six-minute walk test.
Table 1.
Markers of frailty by frailty score category
| Non-frail | Frail | |
|---|---|---|
| Independent in ADLs | 134 (100%) | 38 (35%) |
| Albumin, g/dL median (IQR) | 4.1 [3.8, 4.4] | 3.7 [3.4, 4.0] |
| Gait speed, m/s median (IQR) | 0.5 [0.3, 0.8] | 0.3 [0.0, 0.5] |
| Grip strength, kg (men) median (IQR) | 27.0 [21.3, 31.7] | 18.7 [13.2, 23.2] |
| Grip strength (women) median (IQR) | 14.0 [10.9, 16.9] | 11.0 [9.0, 14.0] |
ADLs, activities of daily living, IQR, interquartile range.
Table 2.
Demographic, clinical, and echocardiographic characteristics by frailty category
| Variable | Non-frail (n = 134) | Frail (n = 110) | p-value |
|---|---|---|---|
| Age (yrs) | 85.4 [79.4, 89.5] | 87.1 [82.7, 90.3] | 0.11 |
| Male gender | 74 (55%) | 52 (47%) | 0.22 |
| Body mass index (kg/m2) | 25.8 [22.2, 29.6] | 24.8 [21.9, 28.3] | 0.46 |
| Transfemoral TAVR | 62 (46%) | 57 (52%) | 0.39 |
| STS Score | 10.5 [8.8, 12.4] | 11.3 [9.6, 13.8] | 0.07 |
| Diabetes mellitus | 43 (32%) | 28 (26%) | 0.26 |
| Hypertension | 122 (91%) | 95 (86%) | 0.25 |
| Angina pectoris | 33 (25%) | 18 (16%) | 0.11 |
| Heart failure | 132 (99%) | 110 (100%) | 0.50 |
| NYHA Class IV | 36 (27%) | 32 (29%) | 0.70 |
| CAD | 114 (85%) | 91 (83%) | 0.62 |
| Previous PCI | 76 (57%) | 45 (41%) | 0.01 |
| Previous coronary bypass | 70 (52%) | 47 (43%) | 0.14 |
| Cerebrovascular disease | 39 (33%) | 26 (26%) | 0.30 |
| Peripheral vascular disease | 55 (41%) | 46 (42%) | 0.89 |
| Previous BAV | 33 (25%) | 35 (32%) | 0.21 |
| Permanent pacemaker | 30 (22%) | 27 (25%) | 0.69 |
| Renal disease | 19 (14%) | 15 (14%) | 0.90 |
| Liver disease | 3 (2%) | 9 (8%) | 0.03 |
| COPD | 57 (43%) | 46 (42%) | 0.91 |
| AV mean gradient (mm Hg) | 40.9 [36.5, 53.9] | 45.2 [34.9, 59.7] | 0.26 |
| AV area (cm2) | 0.63 [0.50, 0.83] | 0.62 [0.51, 0.72] | 0.20 |
| Ejection fraction (%) | 55 [45, 60] | 55 [35, 60] | 0.11 |
| Moderate or severe MR | 9 (7%) | 21 (20%) | 0.004 |
| 6-Minute Walk Test | |||
| Could Not Perform | 23 (17%) | 38 (35%) | 0.002 |
| Total Distance Walked (m)* | 192 [122, 297] | 146 [77, 238] | 0.01 |
Excluding those who could not perform
AV = aortic valve; BAV = balloon aortic valvuloplasty; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; EOA = effective orifice area; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; STS = Society of Thoracic Surgery; TAVR = transcatheter aortic valve replacement; MR = mitral regurgitation.
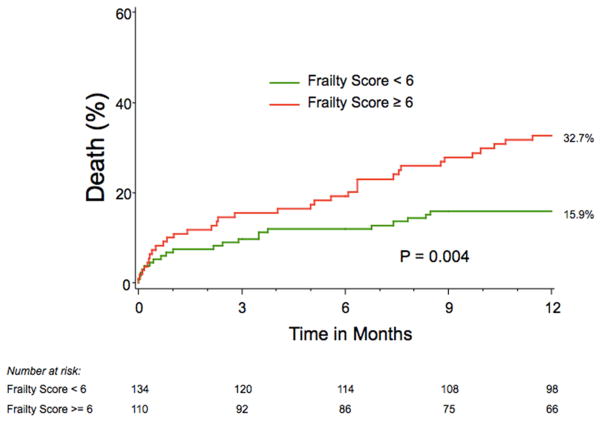
At 30 days, there were no differences in rates of major adverse clinical events according to baseline frailty status, including death, cardiac death, stroke, or repeat hospitalization, (Table 3). At 1 year, the Kaplan-Meier estimated all-cause mortality rate was 32.7% in the frail group and 15.9% in the non-frail group (log-rank p=0.004, Figure 1). In univariable analyses, none of the individual components of the frailty score was associated with mortality over the 1-year after TAVR (Table 4). However, frailty—both as a continuous score and as a categorical variable—was associated with increased mortality after TAVR (Table 4). Frail patients had greater rates of both cardiac death (frail vs. non-frail: 15.0% vs. 8.4%) and non-cardiovascular death (frail vs. non-frail: 9.5% vs. 4.9%) among patients for whom the cause of death could be classified. After adjusting for important clinical and demographic characteristics, frailty remained independently associated with a 2.5-fold increased hazard of 1-year mortality after TAVR (95% CI 1.40–4.35, p=0.002).
Table 3.
Unadjusted 30-day clinical outcomes stratified by baseline frailty score
| Non-Frail (n=134) | Frail (n=110) | p-value | |
|---|---|---|---|
| Death | |||
| Any cause | 10 (8%) | 11 (10%) | 0.49 |
| Cardiovascular cause | 8 (6%) | 8 (7%) | 0.68 |
| Repeat hospitalization* | 9 (7%) | 4 (4%) | 0.29 |
| Major stroke | 2 (2%) | 1 (1%) | 0.68 |
| Major bleeding | 7 (5%) | 10 (9%) | 0.24 |
| Major vascular complications | 6 (5%) | 7 (6%) | 0.51 |
| Permanent pacemaker | 12 (9%) | 10 (9%) | 0.97 |
| Renal failure (dialysis required) | 7 (5%) | 9 (8%) | 0.36 |
Due to aortic stenosis or complications of the valve procedure.
Figure 1.
Kaplan-Meier Survival Estimates Stratified by Frailty Score
Table 4.
Univariable association of markers of frailty and frailty score with 1-year mortality after TAVR
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Gait speed (m/s)* | 1.37 [0.53–3.45] | 0.51 |
| Grip strength (kg)* | 1.02 [0.99–1.05] | 0.28 |
| Albumin (g/dL)* | 1.25 [0.88–1.79] | 0.21 |
| Any ADL limitation | 1.59 [0.93, 2.70] | 0.09 |
| Score (continuous) | 1.12 [1.02, 1.22] | 0.01 |
| Score (≥6 versus < 6) | 2.18 [1.27, 3.75] | 0.005 |
Hazard ratio is per unit decrease
ADL = activities of daily living, HR = hazard ratio, CI = confidence interval, TAVR = transcatheter aortic valve replacement.
Poor outcome at 6-months after TAVR, using a combined mortality and quality of life endpoint, occurred in 41.9% of frail participants and 27.6% of non-frail participants (unadjusted OR of frailty 1.89, 95% CI 1.03–3.46, p=0.04). Similarly, at 12 months, a poor outcome occurred in 50.0% of frail participants and 31.5% of non-frail participants (unadjusted OR for frailty 2.17, 95% CI 1.16–4.07, p=0.02). After adjusting for important clinical and demographic characteristics, frailty remained independently associated with an increased odds of poor outcome after TAVR at both 6 months (OR 2.21, 95% CI 1.09–4.46, p = 0.03) and 1 year (OR 2.40, 95% CI 1.14–5.05, p = 0.02). After excluding those no longer alive, at 6 months poor outcome (KCCQ-OS score <45, or decrease of ≥10 points in the KCCQ-OS score from baseline) was more common in the frail group than in the not frail group (24% versus 14%, p=0.13), however this did not meet statistical significance. This difference was no longer seen at one year.
Discussion
The current report, drawn from a cohort of 244 patients with severe symptomatic AS who underwent TAVR at 3 U.S. sites, evaluated the association between frailty as estimated by the composite of gait speed, grip strength, ADLs, and albumin and outcomes after TAVR. We found that at 30 days the rate of mortality and major complications did not differ between the frail and non-frail groups; however, at 1 year, compared with those with a frailty score below the median value, those with a frailty score above the median value experienced a higher rate of death and poor outcome (which considers both mortality and quality of life) after TAVR.
It is noteworthy that the physical performance of the participants in this study is substantially lower than those seen in other cohorts of older adults, emphasizing how frail many patients enrolled within the PARTNER really were. In the Cardiovascular Health Study, a cohort of community-dwelling adults aged 65 years and older in which the Fried definition of frailty was developed, a gait speed of less than 0.76–0.65 m/s (depending on height) and grip strength of less than 29–32 kg among men (depending on body mass index) and 17–21 kg among women (depending on body mass index) were used to define frailty.5 Similarly, even among cardiac surgery patients age 70 years and older, those with a gait speed of less than 0.65 m/s were considered frail. In both of these studies, these levels represented the lowest quintile of assessments, and even so, are much higher than the median gait speed of 0.38 m/s (men and women; interquartile range 0.23–0.64 m/s) and median grip strength of 23.6 kg (men; interquartile range 17.0–28.3) and12.2 kg (women; interquartile range 10.0–15.7) in our study. Consequently, if traditional gait speed and grip strength cut points for frailty were applied to the population of TAVR patients in this study, approximately 75% would have met criteria for frailty. We suspect the slower gait speed and weaker grip strength in the PARTNER trial population likely reflect a combination of factors including very advanced age, multiple comorbidities, and the disease process of symptomatic AS itself, all affecting both strength and speed. This underscores the need to customize frailty cut points for the population of interest.23 In addition, it is important to understand how the disease process in question could affect the frailty measurements, so as to try to isolate the syndrome of frailty, as opposed to just a more severe version of the disease in question. For this reason, we believe that a more comprehensive assessment of frailty—using all measures in a composite score—is the most effective way to assess frailty in the AS population and is also the reason why the individual frailty components were not as prognostically important as the overall score.
Our study both supports and extends the prior literature investigating the association of frailty with adverse outcomes after TAVR. Ewe and colleagues studied 147 patients undergoing TAVR in 2 centers with a mean follow-up period of 9 ± 5 months. Preoperative frailty, as assessed using the criteria of Fried,5 was an independent predictor of major adverse cardiac events (HR=4.2, 95% CI=2.0–8.8).11 Similarly, in a group of 119 patients from a single center, frailty (using an alternative index) was associated with increased mortality and major adverse cardiovascular events at 30 days and 1 year after TAVR.13 Furthermore, even after controlling for EuroSCORE, frailty was associated with a deterioration in the ability to perform basic ADLs after TAVR.14 In a single-center study of 159 patients who underwent TAVR, we found that frailty (using the same frailty score used in the present study) was independently associated with increased 1-year mortality after TAVR (HR 3.5, 95% CI 1.4–8.5). The current study, performed in the context of a rigorous randomized controlled trial, is the largest multicenter study to demonstrate the relationship between frailty and increased mortality after TAVR.
Furthermore, it is recognized that a successful outcome after TAVR must consider both mortality and quality of life.24 Considering that the goal of TAVR is to prolong life and either improve poor quality or maintain high quality of life, we employed a definition of poor outcome that includes those three aspects. Previous work within the PARTNER cohort has explored the derivation of this definition21 and has identified predictors of poor outcome after TAVR.3 While this antecedent analysis was unable to consider frailty as a complete syndrome, particular domains of frailty—6-minute walk test time and mini-mental status exam—were important predictors of a poor outcome after TAVR.3 The current study thus extends this prior research in determining that frailty, as a comprehensive syndrome, is also an independent multivariable predictor of poor outcome after TAVR, and thus represents a step forward towards identifying those patients who stand to benefit most (and least) from TAVR.
This study adds to the growing body of evidence that an assessment of frailty provides useful prognostic information among older adults at extreme or high risk for surgical aortic valve replacement who are being evaluated for TAVR. Accordingly, practice guidelines have incorporated an assessment of frailty as an essential part of the work up of older adults with aortic stenosis.25 However, it is recognized that the optimal frailty assessment is unknown.23 Some have advocated for gait speed as a single item performance measure, while others have recommended a comprehensive geriatric assessment performed by a trained geriatrics professional that extends well beyond the phenotypic definition of frailty and includes cognition, mood, disability, and clinical factors. Additionally, beyond honing in on the optimal frailty measure, the incremental value of adding frailty to models that predict mortality and/or poor outcome after TAVR must be determined. Ongoing and future research initiatives will provide insight into these issues.
There are several important potential limitations to this study that merit further discussion. First, all patients included in this study were carefully evaluated and deemed to be appropriate candidates for TAVR, meeting the strict inclusion criteria for the first PARTNER Trial. Therefore, the generalizability of these findings to unselected or lower-risk populations or to a population undergoing traditional surgical AVR is unknown. Second, although we believe that our definition of frailty is reasonable for the severe AS patient population and although it has been previously shown to predict outcome in the TAVR population,12 our definition and cut points chosen represent a depart from the one originally operationalized by Fried.5 In addition, we did not include cognitive status in our definition of frailty, as some geriatricians have recommended. For these reasons, it is important to continue to evaluate this definition of frailty, as well as test other possible definitions, in larger cohorts of older adults undergoing TAVR and surgical aortic valve replacement.
Acknowledgments
Funding Source: The PARTNER Trial was funded by Edwards Lifesciences, and the protocol was developed collaboratively by the Sponsor and Steering Committee. The current analysis was designed and completed by the authors through the PARTNER Publications Office, which is co-located at Columbia University Medical Center/The Cardiovascular Research Foundation and The Cleveland Clinic and supported by an unrestricted grant from Edwards Lifesciences, administered by Medstar Health Research Institute. The funding organization had no involvement in the design, analysis, or interpretation of this substudy, or in the decision to publish. Dr. Green (K23 HL12114) and Dr. Arnold (K23 HL116799) are supported by Career Development Grant Awards from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. New Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB. Transcatheter Aortic-Valve Replacement for Inoperable Severe Aortic Stenosis. New Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes-Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ, Investigators P. Predictors of Poor Outcomes After Transcatheter Aortic Valve Replacement: Results From the PARTNER (Placement of Aortic Transcatheter Valve) Trial. Circulation. 2014;129:2682–2690. doi: 10.1161/CIRCULATIONAHA.113.007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 7.Ekerstad N, Swahn E, Janzon M, Alfredsson J, Löfmark R, Lindenberger M, Carlsson P. Frailty Is Independently Associated With Short-Term Outcomes for Elderly Patients With Non-ST-Segment Elevation Myocardial Infarction / Clinical Perspective. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 8.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 10.Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, Mohr FW, Walther T. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2010 doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Ewe SH, Ajmone Marsan N, Pepi M, Delgado V, Tamborini G, Muratori M, Ng AC, van der Kley F, de Weger A, Schalij MJ, Fusari M, Biglioli P, Bax JJ. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, Moses JW, Leon MB, Smith CR, Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stortecky S, Schoenenberger AW, Moser A, Kalesan B, Juni P, Carrel T, Bischoff S, Schoenenberger C-M, Stuck AE, Windecker S, Wenaweser P. Evaluation of Multidimensional Geriatric Assessment as a Predictor of Mortality and Cardiovascular Events After Transcatheter Aortic Valve Implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Schoenenberger AW, Stortecky S, Neumann S, Moser A, Juni P, Carrel T, Huber C, Gandon M, Bischoff S, Schoenenberger CM, Stuck AE, Windecker S, Wenaweser P. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 15.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 16.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. New Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 17.Afilalo J. Frailty in Patients with Cardiovascular Disease: Why, When, and How to Measure. Curr Cardiovasc Risk Rep. 2011;5:467–472. doi: 10.1007/s12170-011-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait Speed and Survival in Older Adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shelkey M, Wallace M. Katz Index of Independence in Activities of Daily Living. J Geront Nurs. 1999;25:8–9. doi: 10.3928/0098-9134-19990301-05. [DOI] [PubMed] [Google Scholar]
- 20.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel M-a, Petersen J, Popma JJ, Takkenberg JJM, Vahanian A, van Es G-A, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation Clinical Trials: A Consensus Report From the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Arnold SV, Spertus JA, Lei Y, Green P, Kirtane AJ, Kapadia S, Thourani VH, Herrmann HC, Beohar N, Zajarias A, Mack MJ, Leon MB, Cohen DJ. How to Define a Poor Outcome After Transcatheter Aortic Valve Replacement: Conceptual Framework and Empirical Observations From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circulation: Cardiovascular Quality and Outcomes. 2013;6:591–597. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 23.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindman BR, Alexander KP, O’Gara PT, Afilalo J. Futility, Benefit, and Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2014 doi: 10.1016/j.jcin.2014.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, 3rd, Thomas JD, Members AATF. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]