Abstract
Patients who survive sepsis display suppressed immune functions, often manifested as an increased susceptibility to secondary infections. Recently, using a cecal-ligation and puncture (CLP) model of sepsis we showed that sepsis induces substantial and long-lasting changes in the available naive CD8+ T cell repertoire affecting the capacity of the host to respond to newly encountered acute infections. However, the extent to which sepsis changes the host susceptibility to chronic infection and affects CD8+ T cell responses is currently unknown. Here, we demonstrate that inbred and outbred mice recovering from a septic event are more susceptible to LCMV clone-13 infection exhibited by mortality and viral burden. Primary virus-specific CD8+ T cells in LCMV clone-13 infected septic mice displayed exacerbated CD8+ T cell exhaustion illustrated by increased inhibitory molecule expression (e.g., PD-1, LAG-3, and 2B4) and diminished Ag-driven cytokine production (e.g. IFNγ, TNFα) compared to similarly infected sham-treated mice. Importantly, therapeutic inhibitory molecule dual-blockade (αPD-L1 and αLAG-3) increased the number of circulating LCMV-specific CD8+ T cells, improved CD8+ T cell function and pathogen control in chronically infected septic mice. Together, these results illustrate that poly-microbial sepsis compromises the overall health of the host leading to increased vulnerability to chronic infection and exacerbated CD8+ T cell exhaustion. Collectively, our findings suggest that septic survivors may be more susceptible and at higher risk of developing exhaustible CD8+ T cells upon encountering a subsequent chronic infection.
Introduction
In the United States, septicemia is the cause of more than 1.6 million hospital cases with an in-hospital mortality rate of approximately 16% (1, 2). A septic event triggers massive apoptosis of immune cells, including T cells, resulting in an initial hyper-inflammatory phase followed by a prolonged hypo-inflammatory immunosuppressive state (3–8). Septic patients exhibit immunoparalysis manifested by the inability to control and eradicate infections that are normally cleared with functioning CD8+ T cell mediated-immunity (3, 6, 7, 9). Furthermore, viral reactivation of latent viruses can occur following a septic event (5, 10–13) and sepsis survivors have an increased risk of death from non-septic causes years after the initial septic insult; e.g. increased heart, lung, renal, liver disease, infection and hematologic disorders experienced in the preceding year are factors associated with increased risk of death in sepsis survivors (14).
CD8+ T cells play a crucial role in the control and eradication of intracellular pathogens (15). The naïve CD8+ T cell repertoire is composed of a small number of unique Ag-specific CD8+ T cell precursors (ranging from 10–1000 cells in an inbred laboratory mouse), which enables the host to respond to a wide range of pathogen-derived epitopes (16–21). Upon recognition of cognate Ag (22, 23), naïve Ag-specific CD8+ T cells proliferate and differentiate into effector CD8+ T cells capable of eliciting effector functions such as cytolysis (cytolytic perforin and granzyme B molecules) and cytokine production (IFNγ and TNFα) that facilitates control and clearance of the invading pathogen. Following the effector stage the expanded Ag-specific CD8+ T cells undergo a contraction phase whereby 90–95% of the responding CD8+ T cells die. The surviving CD8+ T cell population constitutes the primary Ag-specific memory CD8+ T cell pool (24–26).
Lymphocytic choriomeningitis virus (LCMV) (27–29) has been extensively used to study adaptive immune responses to viral infection (22, 23). The Armstrong strain of LCMV (LCMV-Arm) causes an acute system infection, which induces a robust CD8+ T cell response (24) that clears the infection within 8 days (30). A variant of LCMV-Arm, the clone-13 strain (LCMV clone-13), was isolated from the spleen of a mouse infected at birth with LCMV-Arm (31) and differs from the parental LCMV-Arm strain by 2 amino acid functional changes (one change in the polymerase protein (L: K1079Q) and the other in the viral glycoprotein (GP1: L260F)) (32–35). While these mutations increase viral replication and change cell tropism that results in a chronic viral infection (30), they do not alter LCMV-specific CD8+ T cell epitopes allowing for the direct evaluation of CD8+ T cell responses to dominant and subdominant LCMV-specific epitopes (33, 35). As LCMV clone-13 infection persists, CD8+ T cells progress through stages of dysfunction or exhaustion. Certain CD8+ T cell effector functions are lost before others in a stepwise manner (e.g., cytokine production; IL-2 > TNFα > IFNγ) (30, 36, 37). This is accompanied by increased expression of inhibitory molecules (e.g. PD-1, LAG-3, and 2B4) (38–40) and increased viral load (30, 36). Ultimately, deletion of Ag-specific CD8+ T cells occurs that results in an altered CD8+ T cell repertoire and skewed immunodominance hierarchy (30).
Recently, using p:MHC class I tetramer-based enrichment technology we demonstrated that sepsis-induced apoptosis reduces the number of Ag-specific naïve CD8+ T cell precursors, which leads to impairment in primary Ag-specific CD8+ T cell responses to acute systemic bacterial and viral infections (41). In the current study, we utilized the cecal-ligation and puncture (CLP) mouse model of sepsis to investigate both the short and long-term effects of poly-microbial sepsis on primary Ag-specific CD8+ T cell responses to chronic LCMV clone-13 infection. Our data demonstrate that poly-microbial sepsis increases the susceptibility of mice to chronic viral infection and accelerates the path to CD8+ T cell exhaustion.
Material and Methods
Mice and viral infection
Inbred C57BL/6 mice (wild type, Thy1.2/1.2) were bred at the University of Iowa and outbred NIH Swiss mice were purchased from the National Cancer Institute and used at 6–10 weeks of age. Thy1.1/1.1 or Thy1.1/1.2 P14 TCR-transgenic (specific for LCMV-derived GP33 epitope) mice were described previously (41–45). LCMV clone-13 (2 × 106 PFU/mouse; i.v.; non-lethal viral dose) was provided by Dr. Steven M. Varga (Department of Microbiology, University of Iowa) and previously described (31, 46–48). Infected septic mice were housed at the University of Iowa under the appropriate biosafety level. All animal studies were approved by the University of Iowa Institutional Animal Care and Use Committee, and meet the stipulations of the Guide for the Care and Use of Laboratory Animals (NIH).
Cecal-ligation and puncture (CLP)
Poly-microbial sepsis was induced by CLP (41, 49–51). Briefly, mice were anesthetized and the abdomen was shaved and disinfected. A mid-line abdominal incision was made, the cecum was identified and the distal one-third was ligated with 4-0 silk sutures. The ligated portion was punctured once using a 25-gauge needle and a small amount of cecal contents was extruded through the puncture. The cecum was returned into the abdomen and the peritoneum was closed with continuous suture. The skin was glued together with Vetbond tissue adhesive (St. Paul, MN) and 1mL of saline was injected for resuscitation. This level of injury was used to create a chronic septic state characterized by the loss of appetite and body weight, ruffled hair, shivering, diarrhea, and/or periorbital exudates, and with 5–10% mortality. Sham-treated mice underwent the same procedure excluding cecum ligation and puncture. Bupivacaine was administered at the incision site and flunixin meglumine was administered twice for postoperative analgesia to all sham and CLP-treated mice.
mAb, peptides and MHC class I tetramer
The following mAb were purchased from eBioscience or Biolegend and used in an appropriate combination of fluorochromes: CD8 (clone 53-6.7; eBioscience), PD-1 (clone J43; eBioscience), LAG-3 (clone eBioC9B7W; eBioscience), 2B4 (clone eBio244F4; eBioscience), CD11a (clone M17/4; eBioscience), Thy1.1 (clone HIS51; eBioscience), IFNγ (clone XMG1.2; Biolegend) and TNFα (clone MP6-XT22; Biolegend). All LCMV-specific peptides were synthesized by Bio-Synthesis Inc (Bio-Synthesis, Louisville, TX): GP276-286 (SGVENPGGYCL) and GP33-41 (KAVYNFATM) (52). p:MHC class I tetramer H-2Db GP276 and GP33 were made and used as previously described (25, 48).
Adoptive-transfer, quantification/phenotype of CD8+ T cells and intracellular cytokine staining
Thy1.1/1.1 or Thy1.1/1.2 P14 CD8+ T cells were obtained from spleens or peripheral blood of young naïve P14 mice and 103 cells were injected i.v. into naïve C57BL/6 (Thy1.2/1.2) recipients (45). Lymphocytes were isolated from the spleen or peripheral blood as indicated and LCMV-specific CD8+ T cells were identified by tetramer staining for endogenous GP276- and GP33-specific CD8+ T cells or Thy1.1-staining for P14 TCR-transgenic cells in inbred C57BL/6 mice, or CD11a CD8α-staining for CD8+ T cells in outbred NIH Swiss mice (53). Inhibitory molecule expression (PD-1, LAG-3, and 2B4) was determined on virus-specific CD8+ T cells. The frequency of CD8+ T cells producing cytokine after stimulation with indicated peptides was determined using intracellular cytokine staining (IFNγ, TNFα) after 5h incubation at 37°C in the presence of Brefeldin A (BD Biosciences) (54). Cytokine production (IFNγ) from the peripheral blood, was determined after 5h stimulation at 37°C with indicated peptides in the presence of Brefeldin A (BD Biosciences) and EL4 (105) antigen presenting cells as previously described (47). FlowJo software (Tree Star) was used for analysis of samples acquired on a Canto flow cytometer (BD Biosciences).
In vivo antibody blockade
Anti-PD-L1 (200µg; clone 10F.9G2; BioXCell), anti-LAG-3 (200µg; clone C9B7W) or rat IgG (200µg; Sigma) was injected i.p. every 3rd day for 2 weeks (total 5 injections) beginning on day 21 post-LCMV clone-13 infection. For dual-blockade of αPD-L1 and αLAG-3 200µg was used of each antibody as previously described (39, 55). Control groups were injected with PBS or 200µg of isotype antibody.
Virus titers
For analysis of viral burden, mice were infected on the indicated days post-CLP or sham surgery with LCMV clone-13 (2 × 106 PFU/mouse; i.v.) and kidneys, liver, and serum were harvested on indicated days post-LCMV clone-13 infection. Kidneys and livers were homogenized and viral titers were quantified using a Vero cell plaque assay as previously described (31, 46).
Statistical analyses
Data was analyzed with Prism6 GraphPad software and specific tests to determine statistical significance are indicated in figure legends (***p < 0.0001; **p < 0.01; * p < 0.05). Data generated as scatter dot plots are presented as Mean and data generated as bar graphs are presented as Mean + SEM.
Results
Poly-microbial sepsis increases the susceptibility of inbred mice to chronic viral infection
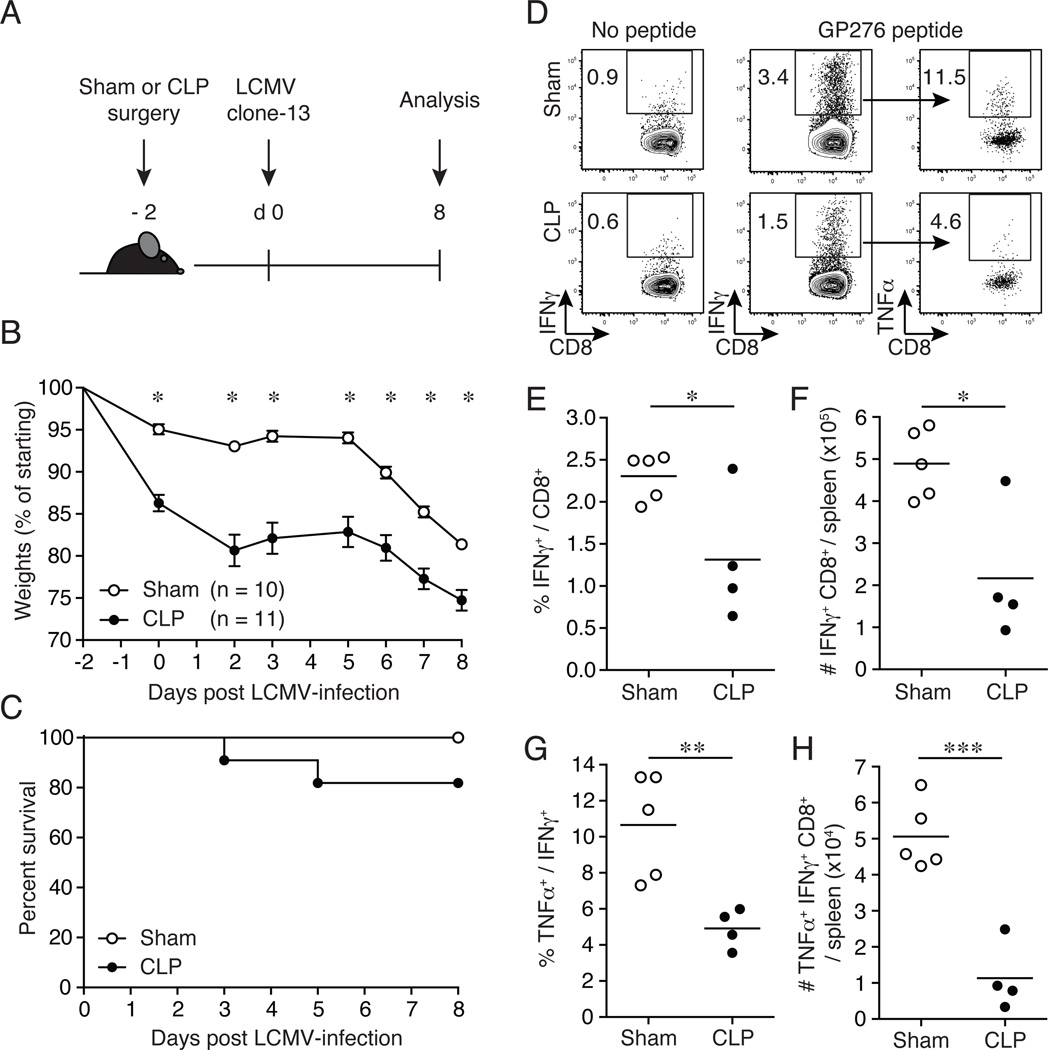
To examine the extent to which sepsis impacts the susceptibility of mice to chronic viral infection, CLP- or sham-treated inbred C57BL/6 mice were infected with LCMV clone-13 (2 × 106 PFU/mouse; i.v.; non-lethal viral dose) early after sepsis induction (day 2 post-surgery). Mice were monitored for morbidity and mortality for 8 days post-infection (Fig. 1A). Two days post-surgery CLP-treated mice displayed significant weight loss compared to sham-treated mice (Fig. 1B). Upon subsequent infection with LCMV clone-13, CLP-treated mice demonstrated sustained weight loss (~25% weight loss; Fig. 1B) and increased mortality (Fig. 1C) compared to sham-treated infected mice. In all of the experiments in which mice were infected with LCMV clone-13 on day 2 post-surgery survival in sham and CLP C57BL/6 mice was 100% (47/47) and 81% (72/89), respectively. These results demonstrate that the induction of sepsis compromises the overall health of the host leading to sustained morbidity and decreased survival with a otherwise non-lethal LCMV clone-13 infection.
Figure 1.
Increased susceptibility to LCMV clone-13 infection and impaired primary Ag-specific CD8+ T cell responses in septic mice. (A) Experimental model. Two days prior to LCMV clone-13 infection (2 × 106 PFU/mouse; i.v.), CLP or sham surgery was performed on inbred C57BL/6 (Thy1.2/1.2) mice. Subsequent CD8+ T cell analyses and viral titers were examined on day 8 post-LCMV clone-13 infection. (B) Morbidity and (C) survival rate were analyzed on indicated days post-CLP or sham surgery. (D) Representative flow cytometry plots illustrating expression of IFNγ+ of CD8+ T cells and TNFα+ of IFNγ+ CD8+ T cells. Numbers within plots represent the frequency of cytokine positive as indicated. Summary data of (E) frequency and (F) total number of IFNγ+ CD8+ T cells. Ex vivo GP276-peptide stimulation of splenocytes on day 8 post-LCMV clone-13 infection. Summary data of (G) frequency and (H) total number of TNFα+ IFNγ+ CD8+ T cells. Dots represent individual mice. Data analyzed in morbidity curve by two-tailed, unpaired Student t test (10–11 mice/group). Data analyzed in survival curve by Gehan-Breslow-Wilcoxon test, no statistical significance found. Ag-specific CD8+ T cell responses analyzed by two-tailed, unpaired Student t test (4–5 mice/group). Data are representative of three to four independent and similar experiments. * p < 0.05, ** p < 0.01, *** p < 0.0001.
LCMV clone-13 infected septic mice demonstrate accelerated CD8+ T cell exhaustion
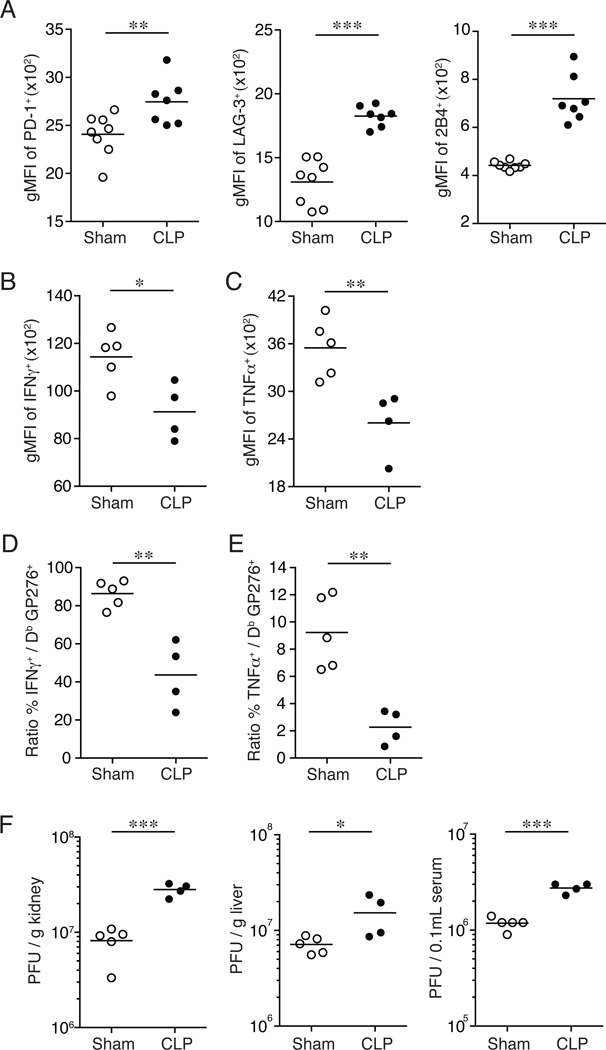
The path to CD8+ T cell exhaustion is a stepwise process resulting in a temporal hierarchal loss in CD8+ T cell functions (30, 36, 37). To investigate the extent to which sepsis impacts CD8+ T cell exhaustion, CLP- or sham-treated inbred C57BL/6 mice were infected with LCMV clone-13 (2 × 106 PFU/mouse; i.v.) on day 2 post-surgery and the endogenous GP276- and GP33-specific CD8+ T cell populations were examined in the spleen at the peak of the primary CD8+ T cell response (day 8 post-infection; Fig. 1 & 2, Supplemental Fig. 1). CLP-treated infected mice had a significant reduction in the frequency (Fig. 1D, 1E) and number (Fig. 1F) of IFNγ+-producing GP276-specific CD8+ T cells after ex vivo GP276-peptide stimulation compared to sham-treated infected mice. This reduction was also observed in the frequency (Fig. 1D, 1G) and number (Fig. 1H) of TNFα+ IFNγ+-producing GP276-specific effector CD8+ T cells from CLP-treated mice compared to sham-treated infected controls. Importantly, analysis of inhibitory molecule surface expression (as measured by PD-1, LAG-3, and 2B4 geometric mean fluorescence intensity (gMFI)) revealed a significant increase in PD-1, LAG-3, and 2B4 expression on Db GP276-specific CD8+ T cells from LCMV clone-13 infected CLP-treated mice compared to sham-treated mice 8 days post-infection (Fig. 2A). Similar data were obtained when endogenous Db GP33-specific CD8+ T cells were analyzed (Supplemental Fig. 1A). The surface expression of inhibitory molecules was significantly increased on Db GP33-specific CD8 T cells from LCMV clone-13 infected CLP-treated mice compared to sham-treated mice (Supplemental Fig. 1B–C). A significant reduction was also observed in the amount of IFNγ (Fig. 2B and Supplemental Fig. 1D–E) and TNFα (Fig. 2C and Supplemental Fig. 1D–F) produced on a per-cell basis after ex vivo peptide stimulation (as measured by IFNγ and TNFα gMFI) suggesting that sepsis not only decreases the overall number of LCMV-specific CD8+ T cells but also affect their functionality.
Figure 2.
CD8+ T cell exhaustion is exacerbated in LCMV clone-13 infected septic mice. Inhibitory molecule expression of splenocytes on day 8 post-LCMV clone-13 infection. (A) Summary data of geometric mean fluorescence intensity (gMFI) of PD-1+, Lag-3+, and 2B4+ expression on tetramer+ GP276-specific CD8+ T cells. Ex vivo GP276-peptide stimulation and staining of splenocytes on day 8 post-LCMV clone-13 infection. Summary data of gMFI of (B) IFNγ+ and (C) TNFα+ expression. Summary data of the percentage of (D) IFNγ and (E) TNFα producing CD8+ T cells of tetramer+ GP276-specific CD8+ T cells. (F) Viral titers were determined from the kidney, liver, and serum at day 8 post-LCMV clone-13 infection. Dots represent individual mice. Data analyzed by two-tailed, unpaired Student t test (4–5 mice/group). Data are representative of two to three independent and similar experiments. * p < 0.05, ** p < 0.01, *** p < 0.0001.
Since the T cell receptor (TCR) on Ag-specific CD8+ T cells is internalized upon peptide recognition in the intracellular cytokine assay used here (56) simultaneous detection of LCMV-specific CD8+ T cells using tetramers and peptide-stimulated intracellular cytokine staining is not feasible. However, the functionality of Ag-specific CD8+ T cells can be measured indirectly on a per-cell basis by calculating the percentage of Db GP276-specific CD8+ T cells capable of producing cytokine (IFNγ, TNFα) (30). Most Db GP276-specific CD8+ T cells were able to produce IFNγ (~86% functional upon stimulation; Fig. 2D) and ~9.2% were capable of producing TNFα (Fig. 2E) from LCMV clone-13 infected sham-treated mice. In contrast, LCMV clone-13 infected CLP-treated mice had a significant reduction in the ability of Db GP276-specific CD8+ T cells to produce IFNγ (~43% functional upon stimulation; Fig. 2D) and TNFα (~2.3% functional upon stimulation; Fig. 2E) compared to sham-treated infected mice.
In addition to the decreased number of virus-specific CD8+ T cells, increased inhibitory molecule expression and loss in Ag-specific CD8+ T cell function, CD8+ T cell exhaustion is associated with an increase in viral burden during chronic infection (30, 36, 38–40). To determine if the sepsis-induced changes in CD8+ T cell responses to chronic infection led to an increase in viral load, LCMV titers were determined in the kidneys, liver, and serum on day 8 post-infection. Importantly, a significant increase in viral burden was detected in all organs of CLP-treated mice tested compared to sham-treated mice (Fig. 2F). Thus, these results collectively demonstrate that septic insult prior to chronic viral infection compromises the host to mount optimal primary Ag-specific CD8+ T cell responses, accelerates the path to CD8+ T cell exhaustion and reduces pathogen control.
Outbred septic animals exhibit increased susceptibility and CD8+ T cell exhaustion following LCMV clone-13 infection
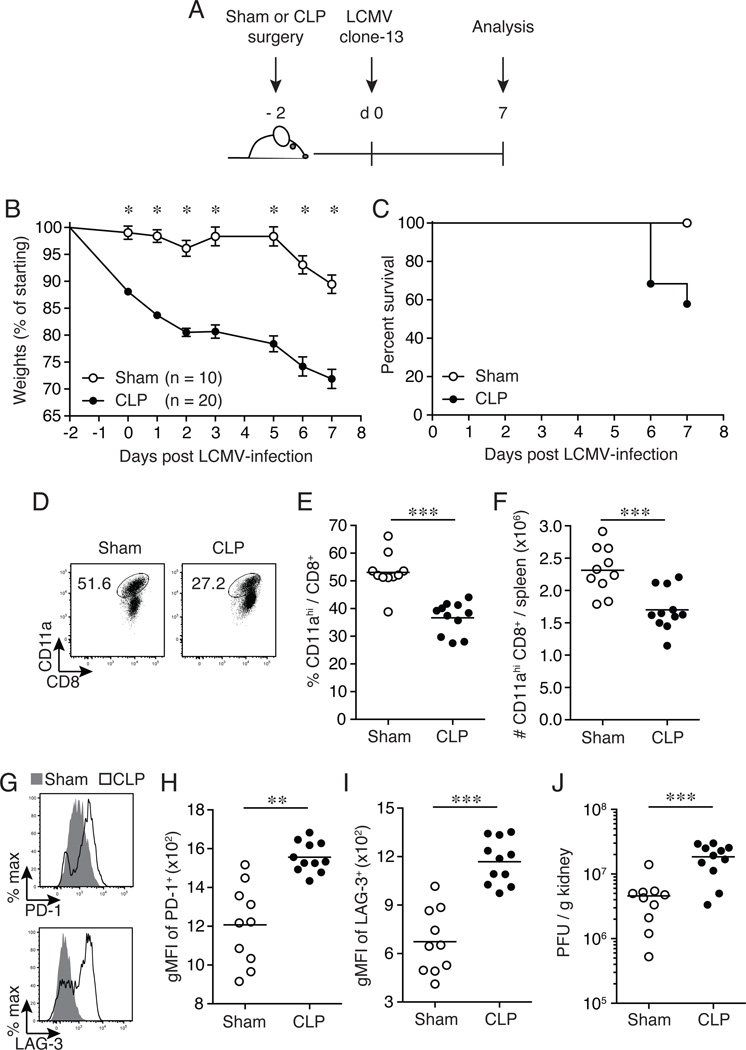
The data presented in Figs 1–2 were generated utilizing inbred C57BL/6 mice and demonstrated that septic mice are more susceptible to chronic viral infection and that CD8+ T cell exhaustion occurs at an accelerated rate. To extend our analysis and verify the results obtained in inbred mice, we performed similar experiments in which endogenous CD8+ T cell responses to chronic LCMV infection were analyzed in sham- and CLP-treated cohorts of outbred NIH Swiss mice. Recently, we demonstrated that all CD8+ T cells responding to pathogen-derived Ag could be detected using a surrogate activation marker approach (up-regulation of CD11a and down-regulation of CD8α surface expression on CD8+ T cells), which enabled identification and analysis of polyclonal CD8+ T cell responses without a priori knowledge of specific epitopes or MHC restriction elements (53, 57–59). CLP- or sham-treated NIH Swiss mice were infected with LCMV clone-13 (2 × 106 PFU/mouse; i.v.) on day 2 post-surgery and morbidity and mortality was monitored for 7 days post-infection (Fig. 3A). CLP-treated mice were highly susceptible to chronic LCMV clone-13 infection as demonstrated by substantial weight loss (~28% weight loss; Fig. 3B) and increased mortality (~55% survival rate; Fig. 3C) compared to sham-treated infected mice (~10% weight loss; Fig. 3B and 100% survival rate; Fig. 3C). Because of the increased susceptibility in the CLP-treated infected NIH Swiss mice CD8+ T cell analysis was performed on day 7 post-LCMV clone-13 infection, as the majority of these mice would not have survived to the day 8 post-infection time point. LCMV clone-13 infected CLP-treated outbred mice displayed decreased frequency (Fig. 3D, 3E) and number (Fig. 3F) of splenic CD11ahi CD8αlo CD8+ T cells compared to sham-treated mice. Furthermore, CLP-treated mice had a significant increase in inhibitory molecule PD-1 (Fig. 3G–H) and LAG-3 (Fig. 3G, 3I) surface expression on splenic CD11ahi CD8αlo CD8+ T cells compared to sham-treated infected controls. Finally, a significant (4-fold) increase in viral burden was detected in the kidneys from LCMV clone-13 infected CLP-treated NIH Swiss mice compared to sham-treated controls (Fig. 3J). These results demonstrate that a septic episode induced in outbred mice significantly increases their susceptibility to chronic viral infection, compromises the host’s ability to mount optimal CD8+ T cell mediated-immunity and decreases pathogen control as observed in inbred septic animals. Collectively, our findings suggest that septic individuals may be more vulnerable to chronic infections and at higher risk of developing exhausted CD8+ T cells.
Figure 3.
Outbred septic mice exhibit increased susceptibility and CD8+ T cell exhaustion. (A) Experimental model. Two days prior to LCMV clone-13 infection (2 × 106 PFU/mouse; i.v.), CLP or sham surgery was performed on outbred NIH Swiss mice. On day 7 post-LCMV clone-13 infection splenocytes and kidneys were harvested for analysis and viral titers, respectively. (B) Morbidity and (C) survival rate were analyzed on indicated days post-CLP or sham surgery. (D) Representative flow cytometry plots illustrating expression of CD11ahi CD8αlo CD8+ T cells. Numbers within plots represent the frequency of CD11ahi CD8αlo expression of CD8+ T cells. Summary data of (E) frequency and (F) total number of CD11ahi CD8αlo CD8+ T cells. (G) Representative histograms illustrating inhibitor molecule PD-1 (top panel) and LAG-3 (bottom panel) expression gated on CD11ahi CD8αlo CD8+ T cells. Sham depicted with gray histograms and CLP depicted with open histograms. Summary data of geometric mean fluorescence intensity (gMFI) of inhibitory molecule of (H) PD-1 and (I) LAG-3 expression. (J) Viral titers were determined from the kidneys from day 7 post-LCMV clone-13 infection. Dots represent individual mice. Data analyzed by two-tailed, unpaired Student t test (10–20 mice/group). Data analyzed in survival curve by Gehan-Breslow-Wilcoxon test, p = 0.0201. Data are representative of two independent and similar experiments. * p < 0.05, ** p < 0.01, *** p < 0.0001.
The timing of sepsis induction impacts the rate of CD8+ T cell exhaustion
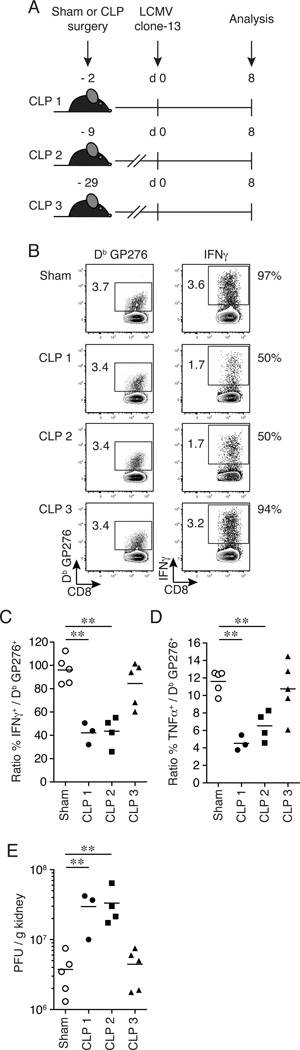
The data presented in Figures 1–3 were all generated when CLP-treated mice were infected with LCMV clone-13 on day 2 post-surgery. To examine the extent to which the time of secondary infection after septic insult impacts CD8+ T cell exhaustion, CLP-treated mice were infected with LCMV clone-13 (2 × 106 PFU/mouse; i.v.) on various days (CLP 1 = 2 days; CLP 2 = 9 days; CLP 3 = 29 days) post-surgery and the endogenous GP276-specific CD8+ T cell response was examined in the spleen on day 8 post-infection (Fig. 4A). Of note, while uninfected CLP mice exhibited weight loss within the first week after surgery, they were able to regain their weight to pre-surgery levels in one to two weeks and had weights above the starting weight at the time of LCMV clone-13 infection (Supplemental Fig. 2 and (41)). The functionality of Ag-specific CD8+ T cells was determined indirectly on a per-cell basis by calculating the frequency of Db GP276-specific CD8+ T cells capable of producing cytokine (IFNγ, TNFα; Fig. 4B–D). The majority of Db GP276-specific CD8+ T cells from sham-treated infected mice were capable of producing IFNγ (~97% functional upon stimulation; Fig. 4B–C). In contrast, CLP-treated infected mice from the CLP 1 and CLP 2 sepsis groups had a significant reduction in the ability of Db GP276-specific CD8+ T cells to produce IFNγ (~50% functional upon stimulation) compared to sham-treated mice. Interestingly, the majority of Db GP276-specific CD8+ T cells from the CLP 3 sepsis group were able to produce IFNγ (~94% functional upon stimulation; Fig 4-BC). Similar results were observed when assessing the ability of Db GP276-specific CD8+ T cells to produce TNFα from all sepsis groups compared to sham mice (Fig. 4D). Consistent with the functional data, there was a significant (8-fold and 8.8-fold) increase in viral burden detected in the kidneys from LCMV clone-13 infected CLP-treated mice from the CLP 1 and CLP 2 sepsis groups compared to sham-treated mice, respectively (Fig. 4E); however, no difference was detected in LCMV clone-13 viral burden from the CLP 3 sepsis group compared to shams (Fig 4E). Together, these results demonstrate that the early events after sepsis (~10 days post-insult) have the most impact on CD8+ T cell exhaustion when CD8+ T cell responses are examined at an early time point (on day 8) post-LCMV clone-13 infection.
Figure 4.
CD8+ T cell exhaustion is pronounced early after septic insult. (A) Experimental model. Two days (CLP 1), 9 days (CLP 2) or 29 days (CLP 3) prior to LCMV clone-13 infection (2 × 106 PFU/mouse; i.v.), CLP or sham surgery was performed on inbred C57BL/6 (Thy1.2/1.2) mice. On day 8 post-LCMV clone-13 infection splenocytes and kidneys were harvested for analysis and viral titers, respectively. (B) Representative flow cytometry plots illustrating expression of tetramer+ GP276-specific CD8+ T cells (left panels) and IFNγ+ (right panels) of CD8+ T cells. Numbers within plots represent the frequency of tetramer+ GP276-specific CD8+ T cells and IFNγ+ of CD8+ T cells, respectively. Numbers right of plots represent the percentage of tetramer+ GP276-specific CD8+ T cells able to produce cytokine (IFNγ). Summary data of the percentage of (C) IFNγ and (D) TNFα producing CD8+ T cells of tetramer+ GP276-specific CD8+ T cells. (E) Viral titers were determined from the kidneys from day 8 post-LCMV clone-13 infection. Dots represent individual mice. Data analyzed by two-tailed, unpaired Student t test (3–5 mice/group). Data are representative of two independent and similar experiments. ** p < 0.01.
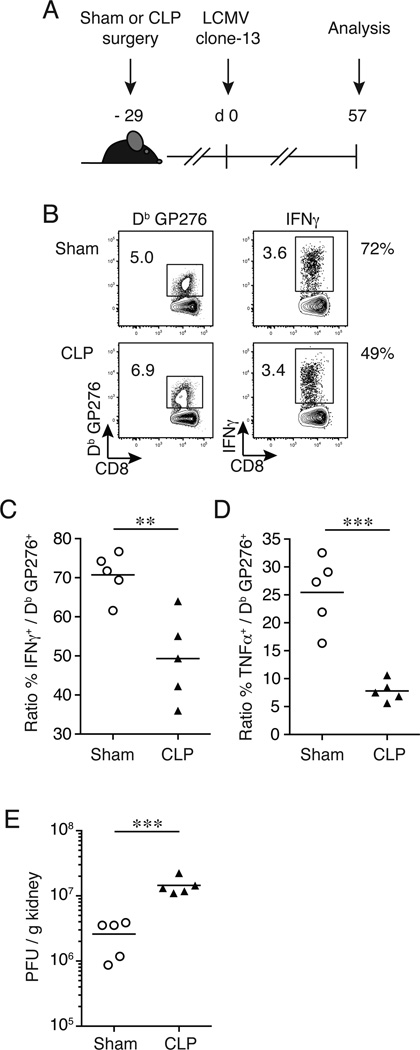
The results in Fig. 4 suggested that LCMV clone-13 infection on day 29 post-surgery (CLP 3 sepsis group) had little impact on CD8+ T cell exhaustion compared to sham-treated controls when CD8+ T cell responses were examined early post-LCMV chronic infection. However, this snapshot analysis performed during the expansion phase of virus-specific CD8+ T cell responses does not exclude the possibility that ‘the path’ or kinetics of exhaustion differs in septic and control groups of mice following chronic LCMV infection. To investigate if CD8+ T cell exhaustion progressed at different rates, CLP-treated mice were infected with LCMV clone-13 (2 × 106 PFU/mouse; i.v.) on day 29 post-surgery and the endogenous Db GP276-specific CD8+ T cell response was examined in the spleen ~2 months post-infection (Fig. 5A). As expected, the ability of Db GP276-specific CD8+ T cells to produce IFNγ after peptide stimulation was reduced in sham-treated mice when responses at days 8 and 57 post-LCMV infection were compared (~94% and 72%, respectively; Fig. 4B, 4C and 5B, 5C). Interestingly, CLP-treated infected mice had a significant reduction in the ability of Db GP276-specific CD8+ T cells to produce IFNγ (~50% functional upon stimulation; Fig. 5B, 5C) compared to sham-treated infected controls at day 57 post LCMV challenge. A significant difference was also observed in Db GP276-specific CD8+ T cells capable of producing TNFα (Fig. 5D). Finally, a significant (5.6-fold) increase in LCMV clone-13 viral burden was detected in the kidneys from CLP-treated mice 2-months post-LCMV infection compared sham-treated mice (Fig. 5E). These results demonstrate that exacerbated CD8+ T cell exhaustion is delayed late after sepsis induction but still occurs at an accelerated rate when CD8+ T cell responses are examined at a later time point post-LCMV clone-13 infection. Collectively, these results suggest that the initial septic insult has sustained negative effects on CD8+ T cell functionality and indicates that sepsis survivors may be at a higher risk of developing exhausted CD8+ T cells upon encountering a subsequent chronic infection. It is important to note that the CLP-treated mice were able to regain their weigh to pre-surgery levels in one to two weeks after surgery (Supplemental Fig. 2 and (41)), suggesting that the increased T cell exhaustion after LCMV- clone-13 infection was not simply due to overall poor health of the septic mice, but instead represent long-term effects on CD8 T cell biology.
Figure 5.
CD8+ T cell exhaustion is delayed late after sepsis induction. (A) Experimental model. Twenty-nine days prior to LCMV clone-13 infection (2 × 106 PFU/mouse; i.v.), CLP or sham surgery was performed on inbred C57BL/6 (Thy1.2/1.2) mice. Two months post-LCMV clone-13 infection splenocytes and kidneys were harvested for analysis and viral titers, respectively. (B) Representative flow cytometry plots illustrating expression of tetramer+ GP276-specific CD8+ T cells + (left panels) and IFNγ+ (right panels) of CD8+ T cells. Numbers within plots represent the frequency of tetramer+ GP276-specific CD8+ T cells and IFNγ+ of CD8+ T cells, respectively. Numbers right of plots represent the percentage of the tetramer+ GP276-specific CD8+ T cells able to produce IFNγ. Summary data of the percentage of (C) IFNγ and (D) TNFα producing CD8+ T cells of tetramer+ GP276-specific CD8+ T cells. (E) Viral titers were determined from the kidneys from day 57 post-LCMV clone-13 infection. Dots represent individual mice. Data analyzed by two-tailed, unpaired Student t test (5 mice/group). Data are representative of two independent and similar experiments. ** p < 0.01, *** p < 0.0001.
Simultaneous PD-L1 and LAG-3 blockade improves CD8+ T cell function in chronically infected septic mice
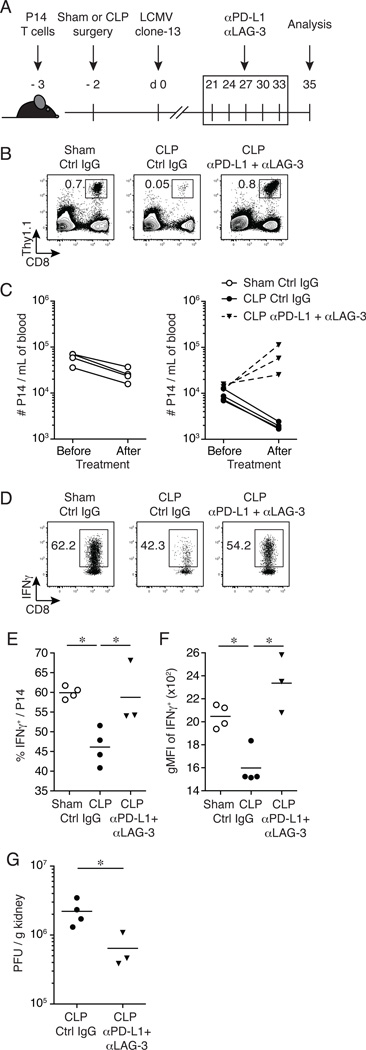
All the data generated thus far have demonstrated that a prior septic event increases the susceptibility of mice to subsequent chronic viral infection and exacerbates CD8+ T cell exhaustion. Therapeutic dual-blockade of inhibitory molecule pathways with αPD-L1 and αLAG-3 blocking antibodies has a synergistic effect in improving CD8+ T cell function in the setting of chronic infections (39, 55). To determine the extent to which CD8+ T cell function could be improved in LCMV clone-13 infected septic mice, a therapeutic inhibitory molecule dual-blockade approach was used. Naïve P14 CD8+ T cells (Thy1.1/1.1; TCR-transgenic CD8+ T cells specific for the GP33 epitope of LCMV) were adoptively transferred into naïve C57BL/6 (Thy1.2/1.2) recipients prior to surgery. Since Thy1.1 expression on T cells is stable and is not influenced during infection and/or Ag recognition, the functionality (ability to produce effector cytokines upon cognate Ag encounter) on Thy1.1 positive virus-specific CD8+ T cells can be assessed directly. One day after adoptive transfer, CLP or sham surgery was performed, and mice were subsequently infected with LCMV clone-13 (2 × 106 PFU/mouse; i.v.) on day 2 post-surgery. Dual-blockade therapy (αPD-L1 + αLAG-3) commenced on day 21 post-infection (a time when chronic infection is established), mice were treated every 3 days for 2 weeks (total of 5 treatments) and CD8+ T cell analyses were performed in the spleen 2 days after the last treatment (day 35 post-infection; Fig. 6A). Prior to dual-blockade therapy, PD-1 expression and P14 CD8+ T cell function (IFNγ production) was evaluated in the peripheral blood on day 14 post-infection (Supplemental Fig. 3). P14 CD8+ T cells from LCMV clone-13 infected CLP-treated mice had a significant increase in PD-1 expression (Supplemental Fig. 3A) compared to sham-treated mice. Importantly, CLP-treated mice had a significant reduction in the frequency (Supplemental Fig. 3B) and total number (Supplemental Fig. 3C) of P14 CD8+ T cells after ex vivo peptide stimulation compared to sham-treated mice. In addition, the capacity of P14 CD8 T cells to produce IFNγ was also reduced in CLP-treated mice (Supplemental Fig. 3D). These data indicate that similar to the experiments when endogenous GP276-specific CD8+ T cell responses were analyzed, CD8+ T cell exhaustion also occurs in GP33-specific TCR-transgenic CD8+ T cells from septic mice.
Figure 6.
αPD-L1 and αLAG-3 dual mAb blockade improves CD8+ T cell function and pathogen control in chronically infected septic mice. (A) Experimental model. Naïve P14 CD8+ T cells (Thy1.1/1.1 or Thy1.1/1.2) were adoptively transferred (103 cells/mouse) into naïve inbred C57BL/6 (Thy1.2/1.2) mice before surgery. Two days prior to LCMV clone-13 infection (2 × 106 PFU/mouse; i.v.), CLP or sham surgery was performed. On day 21 post-LCMV clone-13 infection dual mAb blockade commenced (200µg αPD-L1 + 200µg αLAG-3/mouse i.p. or PBS/rIgG for untreated controls) and mice were treated every 3rd day for 2 weeks (total of 5 treatments). Two days after the last treatment splenocytes and kidneys were harvested for analysis and viral titers, respectively. (B) Representative flow cytometry plots illustrating the frequency of P14 (Thy1.1+) CD8+ T cells from the peripheral blood on day 35 post-LCMV clone-13 infection. (C) Summary data of the number of P14 CD8+ T cells from the peripheral blood before and after dual mAb blockade. (D)Ex-vivo GP33-peptide stimulation of splenocytes on day 35 post-LCMV clone-13 infection. Representative flow cytometry plots illustrating expression of IFNγ+ of CD8+ T cells gated on P14 Thy1.1+ CD8+ T cells. Summary data of (E) frequency of IFNγ+ P14 CD8+ T cells and (F) geometric mean fluorescence intensity (gMFI) of IFNγ+ expression. (G) Viral titers were determined from the kidneys from day 35 post-LCMV clone-13 infection. Dots represent individual mice. Data analyzed by two-tailed, unpaired Student t test (3–4 mice/group). Data are representative of three independent and similar experiments. * p < 0.05.
Following dual-blockade therapy (αPD-L1 + αLAG-3) the frequency (Fig. 6B) and number (Fig. 6C) of P14 CD8+ T cells in the peripheral blood increased in infected CLP-treated mice (16-fold and 4.7-fold, respectively), compared to infected CLP-treated mice that received isotype control antibody. Upon ex vivo GP33-peptide stimulation, P14 CD8+ T cell function improved in infected CLP-treated mice that received dual-blockade exhibited by an increase in frequency (~60% functional upon stimulation; Fig. 6D–E) and amount (Fig. 6F) of IFNγ produced compared to infected CLP-treated mice that received control antibody. Of note, infected CLP-treated mice that did not receive dual-blockade therapy displayed a decline in frequency (Fig. 6B) and number (Fig. 6C) of P14 CD8+ T cells in the peripheral blood (14-fold and 4.5-fold, respectively) as well as a decrease in frequency (~45% functional upon stimulation; Fig. 6D–E) and amount (Fig. 6F) of IFNγ produced following ex vivo GP33-peptide stimulation in the spleen compared to infected sham-treated mice. Finally, a significant (3.4-fold) decrease in LCMV clone-13 viral burden was detected in the kidneys from infected CLP-treated mice that received dual-blockade therapy compared (Fig. 6G) to infected CLP-treated mice that received isotype control antibody. These results demonstrate that inhibitory molecule dual-blockade therapy in chronically infected septic animals increases the number of circulating CD8+ T cells, improves CD8+ T cell function and pathogen control and highlights a potential therapeutic strategy to improve disease outcome.
Discussion
Annually, over 1.6 million hospital cases in the United States are the result of sepsis (1, 2). Sepsis survivors experience long-term negative effects post-sepsis and are at higher risk of acquiring secondary infections that are normally controlled with a functioning immune system (3, 6, 7, 9). Experimental “two-hit” mouse models of sepsis have focused on immune dysfunction post-sepsis to opportunistic pathogens such as Aspergillus, Streptococcus, Pseudomonas and Candida (60–63). Despite these models, there remains a knowledge gap in understanding the immune response to viral infections post-sepsis. In particular, the impact of sepsis on CD8+ T cell responses to chronic viral infection is currently poorly understood. In this study we used a mouse model of poly-microbial sepsis to investigate the primary Ag-specific CD8+ T cell response post-sepsis to chronic viral infection. Our data demonstrate that mice are more susceptible to chronic viral infection after a septic event and CD8+ T cell exhaustion occurs at an accelerated rate in LCMV clone-13 infected septic animals.
The CLP mouse model of sepsis is considered the “gold standard” in experimental sepsis research; however, the septic injury induced varies amongst research groups making data comparisons challenging. Despite the variation of sepsis induction the majority of studies to date demonstrate that a prior septic event increases the susceptibility of mice to secondary infections (60–63). For instance, Muenzer et al. demonstrated that CLP-treated mice had increased mortality when subsequently infected with Streptococcus pneumonia or Pseudomonas aeroginosa (95% and 85% mortality rate, respectively) on day 3 post-surgery compared to S. pneumonia or P. aeroginosa alone (20% mortality rate for both pathogens) (61). Davis et al. demonstrated that CLP-treated mice had increased susceptibility to Candida albicans infection when infected on day 2 post-surgery compared to C. albicans infection alone (91% vs. 11% mortality rate, respectively) (63). Finally, Benjamim et al. also demonstrated that CLP-treated mice infected on day 3 post-surgery succumbed to Aspergillus fumigatus infection compared to sham-treated infected mice (100% vs. 0% mortality rate, respectively) (60). Our findings showed that both inbred C57BL/6 and outbred NIH Swiss CLP-treated mice infected on day 2 post-surgery were more susceptible to LCMV clone-13 infection compared to infected sham-treated mice. In particular, outbred NIH Swiss septic mice were highly vulnerable to LCMV clone-13 infection with 55% survival rate by day 7 post-infection. We have also observed that septic animals infected on day 2 or day 30 post-surgery are highly susceptible to malaria with CLP-treated mice succumbing to Plasmodium yoelii infection compared to P. yoelii infection alone (SA Condotta et al. unpublished observations). Together these data demonstrate, that despite the variability in sepsis induction, that sepsis compromises the overall health of the host leading to increased vulnerability to subsequent infections. However, the potential mechanism(s) for how CLP induction is altering host susceptibility and CD8 T cell responses to chronic viral infection is currently unknown. Interestingly, early treatment with a high dose antibiotic Primaxin ameliorates, but does not prevent, observed exacerbation of CD8 T cell exhaustion after polymicrobial sepsis induction (Khan and Badovinac, unpublished observation), suggesting that decreasing the duration and/or severity of bacterial infection might represent a useful approach in deciphering the factors that control overall susceptibility of a septic host to secondary (unrelated) bacterial or viral infections.
Chronic infections such as Hepatitis B virus, Hepatitis C virus, HIV and protozoan infections (e.g., malaria) affect approximately 10% of the world’s population (64–67). As infection persists during chronic infections, CD8+ T cells progress through stages of dysfunction with a temporal hierarchical loss of functional properties (30, 36, 37, 55). T cell exhaustion has been reported in humans with chronic infections of Hepatitis B virus, Hepatitis C virus, HIV and malaria (55, 68–71). In mouse models of chronic infection, LCMV clone-13 has been extensively utilized as a model pathogen to study CD8+ T cell exhaustion. Wherry et al. demonstrated that during LCMV clone-13 infection the subdominant GP276-specific CD8+ T cell response becomes the dominant population. They showed that on day 8 post-infection the majority of GP276-specific CD8+ T cells from LCMV clone-13 infected mice were able to make IFNγ upon peptide stimulation (~97% functional). However, the capacity to produce IFNγ upon peptide stimulation was diminished on day 30 post-LCMV clone-13 infection (30). Using the established mouse model of CD8+ T cell exhaustion, our data demonstrated that as early as day 8 post-infection GP276-specific CD8+ T cells from LCMV clone-13 infected septic mice lost the ability to produce IFNγ upon peptide stimulation compared to infected sham-treated mice (~43–50% vs. ~86–97% functional, respectively). Additionally, even when septic mice were infected at a time point of sepsis resolution (day 29 post-surgery) CD8+ T cell exhaustion was still accelerated in chronically infected septic mice compared to sham infected controls (50% vs. 72% functional, respectively). Our findings suggest that not only does sepsis increase the susceptibility of mice to chronic viral infection, but it also exacerbates CD8+ T cell exhaustion. It is important to note that this type of analysis has not been done in the setting of sepsis before and our data indicates that sepsis survivors may be at higher risk of developing highly exhaustible CD8+ T cells upon subsequent chronic infection.
While direct secondary viral infection after sepsis is rare, there is a considerable amount of literature describing viral reactivation (e.g., CMV, EBV, or HSV) in the wake of a septic event. The recent publication by Walton et al. (13) presented data showing a strong correlation between viral reactivation and susceptibility to secondary opportunistic bacterial or fungal infections. It is tempting to speculate that the increased susceptibility to these unrelated secondary opportunistic infections is related to the CD8+ T cell exhaustion resulting from chronic viral infection. Recently, we reported that sepsis has long-lasting consequences on the ability of naïve (antigen non-experienced) and memory CD8 T cells to respond to their cognate antigen delivered in the context of newly encountered or repeated infections (41, 59). Thus, a clear and rigorous investigation of the consequences of viral infection after a septic event on the immune system (specifically the ability of non-virus specific naïve and/or memory CD8 T cell responses) becomes quite important and studies will be designed to experimentally address this concept in the future.
Simultaneous inhibitory molecule blockade therapy has been successful in improving CD8+ T cell function in a setting of chronic infection (39, 55). Blackburn et al. demonstrated that co-blockade with αPD-L1 and αLAG-3 blocking antibodies in vivo worked synergistically, increasing the number of GP276-specific CD8+ T cells 5-fold in the peripheral blood and spleen, improving CD8+ T cell function (~80% functional upon stimulation) and reducing viral burden in LCMV clone-13 chronically infected mice compared to isotype control treated mice (39). Butler et al. demonstrated in a mouse model of malaria that dual-blockade with αPD-L1 and αLAG-3 resulted in immediate control of P. yoelii parasite burden, accelerated parasite clearance and improved T cell responses (55). Our data showed that co-administration of αPD-L1 and αLAG-3 blocking antibodies improved CD8+ T cell function (~60% functional upon stimulation) and reduced LCMV clone-13 viral burden in chronically infected septic animals compared to isotype control treated septic mice. These data suggest that exhausted CD8+ T cell function can be improved with therapeutic co-administration of αPD-L1 and αLAG-3 blocking antibodies in chronically infected septic animals. Our findings also illustrate a potential therapeutic strategy to improve CD8+ T cell function in sepsis survivors that have developed exhausted CD8+ T cells from subsequent chronic infections. Finally, our data are in agreement with recent data obtained in human and mouse models of poly-microbial sepsis (72–74) that showed that PD-1 blockade increased survival and lymphocyte function further illustrating the therapeutic potential of inhibitory molecule blockade treatments in septic survivors in the presence or absence of secondary infection.
In summary, we provide evidence that a prior septic event compromises the overall susceptibility of the host to chronic viral infection leading to sustained morbidity, decreased survival and reduced pathogen control. Furthermore, our findings demonstrate that CD8+ T cell exhaustion occurs at an accelerated rate following chronic infection in septic animals. Together, our data suggests that sepsis patients and/or sepsis survivors may be more vulnerable and predisposed to developing exhausted CD8+ T cell when encountering chronic infections. An increased understanding of the immunological consequences of sepsis will help design therapies to improve post-septic patient outcome.
Supplementary Material
Acknowledgements
We like to thank Stacey Hartwig and Dr. Steven M. Varga for LCMV clone-13 virus. Lecia Epping and Dr. John T. Harty for αLAG-3 antibody. We also thank members of the Badovinac Lab and Dr. Martin J. Richer for helpful discussion.
Abbreviations
- CD
cluster of differentiation
- CLP
cecal ligation and puncture
- gMFI
geometric mean fluorescence intensity
- LAG-3
lymphocyte-activation gene 3
- LCMV
Lymphocytic choriomeningitis virus
- PD-1
programmed cell death 1
Footnotes
Supported by National Institutes of Health Grants AI114543 (V.P.B.), P30CA086862, and T32 AI007485, U.S. Department of Veterans Affairs Merit Review Award (T.S.G.), and an award from American Hearth Association (S.A.C.)
References
- 1.Elixhauser A, Friedman B, Stranges E. Septicemia in U. S. Hospital, 2009. Rockville, MD: 2011. A. f. H. R. a. Quality, ed. [PubMed] [Google Scholar]
- 2.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: A challenge for patients and hosptials. Hyattsville, MD: 2011. N. C. f. H. Statistics. [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 4.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 10.Cook CH, Zhang Y, McGuinness BJ, Lahm MC, Sedmak DD, Ferguson RM. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J Infect Dis. 2002;185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 11.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 12.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, Green JM, Storch GA, Hotchkiss RS. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063. [PubMed] [Google Scholar]
- 15.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 16.Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 17.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 22.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 23.Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- 24.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Badovinac VP, Harty JT. CD8(+) T-cell homeostasis after infection: setting the ‘curve’. Microbes Infect. 2002;4:441–447. doi: 10.1016/s1286-4579(02)01558-7. [DOI] [PubMed] [Google Scholar]
- 27.Muckenfuss RS, Armstrong C, Webster L. Etiology of the 1933 epidemic of encephalitis. JAMA. 1934;103:731–733. [Google Scholar]
- 28.Armstrong C, Dickens PF. Benign lymphocytic choriomeningitis (acute aspetic meningitis) Pub. Health Rep. 1935;50:831–842. [Google Scholar]
- 29.Beeman EA. Charles Armstrong MD: A Biography. Bethesda, MD, USA: National Institutes of Health; 2007. [Google Scholar]
- 30.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvato M, Shimomaye E, Southern P, Oldstone MB. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, Clone 13 (CTL-) Virology. 1988;164:517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- 33.Matloubian M, Somasundaram T, Kolhekar SR, Selvakumar R, Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J Exp Med. 1990;172:1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvato M, Borrow P, Shimomaye E, Oldstone MB. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 38.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 39.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22:13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- 41.Condotta SA, Rai D, James BR, Griffith TS, Badovinac VP. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J Immunol. 2013;190:1991–2000. doi: 10.4049/jimmunol.1202379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pircher H, Baenziger J, Schilham M, Sado T, Kamisaku H, Hengartner H, Zinkernagel RM. Characterization of virus-specific cytotoxic T cell clones from allogeneic bone marrow chimeras. Eur J Immunol. 1987;17:159–166. doi: 10.1002/eji.1830170202. [DOI] [PubMed] [Google Scholar]
- 43.Pircher H, Michalopoulos EE, Iwamoto A, Ohashi PS, Baenziger J, Hengartner H, Zinkernagel RM, Mak TW. Molecular analysis of the antigen receptor of virus-specific cytotoxic T cells and identification of a new V alpha family. Eur J Immunol. 1987;17:1843–1846. doi: 10.1002/eji.1830171226. [DOI] [PubMed] [Google Scholar]
- 44.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 45.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 47.Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starbeck-Miller GR, Badovinac VP, Barber DL, Harty JT. Cutting edge: Expression of FcgammaRIIB tempers memory CD8 T cell function in vivo. J Immunol. 2014;192:35–39. doi: 10.4049/jimmunol.1302232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurung P, Rai D, Condotta SA, Babcock JC, Badovinac VP, Griffith TS. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J Immunol. 2011;187:2148–2154. doi: 10.4049/jimmunol.1101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cabrera-Perez J, Condotta SA, James BR, Kashem SW, Brincks EL, Rai D, Kucaba TA, Badovinac VP, Griffith TS. Alterations in Antigen-Specific Naive CD4 T Cell Precursors after Sepsis Impairs Their Responsiveness to Pathogen Challenge. J Immunol. 2015;194:1609–1620. doi: 10.4049/jimmunol.1401711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badovinac VP, Harty JT. Intracellular staining for TNF and IFN-gamma detects different frequencies of antigen-specific CD8(+) T cells. J Immunol Methods. 2000;238:107–117. doi: 10.1016/s0022-1759(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 55.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nolz JC, Rai D, Badovinac VP, Harty JT. Division-linked generation of death-intermediates regulates the numerical stability of memory CD8 T cells. Proc Natl Acad Sci U S A. 2012;109:6199–6204. doi: 10.1073/pnas.1118868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6:e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9:451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J Immunol. 2014;192:3618–3625. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol. 2003;163:2605–2617. doi: 10.1016/S0002-9440(10)63615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock. 2006;26:565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 62.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O’Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis CG, Chang K, Osborne D, Walton AH, Dunne WM, Muenzer JT. Increased susceptibility to Candida infection following cecal ligation and puncture. Biochem Biophys Res Commun. 2011;414:37–43. doi: 10.1016/j.bbrc.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO. Hepatitis B. Fact sheet #204. 2014
- 65.WHO. Hepatitis C. Fact sheet #164. 2014
- 66.WHO. HIV/AIDS. Fact sheet #360. 2014
- 67.WHO. Malaria. Fact sheet #94. 2014
- 68.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 70.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 71.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 72.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, Robbins P, Ulbrandt N, Suzich J, Green J, Patera AC, Blair W, Krishnan S, Hotchkiss R. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18:R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.