Abstract
Background/Aims
Inclusion of liver grafts from cardiac death donors (CDD) would increase the availability of donor livers but is hampered by a higher risk of primary non-function. Here, we seek to determine mechanisms that contribute to primary non-function of liver grafts from CDD with the goal to develop strategies for improved function and outcome, focusing on c-Jun-N-terminal kinase (JNK) activation and mitochondrial depolarization, two known mediators of graft failure.
Methods
Livers explanted from wild-type, iNOS−/−, JNK1−/− or JNK2−/− mice after 45-min aorta clamping were implanted into wild-type recipients. Mitochondrial depolarization was detected by intravital confocal microscopy in living recipients.
Results
After transplantation of wild-type CDD livers, graft iNOS expression and 3-nitrotyrosine adducts increased, but hepatic endothelial NOS expression was unchanged. Graft injury and dysfunction were substantially higher in CDD grafts than in non-CDD grafts. iNOS-deficiency and inhibition attenuated injury and improved function and survival of CDD grafts. JNK1/2 and apoptosis signal-regulating kinase-1 activation increased markedly in wild-type CDD grafts, which was blunted by iNOS-deficiency. JNK inhibition and JNK2-deficiency, but not JNK1-deficiency, decreased injury and improved function and survival of CDD grafts. Mitochondrial depolarization and binding of phospho-JNK2 to Sab, a mitochondrial protein linked to the mitochondrial permeability transition, were higher in CDD than in non-CDD grafts. iNOS-deficiency, JNK inhibition and JNK2-deficiency all decreased mitochondrial depolarization and blunted ATP depletion in CDD grafts. JNK inhibition and deficiency did not decrease 3-nitrotyrosine adducts in CDD grafts.
Conclusion
The iNOS-JNK2-Sab pathway promotes CDD graft failure via increased mitochondrial depolarization and is an attractive target to improve liver function and survival in CDD liver transplantation.
Keywords: c-Jun N-terminal kinase, inducible nitric oxide synthase, liver transplantation, mitochondrial permeability transition, non-heart-beating donors, primary non-function, Sab
INTRODUCTION
Orthotopic liver transplantation remains the only definitive therapy for end-stage liver disease but its use is limited by a severe shortage of donor livers [1]. An urgent task is to seek more usable donor livers for transplantation. While most liver grafts are collected from brain-dead donors before cardiac death, retrieval of liver grafts from cardiac death donors (CDD) could significantly increase the availability of donor organs [2, 3].
Unfortunately, liver CDD grafts exhibit a higher risk of primary non-function (PNF) [2-4]. The mechanisms of poorer clinical outcomes of CDD grafts remain unclear but are likely linked to the extended warm ischemia before explantation [2, 3]. We showed previously that a specific inducible nitric oxide synthase (iNOS) inhibitor 1400W increased the survival of CDD grafts in rats, suggesting that reactive nitrogen species (RNS) production by iNOS plays an important role in CDD graft failure [5]. However, other studies show protection by NO precursors and NO inhalation in injury after hepatic transplantation and warm ischemia/reperfusion (I/R) [6-8]. Thus, the effects of RNS in liver I/R injury and transplantation are controversial, possibly due to different models and selectivity, toxicities, and regimens of NOS inhibitors used in various studies [6]. In this study we attempted to elucidate the role of iNOS in CDD graft injury using a more specific model, namely iNOS-deficient mice.
Preserving bioenergetic status and microcirculation is important for successful transplantation of CDD grafts [9]. Mitochondria provide >90% of the cellular ATP during aerobic liver metabolism. Therefore, recovery/maintenance of mitochondrial function is crucial for liver graft survival and proper function. Recently, growing evidence supports that the onset of the mitochondrial permeability transition (MPT) is a critical step in I/R injury [10, 11]. The MPT collapses mitochondrial membrane potential, leading to ATP depletion and oncotic cell death (necrosis). Moreover, the MPT causes mitochondrial swelling and release of pro-apoptotic factors, which trigger apoptosis [11-13]. Reactive oxygen and nitrogen species (ROS and RNS), c-Jun-N-terminal kinase (JNK) activation, p53 activation, TNFα, pyridine nucleotide oxidation, Bax translocation, Pi, and Ca2+ are linked to mitochondrial dysfunction caused by I/R and other stressors [11-16]. We showed that the MPT occurs in vivo after transplantation of liver grafts subjected to long cold storage [17]. Whether the MPT occurs in CDD grafts remains unclear. Therefore, in this study we further investigated 1) whether the MPT occurs in CDD grafts, 2) whether JNK activation leads to the MPT in CDD grafts, and 3) which subtype of JNK (JNK1 or JNK2) mediates mitochondrial depolarization in CDD grafts.
METHODS
Animals and chemicals
Sources for animals and reagents are listed in the Supplement Table S1.
Liver transplantation
Livers were explanted from male wild-type (WT, C57BL/6J), iNOS−/−, JNK1−/− and JNK2−/− mice (all on a C57BL/6J background, 9-11 weeks) with and without 45 min of aortic clamping, stored in the University of Wisconsin solution at 0-1°C for 3 h and then implanted into male WT recipients. In some studies, 1400W, a specific iNOS inhibitor, was added to the storage solution (5 μM), and SP600125, a pan-JNK inhibitor, was added to the storage solution (20 μM) [5] and/or injected into recipients (10 mg/kg, ip) immediately after implantation [18]. Detailed procedures for aortic clamping, graft harvest and transplantation are described in the Supplement.
Measurement of serum alanine aminotransferase (ALT) and total bilirubin
Blood was collected from the inferior vena cava at 18 h after implantation. Serum ALT and total bilirubin were determined using analytical kits (see Supplement Table S1) according to manufacturer's protocols.
Histology and immunohistochemical staining
Livers were collected at 18 h after sham-operation or transplantation under pentobarbital anesthesia (80 mg/kg, i.p.) for histology [19]. Using hematoxylin and eosin (H&E)-stained slides, necrotic areas were quantified by computerized image analysis [19]. Apoptosis was revealed by TUNEL staining and quantified as described elsewhere [19]. 3-Nitrotyrosine adducts were detected by immunohistochemical staining [5].
Isolation of cell fractions
Some livers were harvested 3 h after transplantation of CDD grafts and cell fractions were isolated as described in the “Supplement”.
Immunoprecipitation and immunoblotting
Immunoblotting of proteins of interest and immunoprecipitation of Sab, a mitochondrial JNK interactive protein, in liver tissue extracts and cell fractions are described in the “Supplement” [19, 20].
Intravital confocal microscopy
The MPT causes mitochondrial depolarization. Mitochondrial polarization status and cell death in livers of living recipients were detected using intravital confocal microscopy at 3 h after transplantation (see the “Supplement”) using rhodamine 123 (Rh123), a cationic fluorophore that is taken up by polarized mitochondria, and propidium iodide (PI), which labels nuclei of non-viable cells, respectively [21, 22].
Measurement of ATP
Liver tissue was snap-frozen in liquid nitrogen, and ATP in liver extracts was detected as described elsewhere [23].
Statistical analysis
Groups were compared using the Kaplan-Meier test and ANOVA as appropriate. Data shown are means±S.E.M. Numbers of animals in each group are shown in figure legends. Differences were considered significant at p<0.05.
RESULTS
Increased RNS production by iNOS in CDD grafts
iNOS was undetectable in livers from sham-operated WT and iNOS−/− mice. At 3 h after transplantation, iNOS expression increased (~17 fold) in WT CDD grafts but was absent in iNOS−/− CDD grafts (Fig. 1A,D). By contrast, eNOS was expressed equally in livers from sham-operated WT and iNOS−/− mice and WT and iNOS−/− CDD grafts (Fig. 1A).
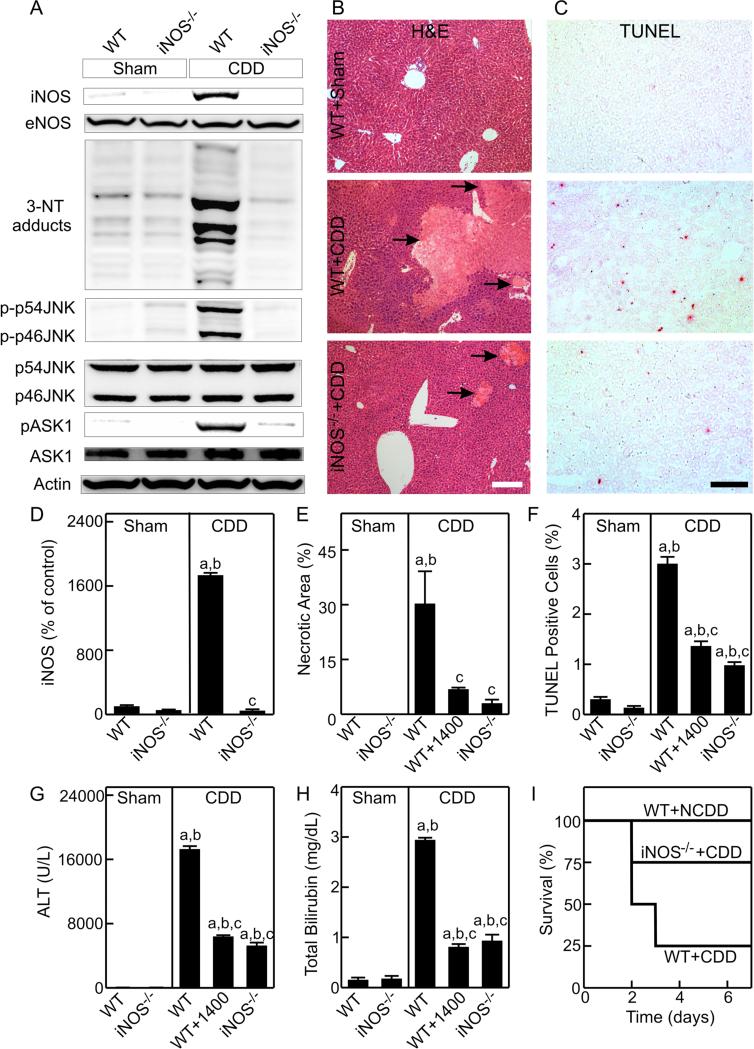
Fig. 1. iNOS deficiency and inhibition decrease RNS production, JNK activation and injury of CDD grafts.
Liver grafts were stored in UW cold storage solution with/without 1400W (1400; 5 μM). Livers were collected at 3 h after sham-operation (Sham) or transplantation of grafts from wild-type (WT) or inducible nitric oxide synthase-deficient (iNOS−/−) cardiac death donors (CDD) for immunoblotting or at 18 h for histology. A: representative immunoblot images of iNOS and eNOS, 3-nitrotyrosine (3-NT), phosphorylated and unphosphorylated c-Jun-N-terminal kinase1/2 (p-p46 JNK and p-p54 JNK) and apoptosis signal-regulating kinase-1 (pASK1 and ASK1); B and C: representative images of H&E stained- and TUNEL-stained slides, respectively. White bar is 100 μm. Black bar is 50 μm. Arrows identify the necrotic areas. D: quantifications of iNOS image by densitometry; E and F: quantifications of necrotic areas and TUNEL-positive cells; G and H: serum ALT and total bilirubin at 18 h after surgery. a, p<0.05 vs sham-operated WT mice; b, p<0.05 vs sham-operated iNOS−/− mice; c, p<0.05 vs WT CDD grafts (mean±SEM, n=4/group). I: 7-day survival after transplantation (n=8/group, p<0.05 by the Kaplan-Meier test).
Multiple bands of 3-nitrotyrosine adducts, an indicator of peroxynitrite formation, were detected in livers from sham-operated WT and iNOS−/− mice. The 3-nitrotyrosine adducts increased markedly in WT CDD grafts but were not increased in iNOS−/− CDD grafts (Fig. 1A).
iNOS-deficiency and inhibition mitigate injury and improve function and survival of CDD grafts
No pathological changes were observed in livers at 18 h in sham-operated WT (Fig. 1B) and iNOS−/− mice (not shown). Large areas of necrosis was observed in WT CDD grafts (~30%), which decreased to only ~3% in iNOS−/− CDD grafts (Fig. 1B,E).
TUNEL-positive cells (red nuclear staining) were rare (0.3% and 0.1%, respectively) in livers from sham-operated WT and iNOS−/− mice (Fig. 1C,F). Apoptosis increased to 3% in WT CDD grafts but was only 1% in iNOS−/− CDD grafts (Fig. 1C,F). Thus, iNOS-deficiency prevented both necrosis and apoptosis in CDD grafts.
Serum ALT increased from ~35 U/L to ~17,500 U/L in recipients of WT CDD grafts, which was blunted ~71% by iNOS-deficiency (Fig. 1G). Total bilirubin increased from 0.16 mg/dL to 2.9 mg/dL in recipients of WT CDD grafts, but was only 0.9 mg/dL in recipients of iNOS−/− CDD grafts (Fig. 1H). Importantly, iNOS-deficiency increased survival of CDD grafts from 25% to 75% (Fig. 1I). Similarly, iNOS inhibition with 1400W decreased necrosis, apoptosis and ALT release, blunted hyperbilirubinemia (Fig. 1E-H) and improved survival to 75% (p<0.05 vs WT CDD grafts, not shown) after WT CDD transplantation.
iNOS-deficiency prevents JNK activation in CDD grafts
JNK activation mediates graft non-function after long cold storage [18]. We investigated whether iNOS-deficiency affects JNK activation in CDD grafts. Phospho-JNK1/2 (phospho-p46 and phospho-p54 JNK) were barely detectable in livers from sham-operated WT and iNOS−/− mice (Fig. 1A) but both increased markedly (~17-20-fold) in WT CDD grafts 3 h after transplantation (Fig. 1A, Supplement Fig. S1A,B). By contrast, phospho-p46 and phospho-p54 JNK did not increase in iNOS−/− CDD grafts (Fig. 1A, Fig. S1A,B). Total JNK1/2 (p46- and p54-JNK) expression was similar in all groups (Fig. 1A).
Apoptosis signal-regulating kinase (ASK)-1 is a mitogen-activated protein kinase kinase kinase (MAP3K) upstream in the JNK pathway. Phospho-ASK1 was barely detectable in livers from sham-operated WT and iNOS−/− mice but increased (~12-fold) in WT CDD grafts (Fig. 1A, Fig. S1B). Increases in phospho-ASK1 were largely blunted by iNOS-deficiency (Fig. 1A).
JNK inhibition improves function and survival of CDD grafts
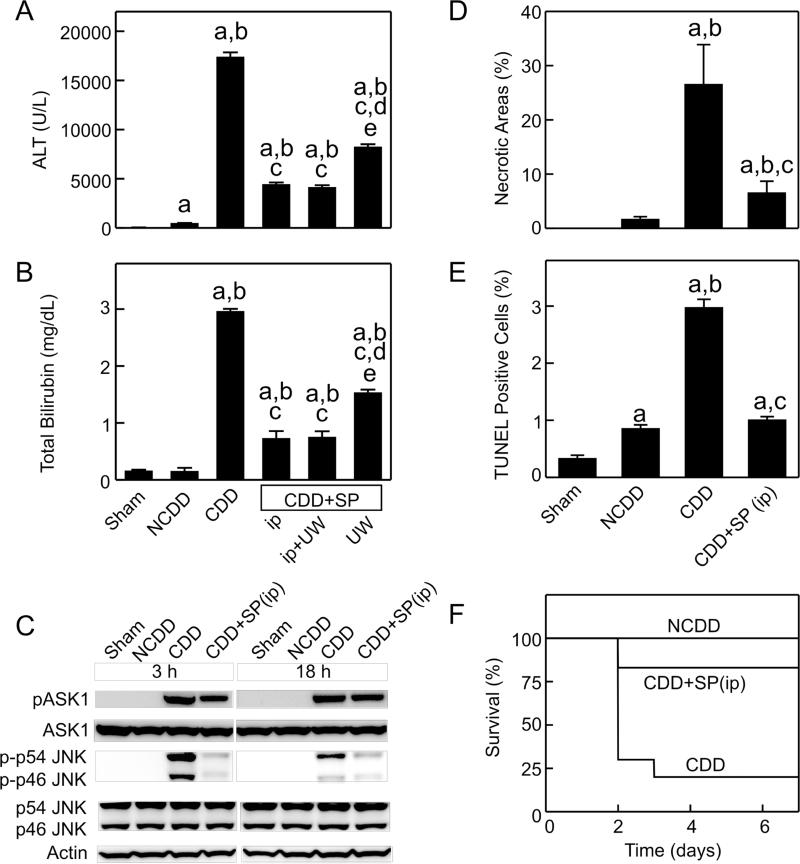
Serum ALT and bilirubin were 37-fold and 20-fold higher, respectively, in the recipients of CDD grafts than in the recipients of non-CDD grafts (Fig. 2A,B). Treatment of recipients alone with SP600125, a pan-JNK inhibitor [18], decreased ALT release and hyperbilirubinemia by 75% after transplantation of CDD grafts (Fig. 2A,B). SP600125 addition to the preservation solution alone also decreased ALT release by 52% and hyperbilirubinemia by 48% (Fig. 2A,B). Combination treatment with SP600125 (recipient injection plus addition to the preservation solution) did not produce better protection than recipient treatment alone (Fig. 2A,B). Therefore, histology was analyzed in slides from the SP600125 recipient treatment alone group. Necrotic areas, TUNEL-positive cells, and cleaved caspase-3 were higher in CDD grafts than in non-CDD grafts (Fig. 2D, Supplement Fig. S2,3). After recipient SP600125 treatment, necrosis, apoptosis and cleaved caspase-3 decreased by 75%, 63%, and 61% in CDD grafts, respectively (Figs. 2D,E and Supplement Fig. S2,3).
Fig. 2. JNK inhibition improves the outcome of transplantation of CDD grafts.
SP600125 (SP) was added to the UW cold storage solution (UW), injected (ip) immediately after transplantation or both. ALT (A) and bilirubin (B), necrotic areas (D) and TUNEL-positive cells (E) were analyzed at 18 h after sham-operation (Sham) or transplantation of grafts from wild-type cardiac-death (CDD) or non-cardiac-death donors (NCDD) (mean±SEM, n=4/group). a. p<0.05 vs sham-operation; b, p<0.05 vs NCDD; c, p<0.05 vs CDD; d, p<0.05 vs CDD+SP600125 ip; e, p<0.05 vs CDD+SP600125 ip+UW. Livers were collected at 3 and 18 h for immunoblotting (C, representative images, n=4/group). Mice were observed 7 days postoperatively for survival (F, n=6-10/group, p<0.05 by the Kaplan-Meier test).
Survival decreased from 100% to 20% after transplantation of CDD grafts (Fig. 2F). Mortality occurred mainly in the first 3 days. SP600125 treatment of recipients increased survival to 83% (Fig. 2F).
Phospho-p46 and phospho-p54 JNK and phospho-ASK1 increased to a greater extent in CDD grafts than in non-CDD grafts at 3 h after transplantation. Phospho-ASK1 remained elevated at 18 h but phospho-p46 and phospho-p54 JNK decreased (Fig. 2C). SP600125 did not alter phospho-ASK1 but markedly inhibited activation of both p46- and p54-JNK in CDD grafts (Fig. 2C).
Deficiency of JNK2, but not JNK1, improves the outcome of transplantation of CDD grafts
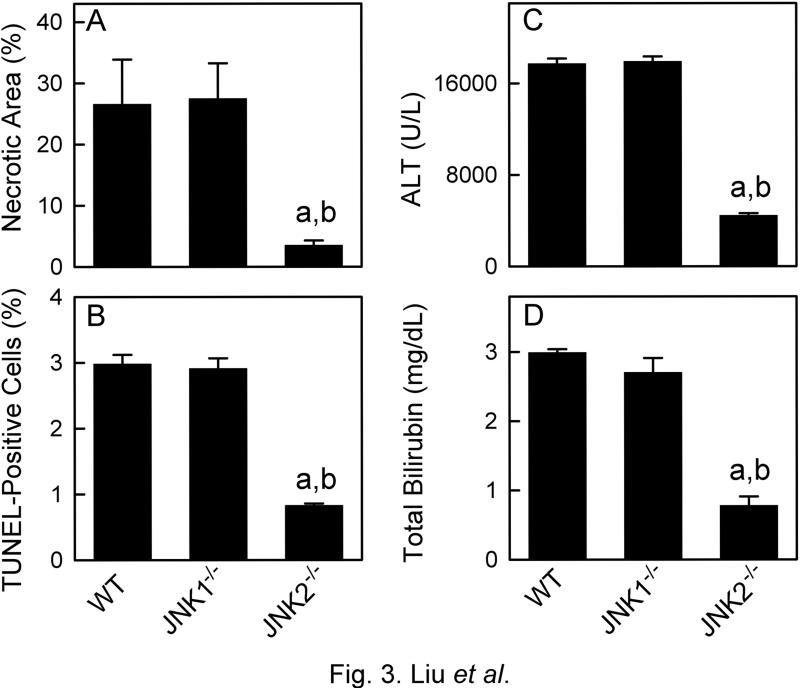
The relative importance of two JNK isoforms in CDD graft injury was investigated using JNK1−/− and JNK2−/− mice. JNK1- and JNK2-deficiency was confirmed in corresponding knockout mice by immunoblotting (not shown). No abnormal histology was observed in the livers of sham-operated JNK1−/− and JNK2−/− mice (Supplement Fig. S2). Necrotic areas, TUNEL-positive cells, ALT release and hyperbilirubinemia were all similar after transplantation of WT and JNK1−/− CDD grafts (Fig. 3, Supplement Fig. S2). By contrast, necrotic areas, TUNEL-positive cells, serum ALT and bilirubin levels were all decreased by 70-75% in the recipients of JNK2−/− CDD grafts compared to recipients of WT CDD grafts (Fig. 3). Survival was 0% in recipients of JNK1 CDD grafts (not statistically different from WT CDD grafts) but increased to 87.5% in recipients of JNK2 CDD grafts (p<0.05 vs WT CDD grafts, not shown). Thus, JNK2-deficiency, but not JNK1deficiency, attenuated CDD graft injury.
Fig. 3. Deficiency of JNK2, but not JNK1, mitigates injury in CDD grafts.
Livers and blood were collected at 18 h after transplantation of grafts from wild-type (WT), c-Jun-N-terminal kinase 1- or 2-deficient (JNK1−/−,, JNK2−/−) cardiac death donors (CDD). A and B: quantifications of necrotic areas and TUNEL-positive cells; C and D: serum ALT and total bilirubin; a, p<0.05 vs grafts from WT CDD; b, p<0.05 vs grafts from JNK1−/− CDD (mean±SEM, n=4/group).
JNK inhibition and deficiency did not alter 3-nitrotyrosine formation in CDD grafts
3-Nitrotyrosine adducts increased in parenchymal and non-parenchymal cells in WT but not in iNOS−/− CDD grafts. 3-Nitrotyrosine adducts in CDD grafts were not decreased by SP600125 or JNK1/2-deficiency (Supplement, Fig. S4).
Blockade of mitochondrial depolarization and ATP depletion in CDD grafts by JNK inhibition, iNOS-deficiency and JNK2-deficiency
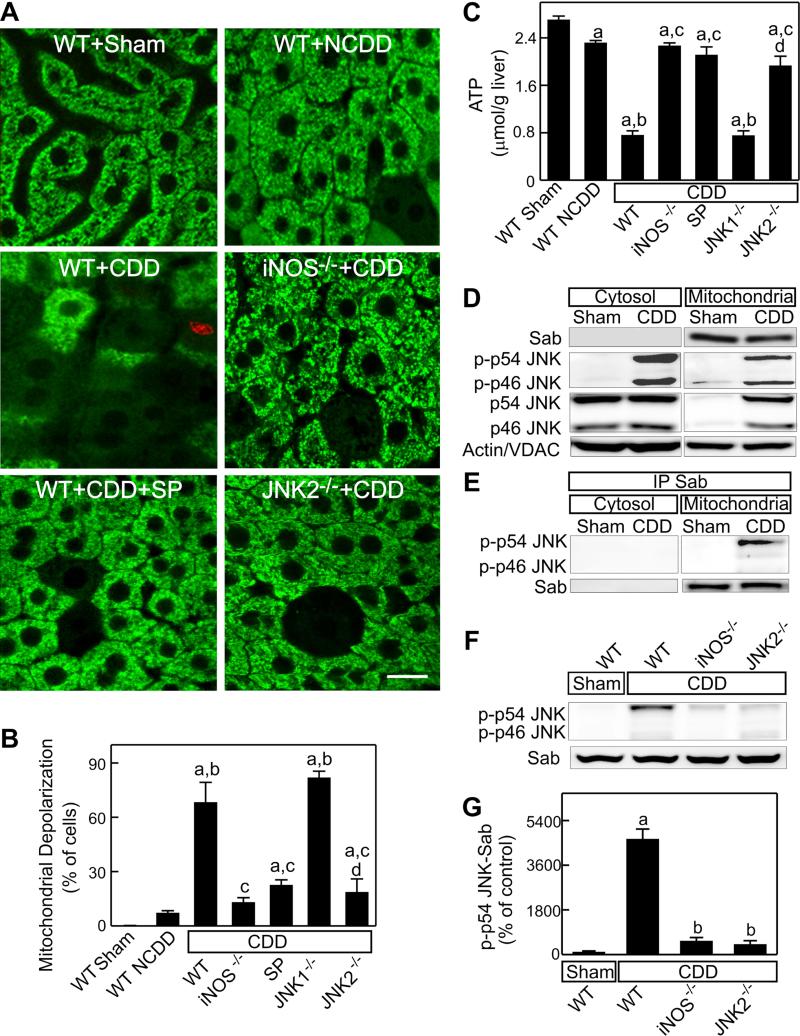
Whether the MPT occurs in CDD grafts remains unknown. Therefore, we examined mitochondrial depolarization, a consequence of the MPT, after transplantation. In livers of sham-operated WT mice, Rh123 green fluorescence was punctate in virtually all hepatocytes, signifying normal mitochondrial polarization (Fig. 4A). Mitochondrial polarization in livers of sham-operated iNOS−/−, JNK1−/− and JNK2−/− mice was similar to WT mice (not shown), whereas more diffuse and dimmer Rh123 fluorescence in hepatocytes after transplantation indicated mitochondrial depolarization (Fig. 4). Such mitochondrial depolarization occurred in ~7% hepatocytes in WT non-CDD grafts, which increased to 68% in WT CDD grafts (Fig. 4A,B). At this early stage, non-viable cells identified by red nuclear PI staining were rare, documenting that mitochondrial depolarization preceded cell death. SP600125 injection decreased mitochondrial depolarization from 68% to 23% of hepatocytes in WT CDD grafts (Fig. 4A,B). iNOS- and JNK2-deficiency also decreased mitochondrial depolarization to 13% and 19%, respectively, whereas mitochondrial depolarization in JNK1−/− CDD grafts was not different than in WT CDD grafts (Fig. 4A,B).
Fig. 4. iNOS-deficiency, JNK-inhibition and JNK2-deficiency decrease mitochondrial depolarization and ATP depletion in CDD grafts.
Mitochondrial depolarization and cell death were detected using intravital confocal microscopy of Rh123 (green) and PI (red) at 3 h after sham-operation (Sham) or transplantation of grafts from wild-type (WT), inducible nitric oxide synthase (iNOS−/−)-, c-Jun-N-terminal kinase 1- or 2-deficient (JNK1−/−,, JNK2−/−) cardiac death donors (CDD) or non-cardiac-death donors (NCDD). Some WT CDD graft recipients were given SP600125 injection (SP, 10 mg/kg, ip). A: representative intravital confocal microscopic images (bar is 10 μm); B: quantification of hepatocytes with depolarized mitochondria. Liver samples were collected at 3 h after transplantation. C: ATP in liver tissue; a, p<0.05 vs sham-operated WT mice; b, p<0.05 vs WT NCDD grafts; c, p<0.05 vs WT CDD grafts; d, p<0.05 vs grafts from JNK1−/− CDD (mean±SEM, n=4/group). D: representative immunoblot images for Sab and phospho-JNK1/2 (p-p46 JNK and p-p54 JNK) in cytosol and mitochondria (n=2 per group); VDAC: voltage-dependent anion channel (a mitochondrial protein); E: representative immunoblot images for phospho-JNK1/2 after immunoprecipitation of Sab in cytosol and mitochondria; F: representative immunoblot images for phospho-JNK1/2 after immunoprecipitation of Sab in liver tissue; G: quantification of phospho-p54 JNK coimmunoprecipitated with Sab in liver tissue. a, p<0.05 vs sham-operated WT mice; b, p<0.05 vs WT CDD grafts (n=3 per group).
ATP in liver tissue decreased ~15% in WT non-CDD grafts and ~74% in WT CDD grafts. SP600125 and deficiency of iNOS and JNK2, but not JNK1, blunted ATP depletion in CDD grafts (Fig. 4C).
JNK binding to Sab in CDD grafts: blockade by iNOS- and JNK2-deficiency
Recent studies show that activated phospho-JNK interacts with Sab (SH3BP5), a novel mitochondrial protein, to promote the MPT onset [24-27]. We explored whether JNK-Sab interactions increase in CDD grafts. Sab was expressed at mitochondria but was not detectable in the cytosol in both sham-operated livers and CDD grafts (Fig. 4D). JNK1 and 2 (p46- and p54-JNK) were expressed in the cytosol at high levels but barely detectable in mitochondria of sham-operated livers. Phospho-p46 and phospho-p54 JNK was barely detectable in the cytosol of sham-operated livers but markedly increased in the cytosol of CDD grafts (Fig. 4D). Both phospho-p46 and phosphop54 JNK translocated to mitochondria in CDD grafts (Fig. 4D). However, immunoblotting of phospho-p46 and phospho-p54 JNK after immunoprecipitation of Sab in mitochondrial fractions showed that only phospho-JNK p54 interacts with Sab (Fig. 4E). These results demonstrate that phospho-p54 JNK interaction with Sab occurs in mitochondria. Similarly, phospho-p46 and phospho-p54 JNK co-immunoprecipitation with Sab in whole liver tissue extracts was undetectable in livers from WT sham-operated mice (Fig. 4F,G). In WT CDD grafts, phosphop46 JNK co-immunoprecipitation with Sab in whole liver extracts was again barely detectable, but phospho-p54 JNK co-immunoprecipitation with Sab increased markedly (46-fold, Fig. 4F,G). This increase in phospho-p54 JNK/Sab interaction was not observed in CDD grafts from iNOS−/− and JNK2−/− mice (Fig. 4F,G), indicating that iNOS-JNK2-Sab pathway plays an important role in mitochondrial dysfunction after CDD liver transplantation.
DISCUSSION
Mitochondrial depolarization occurs in CDD grafts
Higher risk of PNF is a major barrier for use of CDD grafts [2-4]. Kupffer cell activation, parenchymal cell killing, ROS and toxic cytokine production, and increased endothelin may be involved in CDD graft failure [4, 9, 28-31]. Maintaining energy status and microcirculation is crucial for successful transplantation of CDD grafts [9, 32]. The MPT is a key player in compromising energy status [10, 11, 33] and occurs after I/R in cultured hepatocytes, in vivo hepatic warm I/R and extended cold storage/transplantation [12, 17, 21, 22]. In this study, we showed that mitochondrial depolarization increased markedly in CDD compared to non-CDD grafts (Fig. 4). This mitochondrial depolarization caused ATP depletetion, which preceeded cell death and was associated with more severe subsequent injury, poorer graft function and higher mortality in CDD grafts (Fig. 1). Therefore, the MPT most likely also contributes to CDD graft injury and PNF.
iNOS upregulation and JNK2 activation contribute to MPT onset in CDD grafts
Many factors promote the MPT (see Introduction) [15, 16, 26, 34, 35]. The role of RNS in MPT is controversial [8, 36, 37]. In this study we showed that iNOS was upregulated markedly in CDD grafts and that iNOS-deficiency blunted mitochondrial depolarization, ATP depletion, and injury in CDD grafts (Figs. 1,4). These findings provide strong evidence that iNOS-upregulation contributes to mitochondrial depolarization and injury of CDD grafts.
A variety of pathophysiological stresses activate JNK, including RNS [25]. Peroxynitrite causes protein nitration, leading to activation of tyrosine kinases and Rac and increased JNK activity [38]. However, JNK activation also upregulates iNOS in some cells [39]. In this study, iNOS-deficiency markedly decreased JNK activation in CDD grafts (Fig. 1), whereas JNK1/2 deficiency and inhibition did not significantly alter RNS production in CDD grafts (Supplement, Fig. S4), suggesting that increased RNS production in CDD grafts led to JNK activation. Previous work also shows that iNOS inhibition improves function of fatty liver grafts and attenuates hepatic I/R injury [6, 37, 40, 41].
The liver expresses two isoforms of JNK - JNK1 and JNK2, whereas JNK3 is mainly expressed in brain, heart, and testis. Isoform specific effects of JNKs vary in different stresses and diseases [25]. For example, JNK1−/− mice are protected from obesity and methionine- and choline-deficiency-induced steatohepatitis [42, 43], whereas JNK2 contributes to atherosclerosis, tumor growth and TNFα-induced liver injury [44, 45]. In some conditions, such as arthritis, cardiac I/R injury, and concanavalin-A-induced liver injury, both JNK1 and JNK2 activation plays important role [44]. The isoform-specific effects of JNK are possibly related to their different binding partners, tissue and subcellular localization, and isoform-selective action on different substrates.
In this study, both JNK1 and 2 were activated in CDD grafts, and JNK inhibition blocked mitochondrial depolarization and graft injury, consistent with the conclusion that JNK activation is an important mediator of mitochondrial dysfunction in CDD grafts (Figs. 2,4). Furthermore, JNK2-, but not JNK1-deficiency, prevented mitochondrial depolarization, ATP depletion, and graft injury in CDD grafts (Figs. 3,4), consistent with our and others’ studies showing that JNK2-deficiency protects against liver injury after in vivo warm I/R and cold storage/transplantation injury [14, 15, 46].
JNK2 and the MPT
While it is not clear how JNK2 regulates the MPT, JNK has numerous substrates that are located in mitochondria or translocate to mitochondria after exposure to stresses (e.g., Sab, Bcl-xL, Mcl-1, Bax, Bid, and Bim) [25]. For example, activated JNK translocates to mitochondria and binds to the scaffold protein Sab, causing sustained mitochondrial ROS generation [24-27, 47, 48]. ROS can oxidize cyclophilin D, a component of the MPT pore, and lead to the pore opening [49]. In this study, only phospho-p54 JNK was associated with increased binding to Sab in CDD grafts, and phospho-p54 JNK/Sab binding was basically absent in JNK2−/− CDD grafts (Fig. 4), suggesting phospho-JNK2 binds primarily to mitochondrial Sab. We cannot rule out the possibility that phospho-p46 JNK with a lower molecular weight might bind less tightly than higher molecular weight phospho-p54 JNK and therefore be washed away during immunoprecipitation. However, the difference in molecular weights of p46 and p54 JNK is relatively small. In cardiocytes exposed to H2O2, mainly phospho-JNK2 binds to mitochondrial Sab, whereas after acetaminophen treatment both JNK1 and 2 translocate to mitochondria [47]. Thus, our data are consistent with the cardiocyte data and demonstrate that active JNK2 binding to Sab is a key step in JNK2-induced MPT. The mechanisms of this isoform-selective interaction of JNK with Sab in CDD grafts remain unclear. Both phospho-p46 and phospho-p54 JNK translocated to mitochondria in CDD grafts (Fig. 4D). It is possible that phospho-JNK1 and 2 have different binding partners in mitochondria or that different co-binding partners are required for their Sab binding. Another possibility is that Sab binding to phospo-JNK1 and -2 requires different post-transcriptional modifications to Sab. These possibilities will be explored in the future.
iNOS is up-regulated in macrophages during inflammation. JNK activation plays an important role in cell death; however, it is also involved in inflammatory responses. JNK activation in macrophages could lead to M1-like macrophage polarization, thus increasing proinflammatory cytokine production [39]. Therefore, in addition to promotion of mitochondrial dysfunction and cell death, iNOS upregulation and JNK activation may also increase I/R injury by enhancing inflammation.
Together, our data indicate that mitochondrial dysfunction occurs after transplantation of CDD grafts, resulting in graft failure. This mitochondrial dysfunction is due to iNOS upregulation and JNK activation. JNK2 but not JNK1 mediates mitochondrial depolarization in CDD grafts. Therefore, although pan-JNK inhibition can decrease CDD graft injury, selective JNK2 inhibitors may provide more potent protection and avoid other potential side effects.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported, in part, by Grant DK70844 and DK037034 from the National Institute of Health and Grant #81470878 from Chinese National Natural Foundation. The Cell & Molecular Imaging Core of the Hollings Cancer Center at the Medical University of South Carolina supported by NIH Grant 1P30 CA138313 provided instrumentation and assistance for confocal/multiphoton microscopy. The animals were housed in the Animal Resources at Medical University of South Carolina supported by NIH Grant C06 RR015455.
Abbreviations used
- ALT
alanine aminotransferase
- ASK
apoptosis signal-regulating kinase
- CDD
cardiac death donors
- eNOS
endothelial nitric oxide synthase
- H&E
hematoxylin and eosin staining
- hpf
high power field
- iNOS
inducible nitric oxide synthase
- I/R
ischemia/reperfusion
- JNK
c-Jun N-terminal kinase
- MPT
mitochondria permeability transition
- NCDD
non-cardiac death donors
- 3-NT
3-nitrotyrosine adducts
- PNF
primary non-function
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Sab
SH3BP5
- Sham
sham operation
- TNFα
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- 1400W
N-(1-naphtyl)ethylendiamine dihydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
Authors’ contributions: study concept and design, ZZ; acquisition, analysis and interpretation of data; QL, HR, YK, ZZ; drafting and critical revision of the manuscript, ZZ, JJL, RGS
REFERENCES
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major Challenges Limiting Liver Transplantation in the United States. Am J Transplant. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abt PL, Fisher CA, Singhal AK. Donation after cardiac death in the US: history and use. J Am Coll Surg. 2006;203:208–225. doi: 10.1016/j.jamcollsurg.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Neyrinck A, Van RD, Monbaliu D. Donation after circulatory death: current status. Curr Opin Anaesthesiol. 2013;26:382–390. doi: 10.1097/ACO.0b013e328360dc87. [DOI] [PubMed] [Google Scholar]
- 4.Reddy S, Zilvetti M, Brockmann J, McLaren A, Friend P. Liver transplantation from non-heart-beating donors: current status and future prospects. Liver Transpl. 2004;10:1223–1232. doi: 10.1002/lt.20268. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Rehman H, Wright GL, Zhong Z. Inhibition of inducible nitric oxide synthase prevents graft injury after transplantation of livers from rats after cardiac death. Liver Transpl. 2010;16:1267–1277. doi: 10.1002/lt.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah V, Kamath PS. Nitric oxide in liver transplantation: pathobiology and clinical implications. Liver Transpl. 2003;9:1–11. doi: 10.1053/jlts.2003.36244. [DOI] [PubMed] [Google Scholar]
- 7.Lang JD, Jr., Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects hepatocytes against mitochondrial permeability transition-induced reperfusion injury. Hepatology. 2004;39:1533–1543. doi: 10.1002/hep.20197. [DOI] [PubMed] [Google Scholar]
- 9.Miyagi S, Iwane T, Akamatsu Y, Nakamura A, Sato A, Satomi S. The significance of preserving the energy status and microcirculation in liver grafts from non-heart-beating donor. Cell Transplant. 2008;17:173–178. doi: 10.3727/000000008783906874. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di LF, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 11.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol. 2007;22(Suppl 1):S31–S37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3:527–535. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- 13.Kantrow SP, Tatro LG, Piantadosi CA. Oxidative stress and adenine nucleotide control of mitochondrial permeability transition. Free Radic Biol Med. 2000;28:251–260. doi: 10.1016/s0891-5849(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 14.Theruvath TP, Czerny C, Ramshesh VK, Zhong Z, Chavin KD, Lemasters JJ. C-Jun N-terminal kinase 2 promotes graft injury via the mitochondrial permeability transition after mouse liver transplantation. Am J Transplant. 2008;8:1819–1828. doi: 10.1111/j.1600-6143.2008.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theruvath TP, Snoddy MC, Zhong Z, Lemasters JJ. Mitochondrial Permeability Transition in Liver Ischemia and Reperfusion: Role of c-Jun N-Terminal Kinase 2. Transplantation. 2008;85:1500–1504. doi: 10.1097/TP.0b013e31816fefb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, et al. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehara T, Xi P, X, Bennett B, Satoh Y, Friedman G, Currin R, et al. c-Jun N-terminal kinase mediates hepatic injury after rat liver transplantation. Transplantation. 2004;78:324–332. doi: 10.1097/01.tp.0000128859.42696.28. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Rehman H, Shi Y, Krishnasamy Y, Lemasters JJ, Smith CD, et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS One. 2012;7:e41834. doi: 10.1371/journal.pone.0041834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehman H, Krishnasamy Y, Haque K, Thurman RG, Lemasters JJ, Schnellmann RG, et al. Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin a treatment in rats. PLoS One. 2013;8:e65029. doi: 10.1371/journal.pone.0065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, et al. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008;295:G823–G832. doi: 10.1152/ajpgi.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Rehman H, Ramshesh VK, Schwartz J, Liu Q, Krishnasamy Y, et al. Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. J Hepatol. 2012;56:137–145. doi: 10.1016/j.jhep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Z, Ramshesh VK, Rehman H, Liu Q, Theruvath TP, Krishnasamy Y, et al. Acute ethanol causes hepatic mitochondrial depolarization in mice: role of ethanol metabolism. PLoS One. 2014;9:e91308. doi: 10.1371/journal.pone.0091308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiltshire C, Gillespie DA, May GH. Sab (SH3BP5), a novel mitochondria-localized JNK-interacting protein. Biochem Soc Trans. 2004;32:1075–1077. doi: 10.1042/BST0321075. [DOI] [PubMed] [Google Scholar]
- 25.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu HS, Stevenson WC, Pruett TL, Jones RS. Donor lazaroid pretreatment improves viability of livers harvested from non-heart-beating rats. Am J Surg. 1996;171:113–116. doi: 10.1016/S0002-9610(99)80084-1. [DOI] [PubMed] [Google Scholar]
- 29.Astarcioglu H, Karademir S, Unek T, Ozer E, Menekay S, Coker A, et al. Beneficial effects of pentoxifylline pretreatment in non-heart-beating donors in rats. Transplantation. 2000;69:93–98. doi: 10.1097/00007890-200001150-00017. [DOI] [PubMed] [Google Scholar]
- 30.Gu M, Takada Y, Fukunaga K, Ishiguro S, Taniguchi H, Seino K, et al. Pharmacologic graft protection without donor pretreatment in liver transplantation from non-heart-beating donors. Transplantation. 2000;70:1021–1025. doi: 10.1097/00007890-200010150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Currin RT, Peng XX, Lemasters JJ. Ischemic preconditioning of rat livers from non- heart-beating donors decreases parenchymal cell killing and increases graft survival after transplantation. HPB Surg. 2012;2012:236406. doi: 10.1155/2012/236406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CY, Zhang JX, Jones JW, Jr., Southard JH, Clemens MG. Functional recovery of preserved livers following warm ischemia: improvement by machine perfusion preservation. Transplantation. 2002;74:944–951. doi: 10.1097/00007890-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Miura T, Tanno M. The mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc Res. 2012;94:181–189. doi: 10.1093/cvr/cvr302. [DOI] [PubMed] [Google Scholar]
- 34.Sestili P, Tommasini I, Cantoni O. Peroxynitrite promotes mitochondrial permeability transition-dependent rapid U937 cell necrosis: survivors proliferate with kinetics superimposable on those of untreated cells. Free Radic Res. 2001;34:513–527. doi: 10.1080/10715760100300451. [DOI] [PubMed] [Google Scholar]
- 35.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, et al. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann N Y Acad Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 36.Reichert SA, Kim-Han JS, Dugan LL. The mitochondrial permeability transition pore and nitric oxide synthase mediate early mitochondrial depolarization in astrocytes during oxygen-glucose deprivation. J Neurosci. 2001;21:6608–6616. doi: 10.1523/JNEUROSCI.21-17-06608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Rehman H, Krishnasamy Y, Ramshesh VK, Theruvath TP, Chavin KD, et al. Role of inducible nitric oxide synthase in mitochondrial depolarization and graft injury after transplantation of fatty livers. Free Radic Biol Med. 2012;53:250–259. doi: 10.1016/j.freeradbiomed.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natal C, Modol T, Oses-Prieto JA, Lopez-Moratalla N, Iraburu MJ, Lopez-Zabalza MJ. Specific protein nitration in nitric oxide-induced apoptosis of human monocytes. Apoptosis. 2008;13:1356–1367. doi: 10.1007/s10495-008-0263-0. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Isobe M, Katsuramaki T, Hirata K, Kimura H, Nagayama M, Matsuno T. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999;68:803–813. doi: 10.1097/00007890-199909270-00013. [DOI] [PubMed] [Google Scholar]
- 41.He S, Rehman H, Wright GL, Zhong Z. Inhibition of inducible nitric oxide synthase prevents mitochondrial damage and improves survival of steatotic partial liver grafts. Transplantation. 2010;89:291–298. doi: 10.1097/TP.0b013e3181c99185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 43.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 44.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devey L, Mohr E, Bellamy C, Simpson K, Henderson N, Harrison EM, et al. c-Jun terminal kinase-2 gene deleted mice overexpress hemeoxygenase-1 and are protected from hepatic ischemia reperfusion injury. Transplantation. 2009;88:308–316. doi: 10.1097/TP.0b013e3181ae3067. [DOI] [PubMed] [Google Scholar]
- 47.Chambers JW, Pachori A, Howard S, Iqbal S, LoGrasso PV. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. J Biol Chem. 2013;288:4000–4011. doi: 10.1074/jbc.M112.406777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Erauskin J, Galino J, Bianchi P, Fourcade S, Andreu AL, Ferrer I, et al. Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain. 2012;135:3584–3598. doi: 10.1093/brain/aws292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.