Abstract
Evidence now points to the haeme oxygenase (HO) pathway as a possible actor in modulating risk for cardiovascular disease (CVD). In particular, the HO pathway may represent a key endogenous modulator of oxidative, inflammatory, and cytotoxic stress while also exhibiting vasoregulatory properties. In this review, we summarize the accumulating experimental and emerging clinical data indicating how activity of the HO pathway and its products may play a role in mechanisms underlying the development of CVD. We also identify gaps in the literature to date and suggest future directions for investigation. Because HO pathway activity can be influenced not only by genetic traits and environmental stimuli but also by a variety of existing pharmacologic interventions, the pathway could serve as a prime target for reducing the overall burden of CVD. Further work is needed to determine the role of HO pathway products as possible prognostic markers of risk for clinical CVD events and the extent to which therapeutic augmentation or inhibition of HO pathway activity could serve to modify CVD risk.
Keywords: Heme oxygenase pathway, Carbon monoxide, Bilirubin, Biliverdin, Cardiovascular disease
Introduction
Considerable evidence now points to the haeme oxygenase (HO) pathway as a possible central actor in modulating risk for cardiovascular disease (CVD). The ameliorative properties of the HO pathway were first shown in an animal model of haeme protein-induced kidney injury,1 with subsequent work demonstrating that HO induction protects endothelial cells (ECs) in vitro.2 Intriguing early data in humans included the autopsy report of hyperlipidaemia, fatty streaks, and fibrous plaques in the aorta of an HO-1 deficient 6-year-old boy.3 Supporting the concept that intact HO pathway activity is upregulated in response to vascular stress, another study found that HO-1 expression in adults with atherosclerosis was higher in association with worse lesion type and grade of stenosis.4 Research to date now suggests that the HO pathway may represent one of the most important endogenous modulators of oxidative, inflammatory, and cytotoxic stress while also exhibiting vasoregulatory properties. Herein, we review the accumulating experimental and emerging clinical data indicating how activity of the HO pathway and its products may play a key role in mechanisms underlying the development of CVD.
Haeme metabolism and the haeme oxygenase pathway
Haeme forms the prosthetic moiety within haemoproteins [i.e. haemoglobin, myoglobin, cytochrome c, cytochrome P450, catalase, myeloperoxidase, nitric oxide synthase (NOS), and guanylate cyclase] and is involved in numerous biological processes including oxygen transport, cellular respiration, oxidative biotransformations, host defence, and regulation of vascular tone. While haeme is essential for life, free haeme within cells (i.e. cytosolic ‘uncommitted’ haeme that is not a part of haeme proteins) can be pro-inflammatory and cytotoxic, particularly in ECs,5 via generation of reactive oxygen species (ROS) and lipid peroxidation. Efficient degradation of excess haeme is needed to avert such toxicity and, thus, intracellular levels of free haeme are tightly regulated by the HO family of proteins.6
The HO proteins catalyse the oxidative degradation of haeme, producing equimolar amounts of carbon monoxide (CO), iron (Fe2+), and biliverdin-IXα (Figure 1). Biliverdin-IXα is converted to bilirubin-IXα by cytosolic biliverdin reductase, and bilirubin-IXα is a potent endogenous antioxidant7 with recently recognized anti-inflammatory properties.8 Iron induces expression of ferritin, which sequesters iron and also exerts antioxidant2 and anti-apoptotic9 effects. Of the three direct products of haeme metabolism, however, CO has been most extensively studied. Carbon monoxide is a diatomic gas with numerous biological functions including protection against oxidative injury,10–12 inflammation,13 and cell death.12,14,15 Furthermore, CO has been shown to inhibit cellular proliferation,16 suppress matrix production,17 and increase fibrinolysis.18 Notably, CO shares many similarities with nitric oxide (NO), such as the ability to inhibit smooth muscle cell (SMC) proliferation19 and platelet aggregation,20 as well as modulate vascular tone by increasing cyclic guanosine monophosphate (cGMP) levels.6,21
Figure 1.
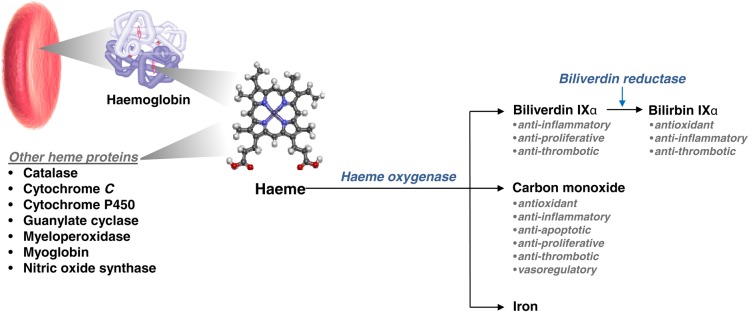
Overview of haeme metabolism and the central role of haeme-oxygenase activity.
Regulation of haeme oxygenase expression
The HO-1 and HO-2 isoforms are encoded by the HMOX1 and HMOX2 genes, respectively. HO-2 is constitutively expressed in multiple tissues, including the vasculature, but its expression is not generally inducible.22,23 HO-1 is also ubiquitous and expressed most strongly in tissues involved in haemoglobin metabolism. Importantly, in other tissues such as the vascular endothelium and SMC, HO-1 is expressed at low levels basally, but is induced in response to diverse stimuli (Figure 2) such as haeme, endotoxin, ROS, NO, cytokines, growth factors, hypoxia, and hyperoxia.23 In particular, HO-1 expression in the vasculature is upregulated in response to oxidized lipids and phospholipids,24 vascular injury,25 and laminar flow.26
Figure 2.
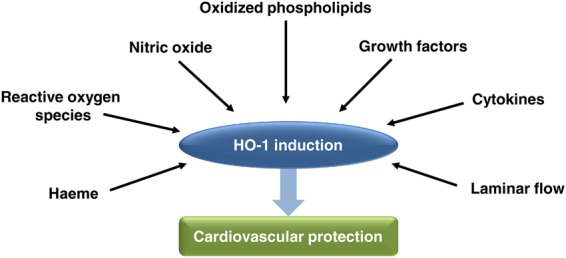
Several stimuli have been shown to induce HO-1 activity and, in turn, upregulated HO-1 expression may provide cardiovascular protection.
Although regulation of HO-1 expression is predominantly at the transcriptional level, extracellular stimuli activate kinase signalling cascades that regulate transcription factor binding to the HO-1 promoter. All three mitogen-activated protein kinase (MAPK) pathways (i.e. extracellular signal-regulated kinases 1/2, c-Jun-N-terminal kinase, and p38 MAPK) have been implicated in regulating HO-1 expression.23 In vascular EC, the selective COX-2 inhibitor, celecoxib, has been shown to induce HO-1 expression via PI3K activation and translocation of nuclear factor erythroid 2-related factor 2 (Nrf2).27 Additionally, HO-1 is induced in EC by TNF-α and IL-1α in a PKC-dependent fashion via activation of arachidonic acid.28
One of the main regulators of HO-1 transcription is Nrf2, an oxidant responsive transcription factor. Nuclear factor erythroid 2-related factor 2 transactivates the HO-1 promoter, while the haeme-binding protein Bach1 represses HO-1 transcription.29 Both Nrf2 and Bach1 have been shown to play key roles in cardiometabolic disease by regulating HO-1 expression.30–37 Defective Nrf2 signalling has been implicated in the pathophysiology of diabetes35,38,39 and coronary artery disease (CAD).37,40,41 Additionally, Nrf2 has been shown to protect against glucose-induced apoptosis in cardiomyocytes.36 Deficiency of Bach1 is also protective in animal models of atherosclerosis,31 myocardial ischaemia-reperfusion injury,34 and vascular injury (Figure 3).30
Figure 3.
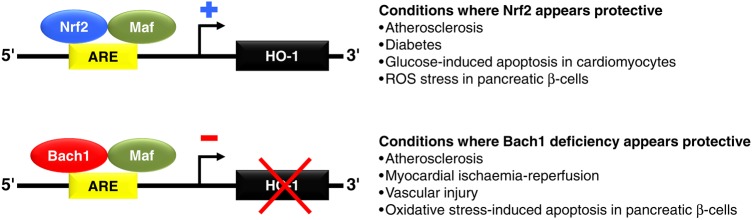
Certain transcription factors, Nrf2 and Bach1, appear to play an important role in regulating HO-1 expression in cardiovascular conditions. Specifically, Nrf2 and Bach1 form heterodimers with Maf proteins and bind to consensus antioxidant response element (ARE) sequences in the HO-1 promoter. Nrf2 transactivates the HO-1 promoter and may provide protection against diabetes and cardiovascular disease. Bach-1 competes with Nrf2/Maf dimers and represses HO-1 transcription. Accordingly, deficiency of Bach1 has been shown to be protective in animal models of atherosclerosis, myocardial ischaemia-reperfusion injury, and vascular injury.
Haeme oxygenase gene expression in humans
Variation in the HO-1 gene has been related to cardiovascular risk in humans. The most extensively studied HO-1 gene variant in humans is the dinucleotide repeat polymorphism, [GT]n.6,42 This variant is the most frequent dinucleotide repeat scattered throughout human and animal genomes, and many repeat regions are length polymorphic. With respect to the HO-1 promoter region, length of the (GT) repeat region in the HO-1 promoter has been related inversely to HO-1 expression.43 Importantly, this finding appears associated with CVD risk in humans. In the presence of pre-existing risk factors such as hypertension, metabolic syndrome, and diabetes, a larger number of (GT) repeats is generally related to increased risk of CVD (Table 1). Conversely, under similar circumstances, a smaller number of (GT) repeats has generally been related to less CVD (Table 1). Interestingly, these promoter polymorphisms have not been associated with CVD risk in the general population, supporting the concept that oxidative and metabolic stress may be required to induce HO-1 expression.
Table 1.
Studies relating haeme oxygenase pathway activity to clinical phenotypes and outcomes in humans
| Phenotype | No. of studies | No. of subjects per study | Study sampling characteristics | Main findings |
|---|---|---|---|---|
| HO-1 promoter polymorphism | ||||
| Hypertension | 5 | 152–1998 | Community sample148,150; Hypertension vs. controls149; MetS patients vs. controls;151 Arsenic-exposed individuals152 |
|
| Metabolic syndrome | 2 | 152–468 | MetS and controls;153 T2DM, MetS, and controls154 | |
| Diabetes | 4 | 189–3089 | T2DM and controls,155,156 T2DM (no controls),157 T2DM, MetS, and controls154 |
|
| Cardiovascular disease | 16 | 70–4596 | CAD vs. controls,43,44,160,162,164 CAD,159,161,163 T2DM158 post-ischaemic stroke vs. controls,165,166 haemodialysis patients vs. controls,167 peripheral arterial disease,168 chronic stable angina169 and arsenic-exposed individuals170,171 |
|
| Restenosis after intervention | 7 | 96–1357 | Coronary stenting,172–174,178 balloon angioplasty or stenting175–177 | |
| Indirect Measures of HO-1 Activity | ||||
| Metabolic syndrome | 8 | 1423–12 342 | Community sample,95,145,179–182 adult women,94 children and adolescents183 | |
| Diabetes | 5 | 417–5960 | T2DM patients vs. controls,132 T2DM patients,184 community sample186 children and adolescents183 |
|
| Cardiovascular disease | 10 | 53–130 052 | T2DM vs. controls,4 community sample,144,145,187,188,190 statin-treated cohort,189 men,191,192 overweight/obese high cardiovascular risk patients193 |
|
CAD, coronary artery disease; MI, myocardial infarction; CVD, cardiovascular disease; MetS, metabolic syndrome; T2DM, type 2 diabetes mellitus.
Although not as thoroughly investigated as the [GT]n polymorphism, there is at least one SNP in the proximal HO-1 promoter, T(-413)A, that has been associated with susceptibility to CVD.6,44,45 The AA genotype of the T(-413)A polymorphism has been correlated with a lower incidence of CAD,44,45 although the recent meta-analysis called this association into question given inconsistencies in the Hardy–Weinberg equilibrium in some studies.46
Haeme oxygenase and mechanisms related to cardiovascular disease
Multiple experimental studies have demonstrated a role for HO-1 and its products in the setting of hypertension, diabetes, vascular injury, atherosclerosis, and ischaemia reperfusion.25,30,47–65 There are numerous mechanisms by which HO-1 activity may impact cardiovascular risk including a variety of antioxidant, anti-inflammatory, anti-apoptotic, anti-proliferative, anti-thrombotic, and vasoregulatory effects.
Antioxidant protection
HO-1 has well-described antioxidant cytoprotective effects in many cell types and disease models.6,10–12 In atherosclerotic lesions, HO-1 is upregulated in EC and SMC with particularly high expression in macrophages and foam cells, where oxidized phospholipids co-localize with HO-1.58 Inhibition of HO-1 enhances atherosclerosis and increases plasma lipid hydroperoxide levels in LDL-receptor knockout mice, suggesting that HO-1 may protect against lipid peroxidation in atherosclerosis.58 In turn, HO-1 overexpression in cardiomyocytes appears to protect against reperfusion injury as well as attenuate cardiac inflammation and oxidative damage to cardiomyocytes.63 Additionally, HO-1 expression correlates with plaque destabilizing factors such as matrix metalloproteinase-9, and overexpression of HO-1 has been shown to prevent progression of atherosclerotic lesions to vulnerable plaques.66
Products of the HO pathway can also exert antioxidant properties. Carbon monoxide can bind to haeme proteins (e.g. NADPH oxidase and cytochrome c oxidase) to inhibit electron transport and ROS generation. Conversely, CO can also bind and inhibit the catalytic function of other haeme proteins, which may lead to pro-oxidant effects under certain conditions.67 Bilirubin is a potent ROS scavenger that can prevent oxidation of LDL and other lipids.68,69 In addition, bilirubin can decrease ROS in EC,70 protect against oxidative damage in ventricular myocytes,71 and reduce infarct size and mitochondrial damage following myocardial ischaemia reperfusion.61 In cerebrovascular EC, both CO and bilirubin appear to attenuate TNF-α-induced apoptosis and inhibit superoxide anion production.72
Interestingly, HO-1 and CO may also crosstalk with NOS enzymes and modulate levels of NO in the vasculature. Although NO-induced HO-1 expression is cytoprotective in EC,73 excess NO can react with ROS to generate peroxynitrite that can promote lipid peroxidation and cell death within the vasculature.74 In turn, HO-1 and CO can bind to the haeme moiety of NOS and may down-regulate NOS expression to reduce NO production75 in the vasculature, which may be protective in certain circumstances. Accordingly, in a rabbit model of atherosclerosis, induction of HO-1 inhibited progression of atherosclerosis and was associated with reduced expression of inducible NOS and NO production, while inhibition of HO-1 had opposite effects.76
Anti-inflammatory activity
HO-1 is a well-recognized modulator of inflammation. Complete absence of HO-1 in mice results in a chronic multi-systemic inflammatory disorder with evidence of vascular and perivascular involvement.77 When exposed to lipopolysaccharide (LPS), HO-1 deficient (HO-1–/–) mice have greater end-organ damage and reduced survival.78 In turn, HO-1 induction by haemoglobin79,80 as well as biliverdin8 can attenuate lung inflammation, decrease pro-inflammatory cytokine expression, and improve survival following LPS exposure.
Notably, CO has been shown to mediate many of the anti-inflammatory effects of HO-1. Administration of CO to LPS-stimulated macrophages inhibits NF-κB activation and secretion of granulocyte macrophage-colony-stimulating factor.81 Carbon monoxide has also been shown to decrease expression of TNF-α, IL-1β, and macrophage inflammatory protein-1β, while increasing expression of the anti-inflammatory cytokine IL-10 in macrophages and in mice.13 Additionally, recent studies suggest that HO-1 may play a role in alternative activation of macrophages towards an M2 anti-inflammatory phenotype.82,83
HO-1 has been shown to exhibit anti-inflammatory effects in the vasculature, as well as globally. In EC, overexpression of HO-1 and bilirubin attenuate TNF-α-induced upregulation of VCAM-1 and E-selectin by inhibiting NF-κB activation.84 Furthermore, overexpression of HO-1 or its products in vivo have led to protective anti-inflammatory as well as anti-proliferative effects in models of vascular injury, in-stent restenosis, and transplant arteriosclerosis.85–87 Overexpression or induction of HO-1, as well as CO administration, have been shown to reduce leukocyte infiltration, pro-inflammatory cytokine expression, NF-κB activation, and apoptosis, in addition to attenuating intimal proliferation in rat aortic allografts and stented arteries.85–87
The anti-inflammatory effects of bilirubin and biliverdin may also protect against CVD risk.88–95 Biliverdin decreases IL-6 secretion in vitro in both macrophages and LPS-stimulated EC.8 In the vasculature, HO-1 induction down-regulates oxidant-induced leukocyte rolling and adhesion, and this finding appears mediated by bilirubin and biliverdin.96 Bilirubin has also been shown to attenuate upregulation of E-selectin, VCAM-1, and ICAM-1, as well as to inhibit neutrophil adhesion in TNF-α-stimulated EC.97
Effects on apoptosis
Whereas oxidative stress and inflammation can lead to apoptosis within the vasculature, HO-1 and its products may counter this process. In EC, CO protects against apoptosis following TNF-α and anoxia-reoxygenation via activation of the p38 MAPK pathway.14,98,99 In vascular smooth muscle cells (VSMCs), absence of HO-1 has been shown to increase susceptibility to oxidant stress and cell death in a vein graft stenosis model.100 Paradoxically, overexpression of HO-1 and bilirubin in VSMC have also been shown to stimulate apoptosis.101 Notably, CO did not have an effect on apoptosis in this study and, in a separate study, was shown to inhibit VSMC apoptosis via soluble guanylate cyclase (sGC) activation and suppression of p53 expression.102 In addition, HO-1 induction by haemin decreases SMC apoptosis and prevents atherosclerotic plaque progression in vivo.103 Furthermore, HO-1 overexpression in the myocardium decreases lipid peroxidation, IL-1β expression, pro-apoptotic signalling, and myocardial infarct size.64 Taken together, HO-1 and the products of haeme metabolism may have differential effects on apoptosis depending on the cell type and mechanism of cellular injury, although most studies suggest that the HO-1-CO pathway confers anti-apoptotic properties in the setting of vascular injury.
Effects on cellular proliferation
HO-1 has potent anti-proliferative effects in the vasculature. HO-1 overexpression in a femoral artery injury model inhibited arterial remodelling by reducing VSMC proliferation and inducing expression of the cell cycle inhibitor p2125; in contrast, absence of HO-1 exaggerated cellular proliferation and enhanced vascular lesion formation.25 Overexpression of HO-1 also decreased VSMC proliferation in models of transplant atherosclerosis and in-stent restenosis.86,87 Carbon monoxide has been shown to mediate these protective effects of HO-1 on VSMC proliferation. In aortic transplant and carotid artery injury models, CO inhibited VSMC proliferation and attenuated intimal hyperplasia in injured vessels and aortic transplant allografts.85,104 In addition to VSMC proliferation, migration of VSMC may contribute to intimal thickening following vascular injury, and the HO-1/CO pathway has recently been shown to attenuate VSMC migration.105 Notably, overexpression of HO-1, CO gas, or treatment with a CO-releasing molecule (CORM) each decreased migration of VSMC via NOX1 inhibition.105
Although CO is best known for modulating the anti-proliferative effects of HO-1, emerging data suggest that biliverdin may have anti-proliferative properties as well.88–90 Biliverdin has been shown to attenuate intimal hyperplasia and decreased EC apoptosis in vein grafting and balloon angioplasty models.90 Biliverdin was also found to decrease SMC migration in vitro.90 Additionally, hyperbilirubinaemic Gunn rats develop minimal intimal hyperplasia following balloon injury.88 In mechanistic in vitro studies, bilirubin attenuates VSMC proliferation and arrests the cell cycle by inhibiting phosphorylation of the retinoblastoma tumour suppressor protein (Rb).88
HO-1 and CO may also play a role in regulating proliferation of EC and angiogenesis. HO-1 overexpression increased proliferation and capillary tube formation in coronary EC,106 while inhibition of HO-1 inhibited VEGF-induced angiogenesis.107 In addition, HO-1 deficient EC have been shown to have reduced angiogenesis that was rescued by CORM.108 HO-1 has also been shown to influence the mobilization of endothelial progenitor cells (EPCs) following vascular injury.109,110 Overexpression of HO-1 or CO inhalation accelerated re-endothelialization of denuded vessels and enhanced EPC mobilization after carotid artery injury.109 In contrast, HO-1–/– animals generated fewer endothelial colony forming cells110 and had reduced EPC mobilization and decreased re-endothelialization following vascular injury.109 Thus, HO-1 may promote EC repair, yet inhibit proliferation and migration of VSMC, thereby preventing the development of intimal lesions at multiple cellular levels. Taken together, the beneficial effects of the HO-1/CO pathway may provide dual vascular protection to promote repair in the setting of vascular injury, further highlighting the central role of HO-1 in cardioprotection.
Anti-thrombotic activity
Induction of HO-1 enzymatic activity and CO have demonstrated beneficial effects on platelet aggregation and thrombus formation.18,20,98,111–116 CO has well-described inhibitory effects on platelet aggregation via activation of sGC and increased platelet cGMP levels.20 In addition, induction of HO-1 and bilirubin have been shown to delay thrombus formation, suggesting that bilirubin has anti-thrombotic properties as well.111 Absence of HO-1 leads to accelerated arterial thrombus formation and EC apoptosis following vascular injury that could be rescued by CO and biliverdin.116 HO-1–/– mice also have increased mortality following aortic allograft transplantation due to graft thrombosis that was attenuated by CORM or adoptive transfer of wild-type platelets.114 Similarly, HO-1 inhibition in rats led to graft rejection following heart transplantation with coronary artery thrombosis, leukocyte infiltration, and myocardial infarction which could be attenuated by CO.113 HO-1–/– mice also have exaggerated venous thrombosis following inferior vena cava ligation, with increased expression of tissue factor, selectins, and pro-inflammatory signaling.115 Furthermore, HO-1 gene transfer into injured carotid arteries of apolipoprotein E null mice leads to earlier thrombolysis, with reduced fibrin deposition and decreased expression of plasminogen activator inhibitor-1.113
Vasoregulation
Although HO-1 has been shown to modulate vascular tone in experimental studies, the physiologic significance of HO-1 on vascular reactivity in vivo remains unknown. In studies where induction of HO-1 decreased blood pressure in hypertensive animals, the vasodilatory effects have been attributed to CO.117,118 Overexpression of HO-1 decreased vasoreactivity of pig arteries ex vivo, in a manner that appeared related to a cGMP-dependent mechanism independent of NO.25 Additional studies have demonstrated that exogenously administered CO relaxes isolated aortas in an endothelium- and NO-independent fashion.119,120 Endogenous CO release has also been shown to dilate blood vessels in the liver, skeletal muscle, and brain.121–123 The mechanism by which CO mediates vasodilation has largely been attributed to sGC activation and increases in cGMP but, compared with NO, CO is a weak activator of sGC.119,124 Additional mechanisms of CO-induced vasorelaxation include stimulation of calcium-activated potassium channels (BKCa) in VSMC,125 as well as modulation of endothelial-derived vasoconstrictors.126 Furthermore, in some vascular tissues under certain conditions, CO has been shown to have vasoconstrictive effects by inhibiting endothelial NO synthase (eNOS) expression and diminishing NO production.127,128
The HO pathway and cardiovascular risk factors
Extending from the experimental data focused on the mechanistic contributors to CVD, HO-1 has been shown to be upregulated in the setting of cardiovascular risk factors such as cigarette smoking,129 hyperglycaemia,130 and hypertension.131 Although increased plasma and monocyte HO-1 levels have been observed in persons with type 2 diabetes,132,133 the role of the HO-1–CO pathway in diabetes and metabolic disease is incompletely understood. Multiple experimental studies have demonstrated a protective role for HO-1 and its products in relation to insulin resistance and diabetes.50–53,91,92,134–138 In rodent obesity models, HO-1 induction decreases weight gain, reduces adiposity, and improves insulin sensitivity and glucose tolerance.134–138 Furthermore, HO-1 induction leads to increased levels of adiponectin and PPAR-γ in adipocytes, reduced adipocyte size,138 and decreased adipogenesis in obese mice.139 However, a recent study suggests that HO-1 activity is paradoxically a maladaptive contributor to obesity-related insulin resistance and diabetes.140 These seemingly contradictory bodies of data could be related to a differential effect of HO-1 metabolism products or a dose-dependent effect of HO pathway activity.
Translating an old paradox into a new paradigm
The dual effects of the HO pathway, having been demonstrated in multiple settings, warrant special attention. Depending on the experimental conditions, HO-1 and its products have been observed to exert differential effects. For instance, HO-1 and CO exert anti-proliferative effects in VSMCs but pro-proliferative effects in ECs.106,107 Most studies suggest that HO-1 and its products confer anti-apoptotic properties in the face of vascular injury, but overexpression of HO-1 and bilirubin has also been shown to stimulate apoptosis in VSMC.101 Similarly, whereas CO administration demonstrates vasodilatory effects in most studies, CO has also been shown to have vasoconstrictive effects under different experimental conditions.127,128
Just as experimental studies have demonstrated variable HO-1 and CO activity in the setting of different experimental conditions, clinical studies have also produced apparently conflicting results (Table 1). On the one hand, genetic polymorphisms leading to increased HO-1 expression have been associated with lower risk for hypertension, diabetes, and CVD in both referral and general population samples. On the other hand, indirect measures of HO-1 activity have been variably associated with increased risk for metabolic traits and CVD in selected and unselected community cohorts. There are several possible reasons for discrepant findings including differences in study design, potential confounders, and limitations of the various indirect measures of HO-1 activity used. It is also likely that while physiologic levels of HO-1 pathway activity are essential for health, measures of increased HO-1 activity reflect a compensatory—and, in some situations, an excessive—response to pathologic stress.
Overall, the apparent paradox of differential effects of HO-1 and its products in experimental models and the both very low and very high levels of HO-1 activity observed in association with adverse clinical outcomes may, in fact, reflect the central biological role of the HO-1 pathway in maintaining cellular and tissue homeostasis (Figure 4). This phenomenon has been demonstrated for well-established markers of cardiovascular stress, including conventional inflammatory markers (i.e. c-reactive protein, interleukins) and natriuretic peptides, for which genetic deficiencies predispose to adverse disease phenotypes even while excess circulating levels are also consistently associated with adverse clinical events.141–143
Figure 4.
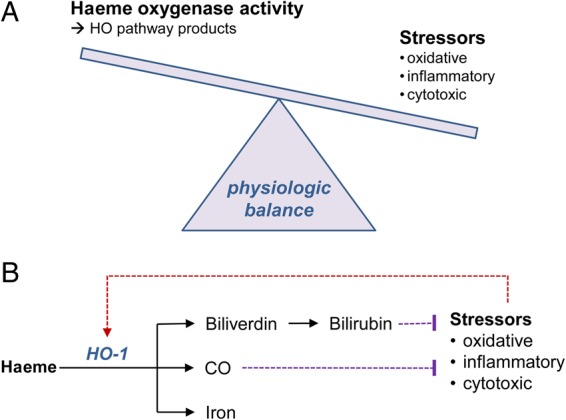
Schematic displaying the possible relationship between HO activity and cardiometabolic stressors, where maintenance of physiologic balance (A) involves HO pathway products countering stressors that activate HO activity (B).
Future directions
Taken together, prior investigations of the HO pathway underscore its potential role in modulating risk for CVD and, in turn, to serve as a therapeutic target with wide ranging implications. To this end, there is more work to be done. For instance, the extent to which measures of HO-1 activity may serve as reliable prognostic markers of clinical cardiovascular risk has yet to be established. Most genetic studies of HO-1 variants have been performed in Asian cohorts and, thus, require validation in other populations. The largest studies of CO and clinical outcomes have relied predominantly on measures of CO in exhaled breath144,145; even though associations with cardiovascular and metabolic endpoints in these studies were significant after accounting for potential confounders (e.g. smoking status and lung disease), additional investigations using more direct measures of endogenous CO are needed. Measures of endogenous CO are preferred in part because circulating levels of the other HO pathway products, biliverdin and iron, are more prone to variation due to the activity of other metabolic pathways. In addition, gaseous or water-soluble tablet delivery of CO (i.e. in the form of CORM) has shown promise as agents for inducing HO-1 activity. Interestingly, HO-1 is also induced by many existing therapeutic agents including statins, rapamycin, paclitaxel, NO, aspirin, and probucol.146,147 Of course, the extent to which certain pre-clinical or clinical disease states could benefit from induction of deficient HO-1 activity, as opposed to inhibition of excess HO-1 activity, remains unknown.
Overall, a large body of accumulating and emerging evidence highlights the need for more research of the HO pathway and its products, particularly endogenous CO, with respect to the development of CVD in humans. Ongoing investigations in the field promise to improve our understanding of how activity of the HO pathway may be harnessed to optimize human health and reduce the global burden of CVD. Accordingly, further discoveries regarding the therapeutic potential of interventions targeting the HO pathway appear to be on the horizon.
Funding
This work was supported by the Ellison Foundation (S.C.) and NIH/NHLBI grants R01HL114839 (L.E.F.), R03HL115106 (L.E.F.), P01HL108801 (L.E.F.), and R00HL107642 (S.C.).
Conflict of interest: none declared.
Acknowledgements
We acknowledge that references to the work of many investigators were omitted due to the word limit for this manuscript.
References
- 1.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 1992;90:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 1992;267:18148–18153. [PubMed] [Google Scholar]
- 3.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 1999;103:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, Sumiyoshi S, Nakashima Y, Doi Y, Iida M, Kiyohara Y, Sueishi K. Overexpression of heme oxygenase-1 in coronary atherosclerosis of Japanese autopsies with diabetes mellitus: Hisayama study. Atherosclerosis 2009;202:573–581. [DOI] [PubMed] [Google Scholar]
- 5.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002;100:879–887. [DOI] [PubMed] [Google Scholar]
- 6.Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Am J Respir Cell Mol Biol 2007;36:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–1046. [DOI] [PubMed] [Google Scholar]
- 8.Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2005;289:L1131–L1137. [DOI] [PubMed] [Google Scholar]
- 9.Choi BM, Pae HO, Jeong YR, Oh GS, Jun CD, Kim BR, Kim YM, Chung HT. Overexpression of heme oxygenase (HO)-1 renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radic Biol Med 2004;36:858–871. [DOI] [PubMed] [Google Scholar]
- 10.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol 1999;276:L688–L694. [DOI] [PubMed] [Google Scholar]
- 11.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol 2003;163:2555–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 2000;279:L1029–L1037. [DOI] [PubMed] [Google Scholar]
- 13.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000;6:422–428. [DOI] [PubMed] [Google Scholar]
- 14.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 2000;192:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem 2003;278:22061–22070. [DOI] [PubMed] [Google Scholar]
- 16.Song R, Mahidhara RS, Liu F, Ning W, Otterbein LE, Choi AM. Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am J Respir Cell Mol Biol 2002;27:603–610. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, Choi AM, Morse D. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol 2005;166:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med 2001;7:598–604. [DOI] [PubMed] [Google Scholar]
- 19.Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem 1997;272:32804–32809. [DOI] [PubMed] [Google Scholar]
- 20.Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol 1987;32:497–504. [PubMed] [Google Scholar]
- 21.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 1995;92:1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 1968;61:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006;86:583–650. [DOI] [PubMed] [Google Scholar]
- 24.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem 2003;278:51006–51014. [DOI] [PubMed] [Google Scholar]
- 25.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 2001;7:693–698. [DOI] [PubMed] [Google Scholar]
- 26.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem 2003;278:703–711. [DOI] [PubMed] [Google Scholar]
- 27.Hamdulay SS, Wang B, Birdsey GM, Ali F, Dumont O, Evans PC, Haskard DO, Wheeler-Jones CP, Mason JC. Celecoxib activates PI-3 K/Akt and mitochondrial redox signaling to enhance heme oxygenase-1-mediated anti-inflammatory activity in vascular endothelium. Free Radic Biol Med 2010;48:1013–1023. [DOI] [PubMed] [Google Scholar]
- 28.Terry CM, Clikeman JA, Hoidal JR, Callahan KS. TNF-alpha and IL-1alpha induce heme oxygenase-1 via protein kinase C, Ca2+, and phospholipase A2 in endothelial cells. Am J Physiol 1999;276:H1493–H1501. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci USA 2004;101:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omura S, Suzuki H, Toyofuku M, Ozono R, Kohno N, Igarashi K. Effects of genetic ablation of bach1 upon smooth muscle cell proliferation and atherosclerosis after cuff injury. Genes Cells 2005;10:277–285. [DOI] [PubMed] [Google Scholar]
- 31.Watari Y, Yamamoto Y, Brydun A, Ishida T, Mito S, Yoshizumi M, Igarashi K, Chayama K, Ohshima T, Ozono R. Ablation of the bach1 gene leads to the suppression of atherosclerosis in bach1 and apolipoprotein E double knockout mice. Hypertens Res 2008;31:783–792. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Zhang Z, Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J 2014;38:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo K, Ishigaki Y, Gao J, Yamada T, Imai J, Sawada S, Muto A, Oka Y, Igarashi K, Katagiri H. Bach1 deficiency protects pancreatic beta-cells from oxidative stress injury. Am J Physiol Endocrinol Metab 2013;305:E641–E648. [DOI] [PubMed] [Google Scholar]
- 34.Yano Y, Ozono R, Oishi Y, Kambe M, Yoshizumi M, Ishida T, Omura S, Oshima T, Igarashi K. Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes Cells 2006;11:791–803. [DOI] [PubMed] [Google Scholar]
- 35.Velmurugan GV, Sundaresan NR, Gupta MP, White C. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc Res 2013;100:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol 2009;46:47–58. [DOI] [PubMed] [Google Scholar]
- 37.Mozzini C, Fratta Pasini A, Garbin U, Stranieri C, Pasini A, Vallerio P, Cominacini L. Increased endoplasmic reticulum stress and Nrf2 repression in peripheral blood mononuclear cells of patients with stable coronary artery disease. Free Radic Biol Med 2014;68:178–185. [DOI] [PubMed] [Google Scholar]
- 38.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 2013;33:2996–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. Nrf2 protects pancreatic beta-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 2014;63:605–618. [DOI] [PubMed] [Google Scholar]
- 40.Ruotsalainen AK, Inkala M, Partanen ME, Lappalainen JP, Kansanen E, Makinen PI, Heinonen SE, Laitinen HM, Heikkila J, Vatanen T, Horkko S, Yamamoto M, Yla-Herttuala S, Jauhiainen M, Levonen AL. The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovasc Res 2013;98:107–115. [DOI] [PubMed] [Google Scholar]
- 41.Ashino T, Yamamoto M, Yoshida T, Numazawa S. Redox-sensitive transcription factor Nrf2 regulates vascular smooth muscle cell migration and neointimal hyperplasia. Arterioscler Thromb Vasc Biol 2013;33:760–768. [DOI] [PubMed] [Google Scholar]
- 42.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 2004;37:1097–1104. [DOI] [PubMed] [Google Scholar]
- 43.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 2002;111:1–8. [DOI] [PubMed] [Google Scholar]
- 44.Ono K, Goto Y, Takagi S, Baba S, Tago N, Nonogi H, Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis 2004;173:315–319. [DOI] [PubMed] [Google Scholar]
- 45.Zhang GH, Chen SM, Wang DM, Huang XS. 413A/T polymorphism in heme oxygenase-1 gene promoter is related to susceptibility of coronary artery disease in patients with dyslipidemia. Chinese Journal of Arteriosclerosis 2010;18:63–66. [Google Scholar]
- 46.Qiao H, Sai X, Gai L, Huang G, Chen X, Tu X, Ding Z. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis. Am J Epidemiol 2014;179:1039–1048. [DOI] [PubMed] [Google Scholar]
- 47.Cheng PY, Chen JJ, Yen MH. The expression of heme oxygenase-1 and inducible nitric oxide synthase in aorta during the development of hypertension in spontaneously hypertensive rats. Am J Hypertens 2004;17:1127–1134. [DOI] [PubMed] [Google Scholar]
- 48.Tiwari S, Ndisang JF. Heme oxygenase system and hypertension: a comprehensive insight. Curr Pharm Des 2014;20:1354–1369. [DOI] [PubMed] [Google Scholar]
- 49.Ndisang JF, Zhao W, Wang R. Selective regulation of blood pressure by heme oxygenase-1 in hypertension. Hypertension 2002;40:315–321. [DOI] [PubMed] [Google Scholar]
- 50.Wang R, Wang Z, Wu L, Hanna ST, Peterson-Wakeman R. Reduced vasorelaxant effect of carbon monoxide in diabetes and the underlying mechanisms. Diabetes 2001;50:166–174. [DOI] [PubMed] [Google Scholar]
- 51.Ndisang JF, Lane N, Jadhav A. Upregulation of the heme oxygenase system ameliorates postprandial and fasting hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2009;296:E1029–E1041. [DOI] [PubMed] [Google Scholar]
- 52.Simon T, Pogu S, Tardif V, Rigaud K, Remy S, Piaggio E, Bach JM, Anegon I, Blancou P. Carbon monoxide-treated dendritic cells decrease beta1-integrin induction on CD8(+) T cells and protect from type 1 diabetes. Eur J Immunol 2013;43:209–218. [DOI] [PubMed] [Google Scholar]
- 53.Nikolic I, Saksida T, Mangano K, Vujicic M, Stojanovic I, Nicoletti F, Stosic-Grujicic S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia 2014;57:980–990. [DOI] [PubMed] [Google Scholar]
- 54.Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis 2001;155:113–122. [DOI] [PubMed] [Google Scholar]
- 55.Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 2001;104:2710–2715. [DOI] [PubMed] [Google Scholar]
- 56.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 57.Ishikawa K, Sugawara D, Goto J, Watanabe Y, Kawamura K, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation 2001;104:1831–1836. [DOI] [PubMed] [Google Scholar]
- 58.Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in LDL-receptor knockout mice. Circ Res 2001;88:506–512. [DOI] [PubMed] [Google Scholar]
- 59.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 2001;104:1519–1525. [DOI] [PubMed] [Google Scholar]
- 60.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J 2003;17:1759–1761. [DOI] [PubMed] [Google Scholar]
- 61.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol 2000;278:H643–H651. [DOI] [PubMed] [Google Scholar]
- 62.Raju VS, Maines MD. Renal ischemia/reperfusion up-regulates heme oxygenase-1 (HSP32) expression and increases cGMP in rat heart. J Pharmacol Exp Ther 1996;277:1814–1822. [PubMed] [Google Scholar]
- 63.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001;89:168–173. [DOI] [PubMed] [Google Scholar]
- 64.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell'Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation 2002;105:602–607. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes 2005;54:778–784. [DOI] [PubMed] [Google Scholar]
- 66.Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, Duckers HJ. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation 2009;119:3017–3027. [DOI] [PubMed] [Google Scholar]
- 67.Knauert M, Vangala S, Haslip M, Lee PJ. Therapeutic applications of carbon monoxide. Oxid Med Cell Longev 2013;2013:360815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol 1996;51:859–862. [DOI] [PubMed] [Google Scholar]
- 69.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem 1994;269:16712–16719. [PubMed] [Google Scholar]
- 70.Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Munzel T, Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol 2010;49:186–195. [DOI] [PubMed] [Google Scholar]
- 71.Wu TW, Wu J, Li RK, Mickle D, Carey D. Albumin-bound bilirubins protect human ventricular myocytes against oxyradical damage. Biochem Cell Biol 1991;69:683–688. [DOI] [PubMed] [Google Scholar]
- 72.Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 2006;291:C897–C908. [DOI] [PubMed] [Google Scholar]
- 73.Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol 1996;270:H107–H114. [DOI] [PubMed] [Google Scholar]
- 74.Chrobak I, Mallarino Haeger C, Maracle M, Fredenburgh L. Pulmonary Arterial Hypertension and Oxidative Stress. “Studies on Respiratory Disorders”. New York: Springer; 2014. p. 259–326. [Google Scholar]
- 75.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 1997;37:517–554. [DOI] [PubMed] [Google Scholar]
- 76.Liu D, He Z, Wu L, Fang Y. Effects of induction/inhibition of endogenous heme oxygenase-1 on lipid metabolism, endothelial function, and atherosclerosis in rabbits on a high fat diet. J Pharmacol Sci 2012;118:14–24. [DOI] [PubMed] [Google Scholar]
- 77.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 1997;94:10919–10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet S-F, Lee M-E, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation 2000;102:3015–3022. [DOI] [PubMed] [Google Scholar]
- 79.Otterbein L, Chin BY, Otterbein SL, Lowe VC, Fessler HE, Choi AM. Mechanism of hemoglobin-induced protection against endotoxemia in rats: a ferritin-independent pathway. Am J Physiol 1997;272:L268–L275. [DOI] [PubMed] [Google Scholar]
- 80.Otterbein L, Sylvester SL, Choi AM. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol 1995;13:595–601. [DOI] [PubMed] [Google Scholar]
- 81.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol 2002;27:739–745. [DOI] [PubMed] [Google Scholar]
- 82.Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 2009;20:1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011;123:1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol 2004;172:3553–3563. [DOI] [PubMed] [Google Scholar]
- 85.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 2003;9:183–190. [DOI] [PubMed] [Google Scholar]
- 86.Bouche D, Chauveau C, Roussel JC, Mathieu P, Braudeau C, Tesson L, Soulillou JP, Iyer S, Buelow R, Anegon I. Inhibition of graft arteriosclerosis development in rat aortas following heme oxygenase-1 gene transfer. Transpl Immunol 2002;9:235–238. [DOI] [PubMed] [Google Scholar]
- 87.Du D, Chang S, Chen B, Zhou H, Chen ZK. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transplant Proc 2007;39:3446–3448. [DOI] [PubMed] [Google Scholar]
- 88.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005;112:1030–1039. [DOI] [PubMed] [Google Scholar]
- 89.Ollinger R, Yamashita K, Bilban M, Erat A, Kogler P, Thomas M, Csizmadia E, Usheva A, Margreiter R, Bach FH. Bilirubin and biliverdin treatment of atherosclerotic diseases. Cell Cycle 2007;6:39–43. [DOI] [PubMed] [Google Scholar]
- 90.Nakao A, Murase N, Ho C, Toyokawa H, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation 2005;112:587–591. [DOI] [PubMed] [Google Scholar]
- 91.Fu YY, Kang KJ, Ahn JM, Kim HR, Na KY, Chae DW, Kim S, Chin HJ. Hyperbilirubinemia reduces the streptozotocin-induced pancreatic damage through attenuating the oxidative stress in the Gunn rat. Tohoku J Exp Med 2010;222:265–273. [DOI] [PubMed] [Google Scholar]
- 92.Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, Takayanagi R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int 2010;78:905–919. [DOI] [PubMed] [Google Scholar]
- 93.Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, Hasegawa G, Nakamura N. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int 2008;74:1197–1201. [DOI] [PubMed] [Google Scholar]
- 94.Kwon KM, Kam JH, Kim MY, Chung CH, Kim JK, Linton JA, Eom A, Koh SB, Kang HT. Inverse association between total bilirubin and metabolic syndrome in rural korean women. J Womens Health (Larchmt) 2011;20:963–969. [DOI] [PubMed] [Google Scholar]
- 95.Wu Y, Li M, Xu M, Bi Y, Li X, Chen Y, Ning G, Wang W. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J Diabetes 2011;3:217–224. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res 1999;85:663–671. [DOI] [PubMed] [Google Scholar]
- 97.Mazzone GL, Rigato I, Ostrow JD, Bossi F, Bortoluzzi A, Sukowati CH, Tedesco F, Tiribelli C. Bilirubin inhibits the TNFalpha-related induction of three endothelial adhesion molecules. Biochem Biophys Res Commun 2009;386:338–344. [DOI] [PubMed] [Google Scholar]
- 98.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 2001;166:4185–4194. [DOI] [PubMed] [Google Scholar]
- 99.Silva G, Cunha A, Gregoire IP, Seldon MP, Soares MP. The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. J Immunol 2006;177:1894–1903. [DOI] [PubMed] [Google Scholar]
- 100.Yet SF, Melo LG, Layne MD, Perrella MA. Heme oxygenase 1 in regulation of inflammation and oxidative damage. Methods Enzymol 2002;353:163–176. [DOI] [PubMed] [Google Scholar]
- 101.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation 2002;105:79–84. [DOI] [PubMed] [Google Scholar]
- 102.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res 2002;55:396–405. [DOI] [PubMed] [Google Scholar]
- 103.Li T, Tian H, Zhao Y, An F, Zhang L, Zhang J, Peng J, Zhang Y, Guo Y. Heme oxygenase-1 inhibits progression and destabilization of vulnerable plaques in a rabbit model of atherosclerosis. Eur J Pharmacol 2011;672:143–152. [DOI] [PubMed] [Google Scholar]
- 104.Togane Y, Morita T, Suematsu M, Ishimura Y, Yamazaki JI, Katayama S. Protective roles of endogenous carbon monoxide in neointimal development elicited by arterial injury. Am J Physiol Heart Circ Physiol 2000;278:H623–H632. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez AI, Gangopadhyay A, Kelley EE, Pagano PJ, Zuckerbraun BS, Bauer PM. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler Thromb Vasc Biol 2010;30:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem 1998;68:121–127. [DOI] [PubMed] [Google Scholar]
- 107.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood 2004;103:761–766. [DOI] [PubMed] [Google Scholar]
- 108.Li Volti G, Sacerdoti D, Sangras B, Vanella A, Mezentsev A, Scapagnini G, Falck JR, Abraham NG. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal 2005;7:704–710. [DOI] [PubMed] [Google Scholar]
- 109.Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost 2009;7:1401–1408. [DOI] [PubMed] [Google Scholar]
- 110.Wu BJ, Midwinter RG, Cassano C, Beck K, Wang Y, Changsiri D, Gamble JR, Stocker R. Heme oxygenase-1 increases endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2009;29:1537–1542. [DOI] [PubMed] [Google Scholar]
- 111.Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler Thromb Vasc Biol 2004;24:601–606. [DOI] [PubMed] [Google Scholar]
- 112.Peng L, Mundada L, Stomel JM, Liu JJ, Sun J, Yet SF, Fay WP. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxid Redox Signal 2004;6:729–735. [DOI] [PubMed] [Google Scholar]
- 113.Chen YH, Tsai HL, Chiang MT, Chau LY. Carbon monoxide-induced early thrombolysis contributes to heme oxygenase-1-mediated inhibition of neointimal growth after vascular injury in hypercholesterolemic mice. J Biomed Sci 2006;13:721–730. [DOI] [PubMed] [Google Scholar]
- 114.Chen B, Guo L, Fan C, Bolisetty S, Joseph R, Wright MM, Agarwal A, George JF. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am J Pathol 2009;175:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Katusic ZS, Nath KA. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol 2008;173:1882–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res 2007;101:893–901. [DOI] [PubMed] [Google Scholar]
- 117.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension 2001;38:210–215. [DOI] [PubMed] [Google Scholar]
- 118.Johnson RA, Lavesa M, DeSeyn K, Scholer MJ, Nasjletti A. Heme oxygenase substrates acutely lower blood pressure in hypertensive rats. Am J Physiol 1996;271:H1132–H1138. [DOI] [PubMed] [Google Scholar]
- 119.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels 1991;28:52–61. [DOI] [PubMed] [Google Scholar]
- 120.Lin H, McGrath JJ. Vasodilating effects of carbon monoxide. Drug Chem Toxicol 1988;11:371–385. [DOI] [PubMed] [Google Scholar]
- 121.Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, Ishimura Y. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest 1995;96:2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol 1999;276:H1641–H1646. [DOI] [PubMed] [Google Scholar]
- 123.Kozma F, Johnson RA, Nasjletti A. Role of carbon monoxide in heme-induced vasodilation. Eur J Pharmacol 1997;323:R1–R2. [DOI] [PubMed] [Google Scholar]
- 124.Graser T, Vedernikov YP, Li DS. Study on the mechanism of carbon monoxide induced endothelium-independent relaxation in porcine coronary artery and vein. Biomed Biochim Acta 1990;49:293–296. [PubMed] [Google Scholar]
- 125.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol 2004;286:H610–H618. [DOI] [PubMed] [Google Scholar]
- 126.Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest 1995;96:2676–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnson FK, Johnson RA. Carbon monoxide promotes endothelium-dependent constriction of isolated gracilis muscle arterioles. Am J Physiol Regul Integr Comp Physiol 2003;285:R536–R541. [DOI] [PubMed] [Google Scholar]
- 128.Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent vasodilatation in the adult rat cerebral microcirculation. Circ Res 2005;97:e104–e114. [DOI] [PubMed] [Google Scholar]
- 129.Favatier F, Polla BS. Tobacco-smoke-inducible human haem oxygenase-1 gene expression: role of distinct transcription factors and reactive oxygen intermediates. Biochem J 2001;353:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jonas JC, Guiot Y, Rahier J, Henquin JC. Haeme-oxygenase 1 expression in rat pancreatic beta cells is stimulated by supraphysiological glucose concentrations and by cyclic AMP. Diabetologia 2003;46:1234–1244. [DOI] [PubMed] [Google Scholar]
- 131.Ishizaka N, de Leon H, Laursen JB, Fukui T, Wilcox JN, De Keulenaer G, Griendling KK, Alexander RW. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation 1997;96:1923–1929. [DOI] [PubMed] [Google Scholar]
- 132.Bao W, Song F, Li X, Rong S, Yang W, Zhang M, Yao P, Hao L, Yang N, Hu FB, Liu L. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS ONE 2010;5:e12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Avogaro A, Pagnin E, Calo L. Monocyte NADPH oxidase subunit p22(phox) and inducible hemeoxygenase-1 gene expressions are increased in type II diabetic patients: relationship with oxidative stress. J Clin Endocrinol Metab 2003;88:1753–1759. [DOI] [PubMed] [Google Scholar]
- 134.Ndisang JF, Jadhav A. Up-regulating the hemeoxygenase system enhances insulin sensitivity and improves glucose metabolism in insulin-resistant diabetes in Goto-Kakizaki rats. Endocrinology 2009;150:2627–2636. [DOI] [PubMed] [Google Scholar]
- 135.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 2008;57:1526–1535. [DOI] [PubMed] [Google Scholar]
- 136.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 2009;53:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ndisang JF, Jadhav A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. Am J Physiol Endocrinol Metab 2009;296:E829–E841. [DOI] [PubMed] [Google Scholar]
- 138.Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, Kappas A, Abraham NG. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension 2010;56:1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 2009;296:C422–C432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, Duvigneau JC, Lindroos-Christensen J, Zapf TC, Amann S, Saluzzo S, Jantscher F, Stiedl P, Todoric J, Martins R, Oberkofler H, Muller S, Hauser-Kronberger C, Kenner L, Casanova E, Sutterluty-Fall H, Bilban M, Miller K, Kozlov AV, Krempler F, Knapp S, Lumeng CN, Patsch W, Wagner O, Pospisilik JA, Esterbauer H. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 2014;158:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Teupser D, Weber O, Rao TN, Sass K, Thiery J, Fehling HJ. No reduction of atherosclerosis in C-reactive protein (CRP)-deficient mice. J Biol Chem 2011;286:6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chida D, Osaka T, Hashimoto O, Iwakura Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 2006;55:971–977. [DOI] [PubMed] [Google Scholar]
- 143.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 2009;41:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng S, Enserro D, Xanthakis V, Sullivan LM, Murabito JM, Benjamin EJ, Polak JF, O'Donnell CJ, Wolf PA, O'Connor GT, Keaney JF, Vasan RS. Association of exhaled carbon monoxide with subclinical cardiovascular disease and their conjoint impact on the incidence of cardiovascular outcomes. Eur Heart J 2014;35:2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng S, Lyass A, Massaro JM, O'Connor GT, Keaney JF, Jr, Vasan RS. Exhaled carbon monoxide and risk of metabolic syndrome and cardiovascular disease in the community. Circulation 2010;122:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation 2006;114:2178–2189. [DOI] [PubMed] [Google Scholar]
- 147.Grosser N, Abate A, Oberle S, Vreman HJ, Dennery PA, Becker JC, Pohle T, Seidman DS, Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem Biophys Res Commun 2003;308:956–960. [DOI] [PubMed] [Google Scholar]
- 148.Ono K, Mannami T, Iwai N. Association of a promoter variant of the haeme oxygenase-1 gene with hypertension in women. J Hypertens 2003;21:1497–1503. [DOI] [PubMed] [Google Scholar]
- 149.Yun L, Xiaoli L, Qi Z, Laiyuan W, Xiangfeng L, Chong S, Jianfeng H, Shufeng C, Hongfan L, Gu D. Association of an intronic variant of the heme oxygenase-1 gene with hypertension in northern Chinese Han population. Clin Exp Hypertens 2009;31:534–543. [DOI] [PubMed] [Google Scholar]
- 150.Lin R, Fu W, Zhou W, Wang Y, Wang X, Huang W, Jin L. Association of heme oxygenase-1 gene polymorphisms with essential hypertension and blood pressure in the Chinese Han population. Genet Test Mol Biomarkers 2011;15:23–28. [DOI] [PubMed] [Google Scholar]
- 151.Haghdoost F, Javanmard SH, Keyhanian K, Samety AA, Loghmani P, Rafiei L, Sarrafzadegan N. Association of heme oxygenase-1 gene promoter polymorphism and blood pressure in an Iranian population. Intern Med J 2014;44:931–932. [DOI] [PubMed] [Google Scholar]
- 152.Wu MM, Chiou HY, Chen CL, Hsu LI, Lien LM, Wang CH, Hsieh YC, Wang YH, Hsueh YM, Lee TC, Cheng WF, Chen CJ. Association of heme oxygenase-1 GT-repeat polymorphism with blood pressure phenotypes and its relevance to future cardiovascular mortality risk: an observation based on arsenic-exposed individuals. Atherosclerosis 2011;219:704–708. [DOI] [PubMed] [Google Scholar]
- 153.Javanmard SH, Keyhanian K, Loghmani P, Samety AA, Haghdoost F, Rafiei L, Talaei M, Asgari S, Jazi MH, Sarrafzadegan N. Association between heme oxygenase-1 gene promoter polymorphisms and metabolic syndrome in Iranians. Mol Biol Rep 2012;39:3355–3360. [DOI] [PubMed] [Google Scholar]
- 154.Arredondo M, Fuentes M, Jorquera D, Candia V, Carrasco E, Leiva E, Mujica V, Hertrampf E, Perez F. Cross-talk between body iron stores and diabetes: iron stores are associated with activity and microsatellite polymorphism of the heme oxygenase and type 2 diabetes. Biol Trace Elem Res 2011;143:625–636. [DOI] [PubMed] [Google Scholar]
- 155.Song F, Li X, Zhang M, Yao P, Yang N, Sun X, Hu FB, Liu L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes in a Chinese population. Am J Epidemiol 2009;170:747–756. [DOI] [PubMed] [Google Scholar]
- 156.Arredondo M, Jorquera D, Carrasco E, Albala C, Hertrampf E. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with iron status in persons with type 2 diabetes mellitus. Am J Clin Nutr 2007;86:1347–1353. [DOI] [PubMed] [Google Scholar]
- 157.Choi SW, Yeung VT, Benzie IF. Heme oxygenase microsatellite polymorphism, oxidative stress, glycemic control, and complication development in type 2 diabetes patients. Free Radic Biol Med 2012;53:60–63. [DOI] [PubMed] [Google Scholar]
- 158.Chen YH, Chau LY, Chen JW, Lin SJ. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care 2008;31:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, Aizawa T, Ishizaka N, Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol 2002;22:1680–1685. [DOI] [PubMed] [Google Scholar]
- 160.Lublinghoff N, Winkler K, Winkelmann BR, Seelhorst U, Wellnitz B, Boehm BO, Marz W, Hoffmann MM. Genetic variants of the promoter of the heme oxygenase-1 gene and their influence on cardiovascular disease (the Ludwigshafen Risk and Cardiovascular Health study). BMC Med Genet 2009;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brydun A, Watari Y, Yamamoto Y, Okuhara K, Teragawa H, Kono F, Chayama K, Oshima T, Ozono R. Reduced expression of heme oxygenase-1 in patients with coronary atherosclerosis. Hypertens Res 2007;30:341–348. [DOI] [PubMed] [Google Scholar]
- 162.Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, Jordanova N, Wojta J, Mannhalter C, Wagner OF, Huber K. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemost 2004;91:155–161. [DOI] [PubMed] [Google Scholar]
- 163.Liang KW, Sheu WH, Lee WL, Lee IT, Lin SY, Ting CT, Lee WJ. Shorter GT repeats in the heme oxygenase-1 gene promoter are associated with a lower severity score in coronary artery disease. J Chin Med Assoc 2013;76:312–318. [DOI] [PubMed] [Google Scholar]
- 164.Chen M, Zhou L, Ding H, Huang S, He M, Zhang X, Cheng L, Wang D, Hu FB, Wu T. Short (GT) (n) repeats in heme oxygenase-1 gene promoter are associated with lower risk of coronary heart disease in subjects with high levels of oxidative stress. Cell Stress Chaperones 2012;17:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bai CH, Chen JR, Chiu HC, Chou CC, Chau LY, Pan WH. Shorter GT repeat polymorphism in the heme oxygenase-1 gene promoter has protective effect on ischemic stroke in dyslipidemia patients. J Biomed Sci 2010;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Funk M, Endler G, Schillinger M, Mustafa S, Hsieh K, Exner M, Lalouschek W, Mannhalter C, Wagner O. The effect of a promoter polymorphism in the heme oxygenase-1 gene on the risk of ischaemic cerebrovascular events: the influence of other vascular risk factors. Thromb Res 2004;113:217–223. [DOI] [PubMed] [Google Scholar]
- 167.Chen YH, Hung SC, Tarng DC. Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients. Clin J Am Soc Nephrol 2013;8:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dick P, Schillinger M, Minar E, Mlekusch W, Amighi J, Sabeti S, Schlager O, Raith M, Endler G, Mannhalter C, Wagner O, Exner M. Haem oxygenase-1 genotype and cardiovascular adverse events in patients with peripheral artery disease. Eur J Clin Invest 2005;35:731–737. [DOI] [PubMed] [Google Scholar]
- 169.Kral A, Kovarnik T, Kralik L, Skalicka H, Horak J, Mintz GS, Uhrova J, Sonka M, Wahle A, Downe R, Aschermann M, Martasek P, Linhart A. Genetic variants in haem oxygenase-1 and endothelial nitric oxide synthase influence the extent and evolution of coronary artery atherosclerosis. Folia Biol (Praha) 2011;57:182–190. [PubMed] [Google Scholar]
- 170.Wu MM, Chiou HY, Lee TC, Chen CL, Hsu LI, Wang YH, Huang WL, Hsieh YC, Yang TY, Lee CY, Yip PK, Wang CH, Hsueh YM, Chen CJ. GT-repeat polymorphism in the heme oxygenase-1 gene promoter and the risk of carotid atherosclerosis related to arsenic exposure. J Biomed Sci 2010;17:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu MM, Chiou HY, Chen CL, Wang YH, Hsieh YC, Lien LM, Lee TC, Chen CJ. GT-repeat polymorphism in the heme oxygenase-1 gene promoter is associated with cardiovascular mortality risk in an arsenic-exposed population in northeastern Taiwan. Toxicol Appl Pharmacol 2010;248:226–233. [DOI] [PubMed] [Google Scholar]
- 172.Chen YH, Chau LY, Lin MW, Chen LC, Yo MH, Chen JW, Lin SJ. Heme oxygenase-1 gene promotor microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J 2004;25:39–47. [DOI] [PubMed] [Google Scholar]
- 173.Gulesserian T, Wenzel C, Endler G, Sunder-Plassmann R, Marsik C, Mannhalter C, Iordanova N, Gyongyosi M, Wojta J, Mustafa S, Wagner O, Huber K. Clinical restenosis after coronary stent implantation is associated with the heme oxygenase-1 gene promoter polymorphism and the heme oxygenase-1 +99G/C variant. Clin Chem 2005;51:1661–1665. [DOI] [PubMed] [Google Scholar]
- 174.Tiroch K, Koch W, von Beckerath N, Kastrati A, Schomig A. Heme oxygenase-1 gene promoter polymorphism and restenosis following coronary stenting. Eur Heart J 2007;28:968–973. [DOI] [PubMed] [Google Scholar]
- 175.Schillinger M, Exner M, Minar E, Mlekusch W, Mullner M, Mannhalter C, Bach FH, Wagner O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: a novel vascular protective factor. J Am Coll Cardiol 2004;43:950–957. [DOI] [PubMed] [Google Scholar]
- 176.Schillinger M, Exner M, Mlekusch W, Ahmadi R, Rumpold H, Mannhalter C, Wagner O, Minar E. Heme oxygenase-1 genotype is a vascular anti-inflammatory factor following balloon angioplasty. J Endovasc Ther 2002;9:385–394. [DOI] [PubMed] [Google Scholar]
- 177.Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther 2001;8:433–440. [DOI] [PubMed] [Google Scholar]
- 178.Li P, Elrayess MA, Gomma AH, Palmen J, Hawe E, Fox KM, Humphries SE. The microsatellite polymorphism of heme oxygenase-1 is associated with baseline plasma IL-6 level but not with restenosis after coronary in-stenting. Chin Med J (Engl) 2005;118:1525–1532. [PubMed] [Google Scholar]
- 179.Guzek M, Jakubowski Z, Bandosz P, Wyrzykowski B, Smoczynski M, Jabloiska A, Zdrojewski T. Inverse association of serum bilirubin with metabolic syndrome and insulin resistance in Polish population. Przegl Epidemiol 2012;66:495–501. [PubMed] [Google Scholar]
- 180.Choi SH, Yun KE, Choi HJ. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutr Metab Cardiovasc Dis 2013;23:31–37. [DOI] [PubMed] [Google Scholar]
- 181.Jo J, Yun JE, Lee H, Kimm H, Jee SH. Total, direct, and indirect serum bilirubin concentrations and metabolic syndrome among the Korean population. Endocrine 2011;39:182–189. [DOI] [PubMed] [Google Scholar]
- 182.Hwang HJ, Kim SH. Inverse relationship between fasting direct bilirubin and metabolic syndrome in Korean adults. Clin Chim Acta 2010;411:1496–1501. [DOI] [PubMed] [Google Scholar]
- 183.Lin LY, Kuo HK, Hwang JJ, Lai LP, Chiang FT, Tseng CD, Lin JL. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis 2009;203:563–568. [DOI] [PubMed] [Google Scholar]
- 184.Choi SW, Lee YH, Kweon SS, Song HR, Ahn HR, Rhee JA, Choi JS, Shin MH. Association between total bilirubin and hemoglobin A1c in Korean type 2 diabetic patients. J Korean Med Sci 2012;27:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sheu WH, Chen YT, Lee WJ, Wang CW, Lin LY. A relationship between serum ferritin and the insulin resistance syndrome is present in non-diabetic women but not in non-diabetic men. Clin Endocrinol (Oxf) 2003;58:380–385. [DOI] [PubMed] [Google Scholar]
- 186.Jung CH, Lee MJ, Kang YM, Hwang JY, Jang JE, Leem J, Park JY, Kim HK, Lee WJ. Higher serum bilirubin level as a protective factor for the development of diabetes in healthy Korean men: a 4 year retrospective longitudinal study. Metabolism 2014;63:87–93. [DOI] [PubMed] [Google Scholar]
- 187.Kim KM, Kim BT, Park SB, Cho DY, Je SH, Kim KN. Serum total bilirubin concentration is inversely correlated with Framingham risk score in Koreans. Arch Med Res 2012;43:288–293. [DOI] [PubMed] [Google Scholar]
- 188.Djousse L, Levy D, Cupples LA, Evans JC, D'Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol 2001;87:1196–1200. [DOI] [PubMed] [Google Scholar]
- 189.Horsfall LJ, Nazareth I, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation 2012;126:2556–2564. [DOI] [PubMed] [Google Scholar]
- 190.Kunutsor SK, Bakker SJ, Gansevoort RT, Chowdhury R, Dullaart RP. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol 2015;35:716–724. [DOI] [PubMed] [Google Scholar]
- 191.Hedblad B, Engstrom G, Janzon E, Berglund G, Janzon L. COHb% as a marker of cardiovascular risk in never smokers: results from a population-based cohort study. Scand J Public Health 2006;34:609–615. [DOI] [PubMed] [Google Scholar]
- 192.Troughton JA, Woodside JV, Young IS, Arveiler D, Amouyel P, Ferrieres J, Ducimetiere P, Patterson CC, Kee F, Yarnell JW, Evans A, Group PS. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Eur J Cardiovasc Prev Rehabil 2007;14:79–84. [DOI] [PubMed] [Google Scholar]
- 193.Jorgensen ME, Torp-Pedersen C, Finer N, Caterson I, James WP, Legler UF, Andersson C. Association between serum bilirubin and cardiovascular disease in an overweight high risk population from the SCOUT trial. Nutr Metab Cardiovasc Dis 2014;24:656–662. [DOI] [PubMed] [Google Scholar]


