Abstract
Background
Gastric cancer is the most prevalent cancer among men and the third most prevalent cancer among women in Iran. Its most important reason for death is its belated diagnosis at the advanced stages of the disease. Various factors can be effective on the survival of these patients after surgery, which are the major concern in this study.
Methods
Data from 330 patients with Gastric cancer who had undergone surgery at Iran Cancer Institute from 1995 to 1999 were analyzed. The Survival Time of patients was determined after surgery and the effect of individual and demographic; clinical and diagnostic; and treatment and post-surgical factors on patients’ survival was studied. For data analysis, Kaplan- Meier, Log-Rank test and Proportional Hazards Model were used.
Results
The median of survival time was 16.33 months. The one-year, three-year, and five-year survival rates were, 0.66, 0.31, and 0.21. Based on univariate analysis results of age(P<0.001), metastases(P=0.012), disease stage(P=0.016), and number of renewed treatments(P<0.001), as well as multivariate analysis which was used to investigate the simultaneous effect of influencing variables on patients’ survival showed that age(61−70:HR=1.40,>70:HR=2.08), marital status(HR=0.39), number of renewed treatments(1:HR=0.54, 2:HR=0.30, 3:HR=0.22), relapse(HR=1.51), type of gastrectomy (Subtotal: HR=1.12, Distal:HR=0.49, Partial:HR=0.94, Proximal:HR=0.52), liver metastases (HR=1.79), distance metastases(HR=1.84), and disease stage(II:HR=1.28, III:HR=2.12, IV:HR=1.90) variables had a significant effect on patients’ survival.
Conclusion
Patients who call on doctors in early stages of disease will have a higher survival rate due to early diagnosis whereas disease progression will increase the risk and will decrease the survival. Identifying factors affecting patients’ survival and improving diagnostic methods can prevent disease progression and increase survival rate.
Keywords: Gastric cancer, Proportional hazards model, Survival rate, Iran
Introduction
Gastric cancer is defined as the uncontrolled growth of malignant cells in the stomach. Most people show no symptoms until the advanced stage of the disease; therefore, Gastric cancer is one of the most common causes of cancer deaths all over the world. Now, Gastric cancer includes 10% of cancers in the world and is one of the most prevalent kinds of cancers. Every year more than 930 thousand new cases are reported all over the world and more than 700 thousand people die from this type of cancer (1). Gastric cancer incidence is completely different in various geographical regions in the world and from this aspect, the world can be divided into 3 regions: high-inci dence regions (ASR>20), middle-incidence regions (ASR>10), and low-incidence regions (ASR<10) (2).
In Iran, northern regions have been identified with a high incidence, central and western regions with a middle incidence, and southern areas with a low incidence (3–6). According to the latest statistics of Iran Cancer Research Center, Gastric cancer is the most common cancer among Iranian men and the third most common cancer among Iranian women after breast cancer (7).
Stomach cancer is usually classified according to the anatomic location of the cancer including Cardia and Non-cardia. Research shows that while regional Non-cardia cancer is declining in the world, regional Cardia cancer is increasing rapidly (6, 8, 9).
Unlike Japan and countries in Western Europe and North America, the incidence of Gastric cancer has been rising over the past 30 years in Iran (3, 9, 10). Studies over this type of cancer in Iran and in high-incidence areas show that the rising rate of Gastric cancer in these regions is due to the high rate of regional Cardia cancer (11).
Gastric cancer is usually treated with surgery, radiotherapy, or chemotherapy. The primary treatment of Gastric cancer in initiative stages is surgery; so it is regarded as the best treatment for cancer. Radiotherapy and chemotherapy will be used as renewed treatments, if necessary. In advanced stages of the disease, surgical procedures, radiotherapy and chemotherapy are also used for the treatment but they do not usually achieve good outcomes. The chances of patients’ full recovery depend on the surgery but the time when the disease passes through the mucous membrane it is possible to spread to the lymph nodes and to causes relapse in spite of the successful surgery which has been performed on the patient (12–15).
One of the most important objectives specified after the right diagnosis and prompt treatment for the patients with Gastric cancer is the survival rate increase especially the 5-year survival rate. Unfortunately, more than 80% of patients with Gastric cancer are diagnosed at a stage when conventional therapies such as gastrectomy, chemotherapy, or radiation therapy are not effective in increasing the patients’ survival (16, 17). For this reason, the 5-year survival rate is low in patients with Gastric cancer after surgery. This rate has been reported to be 29.6% in China, 4.4% in Thailand, 37% in the US, 22% in Switzerland, and 30% in France (18–22). The results of various studies indicate that the type of gastrostomy, age, disease stage, and metastases are the factors affecting the patient’ survival (14, 18–20, 23). The increase in these patients’ survival after surgery involves identifying various factors, including demographic, clinical and diagnostic, therapeutic, and post-surgical factors. Not only do events such as relapses, Metastases, etc. affect survival, they also affect the treatment method and the number of renewed treatments in patients (24, 25).
This study has been designed and carried out to determine the 5-year survival rate in patients with Gastric cancer undergone surgery at the most important Cancer Treatment Center in Iran (Cancer Institute) and to investigate demographic, clinical and diagnostic, therapeutic, and post-surgical factors which affect these patients’ survival.
Materials and Methods
In this historical study, 330 patients with Gastric cancer with the following data were studied: 1) the patients had been hospitalized and had undergone surgery from 1995 to 1999 in surgical wards of Iran Cancer Institute 2) these patients had records in the archives of the hospital, and in their files their addresses and phone numbers were available for subsequent follow-ups. The survival status of these patients in 2011 was determined by reopening the files as well as phone calls. The survival time of these patients was determined after surgery and those patients who were still alive at the end of study period or the ones whose data were not available after a specific time-period were considered right-censored.
The effect of demographic variables such as Age (at the time of surgery), Sex, Marital status, and Smoking history, as well as clinical data such as Tumor location (Cardia - Anterior - other), Type of pathology (Adenocarcinoma - other), Disease stage (I-II-III-IV), Metastases, Location of Metastases (lymph nodes - Liver - other), and the Type and extent of gastrectomy (T.G-S.G-D.G-PT.G-PX.G) was studied. Moreover, the effect of post-surgical and treatment variables including relapse and the number of renewed treatments (chemotherapy - radiotherapy - surgery or a combination of them) on patients’ survival was evaluated.
TNM version 6 was used to determine the stage of the disease. According to this method, four stages were considered for patients.(26) These stages are:
Stage I:
-
–
Tumor has just reached the submucosa layer and the cancerous cells exist in 6 lymph nodes at most.
-
–
Tumor has invaded the muscle layer or subserosa layer and the cancerous cells have not spread to the lymph node or other organs.
Stage II:
-
–
Tumor has just reached the submucosa layer but the cancerous cells have spread to 7 to 15 lymph nodes.
-
–
Tumor has invaded the muscle layer or subserosa layer and the cancerous cells are seen in 1 to 6 lymph nodes.
-
–
Tumor has penetrated the serosa layer of the stomach but lymph nodes or other organs are not involved.
Stage III:
-
–
Tumor has invaded the muscle layer or subserosa layer and the cancerous cells are seen in 7 to 15 lymph nodes.
-
–
Tumor has penetrated the serosa layer and the cancerous cells have spread to 1 to 6 lymph nodes.
-
–
Tumor has involved adjacent organs such as the liver and spleen, but the cancerous cells have not spread to lymph nodes or distant organs.
Stage IV:
-
–
Cancerous cells have reached more than 15 lymph nodes.
-
–
Tumor has invaded adjacent organs and there is at least 1 lymph node.
-
–
Cancerous cells have spread to distant organs.
The significance level was set at 5%. Data analysis was performed using the Kaplan - Meier, Log-Rank Test, and Cox Proportional Hazards Model. Data were put in STATA 11 software for statistical indexes.
Results
The mean and median age at diagnosis time were 65.61 ± 11 and 68 years (range: 32 to 96 years). The mean of age diagnosis was 65.7 ± 11.22 years for men and 65.41 ± 10.56 years for women. 239 patients (72.4%) died by the end of the study and the rest were censored. The survival mean and median of these patients were 24.86 ± 23.73 and 16.33 months, respectively. The patients’ one-year, three-year, and five-year survival rates were 0.66, 0.31, 0.21, consecutively (Figure 1). Analyses showed that 43 patients (13.03%) had a relapse, and in 43.9% of patients Cardia and in 19.1% of them Anterior was involved. In the pathology of 85.2% of patients Adenocarcinoma and for the rest of patients other pathologies (squamous cell carcinoma, small cell carcinoma, carcinoid tumor, carcinoma, malignant lymphoma, stromal tumor, spindle tumor) have been reported.192 patients (58.2%) had metastases out of which 66.67% suffered from lymph nodes metastases only. 52.42% of patients had undergone Total Gastrectomy, 27.27% had undergone Subtotal Gastrectomy, 3.03% had undergone Distal Gastrectomy, 8.79% had undergone Partial Gastrectomy and 8.48% had undergone Proximal Gastrectomy. The analysis of disease stage revealed that 6.67% of patients were in stage I, 18.18% in stage II, 16.36% in stage III and 58.79% in stage IV. 20.3% of patients had not received any renewed treatments whereas 26.06% of the patients had received three renewed treatments. The comparison of survival probability in both sexes demonstrated that the median of survival time was 20.81 months for men and 17.83 months for women. This differ ence was not statistically significant, in addition, marital status and smoking history had no significant effect on patients’ survival. The survival probability decreased significantly with age (P <0.001), and the medians of survival time in the age groups lower than 60 years; 61-70 years; and higher than 70 years were 29.97%, 23.66% and 12.36%, respectively. Analyses showed that the lowest chance of survival was related to those patients whose Cardia was involved (median 16 months) and then those who suffered from Anterior involvement (median 23.9 months) and finally the patients whose other parts were involved (median 21.5 months). These differences, however, were not statistically significant. The survival probability of patients with Adenocarcinoma Pathology was lower than other pathologies (median of survival time 18.7 months vs. 23.14 months). This difference was not statistically significant either. As it was expected, in patients suffering from metastases the survival probability was significantly lower (P=0.012) and their medians of survival time were 16.9 and 22.37 months, respectively.
Fig. 1:
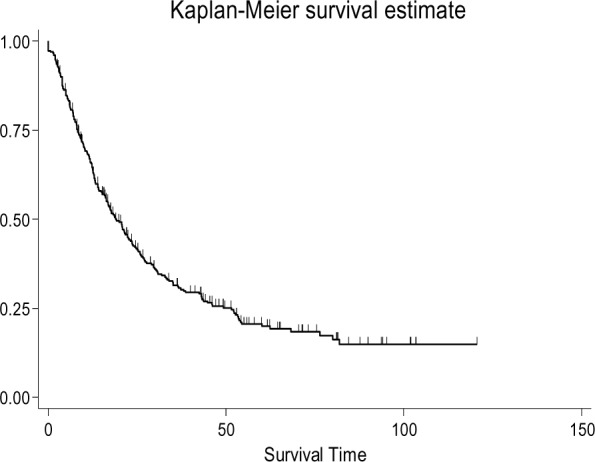
Gastric Cancer Patients Survival Probability
Analyses on patients with metastases disclosed that the median of survival time in patients whose lymph nodes, liver, and other parts were involved, had been 16.9, 17.3, and 16.33 months, respectively, but these differences were not statistically significant. But further investigation showed that Distance Metastases had a significant effect on patients’ survival (P=0.035). The disease stage had also significant effect on patients’ survival (P=0.016) so that the median of survival time has reached from 29.1 months in stage I to 17.3 months in stage IV. Furthermore, the median of survival time for patients with relapse was 17.77 months while it was 19 months for other patients. This difference was not statistically significant. Patients’ survival rate increases with an increase in the number of renewed treatments (P <0.001) so that the median of survival time for patients who received no renewed treatments after surgery was 5 months while it was 33.47 months for patients who received three renewed treatments after surgery (Table 1).
Table 1.
Univariate analysis of Risk factors
| Risk factor | No. of Patient (%) | Median Survival Time(Month) | Log-Rank Test | P-value |
|---|---|---|---|---|
| Sex | 0.35 | 0.870 | ||
| Male | 228(69.1) | 20.81 | ||
| Female | 102(30.9) | 17.83 | ||
| Age | 40.81 | <0.001 | ||
| <61 | 87(26.36) | 29.97 | ||
| 61-70 | 123(37.27) | 23.66 | ||
| >70 | 120(36.36) | 12.36 | ||
| Marital Status | 3.64 | 0.056 | ||
| Married | 315(95.45) | 18.70 | ||
| Single | 15(4.55) | 68.23 | ||
| Smoking History | 0.18 | 0.668 | ||
| NO | 230(69.70) | 21.27 | ||
| Yes | 100(30.30) | 18.30 | ||
| Tumor Location | 2.66 | 0.264 | ||
| Cardia | 145(43.94) | 16 | ||
| Anterior | 63(19.09) | 23.9 | ||
| Other | 122(36.97) | 21.5 | ||
| Pathology | 1.07 | 0.300 | ||
| Adenocarcinoma | 281(85.15) | 18.70 | ||
| Other* | 49(14.85) | 23.14 | ||
| Metastases | 6.36 | 0.012 | ||
| Negative | 138(41.82) | 22.37 | ||
| Positive | 192(58.18) | 16.90 | ||
| Location of Metastases | 2.34 | 0.310 | ||
| Lymph | 128(66.67) | 16.90 | ||
| Liver | 24(12.50) | 17.30 | ||
| Other** | 40(20.83) | 16.33 | ||
| Lymph node Metastases | 1.03 | 0.310 | ||
| Negative | 187(56.67) | 20.81 | ||
| Positive | 143(43.33) | 17.53 | ||
| Liver Metastases | 1.52 | 0.217 | ||
| Negative | 306(92.73) | 19.27 | ||
| Positive | 24(7.27) | 17.30 | ||
| Distance Metastases | 4.47 | 0.035 | ||
| Negative | 287(86.97) | 20.70 | ||
| Positive | 43(13.03) | 16.33 | ||
| Relapse | 1.97 | 0.161 | ||
| No | 287(86.97) | 19.00 | ||
| Yes | 43(13.03) | 17.77 | ||
| Stage | 10.34 | 0.016 | ||
| I | 22(6.67) | 29.10 | ||
| II | 60(18.18) | 25 | ||
| III | 54(16.36) | 13.36 | ||
| IV | 194(58.79) | 17.30 | ||
| Number of Renewed Treatment | 57.48 | <0.001 | ||
| 0 | 67(20.30) | 5.00 | ||
| 1 | 76(23.03) | 12.53 | ||
| 2 | 101(30.61) | 23.54 | ||
| 3 | 86(26.06) | 33.47 | ||
| Type of Gastrectomy | 2.18 | 0.702 | ||
| Total | 173(52.42) | 18.70 | ||
| Subtotal | 90(27.27) | 17.00 | ||
| Distal | 10(3.03) | 43.17 | ||
| Partial | 29(8.79) | 19.00 | ||
| Proximal | 28(8.48) | 24.67 | ||
* Squamous cell carcinoma (SCC), small-cell carcinoma, carcinoid tumor, spindle cell tumor, sarcoma, malignant lymphoma./** Diaphragm, spleen, pancreas, lungs, bone
For multivariate analysis, all variables were entered simultaneously in Cox proportional hazards model. Using backward method and considering the probability of 5% to enter the variables in the model and regarding elimination probability of 10%, the most important variables were identified and their effect on survival time was investigated. The variables of age, marital status, number of renewed treatments, relapse, type of gastrectomy, liver metastases, distance metastases, and disease stage were identified as variables influencing patients’ survival.
The investigation showed that the Hazard Ratios of patients in stages II, III and IV vis-à-vis the patients in stage I, were 1.28 (95%CI: 0.67 - 2.45), 2.12 (95%CI: 1.11 - 4.07) and 1.90 (95%CI: 1.04 — 3.46) times. Relapse will also increase death hazard up to 1.51 (95%CI: 1.03 — 2.22) times. The number of renewed treatments continues to be a positive factor in survival of the patients so that with an increase in the number of renewed treatments, death hazard will reduce in patients who have not received any renewed treatments after gastrectomy. Liver and distance metastases will also increase death hazard up to 1.79 (95%CI: 1.06 — 3.04) and 1.84 (95%CI: 1.24 — 2.75) times, respectively. The analysis also showed that death hazard is lower in single patients and the probability of survival decreases with age (Table 2).
Table 2.
Multivariate analysis of Potential Risks factors
| Risk factor | B | SE | Wald statistic | df | P-value | Hazard Ratio | 95% confidence interval for HR(CI) |
|---|---|---|---|---|---|---|---|
| Age | 15.78 | 2 | <0.001 | ||||
| <61 | |||||||
| 61-70 | 0.34 | 0.18 | 3.43 | 1 | 0.064 | 1.40 | 0.98-1.99 |
| >70 | 0.77 | 0.19 | 15.47 | 1 | <0.001 | 2.08 | 1.44-3.00 |
| Marital Status | |||||||
| Married | |||||||
| Single | -0.94 | 0.43 | 4.76 | 1 | 0.029 | 0.39 | 0.17-0.91 |
| Liver Metastases | |||||||
| Negative | |||||||
| Positive | 0.58 | 0.27 | 6.45 | 1 | 0.030 | 1.79 | 1.06-3.04 |
| Distance Metastases | |||||||
| Negative | |||||||
| Positive | 0.61 | 0.20 | 8.97 | 1 | 0.003 | 1.84 | 1.24-2.75 |
| Relapse | |||||||
| No | |||||||
| Yes | 0.41 | 0.20 | 4.45 | 1 | 0.035 | 1.51 | 1.03-2.22 |
| Stage | 8.97 | 3 | 0.030 | ||||
| I | |||||||
| II | 0.24 | 0.33 | 0.54 | 1 | 0.461 | 1.28 | 0.67-2.45 |
| III | 0.75 | 0.33 | 5.16 | 1 | 0.023 | 2.12 | 1.11-4.07 |
| IV | 0.64 | 0.31 | 4.38 | 1 | 0.036 | 1.90 | 1.04-3.46 |
| Number of Renewed Treatment | 54.242 | 3 | <0.001 | ||||
| 0 | |||||||
| 1 | -0.61 | 0.21 | 8.88 | 1 | 0.003 | 0.54 | 0.36-0.81 |
| 2 | -1.21 | 0.20 | 35.80 | 1 | <0.001 | 0.30 | 0.20-0.44 |
| 3 | -1.52 | 0.23 | 44.87 | 1 | <0.001 | 0.22 | 0.14-0.34 |
| Type of Gastrectomy | 10.388 | 4 | 0.034 | ||||
| Total | |||||||
| Subtotal | 0.12 | 0.16 | 0.58 | 1 | 0.448 | 1.12 | 0.83-1.53 |
| Distal | -0.70 | 0.44 | 2.57 | 1 | 0.109 | 0.49 | 0.21-1.17 |
| Partial | -0.06 | 0.24 | 0.07 | 1 | 0.795 | 0.94 | 0.59-1.50 |
| Proximal | -0.66 | 0.26 | 6.45 | 1 | 0.011 | 0.52 | 0.31-0.86 |
It is worth noting that the most important condition to use Cox Proportional Hazard Model is to make the assumption of proportional hazard (PH) for all independent variables included in the model. So, in order to test this assumption, Schoenfeld Residual(27) is used(28, 29). This test can help assess proportional hazard for each of the independent variables as well as the final model. The results of these assessments showed that multivariate analysis model enjoys the assumption of proportional hazards.
Discussion
In this study, 5-year survival rate of patients with Gastric cancer was estimated at 21% which was lower than the estimation obtained in the studies conducted in countries like the US, Switzerland, France, and China(18–22, 30). Unfortunately, most patients with Gastric cancer are diagnosed at a stage when conventional therapies such as gastrectomy, chemo-therapy, or radiation therapy are not effective in increasing the patients’ survival. For this reason, the 5-year survival rate is low in patients with Gastric cancer after surgery. In the present study, men had higher survival rates than women, but this difference was not statistically significant. The results of this study are consistent with the results of some studies which reported better survival rates for men and a higher death hazard for women(31–34). Some studies, however, have statistically reported the significant effect of gender(35–37). In this study, the survival rate decreased significantly with age, which is in harmony with the studies carried out in the US, Japan, and Italy(32, 38, 39). 43 patients (13.03%) had a relapse, but their survival rate was not significantly different from other patients statistically. Also, 192 patients (58.18%) had metastases whose survival was far less than other patients. This finding has been confirmed in all other studies too(32–34, 40). Of 192 patients with metastases, 128 patients (66.67%) had lymph nodes involvement, 24 patients (12.5%) had liver metastases and 40 patients (20.83%) had distance metastases, but no significant difference was observed between the survival rates of patients and the location of involvement. Distance metastases reduced patients’ survival. As these patients were classified in stage IV, this result was already expected.
The disease stage highly influenced the patients’ survival so that the median of survival time was 29.10 months in stage I and 17.30 months in stage IV. The effect of disease stage on patients’ survival was also observed in other studies done in developed and Western countries(19, 41, 42). As expected, the survival rate increased with an increase in the number of renewed treatments. Due to the fact that patients with low survival rate had practically no chance to receive renewed treatment or in more severe cases doctors had anticipated little chance of recovery leading to less attention, these findings could be justified. The study also revealed that the survival rate of married patients was lower than single ones, and the survival rate of those who had not a history of smoking was higher than those who had. But none of these differences were statistically significant.
Multivariate analysis which was used to investigate the simultaneous effect of influencing variables on patients’ survival showed that marital status, age, disease stage, type of gastrectomy, relapse, liver metastases, and distance metastases variables had a significant effect on patients’ survival. Having liver and distance metastases, relapse, and aging would decrease patients’ chance of survival. These results are consistent with the findings of most studies, but in some studies the location of a tumor has also pointed out to be influential in addition to the aforementioned variables (42).
Conclusion
The results of this study showed that people who call on doctors in the early stages of the disease will have a higher survival rate due to early diagnosis. Delayed diagnosis causes disease progression and increases risk and decreases patients’ survival. Identifying more affecting factors on the survival of patients with Gastric cancer undergone surgery and improving diagnostic methods can prevent disease progression and increase patients’ survival receiving effective treatment. In the end, it should be noted that due to data limitation of the study to Iran Cancer Institute, the survival estimation of patients with gastric cancer undergone surgery in this study can not necessarily be an accurate estimation of these patients’ survival rate. Achieving a true estimation of the survival status of such patients requires studies in lager scales.
Ethical considerations
Ethical issues (Including plagiarism, Informed Con-sent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors gratefully acknowledge the financial support for this work provided by Tehran University of Medical Sciences. The authors declare that there is no conflict of interests.
Reference
- Parkin DM, Bray F, Ferlay J, Pisani P (2005). Global cancer statistics, 2002. CA:Ca-Cancer J Clin, 55: 74. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011). Global cancer statistics. CA: Ca-Cancer J Clin, 61: 69–90. [DOI] [PubMed] [Google Scholar]
- Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, Yazdanbod A, Shokoohi B, Mashayekhi A, Arshi S (2003). Cancer occurrence in Ardabil: Results of a population‐based Cancer Registry from Iran. Int J Cancer, 107: 113–118. [DOI] [PubMed] [Google Scholar]
- Sadjadi A, Zahedi M, Nouraie M, Alimohammadian M, Ghorbani A, Bahmanyar S, Mohagheghi M, Malekzadeh R (2007). The first population-based cancer survey in Kerman Province of Iran. Iranianin J Publ Health, 36(4): 26–34. [Google Scholar]
- Semnani S, Sadjadi A, Fahimi S et al. (2006). Declining incidence of esophageal cancer in the Turkmen Plain, eastern part of the Caspian Littoral of Iran: a retrospective cancer surveillance. Cancer Detect Prev, 30: 14–19. [DOI] [PubMed] [Google Scholar]
- Alireza S, Mehdi N, Ali M (2005). Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev, 6: 359. [PubMed] [Google Scholar]
- Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z (2009). Cancer incidence and mortality in Iran. Ann Oncol, 20: 556–563. [DOI] [PubMed] [Google Scholar]
- Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, Rakhshani N, Didevar R, Sotoudeh M, Zolfeghari A (2008). Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut, 57: 298–305. [DOI] [PubMed] [Google Scholar]
- Babaei M, Mousavi S, Malek M, Tosi G, Masoumeh Z, Danaei N, Gafar G (2005). Cancer occurrence in Semnan Province, Iran: results of a population-based cancer registry. Asian Pac J Cancer Prev, 6: 159–164. [PubMed] [Google Scholar]
- Yazdizadeh B, Jarrahi AM, Mortazavi H, Mohagheghi MA, Tahmasebi S, Nahvijo A (2005). Time trends in the occurrence of major GI cancers in Iran. Asian Pac J Cancer Prev, 6: 130–134. [PubMed] [Google Scholar]
- Derakhshan M, Yazdanbod A, Sadjadi A, Shokoohi B, McColl K, Malekzadeh R (2004). High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut, 53: 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson LL, Sosin H (1982). Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol, 8: 1–11. [DOI] [PubMed] [Google Scholar]
- Wisbeck WA, Becker EM, Russell AH (1986). Adenocarcinoma of the stomach: Autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol, 7: 13–18. [DOI] [PubMed] [Google Scholar]
- Sadighi S, Mohagheghi M, Haddad P, Omranipoor R, AR MJ, Meemari F, Raafat J, Shahbazkhani B, Khalili N (2008). Life expectancy with perioperative chemotherapy and chemora-diotherapy for locally advanced gastric adenoc-arcinoma. Tehran University Medical Journal (TUMJ), 66(9): 664–669. [Google Scholar]
- Association JGC (2011). Japanese gastric cancer treatment guidelines 2010 (ver. 3). GastricCancer, 14: 113–123. [DOI] [PubMed] [Google Scholar]
- Samadi F, Babaei M, Yazdanbod A, Fallah M, Nouraie M, Nasrollahzadeh D, Sadjadi A, Derakhshan MH, Shokuhi B, Fuladi R (2007). Survival rate of gastric and esophageal cancers in Ardabil province, North-West of Iran. Arch Iran Med, 10(1): 32–37. [PubMed] [Google Scholar]
- Sadighi S, Raafat J, Mohagheghi M, Meemary F (2005). Gastric carcinoma: 5 year experience of a single institute. Asian Pac J Cancer Prev, 6: 195–196. [PubMed] [Google Scholar]
- Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L, Liu YX (2004). Correlation of tumor-positive ratio and number of perigastric lymph nodes with prognosis of patients with surgically-removed gastric carcinoma. World J Gastroentero, 10: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong-Ngam D, Tangkijvanich P, Mahachai V, Kullavanijaya P (2001). Current status of gastric cancer in Thai patients. J Med Assoc Thai, 84: 475–482. [PubMed] [Google Scholar]
- Schwarz RE, Zagala-Nevarez K (2002). Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol, 9: 394–400. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S (2003). AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology, 65: 95–101. [DOI] [PubMed] [Google Scholar]
- Triboulet J, Fabre S, Castel B, Toursel H (2001). Adenocarcinomas of the distal esophagus and cardia: Surgical management. Cancer Radither, 5: 90–97. [PubMed] [Google Scholar]
- Biglarian A, Hajizadeh E, Kazemnejad A, Zali M (2009). Survival analysis of gastric cancer patients using Cox model: afive year study. Tehran University Medical Journal (TUMJ), 14: 6369–6370. [Google Scholar]
- Zeraati H, Mahmoudi M, Kazemnejad A, Mohammad K (2005). Postoperative Survival in Gastric Cancer Patients and its Associated Factors: A Method Based on a Non-homogenous Semi-Markovian Process. Int J Cancer Res, 1: 87–93. [Google Scholar]
- Zeraati H, Mahmoudi M, Kazemnejad A, Mohammad K (2006). Postoperative survival in gastric cancer patients and its associated factors: A time dependent covariates model. Iranianin J Publ Health, 35 (3)40–46. [Google Scholar]
- Greene FL, Page DL, Fleming ID, Balch CM, Fritz AG (2002). AJCC cancer staging handbook: from the AJCC cancer staging manual. ed. Springer Verlag. [Google Scholar]
- Schoenfeld D (1982). Partial residuals for the proportional hazards regression model. Biometrika, 69: 239–241. [Google Scholar]
- Klein JP, Moeschberger ML (2003). Survival analysis: techniques for censored and truncated data. ed. Springer. [Google Scholar]
- Hosmer DW, Lemeshow S, May S (2011). Applied survival analysis: regression modeling of time to event data. ed. Wiley-Interscience. [Google Scholar]
- Wang CS, Hsieh CC, Chao TC, Jan YY, Jeng LB, Hwang TL, Chen MF, Chen PC, Chen JS, Hsueh S (2002). Resectable gastric cancer: operative mortality and survival analysis. Chang Gung Medical Journal, 25: 216–227. [PubMed] [Google Scholar]
- Sigon R, Canzonieri V, Rossi C (2003). Early gastric cancer: a single-institution experience on 60 cases. Tumori-Supplementi-, 2: 23–26. [PubMed] [Google Scholar]
- Otsuji E, Yamaguchi T, Sawai K, Sakakura C, Okamoto K, Takahashi T (1999). Regional lymph node metastasis as a predictor of peritoneal carcinomatosis in patients with Borrmann type IV gastric carcinoma. Am J Gastroenterol, 94: 434. [DOI] [PubMed] [Google Scholar]
- Koizumi W, Kurihara M, Tanabe S, Kondo I, Yamazaki I, Nonaka M, Shimamura Y, Saigenji K (1999). Advantages of Japanese response criteriafor estimating the survival of patients with primary gastric cancer. Gastric Cancer, 2: 14–19. [DOI] [PubMed] [Google Scholar]
- Yagi Y, Seshimo A, Kameoka S (2000). Prognostic factors in stage IV gastric cancer: univariate and multivariate analyses. Gastric Cancer, 3: 71–80. [DOI] [PubMed] [Google Scholar]
- Feuer EJ, Wun LM, Boring CC (1992). Probability of developing cancer in USA: Cancer review : 1973–1989. ed. USA: NIH pub [Google Scholar]
- Curtis RE, Kennedy B, Myers MH, Hankey BF (1985) Evaluation of AJC stomach cancer staging using the SEER population. Seminars in oncology, pp. 21. [PubMed] [Google Scholar]
- Bako G, Ferenczi L, Hanson J, Hill G, Dewar R (1985). Factors influencing the survival of patients with cancer of the stomach. Clinical and investigative medicine. Medecine Clinique Et Experimentale, 8: 22. [PubMed] [Google Scholar]
- Saidi RF, Bell JL, Dudrick PS (2004). Surgical resection for gastric cancer in elderly patients: is there a difference in outcome? J Surg Res, 118: 15. [DOI] [PubMed] [Google Scholar]
- Bucchi L, Nanni O, Ravaioli A, Falcini F, Ricci R, Buiatti E, Amadori D (2004). Cancer mortality in acohort of male agricultural workers from northern Italy. J Occup Environ Med, 46: 249. [DOI] [PubMed] [Google Scholar]
- Noguchi Y, Yamamoto Y, Morinaga S, Amano T, Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H (2002). Does pancreaticosplenectomy contribute to better survival? Hepato-Gastroenterol, 49: 1436–1440. [PubMed] [Google Scholar]
- Kandasami P, Tan W, Norain K (2003). Gastric cancer in Malaysia: the need for early diagnosis. Med J Malaysia, 58: 758. [PubMed] [Google Scholar]
- Buonadonna A, Lombardi D, De Paoli A, Bidoli E, Frustaci S (2003). Adenocarcinoma of the stomach: univariate and multivariate analyses of factors associated with survival. I supplementi di Tumori: official journal of Società italiana di cancerologia…[et al.], 2:S31. [PubMed] [Google Scholar]


