Abstract
Background
Several translational studies have identified the differential role between saturated and unsaturated fatty acids at cardiovascular level. However, the molecular mechanisms that support the protective role of oleate in cardiovascular cells are poorly known. For these reasons, we studied the protective role of oleate in the insulin resistance and in the atherosclerotic process at cellular level such as in cardiomyocytes (CMs), vascular smooth muscle cells (VSMCs) and endothelial cells (ECs).
Methods
The effect of oleate in the cardiovascular insulin resistance, vascular dysfunction, inflammation, proliferation and apoptosis of VSMCs were analyzed by Western blot, qRT-PCR, BrdU incorporation and cell cycle analysis.
Results
Palmitate induced insulin resistance. However, oleate not only did not induce cardiovascular insulin resistance but also had a protective effect against insulin resistance induced by palmitate or TNFα. One mechanism involved might be the prevention by oleate of JNK-1/2 or NF-κB activation in response to TNF-α or palmitate. Oleate reduced MCP-1 and ICAM-1 and increased eNOS expression induced by proinflammatory cytokines in ECs. Furthermore, oleate impaired the proliferation induced by TNF-α, angiotensin II or palmitate and the apoptosis induced by TNF-α or thapsigargin in VSMCs.
Conclusions
Our data suggest a differential role between oleate and palmitate and support the concept of the cardioprotector role of oleate as the main lipid component of virgin olive oil. Thus, oleate protects against cardiovascular insulin resistance, improves endothelial dysfunction in response to proinflammatory signals and finally, reduces proliferation and apoptosis in VSMCs that may contribute to an ameliorated atherosclerotic process and plaque stability.
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-015-0237-9) contains supplementary material, which is available to authorized users.
Keywords: Fatty acids, Atherosclerosis, Insulin resistance
Background
Elevated circulating levels of saturated free fatty acids (SFAs; e.g. palmitate) induce inflammatory responses and cause insulin resistance in peripheral tissues. By contrast, mono- or poly-unsaturated FFAs (MUFAs or PUFAs) protects against SFAs. Elevated saturated FFAs can induce inflammation and insulin resistance, through several mechanisms including diacylglycerol-mediated activation of protein kinase Cθ [1] and activation of Toll-like receptors (TLR) [2]. Both mechanisms lead to the activation of the proinflammatory transcription factor nuclear factor-kappa B (NF-κB), which has been linked to fatty acid-induced impairment of insulin action in skeletal muscle [3, 4]. Once activated, NF-κB regulates the expression of multiple inflammatory mediators, including IL-6, TNF-α and other factors implicated in the metabolic alterations. IL-6 strongly correlates with insulin resistance and type 2 diabetes and its plasma levels are increased in patients with obesity and type 2 diabetes [5]. Moreover, NF-κB also regulates the expression of genes involved in the early and late atherosclerotic process and its instability, such as endothelial nitric oxide synthase (eNOS), adhesion molecules (e.g. ICAM-1), monocyte chemotactic protein 1 (MCP-1), and plasminogen activator inhibitor-1 (PAI-1) [6–8]. On the other hand, activated NF-κB also might be implicated in JNK-1/2 activation and induces insulin resistance in several tissues [9].
Various translational studies have identified the differential role of saturated and unsaturated fatty acids and their effects at cardiovascular level [10, 11]. MUFAs, as oleic acid, improve lipid profile [12], maintain a balance of body weight [13] and prevent palmitate-induced mitochondrial dysfunction, insulin resistance and inflammatory signalling in neuronal cells [14] and skeletal muscle [15]. However, the underlying molecular mechanisms of the protective role of oleate in cardiovascular cells are poorly known. Thus, we have studied the protective role of oleate in insulin resistance and in the early and late atherosclerotic process and its instability at the cellular level. To assess this objective, we analyzed the insulin signalling in cardiovascular cells such as endothelial cells (ECs), vascular smooth muscle cells (VSMCs) and cardiomyocyte cells (CMs), the latter two cell lines were generated by our laboratory. Secondly, we have explored the differential molecular mechanisms of oleate and palmitate on JNK-1/2 or NF-κB signalling pathways. Furthermore, we analysed the protective role of oleate in the expression of inflammatory and proatherogenic markers as well as in the proliferation and apoptosis of VSMCs. Our data provide a new insight on the differential effect of oleate versus palmitate giving support to recent findings that highlight the beneficial effects of oleic acid as an essential component of the Mediterranean diet at cardiovascular level.
Methods
Cell lines
Primary VSMCs were obtained from thoracic aorta arteries of three male of 8 week-old wild-type mice. Anesthetized mice (Avertin, 250 mg/kg, ip) were saline perfused and thoracic aorta arteries were submitted to collagenase dispersion and primary culture as previously described [16]. Other cell line also generated in our laboratory was neonatal cardiomyocytes from hearts of eight neonatal mice (WT CMs). Hearts were dispersed with collagenase type II (1.2 mg/ml, Whorthington) and treated with DNAsa I, grade II (10 μg/ml, Roche) at 37 °C for 20 min. Cells were grown with high glucose DMEM (Life Technologies), Fetal Bovine Serum (5 %), Horse Serum (10 %), HEPES (20 mM), cardiotrophin I (R&D Systems 0.2 ng/ml), insulin (1 μM) and a mixture of antibiotics and antifungals. Cells were seeded in plates pretreated with collagen type I (Collagen from rat tail, Roche Applied Sciences) to facilitate adhesion of cardiomyocytes. So, both primary cultures of WT VSMCs or WT CMs were infected by retroviral infection (viral particles containing pBabe retroviral vector encoding of SV40 Large T antigen) and selected with 1 μg/mL puromycin for 3 weeks.
Mouse endothelial cells (ECs) were purchased from ATCC (CRL-2167). These cells were cultured with high-glucose DMEM (ATCC), with L-glutamine (4 mM), NaHCO3 (1.5 g/L) and was supplemented with inactivated Horse Serum (10 %).
Cell lines were cultured with 10 % FBS-DMEM and were serum-starved for 4–5 h for insulin signaling and proliferation studies or 18 h for studies of mRNA expression, and then incubated with the corresponding stimuli. For in vitro experiments, we have used insulin (1, 10 and 100 nmol/L), TNF-α (10 ng/mL, Sigma), angiotensin II (1 μmol/L, Sigma), oleic acid (0.6 -1 mmol/L, Sigma) and palmitic acid (0.4 - 1 μmol/L, Sigma). Moreover, we used an NF-κB inhibitor as parthenolide (10−5 M for 90 min).
Anna-Maria Ordelheide (Helmholtz Center Munich, Germany) kindly provided the protocol for the preparation and dissolution of fatty acids used in this study. First, a standard solution of palmitic acid (PA, 200 mM (Sigma)) was dissolved in absolute ethanol and a standard oleic acid (OA, 100 mM (Sigma)) dissolved in NaOH solution (0.1 M) was performed. Additionally, a buffer containing 20 % bovine serum albumin (BSA) / Krebs-Ringer-HEPES pH 7.4, with which the intermediate fatty acid solution is prepared to 5 mM. The solution of conjugated fatty acids is prepared minutes before experimentation left stirring at 37 °C until complete dissolution of the fatty acids are observed to prevent oxidation of these. Finally, the necessary amount of the conjugate acid (5 mM)-20 % BSA, the average fatty acid conjugation which involves low glucose DMEM, BSA (2.5 %) and FBS (0.5 %) is added. This protocol maintain the proportions 5:1 BSA-fatty acid which allows adequate transport and bioavailability of the fatty acid to the cell. In all control cells, we used BSA (2.5 %) and FBS (0.5 %) to be able to compare with OA/PA stimulations.
Western blot
Western blot analyses were performed on protein extracts from CMs, ECs and VSMCs as previously described [17]. The antibodies used were, SV40 TAg and IκBα from Santa Cruz Biotechnology, p53 and TnT from Calbiochem, phospho-AKT (T308), AKT, p-p70S6K (Ser389), p70S6K, p-AMPK (T172), AMPK, p-JNK-1/2 (T183/Y185), Cleaved Caspase 3, p-p42/44 (S202/T204) and p42/44 from Cell Signalling, p-IRS-1 from Millipore and p-Tyr, PAI-1, α- and β-actin and α-tubulin from Sigma Corp.
Immunoprecipitation
To obtain total cell lysates, cells from supernatants were collected by centrifugation at 2000 × g for 5 min at 4 °C. Attached cells were scrapped off in ice-cold PBS, pelleted by centrifugation at 4000 × g for 10 min at 4 °C, and resuspended in lysis buffer. Samples of cell lysates of VSMCs were sonicated 30 s at 1.5 mA, and lysates were clarified by centrifugation at 12,000 × g for 10 min. For immunoprecipitation, 150–200 μg of protein were immunoprecipitated at 4 °C with the IRS-1 antibody and isotype control serum. The immune complexes were collected on protein A-agarose and submitted to SDS-PAGE to check its phosphorylation in Tyr and Ser residues.
Proliferation assays
Briefly, 104 cells in 1 mL of complete medium were seeded into each well of an uncoated 96-well plate. The following day, the cells were serum deprived for 5 h and stimulated with TNF-α, angiotensin II or palmitate for 24 h with or without pretreatment with oleic acid for 2 h. After that, the rate of cellular proliferation was evaluated using a cell proliferation ELISA BrdU kit (Roche Applied Science). The incubation with BrdU labeling solution was for 18 h.
Cell cycle
After treating cells in culture with the corresponding stimulus, the supernatant cells were collected and the attached cells were lifted with trypsin. The cells were centrifuged (5 min, 110 × g, 4 °C) and washed twice with PBS. The precipitate containing the cells was resuspended in 300 μl of ice-cold PBS, and added 700 μl of absolute ethanol at −20 °C, for fixing for one minute. Then the cells were washed twice with cold PBS. Cells were then incubated with 500 μl of warm PBS with RNase (Roche) for 30 min at 37 °C. Finally, 25 μl of propidium iodide (PI) (Sigma, 0.1 % (w/v)) in PBS were added. Cellular DNA content by PI incorporation was assessed (fluorescent emission between 562 and 588 nm) and can differentiate apoptotic cells (<2n), cells in the G0/G1, and cell proliferation (phase S / G2-M, > 2n). For this a FACScalibur cytometer and Cell Quest Pro software (Becton Dickinson) was used.
Immunofluorescence
Cells were seeded at low density on circular crystals of 1 cm in diameter. Once treatment is completed, the cells were washed once with PBS and fixed with paraformaldehyde solution (4 % w/v), for 10 min at room temperature. Then washed with PBS, and incubated 5 min at room temperature with Triton X-100 (0.2 % v/v) in PBS in order to permeabilize the cells and permit labeling of intracellular antigens. To cushion the cellular autofluorescence, the cells were washed and incubated with a solution of NH4Cl (50 mM) for 15 min. at room temperature. After further washing, the nonspecific binding sites are blocked by incubation in a blocking solution of BSA (1 % w/v), 0.2 % Triton X-100 (v/v) in PBS supplemented with Tween-20 (0.05 % w/v) (PBST) for 45 min at room temperature. Incubation with primary antibodies was performed for 1.5 h at room temperature in a humid chamber. After washing with PBST, we incubated with secondary antibody for 45 min. After a final washing with PBST, the preparations were mounted on a slide with SlowFade mounted liquid Gold (Life Technologies) containing DAPI to visualize nuclei. Samples were generally observed to stabilize the following mounting liquid day, although stable preparations are stored at 4 °C in the dark for several weeks.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted from ECs by TRIzol method (Invitrogen, Barcelona, Spain) and quantified by absorbance at 260 nm in duplicate. Twenty nanogram of RNA were necessary to perform the reverse transcription reaction, for 15 min at 25 °C and 2 h at 37 °C, with the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). We evaluated the mRNA expression of genes involved in vascular dysfunction (eNOS and ICAM-1) and inflammation (MCP-1) on ECs. Amplification of GAPDH was used in the same reaction of all samples as an internal control. Gene-specific mRNA was subsequently normalized to GAPDH RNA. Quantitative reverse transcription-PCR was performed in 7500 Real-Time PCR device, and the relative quantification was performed with the Prism 7000 system SDS software (Applied Biosystems). The expression of these genes was analyzed by real-time quantitative PCR as described [17]. Thus, the amount of target, normalized to endogenous gene and relative to the control is given by 2-ΔΔCt [ΔCt = Ct (target gene) – Ct (endogenous gene); ΔΔCt = ΔCt for any sample - ΔCt for the control].
Statistical analysis
All values are expressed as mean +/− SEM. Data were analyzed using one-way analysis of variance, followed by a Bonferroni test if differences were noted (SPSS 15.0 program). The null hypothesis was rejected when the p value was less than 0.05.
Results
Characterization of cardiovascular cell lines
Firstly, we assured the CMs immortalization by Western blot analysis of T-antigen and p53 (Fig. 1a). Subsequently, we demonstrated the presence of the cardiomyocytes specific protein, troponin T (TnT), by Western blot and immunofluorescence (Fig. 1b). Moreover, we also characterized the ECs with CD31 antibody or PECAM-1 (Fig. 1c). By immunofluorescence, we detected the ECs specific protein, von Willebrand factor (vWF) (Fig. 1d).
Fig. 1.
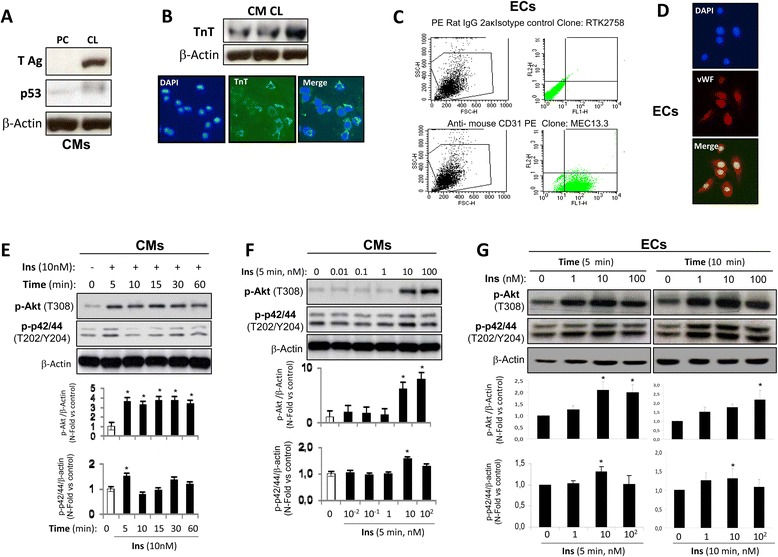
Characterization of cardiovascular lines. a Western blot analysis against AgT and p53 to check the immortalization performed in neonatal cardiomyocyte lines. β-actin was used as charge control. b Characterization of neonatal cardiomyocytes with a specific marker as TnT by Western blot or by immunofluorescence. Characterization of ECs with specific markers as PECAM-1 or CD31 by flow cytometry (c) or vWF by immunofluorescence (d). Western blot analysis of insulin signalling in CMs (e, f) and in ECs (g). *p < 0.05 vs. control
Secondly, we analyzed insulin signaling in both cardiovascular cell lines. We observed that 10 nmol/L insulin induced an increase in Akt (T308) phosphorylation at 5 min. On the other hand, a significant increase of p42/44 (T202/Y204) phosphorylation was activated by 10 nmol/L insulin (Fig. 1e). Subsequently, an insulin dose–response curve showed that at 5 min the maximal activation of AKT was found at 100 nM insulin (Fig. 1f). Moreover, with the lowest insulin dose (1 nmol/L), the phosphorylation was induced in AKT and p42/44 at 5 and 10 min in ECs. A significant increase in AKT phosphorylation was observed at 10 and 100 nmol/L (Fig. 1g). VSMC lines used in this work were characterized and its insulin signaling shown in a previous work [18].
Differential effect of oleic or palmitic acid on the cardiovascular cell insulin sensitivity
Firstly, we studied the oleate effect on insulin sensitivity. Thus, cardiomyocytes were treated with oleate at 0.6, 0.8 or 1 mmol/L for 2 h before insulin stimulation. We observed that insulin stimulation significantly increased Akt phosphorylation for each dose of oleate used. Under the same conditions, Akt activation was accompanied by the inactivation of AMPK phosphorylation (Fig. 2a). However, we observed AMPK activation in absence of insulin (Fig. 2a). In vascular cells, 0.8 mmol/L oleate did not prevent Akt, p42/44 and p70S6K phosphorylation or AMPK dephosphorylation in response to insulin (Fig. 2b, c). In addition, oleate induced AMPK activation in the absence of insulin in vascular cells (Fig. 2b, c). Overall, oleate treatment for 2 h did not produce insulin resistance in the cardiovascular cells studied. These results were confirmed by the dose–response effect of oleate at 18 h on Akt phosphorylation in the presence of insulin (Additional file 1: Figure S1). At this stage, we confronted these data regarding oleate with palmitate effect on insulin sensitivity. For that purpose, we chose 0.4 mmol/L palmitate dose as previously described in myocytes [15]. Palmitate (0.4 mmol/L) treatment did not prevent Akt or p42/44 phosphorylation induced by insulin for 2 or 6 h in CMs. However, prolonged palmitate treatment for 18 or 24 h induced insulin resistance (Additional file 2: Figure S2). At this stage, 0.4 mmol/L palmitate for 18 h significantly induced insulin resistance as revealed by Akt, p42/44 and p70S6K phosphorylation in response to insulin in all the vascular cells studied (Fig. 3).
Fig. 2.
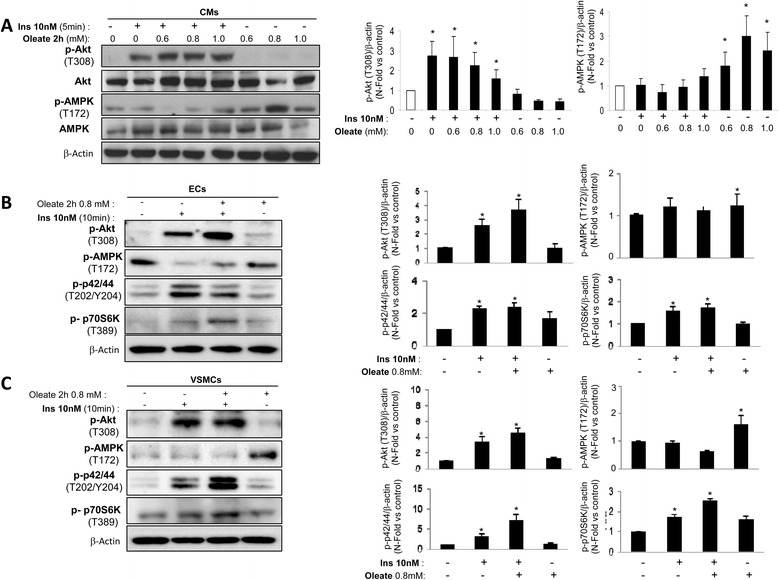
Oleate did not induce cardiovascular insulin resistance. Western blot analysis of phosphorylation de Akt (T308), AMPK (T172), p42/44 (T202/Y204) and p70S6K (T389) induced by insulin (10nM, 10 min) in presence or absence of oleate (2 h) in CMs (a), ECs (b) and VSMCs (c). β-actin was used as charge control. *p < 0.05 vs. control
Fig. 3.
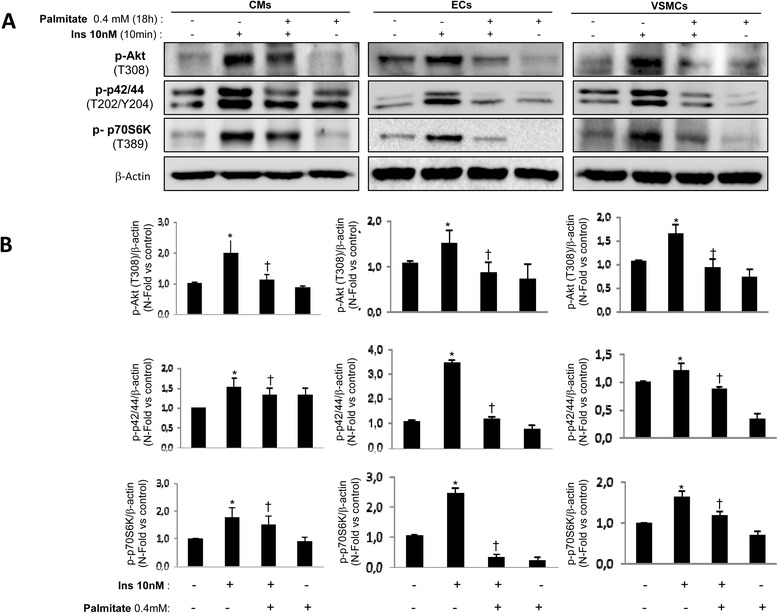
Palmitate induced cardiovascular insulin resistance. Western blot analysis (a) and its quantifications (b) of Akt phosphorylation (T308), p42/44 (T202/Y204) and p70S6K (T389) induced by insulin (10nM, 10 min) in presence or absence of oleate (2 h) in CMs, ECs and VSMCs. β-actin was used as charge control. *p < 0.05 vs. control; †p < 0.05 vs. stimulus
We also demonstrate that TNF-α at 2 h induced vascular insulin resistance as palmitate did at 18 h (Fig. 4). The pretreatment with oleate prevented the inhibition by TNF-α or palmitate on Akt phosphorylation induced in response to insulin in cardiovascular cells (Fig. 4). Beside the previously proposed mechanism, according to which oleate may be protecting against cardiovascular damage induced by TNF-α by UCP-2 expression [19], we studied the NF-κB signaling pathway. Thus, we observed that TNF-α as well as palmitate increased JNK1/2 phosphorylation in all the vascular cells studied. However, the pretreatment with oleate reduced JNK phosphorylation induced by TNF-α or palmitate (Fig. 5a). When JNK1/2 was activated by TNF-α, there was an increase in IRS-1 serine phosphorylation and a decrease in IRS-1 tyrosine phosphorylation. Pretreatment with oleate prevented serine phosphorylation and maintained Tyr phosphorylation on IRS-1 induced by TNF-α or palmitate (Fig. 5b). Another mechanism that might be modulated by oleate, could be mediated by NF-κB. Thus, we observed that TNF-α, from 10 to 30 min and palmitate at 30 min were able to reduce the levels of IκB-α (Fig. 5c). Subsequently, we observed that oleate was able to partially prevent IκB-α degradation induced by TNF-α or palmitate. More importantly, parthenolide, an inhibitor of NF-κB [20], prevented IκB-α degradation induced by TNF-α (Fig. 5d) and precluded insulin resistance on Akt phosphorylation induced by TNF-α or palmitate in VSMCs (Fig. 5e).
Fig. 4.
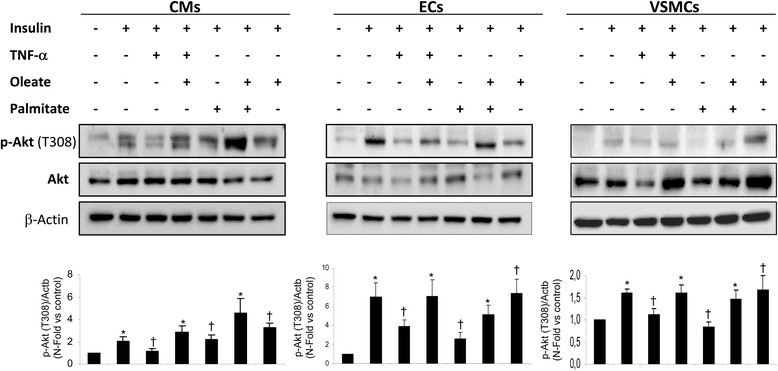
Oleate protected cardiovascular insulin resistance induced by TNF-α or palmitate. Western blot analysis of phosphorylation of Akt (T308) induced by insulin (10nM, 10 min) and /or palmitate (0.4 mM, 18 h) or TNF-α (10 ng/mL, 2 h) in presence or absence of oleate in CMs, ECs and VSMCs. β-actin was used as charge control. *p < 0.05 vs. control; †p < 0.05 vs. stimulus
Fig. 5.
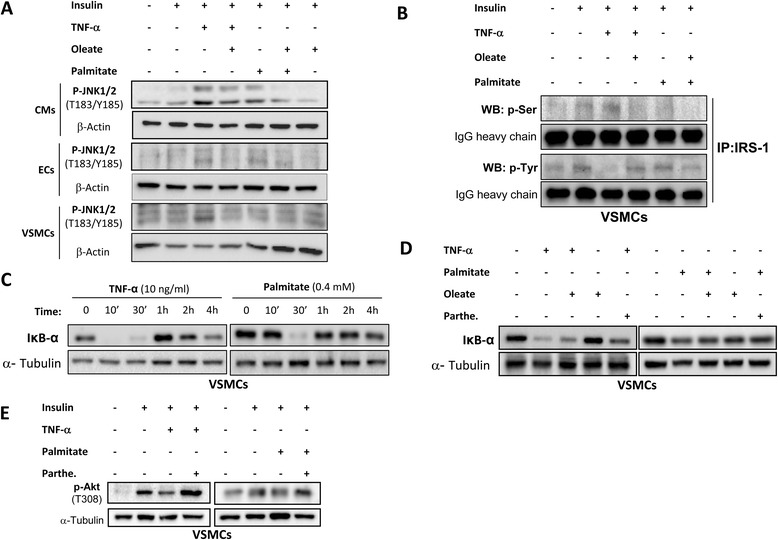
Modulation of JNK-1/2 and NF-κB pathway by oleate. a Western blot analysis of JNK-1/2 phosphorylation in CMs, ECs and VSMCs. b Effect of oleate in the Ser and Tyr phosphorylation of IRS-1 in VSMCs. Effect of TNF-α or palmitate in IκBα levels in absence (c) or presence (d) of oleate in VSMCs. e Effect of parthenolide in the phosphorylation of Akt in VSMCs. β-actin was used as charge control
Protective role of oleate in the endothelial dysfunction markers and in the inflammatory response
We decided to study the effect of several cytokines involved in the inflammatory process, such as TNF-α, IL-6 and IL-1β on some markers of endothelial dysfunction and activation in ECs and the possible protective effect of the oleate against those stimuli.
First, we observed that the treatment with oleate prevented eNOS mRNA reduction induced by TNF-α, IL-6 or IL-1β in ECs (Fig. 6a). We also observed that the pretreatment with oleate significantly reduced ICAM-1, and MCP-1 expression induced by the pro-inflammatory cytokines in ECs (Fig. 6b, c).
Fig. 6.
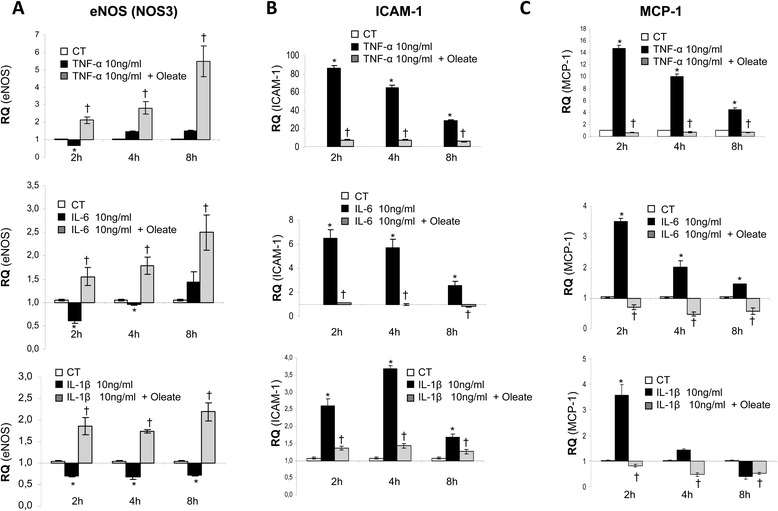
Effect of oleate in eNOS, ICAM-1 and MCP-1 mRNA expression in ECs. qRT-PCR analysis of eNOS (a), ICAM-1 (b) and MCP-1 (c) mRNA expression induced by TNF-α, IL-6 and IL-1β in presence or absence of oleate in ECs. *p < 0.05 vs. control; †p < 0.05 vs. stimulus
Protective effect of the oleate on the VSMCs proliferation
One of the factors that contribute to the early growth of atherosclerotic plaque is the migration and proliferation of VSMCs. On this regard, pretreatment with oleate mostly prevented the rate of proliferation induced by TNF-α, Ang II or palmitate (Fig. 7).
Fig. 7.
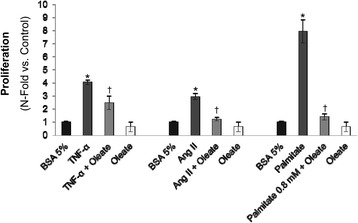
Effect of oleate in VSMCs proliferation. Rates of proliferation measured by BrdU incorporation in response to TNF-α, angiotensin II or palmitate in presence or absence of oleate. *p < 0.05 vs. control; †p < 0.05 vs. stimulus
Protective effect of oleate on the VSMCs apoptosis
One of the described mechanisms and directly involved in the instability and rupture of atherosclerotic plaque, is the increase of fibrous cap VSMCs apoptosis, weakening it and triggering the acute event [21, 22]. For this purpose, we explored if oleate could improve the plaque stability, reducing VSMCs apoptosis. First, we observed that the treatment with TNF-α significantly increased the percentage of cells in G0/G1 phase in comparison to the control. In addition, oleate per se does not alter the cellular cycle in VSMCs. More importantly, the pre-treatment with oleate significantly reduced the percentage of apoptotic cells induced by TNF-α (Fig. 8a). On this regard, we analysed active caspase-3 protein levels induced by several stimuli. As a positive control of apoptosis, we used 1 μg/ml thapsigargin for 30 min that induced a high expression of active caspase-3. Thus, TNF-α increased the expression of active caspase-3 as compared with the control. In the presence of oleate, the expression of caspase-3 induced by TNF-α was significantly impaired (Fig. 8b). In addition, oleate also impaired active caspase-3 levels induced by thapsigargin (Fig. 8c). However, palmitate did not show any protective effect on the expression of active caspase-3 induced by thapsigargin. Conversely, oleate impaired the rate of apoptosis induced by thapsigargin in the presence of palmitate (Fig. 8c).
Fig. 8.
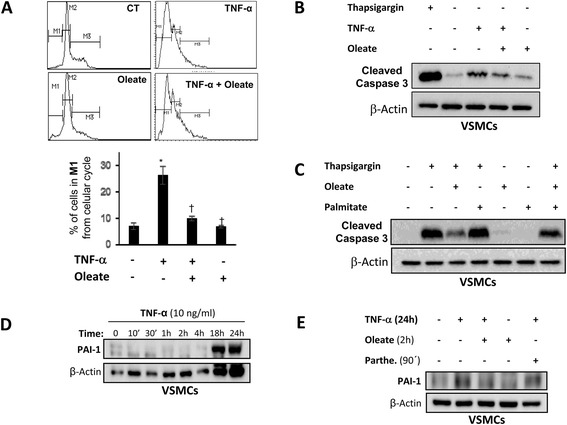
Effect of oleate in VSMCs apoptosis. Effect of oleate in VSMCs apoptosis induced by TNF-α measured by cellular cycle (a) or by Western blot analysis of active caspase 3 (b) or induced by thapsigargin by Western blot analysis (c). *p < 0.05 vs. control; †p < 0.05 vs. stimulus. d Western blot analysis of PAI-1 levels induced by TNF-α at different time points. e Effect of oleate and parthenolide in PAI-1 levels induced by TNF-α. β-actin was used as charge control
Protective effect of oleate on thrombogenesis in vascular cells
Elevated levels of PAI-1 are associated with increased cardiovascular and thrombotic events [23]. On this regard, TNF-α induces the expression of PAI-1 between 18 and 24 h in VSMCs (Fig. 8d). At this stage, we explored whether or not TNF-α could be increasing the production of PAI-1 in VSMCs through the activation of NF-κB. For this purpose, we observed that when VSMCs were pretreated with parthenolide and therefore NF-κB pathway was inhibited, the production of PAI-1 induced by TNF-α was partially reduced (Fig. 8e). Next, we decided to explore the effect of oleate on the expression of PAI-1 induced by TNF-α in VSMCs. First, we observed that oleate per se did not increase PAI-1 protein levels. However, treatment with oleate mostly prevented the elevation of PAI-1 induced by TNF-α (Fig. 8e).
Discussion
Over the past decades, the prevalence of obesity and metabolic syndrome (MetS), and its underlying risk of developing cardiovascular disease (CVD), has increased worldwide becoming a public health problem [24]. It is known that subjects with MetS have an increased risk of developing type 2 diabetes, in turn, these people tend to be overweight or obese, with high levels of circulating FFA. This increase contributes to the complications of obesity, such as insulin resistance, excessive fat accumulation in peripheral tissues [25] and alterations in cardiac function [26, 27], among others. Additionally, increased levels of circulating FFAs are recognized as a predictor of myocardial infarction [28].
FFAs may act as either pro- or anti-inflammatory agents depending on the chemical structure [29]. SFAs, such as palmitic acid, have been associated with adverse cardiovascular effects [30, 31]. However, PUFAs, such omega-3 FAs, improve triglyceride levels and reduce the risk of CVD by their anti-inflammatory properties [28]. Moreover, it has recently been reported that there is not association between dietary omega-3 FA and the risk of MetS [32]. Similarly to PUFAs, MUFAs, such as oleic acid, has been associated with antiproliferative effects on cancer and decreased risk of CVD [13, 33]. Translational studies as PREDIMED have recommended from Mediterranean diet, the use of virgin olive oil where its main fatty acid is oleate due to decreased CVD and improve irrigation in the treatment of Type 2 Diabetes Mellitus [10, 33]. However, the molecular mechanisms by which oleate exerts its protective role in vascular cells is not fully understood. Our results show that oleate has a differential beneficial cardiovascular effect with respect to other SFAs as palmitate. So far the evidence pointed to the long-chain SFA palmitate induced insulin resistance in tissues such as adipocytes and skeletal muscle [34, 35]. We showed that in cardiovascular cells, palmitate also induces insulin resistance. In this regard, others authors also demonstrated that SFAs stimulated the fatty-acid uptake in detriment of glucose assimilation in cardiomyocytes [36] and in vivo, SFAs intake is associated with most cardiac remodeling [37]. However, we have shown that oleate did not generate insulin resistance in any studied cardiovascular cell lines. Similarly to our results in cardiovascular cells, oleate was also able to prevent insulin resistance in the myotubes through activation of PI3K [35] or by a mechanism dependent of AMPK [15]. In addition, in the pancreatic β-cells, it has been described that oleate induced activation PI3K and PKB pathway and prevented apoptosis [38]. On the other hand, other authors have described that both oleate and palmitate induced insulin resistance in primary hepatocytes, associated with the accumulation of dyacilglycerols and/or ceramide [39] or a diet rich in UFAs increased total adiposity without impairing cardiovascular parameters [40].
In the present work, we have shown that oleate did not induce insulin resistance in the context of cardiovascular cells and moreover it is capable of protecting against insulin resistance induced by palmitate or TNF-α. Just recently, we proposed that oleate induced an increase of UCP-2 levels and this might be one of the mechanisms by which oleate protects against the deleterious action of palmitate on vascular cells [19]. Additionally, we have now hypothesized that palmitate or TNF-α could be activating different pathways such as JNK and NF-κB in cardiovascular cells. One of the mechanisms that may be involved in the deleterious effects of SFAs is that they can act as ligands for TLR, and activate different signaling pathways involved in the inflammatory response [41, 42]. Thus, it has been described that palmitate through TLR2 may induce insulin resistance in myotubes, inducing activation of NF-κB, JNK and p38 [2] and the impairment of vasodilator actions of insulin [43]. In our work, both palmitate and TNF-α activated JNK pathway in all three cardiovascular cell lines. However, pretreatment with oleate substantially decreased activation of JNK1/2. These results confirm that oleate prevents insulin resistance avoiding IRS-1 Ser phosphorylation and maintaining IRS-1 Tyr phosphorylation in presence of TNF-α or palmitate, favoring the activation of the PI3K pathway.
Our results also suggest that other mechanism by which oleate prevents insulin resistance in VSMCs is the modulation of NF-κB activation. We demonstrate that TNF-α or palmitate activate NF-κB, decreasing the levels of IκB-α [44]. However, the reduction of NF-κB by oleate might be implicated in the protective role of oleate against insulin resistance induced by the TNF-α or palmitate. We also checked that parthenolide, an inhibitor of NF-κB [20], prevented IκB-α degradation and therefore the activation and translocation of NF-κB to the nucleus in addition to prevent insulin resistance induced by TNF-α or palmitate.
In the literature, it has also been described the differential correlation among oleate and palmitate with inflammasomes. Thus, the proinflammatory effect of SFAs through a caspase-1/ASC/NLRP3-dependent pathway [45] might justify that SFA-rich diets increase IL-1β production and other inflammatory processes related to IL-1β [45, 46]. However, anti-inflammatory actions of MUFAs prevent activation of NLRP3 inflammasome induced by SFAs in human monocytes/macrophages [47], supporting the beneficial effects of MUFAs in Mediterranean diet [10]. Moreover, high MUFAs diet or replacement of SFAs for MUFAs induce changes in abdominal fat distribution, improve insulin sensitivity [48, 49], and postprandial oxidative stress in patients with metabolic syndrome [50].
It is known that during processes such as obesity or atherosclerosis, there are increased levels of proinflammatory cytokines such as IL-6 and TNF-α, which are capable to activate NF-κB in different tissues. At the same time, this proinflammatory cytokines activate other pro-inflammatory and chemotactic agents creating a cycle of self-maintaining inflammation [51]. The permanence of the inflammatory condition in the vasculature can induce endothelial dysfunction and activation with an increase in the expression of chemotactic factors and adhesion molecules such as ICAM-1 and VCAM-1 [52]. Thus, our work suggests that oleate has also a protective role in the activation and endothelial function in addition to the inflammatory response. These findings correlate with the positive effect of oleate found in human VSMCs and ECs [53, 54].
TNF-α is capable of activating two opposite mechanisms, cell survival and cell death simultaneously [55, 56]. Thus, we found that TNF-α, but not palmitate, significantly induced an increase in the percentage of cells found in the G0/G1 phase of the cell cycle. However, treatment with palmitate has a different behavior between the two vascular lines: decreases the viability of ECs, but favors the proliferation of VSMCs, and may play an important role in the initiation and progression of atherosclerosis. On this regard, saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation [57]. On the other hand, we demonstrated that oleate exerted a protective role against the proliferation of VSMCs stimulated by TNF-α, Ang II, or palmitate, contributing to the prevention of the growth of atherosclerotic plaque. Converserly, palmitate induced changes in VSMCs phenotype promoting formation of atherosclerotic plaque [31].
Increased apoptosis of VSMCs is one of the mechanisms directly involved in the instability and rupture of atherosclerotic plaques, and underlying complications such as thrombosis [22, 23]. We propose that pretreatment with oleate has a protective role against apoptosis in VSMCs owing to oleate pretreatment significantly reduced the percentage of apoptotic cells and active caspase-3 protein levels induced by TNF-α. It has been previously described that oleate modulates proapoptotic proteins expression as Bax, caspase-3 and PARP cleavage in cardiomyocytes [58]. Thus, we confirm that oleate exerts a protective role against apoptosis in VSMCs, decreasing the activation of caspase-3, induced by TNF-α or thapsigargin that induces stress in the endoplasmic reticulum [59].
Elevated levels of PAI-1 are associated with increased cardiovascular and thrombotic events [23]. We found that oleate reduces PAI-1 protein levels induced by TNF-α in VSMCs, and we show that increased PAI-1 induced by TNF-α is through activation of NF-κB, given that parthenolide decreases PAI-1 levels induced by TNF-α in VSMCs. We suggest that oleate exerts its protective effects through inhibition of NF-κB pathway, and also improves the thrombogenesis promoting the fibrinolysis by inhibition of PAI-1.
Conclusions
In conclusion, our results show that oleic acid has a beneficial effect at cardiovascular level as compared with saturated fatty acids such as palmitic acid. Thus, oleate protects against cardiovascular insulin resistance, improves endothelial dysfunction, inflammation and finally, reduces proliferation and apoptosis in VSMCs that may contribute to an ameliorated atherosclerotic process and its stability. These processes may be mediated by inhibition of JNK-1/2 and NF-κB pathways.
Acknowledgements
The authors thank Gema García and Silvia Fernandez for technical assistance. This work was supported by grants SAF2007/60058, SAF2008/00031 and SAF2011/22555 from Ministerio de Ciencia e Innovación, Comunidad de Madrid (S2010/BMD-2423) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM, ISCIII, Spain), Spain.
Abbreviations
- AKT
Protein kinase A
- CMs
Cardiomyocyte cells
- ECs
Endothelial cells
- eNOS
Endothelial nitric oxide synthase
- ERK-1/-2
Extracellular signal-regulated kinase
- ICAM-1
Intercellular adhesion molecule 1
- iNOS
Inducible nitric oxide synthase
- IRS-1
Insulin receptor substrate 1
- MCP-1
Monocyte chemoattractant protein-1
- NF-κB
Nuclear factor kappa B
- OA
Oleic acid
- PA
Palmitic acid
- PAI-1
Plasminogen activator inhibitor-1
- qRT-PCR
Quantitative real time reverse transcription polymerase chain reaction
- VSMCs
Vascular smooth muscle cells
Additional files
Oleate did not induce vascular insulin resistance for a long time. Western blot analysis of Akt (T308) phosphorylation in ECs and VSMCs stimulated with insulin (10nM, 10 min) with or without oleate (0.6, 0.8 or 1 mM for 18 h). β-actin was used as charge control.
Effect of palmitate for different times in insulin signaling in neonatal cardiomyocytes. Representative gels (A) and its quantifications (B and C) of Western blot analysis of Akt (T308) and p42/44 (T202/Y204) phosphorylation in cardiomyocytes (CMs) stimulated with insulin (10nM, 10 min) with or without palmitate (0.4 mM for 2, 6,18 or 24 h). β-actin was used as charge control. *p < 0.05 vs. control; †p < 0.05 vs. stimulus.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LP: has participated in the acquisition of data, analysis and interpretation of data, and helped to draft the manuscript. NB, YFO, OE and SD-C: have participated in the acquisition of data, analysis and interpretation of data. AG-H: has participated in the acquisition of data, analysis and interpretation of data and in the design of study and its coordination and in the statistical analysis as well as writing the paper. MB: has participated in the design of the study and its coordination and writing the manuscript. All authors have read and approved the final manuscript.
References
- 1.Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283:11107–16. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 2.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 3.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–46. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Wu W, Li D, Guo Y, Ding H. Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine. 2010;37:157–66. doi: 10.1007/s12020-009-9283-y. [DOI] [PubMed] [Google Scholar]
- 5.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 6.Lee KS, Kim J, Kwak SN, Lee KS, Lee DK, Ha KS, et al. Functional role of NF-κB in expression of human endothelial nitric oxide synthase. Biochem Biophys Res Commun. 2014;448:101–7. doi: 10.1016/j.bbrc.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 7.Yeh M, Leitinger N, de Martin R, Onai N, Matsushima K, Vora DK, et al. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:1585–91. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi E, Takamura T, Shimizu A, Nagai Y. Tumor necrosis factor-alpha and troglitazone regulate plasminogen activator inhibitor type 1 production through extracellular signal-regulated kinase- and nuclear factor-kappaB-dependent pathways in cultured human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2003;307:987–94. doi: 10.1124/jpet.103.054346. [DOI] [PubMed] [Google Scholar]
- 9.Gorgani-Firuzjaee S, Ahmadi S, Meshkani R. Palmitate induces SHIP2 expression via the ceramide-mediated activation of NF-κB, and JNK in skeletal muscle cells. Biochem Biophys Res Commun. 2014;450:494–9. doi: 10.1016/j.bbrc.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 11.Mayneris-Perxachs J, Sala-Vila A, Chisaguano M, Castellote AI, Estruch R, Covas MI, et al. Effects of 1-year intervention with a mediterranean diet on plasma Fatty acid composition and metabolic syndrome in a population at high cardiovascular risk. PLoS One. 2014;9(3):e85202. doi: 10.1371/journal.pone.0085202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hlais S, El-Bistami D, El Rahi B, Mattar MA, Obeid OA. Combined fish oil and high oleic sunflower oil supplements neutralize their individual effects on the lipid profile of healthy men. Lipids. 2013;48:853–61. doi: 10.1007/s11745-013-3819-x. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Martínez P, García-Ríos A, Delgado-Lista J, Pérez-Jiménez F, López-Miranda J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr Pharm Des. 2011;17:769–77. doi: 10.2174/138161211795428948. [DOI] [PubMed] [Google Scholar]
- 14.Kwon B, Lee HK, Querfurth HW. Oleate prevents palmitate-induced mitochondrial dysfunction, insulin resistance and inflammatory signaling in neuronal cells. Biochim Biophys Acta. 1843;2014:1402–13. doi: 10.1016/j.bbamcr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Salvadó L, Coll T, Gómez-Foix AM, Salmerón E, Barroso E, Palomer X, et al. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 2013;56:1372–82. doi: 10.1007/s00125-013-2867-3. [DOI] [PubMed] [Google Scholar]
- 16.Hernández-Presa M, Bustos C, Ortego M, Tuñon J, Renedo G, Ruiz-Ortega M, et al. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.CIR.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 17.Escribano O, Guillén C, Nevado C, Gómez-Hernández A, Kahn CR, Benito M. Beta-Cell hyperplasia induced by hepatic insulin resistance: role of a liver-pancreas endocrine axis through insulin receptor A isoform. Diabetes. 2009;58:820–8. doi: 10.2337/db08-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez-Hernández A, Escribano Ó, Perdomo L, Otero YF, García-Gómez G, Fernández S, et al. Implication of insulin receptor A isoform and IRA/IGF-IR hybrid receptors in the aortic vascular smooth muscle cell proliferation: role of TNF-α and IGF-II. Endocrinology. 2013;154:2352–64. doi: 10.1210/en.2012-2161. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Hernández A, Perdomo L, de las Heras N, Beneit N, Escribano O, Otero YF, et al. Antagonistic effect of TNF-alpha and insulin on uncoupling protein 2 (UCP-2) expression and vascular damage. Cardiovasc Diabetol. 2014;13:108. doi: 10.1186/s12933-014-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan M, Wong HR, Hake PW, Malhotra V, O’Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol. 2002;61:953–63. doi: 10.1124/mol.61.5.953. [DOI] [PubMed] [Google Scholar]
- 21.Martín-Ventura JL, Blanco-Colio LM, Muñoz-García B, Gómez-Hernández A, Arribas A, Ortega L, et al. NF-kappaB activation and Fas ligand overexpression in blood and plaques of patients with carotid atherosclerosis: potential implication in plaque instability. Stroke. 2004;35:458–63. doi: 10.1161/01.STR.0000114876.51656.7A. [DOI] [PubMed] [Google Scholar]
- 22.Harari OA, Liao JK. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157:291–8. doi: 10.1111/j.1365-2141.2012.09074.x. [DOI] [PubMed] [Google Scholar]
- 24.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–10. [PubMed] [Google Scholar]
- 25.Chavez JA, Holland WL, Bär J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148–53. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 26.Lima-Leopoldo AP, Leopoldo AS, da Silva DC, do Nascimento AF, de Campos DH, Luvizotto RA, et al. Long-term obesity promotes alterations in diastolic function induced by reduction of phospholamban phosphorylation at serine-16 without affecting calcium handling. J Appl Physiol. 2014;117:669–78. doi: 10.1152/japplphysiol.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leopoldo AS, Lima-Leopoldo AP, Sugizaki MM, do Nascimento AF, de Campos DH, Luvizotto Rde A, et al. Involvement of L-type calcium channel and SERCA2a in myocardial dysfunction induced by obesity. J Cell Physiol. 2011;226:2934–42. doi: 10.1002/jcp.22643. [DOI] [PubMed] [Google Scholar]
- 28.Roy VK, Kumar A, Joshi P, Arora J, Ahanger AM. Plasma free Fatty Acid concentrations as a marker for acute myocardial infarction. J Clin Diagn Res. 2013;7:2432–4. doi: 10.7860/JCDR/2013/7682.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpe CM, Nogueira-Machado JA. The dual role of free fatty acid signaling in inflammation and therapeutics. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:189–97. doi: 10.2174/18715303113139990041. [DOI] [PubMed] [Google Scholar]
- 30.Shen H, Eguchi K, Kono N, Fujiu K, Matsumoto S, Shibata M, et al. Saturated fatty acid palmitate aggravates neointima formation by promoting smooth muscle phenotypic modulation. Arterioscler Thromb Vasc Biol. 2013;33:2596–607. doi: 10.1161/ATVBAHA.113.302099. [DOI] [PubMed] [Google Scholar]
- 31.Harvey KA, Walker CL, Pavlina TM, Xu Z, Zaloga GP, Siddiqui RA. Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clin Nutr. 2010;29:492–500. doi: 10.1016/j.clnu.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Lai YH, Petrone AB, Pankow JS, Arnett DK, North KE, Ellison RC, et al. Association of dietary omega-3 fatty acids with prevalence of metabolic syndrome: the National Heart, Lung, and Blood Institute Family Heart Study. Clin Nutr. 2013;32:966–9. doi: 10.1016/j.clnu.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon HS, Batirel S, Mantzoros CS. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism. 2014;63:1447–54. doi: 10.1016/j.metabol.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhou YJ, Tang YS, Song YL, Li A, Zhou H, Li Y. Saturated fatty acid induces insulin resistance partially through nucleotide-binding oligomerization domain 1 signaling pathway in adipocytes. Chin Med Sci J. 2013;28:211–7. doi: 10.1016/S1001-9294(14)60004-3. [DOI] [PubMed] [Google Scholar]
- 35.Gao D, Griffiths HR, Bailey CJ. Oleate protects against palmitate-induced insulin resistance in L6 myotubes. Br J Nutr. 2009;102:1557–63. doi: 10.1017/S0007114509990948. [DOI] [PubMed] [Google Scholar]
- 36.Ramírez E, Klett-Mingo M, Ares-Carrasco S, Picatoste B, Ferrarini A, Rupérez FJ, Caro-Vadillo A, Barbas C, Egido J, Tuñón J, Lorenzo Ó. Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc Diabetol. 2013;12:172. doi: 10.1186/1475-2840-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira Junior SA, Padovani CR, Rodrigues SA, Silva NR, Martinez PF, Campos DH, Okoshi MP, Okoshi K, Dal-Pai M, Cicogna AC. Extensive impact of saturated fatty acids on metabolic and cardiovascular profile in rats with diet-induced obesity: a canonical analysis. Cardiovasc Diabetol. 2013;12:65. doi: 10.1186/1475-2840-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J Biol Chem. 2002;277:49676–84. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 39.Chabowski A, Żendzian-Piotrowska M, Konstantynowicz K, Pankiewicz W, Mikłosz A, Łukaszuk B, Górski J. Fatty acid transporters involved in the palmitate and oleate induced insulin resistance in primary rat hepatocytes. Acta Physiol (Oxf) 2013;207:346–57. doi: 10.1111/apha.12022. [DOI] [PubMed] [Google Scholar]
- 40.Medei E, Lima-Leopoldo AP, Pereira-Junior PP, Leopoldo AS, Campos DH, Raimundo JM, et al. Could a high-fat diet rich in unsaturated fatty acids impair the cardiovascular system? Can J Cardiol. 2010;26:542–8. doi: 10.1016/S0828-282X(10)70469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–9. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 42.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–86. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Jang HJ, Kim HS, Hwang DH, Quon MJ, Kim JA. Toll-like receptor 2 mediates high-fat diet-induced impairment of vasodilator actions of insulin. Am J Physiol Endocrinol Metab. 2013;304:E1077–88. doi: 10.1152/ajpendo.00578.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 45.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–63. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 47.L’homme L, Esser N, Riva L, Scheen A, Paquot N, Piette J, Legrand-Poels S. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J Lipid Res. 2013;54:2998–3008. doi: 10.1194/jlr.M037861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finucane OM, Lyons CL, Murphy AM, Reynolds CM, Klinger R, Healy NP, et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–28. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 49.Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, Moore NR, Frayn KN. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia. 2002;45:369–77. doi: 10.1007/s00125-001-0768-3. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Martinez P, Garcia-Quintana JM, Yubero-Serrano EM, Tasset-Cuevas I, Tunez I, Garcia-Rios A, Delgado-Lista J, Marin C, Perez-Jimenez F, Roche HM, Lopez-Miranda J. Postprandial oxidative stress is modified by dietary fat: evidence from a human intervention study. Clin Sci (Lond) 2010;119:251–61. doi: 10.1042/CS20100015. [DOI] [PubMed] [Google Scholar]
- 51.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 52.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 53.Lamers D, Schlich R, Horrighs A, Cramer A, Sell H, Eckel J. Differential impact of oleate, palmitate, and adipokines on expression of NF-κB target genes in human vascular smooth muscle cells. Mol Cell Endocrinol. 2012;362:194–201. doi: 10.1016/j.mce.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, et al. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175. doi: 10.1186/1476-511X-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–64. doi: 10.1023/A:1020546615229. [DOI] [PubMed] [Google Scholar]
- 56.Fan JH, Feng GG, Huang L, Tang GD, Jiang HX, Xu J. Naofen promotes TNF-α-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J Gastroenterol. 2014;20:4963–71. doi: 10.3748/wjg.v20.i17.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staiger K, Staiger H, Weigert C, Haas C, Häring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;551:3121–6. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 58.Al-Shudiefat AA, Sharma AK, Bagchi AK, Dhingra S, Singal PK. Oleic acid mitigates TNF-α-induced oxidative stress in rat cardiomyocytes. Mol Cell Biochem. 2013;372:75–82. doi: 10.1007/s11010-012-1447-z. [DOI] [PubMed] [Google Scholar]
- 59.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–71. [PubMed] [Google Scholar]


