Abstract
Metabolic syndrome (MetS) as a collection of obesity-associated disorders is associated with inflammation, oxidative stress, pro-thrombotic state, elevated risk of developing cardiovascular disease and type 2 diabetes. Adiponectin is one of the most abundant peptide hormones derived from adipose tissue. This protein plays a major role in glucose and lipid metabolism and prevents development of vascular changes. Anti-oxidative and anti-inflammatory effects are the other features of adiponectin. Hypoadiponectinemia is associated with hypertension and pro-thrombotic state. In this review, we discuss the crucial role of adiponectin in prevention of metabolic syndrome considering its effects on the components of this syndrome. Pharmacological interventions and lifestyle modification may increase plasma adiponectin level or tissue sensitivity which seems to be a promising target for prevention and therapeutic approaches of MetS and related diseases.
Keywords: Adiponectin, Atherosclerosis, Hypertension, Inflammation, Metabolic syndrome, Oxidative stress
Introduction
Metabolic syndrome (MetS), also known as syndrome X or insulin resistance syndrome, is a collection of obesity-associated disorders that comprises dyslipidemia (triglyceride (TG) >150 mg/dl, high-density lipoprotein (HDL) cholesterol <40 mg/dl in males and <50 in females), impaired fasting glucose (fasting glucose ≥100) and visceral adiposity (waist circumference >102 cm in men and >88 cm in woman) (1-3). Also, this syndrome is associated with prothrombotic state (4), inflammation, oxidative stress (5), elevated risk of developing cardiovascular disease (CVD) like atherosclerosis (6) and type 2 diabetes (T2D) (4). MetS and atherosclerosis are the main causes of morbidity and mortality worldwide (7).
In past, adipose tissue was considered only as a storage depot of extra energy, but is now regarded to be a highly active endocrine gland secreting several bioactive molecules known as adipokines or adipocytokines. One of the most abundant peptide hormones derived from adipose tissue is known as a new adipokine with anti-atherogenic and anti-inflammatory features (8). In this review article, focusing on adiponectin effects on each component of MetS, its crucial roles in the prevention of this syndrome are reviewed.
Adiponectin, structure and its receptors
In human, adiponectin gene is located on chromosome 3q27 (9) and encodes a 244 amino acids protein (10, 11). This locus has been associated with diabetes and cardiovascular diseases (12). Primarily three isoforms are detected in plasma: a low molecular weight trimer (LMW), a medium molecular weight hexamer (MMW) and a high molecular weight (HMW) 12- to 18-mers (9, 13). HMW form may be the most active form of adiponectin (14). Also, HMW to total adiponectin ratio is more useful than total adiponectin in metabolic syndrome diagnosis (15). Adiponectin is mainly produced in white adipose tissue (WAT) and particularly in mature adipocytes. Also, epicardial fat expresses adiponectin (7, 16). Adiponectin plasma levels are more nearly associated with the amount of visceral than total body fat (17). This specifically indicated a close relationship between visceral obesity and metabolic or cardiovascular diseases (15). Liver, cardiomyocytes, skeletal muscle, colon, salivary glands, placenta and pituitary express adiponectin at lower levels (7). Adiponectin performs its physiological effects mainly via AdipoR1 and AdipoR2 receptors. Both receptors are ubiquitously expressed (7) especially in monocytes and macrophages (18). AdipoR1 is principally expressed in skeletal muscle (19), but it is also expressed in endothelial cells (20), cardiomyocytes (21) and pancreatic-β cells (22). AdipoR2 is primarily expressed in the liver (19) and partly in endothelial cells (23) and cardiomyocytes (24). In muscle and adipose tissue in hyperinsulinemic and hyperglycemic states, the expression of both receptors is meaningfully reduced (25). AdipoR1 and AdipoR2 effects are mediated through APPL1, an adaptor protein which has a prominent role in metabolic effects of adiponectin (26). In addition, APPL1 interactions with insulin-signaling molecules indicated a molecular link between adiponectin and downstream insulin occurrence (27). The studies revealed that AdipoR1 and AdipoR2 may be firmly associated with activation of AMPK and PPAR-α signaling pathway in the liver, respectively (28). Activation of AMP-activated protein kinase (AMPK), PPAR-α and p38 mitogen-activated protein kinase (MAPK)-signaling pathways are involved in molecular signaling of adiponectin (29).
The third adiponectin receptor is T-cadherin, a glycosylphosphatidylinositol (GPI) which is anchored to the surface membrane and lacks cytoplasmic domain. This receptor is not effectively expressed in muscles and liver, but is expressed in vascular endothelial and smooth muscle cells (27, 30).
The Effect of adiponectin on glucose metabolism
AMPK, a stress-responsive kinase, has an important role in the regulation of cellular and whole body energy balance. AMPK has several functions such as inhibition of hepatic gluconeogenesis, increasing muscle glucose transport (31), phosphorylation of phosphor-fructokinase-2 (PFK-2) and increasing glycolytic glucose disposal (32). Adiponectin signaling causes an increase in the phosphorylation of P-38MAPK and AMPK in skeletal muscle (33, 34), liver (27) and adipocytes (35). Adiponectin knockout (KO) mice showed decreased expression of glucokinase, phosphofruktokinase and pyruvate dehydrogenase (important enzymes that control glycolysis), also isocitrate dehydrogenase which is a major enzyme of tricarboxilic acid (TCA) cycle (36).
Adiponectin directly sensitizes body tissues to insulin. There is a significant inverse relationship of adiponectin and its receptors with insulin resistance. Hemoglobin A1c (HbA1C), an indicator of glycemic control, showed a negative correlation with serum adiponectin (8). Serum adiponectin level is a precise index that can predict insulin resistance in patients with T2D and may have an important role in the pathogenesis of diabetes (37, 38). Therefore, hypoadiponectinemia may place individuals at risk for developing diabetes (9)and MetS patients are at high risk for developing T2D (39).
Adiponectin and lipid metabolism
Proteins such as CD-36 (fatty acid transporter/-scavenger receptor), acyl-coenzyme A (acyl-CoA) oxidase and uncoupling protein-2 are involved in fatty acid transport and oxidation. Adiponectin can stimulate these proteins in muscle and increase fat combustion and energy waste (19, 40). Liver lipid metabolism is regulated by AMPK activation, which is involved in the regulation of lipogenesis and cholesterol synthesis (41) and can be directly activated by adiponectin (42).
Expression of several genes which are involved in proximal and mitochondrial β-oxidation are regulated by PPAR-α (43, 44). Adiponectin induces PPAR-α transcriptional activity through AMPK and P38-MAPK activation (45). Adiponectin induces phosphorylation and inactivation of acetyl Co-A carboxylase (via AMPK activation) and enhances fatty acid oxidation rates (46). This suggested that adiponectin can initiate long and short-term stimulation of fatty acid oxidation (45).
Hepatocyte nuclear factors (Hnf1, Hnf3, Hnf4a, Hnf6) are principally involved in the regulation of liver complex function (47, 48) in which especially Hnf1a, Hnf6 and Hnf4a have central roles (49). Hnf4a regulates the expression of transcription factors such as Hnf1, a chief regulator in hepatocytes, SREBP1 and ChREBP1, these two factors participate in hepatic lipogenesis. The expression of Hnf4a is reduced in adiponectin KO mice (36). Adiponectin regulates the expression of several important hepatic metabolic genes through Hnf4a (36). Whilst AMPK activation suppresses the expression of SREBP1c; this transcription factor is involved in the expression of genes which encode proteins that are involved in liver fatty acid synthesis (42).
The ATP binding cassette A1 (ABCA1) is a key participant in the reverse cholesterol process whereby it mediates cholesterol efflux directly to HDL particles (50). Adiponectin enhances Apo-A1 and ABCA-1 expression in hepatocytes that are involved in HDL formation (51). Adiponectin meaningfully enhances mRNA transcription of ABCA-1 in the liver (52). Hypoadiponectinemia may be associated with smaller low-density lipoprotein (LDL) size, reduction of lipoprotein lipase (LPL) activity, decrease in HDL and increase in TG levels (53). Plasma adiponectin level has negative relevance with apolipoprotein B-100 and triglyceride in T2D patients (53).
Anti-Inflammatory effects
MetS patients often have a pro-inflammatory state, as displayed by increased levels of cytokines such as tumor necrosis factor alpha (TNF-α), IL-6 and C-reactive protein (CRP) (4), an important marker for systemic inflammation (53). MetS is closely associated with systemic inflammation and it is suggested that CRP could be considered as an appropriate marker for MetS diagnosis (54). However, liver is an important source of CRP, human adipose tissue can express CRP mRNA (55). Human studies reported an inverse association between adiponectin level and CRP (56), TNF-α and IL-6 (54). Also, there is a reverse association between adiponectin mRNA and CRP mRNA in human adipose tissue (55). It is suggested that adiponectin can control the expression of CRP in adipose tissue (57).
Monocyte chemo-attractant protein-1 (MCP-1) has a great role in the recruitment of monocytes and regulation of migration and infiltration of monocyte/-macrophages (58), hence it induces inflammation and insulin resistance (14). It is reported that concurrent adipoR1 and adipoR2 disturbances caused an increase in MCP-1 expression in WAT. It seems that reduced adiponectin signaling increases inflammation in WAT (28).
In macrophages, the production of pro-inflammatoy cytokines such as TNF-α and IL-6 is inhibited by chronic treatment with adiponectin which can be associated with nuclear factor kappa B (NF-kβ) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation by adiponectin (59-61). Also, this protein suppresses TNF-α mRNA expression in adipocytes (62). Following stimulation with adiponectin, human monocytes are primed into M2 macrophages with anti-inflammatory properties (63).
In vitro studies showed that adiponectin induces the production of anti-inflammatory cytokines such as interleukin 10 (IL-10), interleukin-1 receptor antagonist (IL-1RA) in primary human monocytes and monocyte-derived dendritic cells (Mo-DCs) and macrophages. Low circulating level of IL-10 is associated with metabolic syndrome in women (64).
Perivascular adipose tissue has a significant role in vascular inflammation (65). The experiments suggested that MetS is associated with perivascular adipose inflammation and endothelial dysfunction due to reduced NO (66). Insulin resistance and hyperglycemia, as major factors involved in MetS, have negative effects on the synthesis and release of NO (67). NO has vasodilatory effect and it can decrease vascular permeability, reduce LDL oxidation rate and suppress vascular smooth muscle cell proliferation (68, 69). Adiponectin activates endothelial nitric oxide synthase (eNOS) in endothelial cells and stimulates NO production (61) via activation of AMPK signaling and phosphoinositide-3-kinase (PI3K)-Akt pathway (7, 64) and increases eNOS expression in endothelial cells. Adiponectin reverts the inhibition of eNOS activity by oxidized LDL (ox-LDL) (70) and hyperglycemia (71). Also, CRP has direct pro-inflammatory effect on vessel wall (72, 73). An experiment using human recombinant CRP showed that this protein causes down-regulation of eNOS, up-regulation of adhesion molecules and simplification of endothelial cell apoptosis and increases angiotensin type I receptor and neointima formation (73). As noted above, adiponectin has negative effect on CRP production.
In summary, Adiponectin suppresses endothelial cell activation and monocyte attachment and inhibits interplay between leukocyte and endothelial cells (67).
Anti-oxidative effect of Adiponectin
MetS patients have increased oxidation damage in the form of elevated malondialdehyde (a product of lipid peroxidation), protein carbonyls and xanthine oxidase activity. On the other hand, in these patients antioxidant defense, vitamin C and E concentrations, superoxide dismutase activity (74, 75), and adiponectin level have decreased (76). Animal models indicated that oxidative stress promoted insulin resistance (77). It should be noted that ROS have important role in oxidative stress (78). Human studies showed that plasma adiponectin level had inverse association with oxidative stress markers e.g. plasma thiobarbituric acid reactive substance (TBARS) and urinary 8-epi-prostaglandin-F2α (8-epi-PGF2α) (79). Also, another study indicated that in MetS patients, adiponectin has positive association with reduced glutathione (GSH) (80). The production of ROS generated by high glucose (81), ox-LDL (82) (by cAMP/PKA activation) (83) and palmitate (84), is inhibited by adiponectin in endothelial cells. Decreased adiponectin levels are indicators of increased oxidative state in the arterial wall and are associated with high ox-LDL levels in patients with type 2 diabetes mellitus and coronary artery disease (85). The studies suggested that adiponectin suppresses vascular endothelial growth factor (VEGF)-induced ROS production which -when considered with anti-inflammatory effect of adiponectin- denoted an important antioxidant role of adiponectin in the vasculature (83). As mentioned above, adiponectin has negative effect on the production of CRP, a protein that stimulates ROS production (73). Homocysteine has pro-oxidant activity (86) and there is an inverse association between adiponectin and homocysteine levels in MetS patients (87). Therefore, hypoadiponectinemia has been closely associated with oxidative stress in these patients.
Adiponectin and hypertension
Hypertension is a significant health problem worldwide and affects more than 20% of the adult population (88). This disorder is regarded as an important feature of MetS (89). Arterial stiffness is associated with hypertension development (90) and MetS (89). Hyperglycemia, a significant constituent of MetS, enhances arterial stiffness (91). Increased collagen deposition is correlated with intensified arterial stiffness. Collagen deposition in hypertension is related to serum level of procollagen type I carboxyterminal propeptide (PICP) (92). Serum adiponectin level has an important negative association with PICP (93). Hypoadiponectinemia is a determinant of elevated peripheral stiffness (94). Low levels of adiponectin can predispose to hypertension through several mechanisms like insulin sensitizing, involvement in fatty acid metabolisms and vasoprotective effects (95).
Renin-angiotensin system (RAS) has an important role in the regulation of blood pressure and cardiovascular function. This system is activated in MetS and leads to elevation of angiotensin II levels, arterial wall inflammation, oxidative stress and development of atherosclerosis (96, 97). New finding indicated that elevating adiponectin level may be an efficient strategy to suppresses RAS activation related disorders in MetS (52).
The relationship of oxidative stress and inflammation (both are involved in MetS) with hypertension is well recognized (98). As noted above, adiponectin has antioxidative and anti-inflammatory effects. Cross-sectional studies revealed that hypoadiponectinemia may be an independent risk factor for hypertension (99, 100).
Adiponectin and prothrombotic state in metabolic syndrome
Several studies indicated the association between MetS and higher risk of pro-thrombotic state which includes elevated plasmatic coagulation, decreased fibrinolysis, reduced endothelial thrombo resistance and platelet hyperactivity (101, 102). Prothrombotic markers have positive correlations with various component of MetS (103). P-selectin, an integral membrane glycoprotein, and sCD40L, a soluble form of CD40 Ligand, are markers of platelets activation. MetS patients have higher levels of these markers than control subjects (104, 105). It should be noted that continual platelet activation is important for progression of acute vascular events (106). AdipoR1/R2 is expressed on platelets and adiponectin reduces platelet aggregation and sCD40L release from platelet. (107). It is indicated a proagregatory platelet phenotype in adiponectin-null mice (108). Also, adiponectin acts as anti-thrombotic agent via NO which inhibits platelet aggregation and adhesion to vascular walls (68, 71).
Plasminogen activator inhibitor type-1 (PAI-1) antigen, a single chain glycoprotein with proinflammatory effect, has an important function in the fibrinolysis (109). PAI-1 is closely associated with MetS (110, 111) and atherosclerosis development (112). It has been suggested that in overweight hypertensive patients, adiponectin is independently and negatively associated with PAI-1 antigen, (113) (Figure 1).
Figure 1.
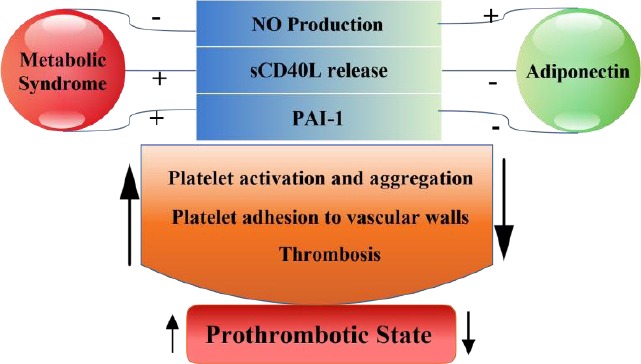
The suppression of prothrombotic state by adiponectin
NO: nitric oxide; sCD40L: soluble form of CD40 Ligand; PAI-1: Plasminogen activator inhibitor type-1
Adiponectin and atherosclerosis
MetS is an important predictor of CVD (114). Particularly, MetS has independent association with atherosclerosis (115). Hypoadiponectinemia is correlated with elevated risk factors of atherosclerotic cardiovascular disease. Plasma adiponectin level is associated with atherosclerosis markers such as inflammation, oxidative stress, and endothelial dysfunction (116). Adiponectin functions as a molecular regulator of atherosclerosis. Lipid laden macrophages or foam cells illustrating early atherosclerotic lesions. Adiponectin inhibits foam cell formation by weakening the attachment of monocytes to endothelial cells via inhibiting synthesis of adhesion molecules such as intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM) and E-selectin (12). Also, adiponectin represses the class A macrophage scavenger receptor (SR-A) expressing (117), down-regulates acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) (118) and induces IL-10 secretion from macrophages (119). Matrix metalloproteinase (MMP) enzymes which are involved in degradation of supportive collagen and fibrous cap thinning can increase the risk of plaque rupture and eventual thrombosis (120). Adiponectin enhances the expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in human monocytes-derived macrophages, which results in plaque stabilization (121) (Figure 2). Adiponectin null mice showed increased thrombus formation and platelet aggregation at locations of vascular damage (108). Besides, apolipoprotein E-null (apoE-null) mice overexpressing adiponectin had fewer atherosclerotic lesion than control apoE-null mice (122).
Figure 2.
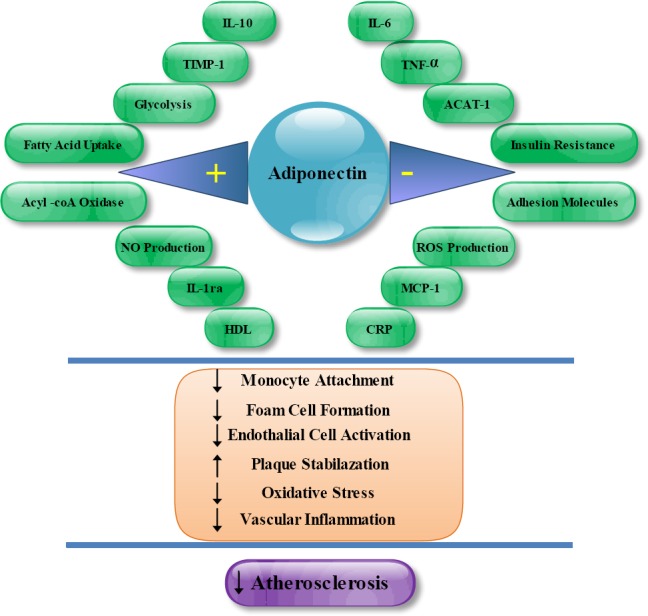
MetS has independent association with Atherosclerosis. Adiponectin reduces the progression of atherosclerosis through several mechanisms.
TIMP-1: tissue inhibitor of metalloproteinase-1; ACAT-1: acyl-CoA:cholesterol acyltransferase-1; MCP-1: Monocyte chemo attractant protein-1; IL-: interleukin-; NO: nitric oxide; CRP: C-reactive protein; TNF-α: tumor necrosis factor alpha; HDL: high-density lipoprotein
Adiponectin and lifestyle modification
It has been indicated that lifestyle modification has important effects on MetS as it reduces the intensity of related abnormalities (123). Obesity is closely associated with an increase in adipocytes size and number (124). Furthermore animal studies indicated that enlarged adipocyte cells in obese mice caused similar condition with T2D and insulin resistance (125). A negative correlation has been reported between adipocyte size and adiponectin level (126). Adiponectin plasma level decreases with weight gain and increases with weight loss (127, 128) and has inverse correlation with body mass index (BMI), intra-abdominal fat and insulin resistance (129). In coronary artery disease, considerable weight reduction is correlated with elevation of HMW adiponectin (130). It was shown that acute weight loss (4-6 weeks) did not change adiponectin level (131).
Diet-induced weight loss increased different forms of adiponectin (HMW, MMW and LMW) in plasma (132). Also, dietary changes affects adiponectin level as it was seen in animal studies that fish oil (rich in n-3 fatty acids) incrementally raised adiponectin level in a dose dependent manner. Fish oil induces adiponectin gene expression in epididymal fat in a PPAR-γ dependent fashion (133). Also, low glycemic load and fiber rich diets may increase plasma adiponectin level in diabetic patients (134). However, it has been reported that there is no association between total energy intake, protein, fat or carbohydrate intake and adiponectin levels (135).
Exercise training or increased physical activity, especially with reduction in fat mass, increased adiponectin in adipose tissue and decreased production of inflammatory cytokines (136, 137).
Visceral adipose tissue (VAT) secretes high levels of PAI-1 and accumulation of VAT is associated with hypo-secretion of adiponectin (138). Regular exercise training can reduce VAT with minimum change in weight (139, 140). It has been shown that 12 weeks of aerobic exercise training in older obese adults, independent of dietary glycemic index, significantly increased HMW adiponectin secretion that is inversely correlated with reduction in VAT. It was concluded that VAT is a key factor in the regulation of HMW adiponectin (141).
Acute sessions of low or moderate exercise do not affect adiponectin levels in healthy lean subjects (142-144), even a decrease in adiponectin levels was seen in young athletes after acute strenuous rowing. However, adiponectin levels do not change in response to low and moderate running or cycling (145). But in case of obese individuals, a recent study demonstrated that in inactive, abdominally obese men, acute and short-term (one week) aerobic exercise training significantly increased plasma adiponectin levels independent of intensity (146). Also, seven consecutive days of vigorous aerobic exercise improves insulin sensitivity, fat oxidation and HMW adiponectin, independent of changes in body weight and composition (147). The effect of various intensities of acute exercise on changes in total adiponectin level and its oligomers in middle-aged abdominally obese men has been studied. Moderate-intensity exercise has no effect on total adiponectin level but high-intensity exercise decreases it without changing HMW form and mainly by reduction of MMW and LMW form (148). The researchers indicated that epinephrine may partially regulate the decrease in total and MLMW adiponectin levels during high-intensity exercise (148).
Chronic exercise training can improve MetS by several mechanisms as it increases insulin sensitivity, reduces body weight and improves fitness levels. Also, it can increase adiponectin resting levels and expression of adiponectin mRNA and AdipoR1/R2 mRNA in skeletal muscle (145, 149, 150). The studies showed 12 weeks of regular aerobic training decreases the potential risk of coronary heart disease by increasing adiponectin levels and decreasing inflammatory markers in non-athlete obese men (151).
Different results may be attributed to the nature of exercise program i.e. type (endurance, resistance, combined), intensity (high, moderate, low), duration (short- vs. long-term), and subject status (healthy vs. patient; trained vs. untrained; overweight and obese vs. lean) while the form of measured adiponectin (total or multimers) is another issue (152). However, it seems that overweight and obese individuals may benefit more than normal-weight persons from exercise training. Hence, further and better controlled studies are required.
Smoking has an important role in health status. Hypoadiponectinemia is associated with smoking habits (153- 155). An important dose-response association was detected between the number of cigarettes and plasma adiponectin level (153). Hypoadiponectinemia in smokers may be associated with smoking not to concurrent presence of insulin resistance (156). Nicotine enhances inflammation and directly affects human adipose tissue (157). In vitro studies in mice 3T3-L1 showed that nicotine and H2O2 decreased mRNA expression and secretion of adiponectin in a dose-dependent fashion (158).
Lifestyle controls synthesis and secretion of adiponectin via several mechanisms: PPAR-γ and AMPK activation, post-translational modification, modification of adipose tissue morphology, infiltration of macrophages and inflammation (159).
Adiponectin and pharmacological intervention
PPAR γ agonists such as TZDs (thiazolidinediones) enhance insulin sensitivity. In vivo and in vitro studies showed that TZDs can normalize or increase adiponectin mRNA expression and adiponectin secretion in a dose-time dependent fashion (160). Natural compounds may increase adiponectin level (161). Recent studies indicated that Zataria multiflora can increase adiponectin level, which may be due to increase in PPAR γ protein (162). A recent study showed that using aged garlic extract for 12 weeks can increase adiponectin level in MetS patients (163). Also, resveratral, a natural polyphenol, can regulate adiponectin expression and multimerization in adipocytes. This is caused by activation of FOX1, a transcription factor involved in the regulation of adiponectin gene expression and AMPK signaling pathways (164). It seems that the expression of AdipoR1 and AdipoR2 in skeletal muscle and adipose tissue are increased by PPAR-γ agonists and this may activate adiponectin intracellular signaling pathway (9). Also, another study showed that dual PPAR α/γ agonists like MK-0767 increases plasma adiponectin level in healthy subjects (165). Troglitazone therapy for 3 months up-regulates adiponectin synthesis and secretion in obese T2D patients (166). An in vitro study indicated that PPAR-α agonists enhance insulin sensitivity and increase serum total adiponectin (167). Fenofibrate increases HMW adiponectin in hypertriglyceridemic patients (168). RAS blockers such as ACEIs (angiotensin converting-enzyme inhibitors) and ARBs (angiotensin II receptor blockers) increase adiponectin level in MetS patients (169). Also, temocapril (an ACEI) or candesartan (an ARB) increases adiponectin level in insulin-resistant essential hypertensives and losartan (an ARB) increases adiponectin level in type-1 diabetes or hypertensive patients (53).
Conclusion
MetS is considered as an important world health problem, which has close association with T2D and CVD. A large body of evidence suggested that adiponectin has an important role in the prevention of MetS. Adiponectin is known as an anti-inflammatory, antioxidative, anti-atherogenic adipokine and it has insulin sensitizing effect. Several clinical and experimental studies have emphasized these biological functions. It is suggested that pharmacological approaches and lifestyle modification may increase plasma adiponectin level or tissue sensitivity which could be a promising target for prevention and treatment of MetS and related diseases.
Footnotes
Conflict of interest
Authors have no conflict of interest to declare.
References
- 1.Alberti K, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and Management of the Metabolic Syndrome An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation. 2005;112:e285–e290. [Google Scholar]
- 5.Pop-Busui R, Pietropaolo M. Metabolic Syndrome and Inflammation. In: Eisenbarth GS, editor. Immunoendocrinology: Scientific and Clinical Aspects. Totowa, NJ: Humana Press; 2011. pp. 69–92. [Google Scholar]
- 6.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 7.Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M. The role of adiponectin in human vascular physiology. Int J Cardiol. 2012;155:188–193. doi: 10.1016/j.ijcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Izadi M, Goodarzi MT, Khorshidi D, Doaly H, Samarikhalaj H. Relationship of serum adiponectin and beta-cell function in obese males with type 2 diabetes. Asian J Pharm Biol Res. 2012;2:40–44. [Google Scholar]
- 9.Szmitko P, Teoh H, Stewart D, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol. 2007;292:H1655–1663. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2010;425:41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- 11.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu W, Cheng KKY, Vanhoutte PM, Lam KSL, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci. 2008;114:361. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 13.Villarreal-Molina M-T, Antuna-Puente B. Adiponectin: anti-inflammatory and cardiopro-tective effects. Biochimie. 2012;94 doi: 10.1016/j.biochi.2012.06.030. 2143e2149. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes. 2008;32:S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high–molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–445. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 17.Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: From pathophysiology to risk stratification. Int J Cardiol. 2010;138:3–8. doi: 10.1016/j.ijcard.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 18.Pang TTL, Narendran P. The distribution of adiponectin receptors on human peripheral blood mononuclear cells. Ann N Y Acad Sci. 2008;1150:143–145. doi: 10.1196/annals.1447.021. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 20.Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Piñeiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–5269. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 22.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic βcells. Biochem Biophys Res Commun. 2003;312:1118–1122. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Tan KCB, Xu A, Chow WS, Lam MCW, Ai VHG, Tam SCF, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Rev Cardiol. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 26.Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol - Endocrinol Metab. 2009;296:E22–E36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34:12–18. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 29.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol. 2007;40:115–120. doi: 10.1007/s00795-007-0364-9. [DOI] [PubMed] [Google Scholar]
- 31.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci. 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 32.Marsin A-S, Bertrand† L, Rider MH, Deprez J, Beauloye C, Vincent MF, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 33.Tomas E, Tsao T-S, Saha AK, Murrey HE, Zhang C cheng, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: Acetyl–CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U A. 2012;109:14568–14573. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izadi M, Goodarzi MT, Khalaj HS, Khorshidi D, Doali H. Serum adiponectin levels are inversely correlated with insulin resistance in obese men with type 2 diabetes. Int J Endocrinol Metab. 2011;9:253–257. [Google Scholar]
- 38.Goodarzi MT1, Babaahmadi-Rezaei H, Kadkhodaei-Eliaderani M, Haddadinezhad S. Relationship of serum adiponectin with blood lipids, HbA(1)c, and hs-CRP in type II diabetic postmenopausal women. J Clin Lab Anal. 2007;21:197–200. doi: 10.1002/jcla.20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PWF, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the framingham offspring cohort. Diabetes Care. 2004;27:538–546. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 41.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: focus on adiponectin. Curr Opin Pharmacol. 2006;6:580–585. doi: 10.1016/j.coph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B, Marcus SL, Sajjadi FG, Alvares K, Reddy JK, Subramani S, et al. Identification of a peroxisome proliferator-responsive element upstream of the gene encoding rat peroxisomal enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydro-genase. Proc Natl Acad Sci U A. 1992;89:7541–7545. doi: 10.1073/pnas.89.16.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez JC, Gil-Gómez G, Hegardt FG, Haro D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J Biol Chem. 1994;269:18767–18772. [PubMed] [Google Scholar]
- 45.Yoon MJ, Lee GY, Chung J-J, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator–activated receptor α. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 46.Naomi Kudo JGG. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta BBA - Lipids Lipid Metab. 1996:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 47.Cereghini S. Liver-enriched transcription factors and hepatocyte differentiation. FASEB J. 1996;10:267–282. [PubMed] [Google Scholar]
- 48.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.dom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darabi M, Rabbani M, Ani M, Zarean E, Panjehpour M, Movahedian A. Increased leukocyte ABCA1 gene expression in post-menopausal women on hormone replacement therapy. Gynecol Endocrinol. 2011;27:701. doi: 10.3109/09513590.2010.507826. [DOI] [PubMed] [Google Scholar]
- 51.Matsuura F, Oku H, Koseki M, Sandoval JC, Yuasa-Kawase M, et al. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem Biophys Res Commun. 2007;358:1091–1095. doi: 10.1016/j.bbrc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 52.Van Stijn CMW, Kim J, Barish GD, Tietge UJF, Tangirala RK. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PLoS One. 2014;9:e86404. doi: 10.1371/journal.pone.0086404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006;110:267. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 55.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 56.Kriketos AD, Greenfield JR, Peake PW, Furler SM, Denyer GS, Charles-worth JA, et al. Inflammation, insulin resistance, and adiposity: a study of first-degree relatives of type 2 diabetic subjects. Diabetes Care. 2004;27:2033–2040. doi: 10.2337/diacare.27.8.2033. [DOI] [PubMed] [Google Scholar]
- 57.Winer JC, Zern TL, Taksali SE, Dziura J, Dziura J, Cali AM, Wollschlager M, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4415–4423. doi: 10.1210/jc.2006-0733. [DOI] [PubMed] [Google Scholar]
- 58.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 61.Xu A, Vanhoutte PM. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;302:H1231–H1240. doi: 10.1152/ajpheart.00765.2011. [DOI] [PubMed] [Google Scholar]
- 62.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol - Regul Integr Comp Physiol. 2005;288:R1220–R1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 63.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esposito K, PontiUo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88:1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 65.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivas- cular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 67.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 69.Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007;18:58–65. doi: 10.1097/MOL.0b013e328012b627. [DOI] [PubMed] [Google Scholar]
- 70.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franchini M, Targher G, Montagnana M, Lippi G. The metabolic syndrome and the risk of arterial and venous thrombosis. Thromb Res. 2008;122:727–735. doi: 10.1016/j.thromres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 72.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 73.Szmitko PE, Wang C-H, Weisel RD, Almeida JR de, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation part I. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 74.Armutcu F, Ataymen M, Atmaca H, Gurel A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med. 2008;46:785–790. doi: 10.1515/CCLM.2008.166. [DOI] [PubMed] [Google Scholar]
- 75.Palmieri VO, Grattagliano I, Portincasa P, Palasciano G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr. 2006;136:3022–3026. doi: 10.1093/jn/136.12.3022. [DOI] [PubMed] [Google Scholar]
- 76.Chen SJ, Yen CH, Huang YC, Lee BJ, Hsia S, Lin PT. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7:e45693. doi: 10.1371/journal.pone.0045693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radic Biol Med. 2009;47:213–218. doi: 10.1016/j.freeradbiomed.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 78.Noh H, Ha H. Reactive Oxygen Species and Oxidative Stress. In: Lai KN, Tang SCW, editors. Contributions to Nephrology. Basel: KARGER; 2011. pp. 102–112. [DOI] [PubMed] [Google Scholar]
- 79.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Q-R, Yu T, Li P. Association of oxidative stress and serum adiponectin in patients with metabolic syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:623–627. [PubMed] [Google Scholar]
- 81.Ouedraogo R, Wu X, Xu S-Q, Fuchsel L, Motoshima H, Mahadev K, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 82.Plant S, Shand B, Elder P, Scott R. Adiponectin attenuates endothelial dysfunction induced by oxidised low-density lipoproteins. Diabetes Vasc Dis Res Off J Int Soc Diabetes Vasc Dis. 2008;5:102–108. doi: 10.3132/dvdr.2008.017. [DOI] [PubMed] [Google Scholar]
- 83.Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res. 2008;78:376–384. doi: 10.1093/cvr/cvn034. [DOI] [PubMed] [Google Scholar]
- 84.Kim J-E, Song SE, Kim Y-W, Kim J-Y, Park S-C, Park Y-K, et al. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010;207:35–44. doi: 10.1677/JOE-10-0093. [DOI] [PubMed] [Google Scholar]
- 85.Basati G, Pourfarzam M, Movahedian A, Samsamshariat SZ, Sarrafzadegan N. Reduced plasma adiponectin levels relative to oxidized low density lipoprotein and nitric oxide in coronary artery disease patients. Clinics. 2011;66:1129–1135. doi: 10.1590/S1807-59322011000700002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobsen DW. Hyperhomocysteinemia and oxidative stress time for a reality check? Arterioscler Thromb Vasc Biol. 2000;20:1182–1184. doi: 10.1161/01.atv.20.5.1182. [DOI] [PubMed] [Google Scholar]
- 87.Simão TNC, Lozovoy MAB, Simão ANC, Oliveira SR, Venturini D, Morimoto HK, et al. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br J Nutr. 2013;110:1885–1894. doi: 10.1017/S0007114513001207. [DOI] [PubMed] [Google Scholar]
- 88.Tavilani H, Esfahani M. Gene polymorphism and hypertension. ARYA Atheroscler. 2012;0:S212–S216. [Google Scholar]
- 89.Schillaci G, Pirro M, Vaudo G, Mannarino MR, Savarese G, Pucci G, et al. Metabolic syndrome is associated with aortic stiffness in untreated essential. Hypertension. 2005;45:1078–1082. doi: 10.1161/01.HYP.0000165313.84007.7d. [DOI] [PubMed] [Google Scholar]
- 90.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension the ARIC study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 91.Lee AT, Cerami A. Role of glycation in aging. Ann N Y Acad Sci. 1992;663:63–70. doi: 10.1111/j.1749-6632.1992.tb38649.x. [DOI] [PubMed] [Google Scholar]
- 92.Querejeta R, Varo N, López B, Larman M, Artiñano E, Etayo JC, et al. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729–1735. doi: 10.1161/01.cir.101.14.1729. [DOI] [PubMed] [Google Scholar]
- 93.Tsai W-C, Lin C-C, Chen J-Y, Huang Y-Y, Lee C-H, Li W-T, et al. Association of adiponectin with procollagen type I carboxyterminal propeptide in non-diabetic essential hypertension. Blood Press. 2008;17:233–238. doi: 10.1080/08037050802308895. [DOI] [PubMed] [Google Scholar]
- 94.Snijder MB, Flyvbjerg A, Stehouwer CDA, Frystyk J, Henry RMA, Seidell JC, et al. Relationship of adiposity with arterial stiffness as mediated by adiponectin in older men and women: the Hoorn Study. Eur J Endocrinol. 2009;160:387–395. doi: 10.1530/EJE-08-0817. [DOI] [PubMed] [Google Scholar]
- 95.Chow W-S, Cheung BMY, Tso AWK, Xu A, Wat NMS, Fong CHY, et al. Hypoadiponectinemia as a predictor for the development of hypertension A 5-year prospective study. Hypertension. 2007;49:1455–1461. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- 96.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 97.De Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav. 2010;100:525–534. doi: 10.1016/j.physbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 99.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 100.Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002;25:971–976. doi: 10.2337/diacare.25.6.971. [DOI] [PubMed] [Google Scholar]
- 101.Kok MGM, Meijers JCM, Pinto-Sietsma S-J. Individuals with coronary artery disease at a young age and features of the metabolic syndrome have an increased prothrombotic potential. Thromb Haemost. 2014;111:458–464. doi: 10.1160/TH13-07-0587. [DOI] [PubMed] [Google Scholar]
- 102.Russo I. The prothrombotic tendency in metabolic syndrome: focus on the potential mechanisms involved in impaired haemostasis and fibrinolytic balance. Scientifica (Cairo) 2012. 2012 doi: 10.6064/2012/525374. 525374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mauras N, Delgiorno C, Kollman C, Bird K, Morgan M, Sweeten S, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. 2010;95:1060–1068. doi: 10.1210/jc.2009-1887. [DOI] [PubMed] [Google Scholar]
- 104.Natal C, Restituto P, Inigo C, Colina I, Diez J, Varo N. The proinflammatory mediator CD40 ligand is increased in the metabolic syndrome and modulated by adiponectin. J Clin Endocrinol Metab. 2008;93:2319–2327. doi: 10.1210/jc.2007-2491. [DOI] [PubMed] [Google Scholar]
- 105.Gokulakrishnan K, Deepa R, Mohan V, Gross MD. Soluble P-selectin and CD40L levels in subjects with prediabetes, diabetes mellitus, and metabolic syndrome—the chennai urban rural epidemiology study. Metabolism. 2006;55:237–242. doi: 10.1016/j.metabol.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 106.Serebruany VL, Malinin A, Ong S, Atar D. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. J Thromb Thrombolysis. 2008;25:207–213. doi: 10.1007/s11239-007-0047-3. [DOI] [PubMed] [Google Scholar]
- 107.Restituto P, Colina I, Varo JJ, Varo N. Adiponectin diminishes platelet aggregation and sCD40L release. Potential role in the metabolic syndrome. Am J Physiol - Endocrinol Metab. 2010;298:E1072–E1077. doi: 10.1152/ajpendo.00728.2009. [DOI] [PubMed] [Google Scholar]
- 108.Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, Ujiie H, et al. Adiponectin acts as an endogenous antithrombotic factor. Arter Thromb Vasc Biol. 2006;26:224–230. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 109.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 110.Alessi M-C, Juhan-Vague I. PAI-1 and the metabolic syndrome links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 111.Corgosinho FC, Piano A de, Sanches PL, Campos RM, Silva PL, Carnier J, et al. The role of PAI-1 and adiponectin on the inflammatory state and energy balance in obese adolescents with metabolic syndrome. Inflammation. 2012;35:944–951. doi: 10.1007/s10753-011-9397-2. [DOI] [PubMed] [Google Scholar]
- 112.Maruyoshi H, Kojima S, Funahashi T, Miyamoto S, Hokamaki J, Soejima H, et al. Adiponectin is inversely related to plasminogen activator inhibitor type 1 in patients with stable exertional angina. Thromb Haemost. 2004;91:1026–1030. doi: 10.1160/TH03-12-0731. [DOI] [PubMed] [Google Scholar]
- 113.Skurk T, van Harmelen V, Lee YM, Wirth A, Haune H. Relationship between IL-6, leptin and adiponectin and variables of fibrinolysis in overweight and obese hypertensive patients. horm metab res. 2002;34:659–663. doi: 10.1055/s-2002-38253. [DOI] [PubMed] [Google Scholar]
- 114.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med J Br Diabet Assoc. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 115.Chiu T-Y, Chen C-Y, Chen S-Y, Soon C-C, Chen J-W. Indicators associated with coronary atherosclerosis in metabolic syndrome. Clin Chim Acta. 2012;413:226–231. doi: 10.1016/j.cca.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 116.Ekmekci H, Ekmekci OB. The role of adiponectin in atherosclerosis and thrombosis. Clin Appl Thromb. 2006;12:163–168. doi: 10.1177/107602960601200203. [DOI] [PubMed] [Google Scholar]
- 117.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 118.Furukawa K, Hori M, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, et al. Adiponectin down-regulates acyl-coenzyme A: cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun. 2004;317:831–836. doi: 10.1016/j.bbrc.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 119.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 120.Watanabe N, Ikeda U. Matrix metalloproteinases and atherosclerosis. Curr Atheroscler Rep. 2004;6:112–120. doi: 10.1007/s11883-004-0099-1. [DOI] [PubMed] [Google Scholar]
- 121.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 122.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 2012;10:138. doi: 10.1186/1741-7015-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes 2005. 2006;30:899–905. doi: 10.1038/sj.ijo.0803219. [DOI] [PubMed] [Google Scholar]
- 125.Tahergorabi Z, Rashidi B, Khazaei M. Ghrelin does not alter aortic intima-media thickness and adipose tissue characteristics in control and obese mice. Iran J Basic Med Sci. 2013;16:942–945. [PMC free article] [PubMed] [Google Scholar]
- 126.Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–214. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 127.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 128.Mavri A, Poredoš P, Suran D, Gaborit B, Juhan-Vague I, Poredoš P. Effect of diet-induced weight loss on endothelial dysfunction: early improvement after the first week of dieting. Heart Vessels. 2011;26:31–38. doi: 10.1007/s00380-010-0016-1. [DOI] [PubMed] [Google Scholar]
- 129.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol Eur Fed Endocr Soc. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 130.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xydakis AM, Case CC, Jones PH, Hoogeveen RC, Liu M-Y, Smith EO, et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004;89:2697–2703. doi: 10.1210/jc.2003-031826. [DOI] [PubMed] [Google Scholar]
- 132.Polak J, Kovacova Z, Jacek M, Klimcakova E, Kovacikova M, Vitkova M, et al. An increase in plasma adiponectin multimeric complexes follows hypocaloric diet-induced weight loss in obese and overweight pre-menopausal women. Clin Sci. 2007;112:557. doi: 10.1042/CS20060296. [DOI] [PubMed] [Google Scholar]
- 133.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator–activated receptor-γ–dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 134.Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care. 2005;28:1022–1028. doi: 10.2337/diacare.28.5.1022. [DOI] [PubMed] [Google Scholar]
- 135.Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. Clin Endocrinol Metab. 2003;88:1730–1736. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]
- 136.Kim SH, Lee SH, Ahn KY, Lee DH, Suh YJ, Cho SG, et al. Effect of lifestyle modification on serum chemerin concentration and its association with insulin sensitivity in overweight and obese adults with type 2 diabetes. Clin Endocrinol (Oxf) 2014;80:825–833. doi: 10.1111/cen.12249. [DOI] [PubMed] [Google Scholar]
- 137.Bradley RL, Jeon JY, Liu F-F, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586–594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes. 2008;32:S83–92. doi: 10.1038/ijo.2008.243. [DOI] [PubMed] [Google Scholar]
- 139.Ross R, Janssen I, Dawson J, Kungl A-M, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 140.Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA J Am Med Assoc. 2003;289:323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 141.Kelly KR, Navaneethan SD, Solomon TPJ, Haus JM, Cook M, Barkoukis H, et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med Sci Sports Exerc. 2014;46:920–926. doi: 10.1249/MSS.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ferguson MA, White LJ, McCoy S, Kim H-W, Petty T, Wilsey J. Plasma adiponectin response to acute exercise in healthy subjects. Eur J Appl Physiol. 2004;91:324–329. doi: 10.1007/s00421-003-0985-1. [DOI] [PubMed] [Google Scholar]
- 143.Punyadeera C, Zorenc AHG, Koopman R, McAinch AJ, Smit E, Manders R, et al. The effects of exercise and adipose tissue lipolysis on plasma adiponectin concentration and adiponectin receptor expression in human skeletal muscle. Eur J Endocrinol Eur Fed Endocr Soc. 2005;152:427–436. doi: 10.1530/eje.1.01872. [DOI] [PubMed] [Google Scholar]
- 144.Bobbert T, Wegewitz U, Brechtel L, Freudenberg M, Mai K, Möhlig M, et al. Adiponectin oligomers in human serum during acute and chronic exercise: relation to lipid metabolism and insulin sensitivity. Int J Sports Med. 2007;28:1–8. doi: 10.1055/s-2006-924028. [DOI] [PubMed] [Google Scholar]
- 145.Kraemer RR, Castracane VD. Exercise and humoral mediators of peripheral energy balance: ghrelin and adiponectin. Exp Biol Med Maywood NJ. 2007;232:184–194. [PubMed] [Google Scholar]
- 146.Saunders TJ, Palombella A, McGuire KA, Janiszewski PM, Després J-P, Ross R. Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab 2012. 2012:148729. doi: 10.1155/2012/148729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kelly KR, Blaszczak A, Haus JM, Patrick-Melin A, Fealy CE, Solomon TPJ, et al. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc. 2012;44:69–74. doi: 10.1249/MSS.0b013e318228bf85. [DOI] [PubMed] [Google Scholar]
- 148.Numao S, Katayama Y, Hayashi Y, Matsuo T, Tanaka K. Influence of acute aerobic exercise on adiponectin oligomer concentrations in middle-aged abdominally obese men. Metabolism. 2011;60:186–194. doi: 10.1016/j.metabol.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 149.Blüher M, Bullen JW, Jr, Lee JH, Kralisch S, Fasshauer M, Klöting N, et al. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab. 2006;91:2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- 150.Oberbach A, Tönjes A, Klöting N, Fasshauer M, Kratzsch J, Busse MW, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol Eur Fed Endocr Soc. 2006;154:577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 151.Akbarpour M. The effect of aerobic training on serum adiponectin and leptin levels and inflammatory markers of coronary heart disease in obese men. Biol Sport. 2013;30:21–27. doi: 10.5604/20831862.1029817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res 2014. 2014:726861. doi: 10.1155/2014/726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takefuji S, Yatsuya H, Tamakoshi K, Otsuka R, Wada K, Matsushita K, et al. Smoking status and adiponectin in healthy Japanese men and women. Prev Med. 2007;45:471–475. doi: 10.1016/j.ypmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 154.Sull JW, Kim HJ, Yun JE, Park EJ, Kim G, Jee SH. Serum adiponectin is associated with smoking status in healthy Korean men. Endocr J. 2009;56:73–78. doi: 10.1507/endocrj.k08e-231. [DOI] [PubMed] [Google Scholar]
- 155.Kotani K, Hazama A, Hagimoto A, Saika K, Shigeta M, Katanoda K, et al. Adiponectin and smoking status: a systematic review. J Atheroscler Thromb. 2012;19:787–794. doi: 10.5551/jat.11833. [DOI] [PubMed] [Google Scholar]
- 156.Abbasi F, Farin HMF, Lamendola C, McLaughlin T, Schwartz EA, Reaven GM, et al. The relationship between plasma adiponectin concentration and insulin resistance is altered in smokers. J Clin Endocrinol Metab. 2006;91:5002–5007. doi: 10.1210/jc.2006-0419. [DOI] [PubMed] [Google Scholar]
- 157.Andersson K, Arner P. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int J Obes Relat Metab Disord. 2001;25:1225–1232. doi: 10.1038/sj.ijo.0801654. [DOI] [PubMed] [Google Scholar]
- 158.Iwashima Y, Katsuya T, Ishikawa K, Kida I, Ohishi M, Horio T, et al. Association of hypoadiponectinemia with smoking habit in men. Hypertension. 2005;45:1094–1100. doi: 10.1161/01.HYP.0000169444.05588.4c. [DOI] [PubMed] [Google Scholar]
- 159.Tishinsky JM, Dyck DJ, Robinson LE. Chapter One-Lifestyle Factors Increasing Adiponectin Synthesis and Secretion. In: Gerald Litwack., editor. Vitamins & Hormones. Academic Press; 2012. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 160.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 161.Xu A, Wang H, Hoo RLC, Sweeney G, Vanhoutte PM, Wang Y, et al. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology. 2009;150:625–633. doi: 10.1210/en.2008-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mohammadi A, Gholamhoseinian A, Fallah H. Zataria multiflora increases insulin sensitivity and PPARγ gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014;17:263–270. [PMC free article] [PubMed] [Google Scholar]
- 163.Gómez-Arbeláez D, Lahera V, Oubiña P, Valero-Muñoz M, de Las Heras N, Rodríguez Y, et al. Aged garlic extract improves adiponectin levels in subjects with metabolic syndrome: a double-blind, placebo-controlled, randomized, crossover study. Mediators Inflamm 2013. 2013 doi: 10.1155/2013/285795. 285795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang A, Liu M, Liu X, Dong LQ, Glickman RD, Slaga TJ, et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J Biol Chem. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Decochez K, Rippley RK, Miller JL, Smet MD, Yan KX, Matthijs Z, et al. A dual PPAR α/γ agonist increases adiponectin and improves plasma lipid rofiles in healthy subjects. Drugs RD. 2006;7:99–110. doi: 10.2165/00126839-200607020-00004. [DOI] [PubMed] [Google Scholar]
- 166.Phillips SA, Ciaraldi TP, Kong APS, Bandukwala R, Aroda V, Carter L, et al. Modulation of Circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–674. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]
- 167.Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, et al. Peroxisome proliferator–activated receptor (PPAR)α activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue comparison of activation of PPARα, PPARγ, and their combination. Diabetes. 2005;54:3358–3370. doi: 10.2337/diabetes.54.12.3358. [DOI] [PubMed] [Google Scholar]
- 168.Oki K, Koide J, Nakanishi S, Nakashima R, Yamane K. Fenofibrate increases high molecular weight adiponectin in subjects with hypertrigly-ceridemia. Endocr J. 2007;54:431–435. doi: 10.1507/endocrj.k06-172. [DOI] [PubMed] [Google Scholar]
- 169.Tian F, Luo R, Zhao Z, Wu Y, Ban D. Blockade of the RAS increases plasma adiponectin in subjects with metabolic syndrome and enhances differentiation and adiponectin expression of human preadipocytes. Exp Clin Endocrinol Amp Diabetes. 2009;118:258–265. doi: 10.1055/s-0029-1237706. [DOI] [PubMed] [Google Scholar]


