Abstract
Objective(s):
Due to unsatisfactory response or intolerable side effects of current drugs, treatment of essential tremor remains inadequate. Thus, we aimed to investigate the protective and therapeutic effects of aqueous and ethanolic extracts of Crocus sativus (saffron), and its active consistent, safranal, on the harmaline-induced tremor in mice.
Materials and Methods:
To induce tremor, harmaline (30 mg/kg) was injected intraperitoneally. Test groups were also given the aqueous and ethanolic extracts of saffron (40, 80, and 160 mg/kg) as well as safranal (0.1, 0.3, and 0.5 ml/kg), intraperitoneally, 10 min before harmaline administration (prophylactic study) or 10 min after the onset of tremors (curative study). The latency of onset, duration, and intensity of tremor were recorded.
Results:
The extracts (80 and160 mg/kg) dose dependently attenuated duration of harmaline-induced tremors as did reference drug, propranolol (2 and 5 mg/kg). Only the highest dose of extracts (160 mg/kg) attenuated intensity of harmaline-induced tremors throughout the study. Safranal at the doses of (0.1 and 0.3 ml/kg) but not 0.5 ml/kg attenuated duration and intensity of tremor. Onset of tremor increased with the extracts (80 and 160 mg/kg) in prophylactic study, as the effect observed with propranolol at the dose of 5 mg/kg. Safranal did not affect the latency of tremor.
Conclusion:
Both aqueous and ethanolic extracts of saffron and with a less effect, low doses of safranal, have relatively protective and suppressive effects on the harmaline-induced tremor and different constituents of extracts seem to participate in the protective effects against harmaline induced tremor.
Keywords: Crocus sativus, Essential tremor, Harmaline, Safranal
Introduction
The primary manifestations of essential tremor (ET), one of the most frequent movement disorders, are postural and kinetic tremors. Due to the limited understanding of exact mechanisms involved in the pathogenesis of this disorder, developing new pharmacological agents with higher efficacy and lower side effects is required (1). Current approved drugs for the treatment of ET with the established efficacy (level A), are primidone and propranolol; however, these drugs have limited efficacy (a mean tremor reduction of about 50%), common side effects, and they often lead to patient noncompliance (2, 3). Harmaline, a β-carboline alkaloid, from the seeds of Peganum harmala, exhibits action tremor in mammals resembling centrally induced tremors, and is regarded as a known model for postural tremor and finding new therapies (4, 5).
From ancient times, the old-aged spice saffron (Crocus sativus L. stigma), has been used for flavoring and coloring food preparations. There is much evidence regarding the use of saffron in folk medicine for the treatment of many ailments such as an anticonvulsant remedy (6, 7). Up to now, anti-cancer (8, 9), anti-oxidant (10), cardioprotective effects (11, 12), anti-depressant (13, 14), anti-anxiety (15), memory improvement properties (16, 17), anti-nociceptive, and anti-inflammatory properties (18-20) of ethanolic and aqueous extracts of saffron as well as active ingredients including safranal and crocin, have been shown in advanced pharmacological studies. In addition, neuroprotective effects of saffron against focal cerebral ischemia (21), Alzheimer’s disease (22), as well as anticonvulsant effects have been verified (23). The beneficial medicinal effects of extracts typically result from the combinations of man ingredients, which could act synergistically. The present study was therefore taken up to investigate if the systemic administration of ethanolic and aqueous extracts of saffron and its active constituent, safranal, could suppress tremors induced by harmaline in mice.
Animals
Albino mice weighing 25–30 g were obtained from the animal center of School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran and were housed under standard environmental conditions (12–12-hr light/dark cycle at 22 °C) with free access to food pellets and water. Experimental protocol was approved by Mashhad University of Medical Sciences and conformed to internationally accepted principles for laboratory animal use and care (24).
Materials and Methods
Materials
The stigmas of C. sativus L. was purchased from Novin Saffron Co. (Mashhad, Iran) and analyzed in accordance to ISO/TS 67321–2. Aqueous and ethanolic extracts were extracted from saffron in our laboratory and administered at the doses of 40, 80, and 160 mg/kg, IP. Safranal (0.1, 0.3 and 0.5, ml/kg, IP) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dosage selection was based on our previous study (12). Harmaline was purchased from Fluka Chemie GmbH; Buchs, Switzerland and administered at the dose of 30 mg/kg via intraperitoneal rout (25). Propranolol was donated by Tehran Darou Pharmaceutical Co. (Tehran, Iran) and administered at the doses of 1, 2, and 5 mg/kg, IP (26). Both extracts and propranolol were prepared freshly in normal saline (0.9%). Paraffin was used as a vehicle for Safranal. All compounds were administered at the intraperitoneal rout.
Preparation of extracts
Ethanolic extract
An amount of 10 g of saffron powder was mixed with 25 ml of ethanol 80% at 0 °C and shaked by vortex for 2 min. After centrifugation at 4000 g for 10 min, the supernatant was separated. Extraction by ethanol from sediment was repeated for 6 other times. The resulting solution was dried in a rotary evaporator system in darkness at 35 °C.
Aqueous extract
Saffron powder was mixed with distilled water (1:50 w/v) and left on a shaking incubator at 8 °C for 48 hr. The solution was centrifuged (4000 g for 10 min). The yielded supernatant was retained and sediment was suspended in the half amount of mentioned distilled water and placed on the shaking incubator for another 24 hr. The centrifugation was repeated again and resulting supernatant was separated and stored at −20 °C in a freezer. Sublimation of solvent was performed by freeze drying (20).
Methods
Animals randomly were selected and divided into twenty eight groups of 6 animals each as follows:
All groups received a single injection of harmaline (30 mg/kg) IP.
The mice in group 1 served as control which was treated by harmaline only.
Prophylactic study
Animals in groups 2, 3, and 4 were given aqueous extract at the doses of 40, 80, and 160 mg/kg, respectively. Injection was given 10 min before harmaline.
Mice in groups 5, 6, and 7 were given ethanolic extract at the same doses, 10 min before harmaline. Animals in groups 8-10 received safranal (0.1. 0.3, and 0.5 ml/kg respectively), 10 min before harmaline. As a positive control, groups 11-13 received IP administration of propranolol (1, 2, and 5 mg/kg) respectively.
Curative study
Aqueous extract (groups 14-16), ethanolic extract (groups 17-19), safranal (groups 20-22), and propranolol (23-25) were given 10 min after the induction of tremor, in the same dose regimen as mentioned above.
The period between the injection of harmaline and the appearance of the first symptoms of tremors was recorded as the time of onset of tremors. The time between onset and complete disappearance of tremors was considered as the duration of tremors. The intensity of tremors was evaluated at the respective intervals (10, 30, 60, 120, 300, and 360 min after the start of experiment) until the tremors completely subsided and the mice became normal.
Animals were given a score previously described (27), as follows:
No tremor: 0, mild tremor: 1, moderate intermittent tremor: 2, moderate persistent tremor: 3, and pronounced severe tremor: 4.
Statistical methods
Data were expressed as means±SEM for 6 mice in each group. Tremor duration and onset statistically were analyzed by one-way ANOVA followed by Tukey’s post hoc tests, using SPSS version 13. Difference in the tremor intensity of treated and untreated groups during the time course of study was determined by a two-way analysis of variance with repeated measure followed by Bonferroni’s for multiple comparisons. A P-value of <0.05 was considered to be significant.
Results
Effects of ethanolic and aqueous extracts of C. sativus on the onset of tremor
At first we used harmaline at the dose of 50 mg/kg (28); however, animals showed a course non intermittent tremor with this dose during the observation (data not shown); as a result, the dose of 30 mg/kg of harmaline was assessed (25). The onset of tremor was observed at 2.7±0.3 min following treatment of mice with harmaline. A fine tremor appeared first in the head and spread later to the body and tail accompanied by slight piloerection. The tremor persisted at rest and during movement. After about 10 min, the tremor became coarse and intermittent, resembling shivering. This coarse episodic tremor lasted as long as 3 hr in some animals.
Pretreatment of animals with the aqueous extract of saffron at the doses of 80 and 160 but not the low dose of 40 mg/kg delayed the onset of harmaline-induced tremor to 5.2±0.32 min (P <0.001) and 6.4±0.34 min (P <0.001), respectively (Figure 1A).
Figure 1.
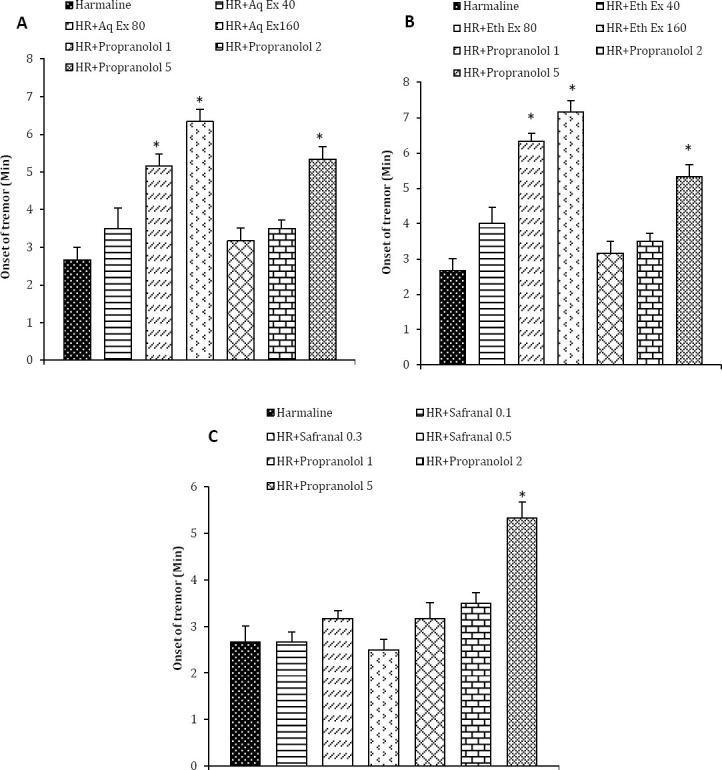
Effects of A: aqueous extract (Aq Ex, 40, 80, and 160 mg/kg), B: ethanolic extract (Eth Ex, 40, 80, and 160 mg/kg), and C: safranal (0.1, 0.3, and 0.5 ml/kg) on the onset of harmaline-induced tremor. Values are mean±SEM of six mice, *P <0.05 as compared with harmaline alone treated animals by ANOVA followed by post-hoc comparison using Tukey’s test. HR: harmaline. Times in x-line indicate time after harmaline treatment
As shown in Figure 1B, the latency observed with ethanolic extract of saffron at the doses of 80 and 160 mg/kg body weight was 6.4±0.24 min (P <0.001) and 7.2±0.31 min (P <0.001), respectively which was significantly higher than harmaline treated group. Safranal did not alter onset of harmaline-induced tremor at any doses tested in this study. Propranolol as a positive control increased onset of tremor, only at the dose of 5 mg/kg.
Effects of ethanolic and aqueous extracts of C. sativus on the total duration of tremor
The mean duration of the tremor for mice receiving harmaline alone was 295.6±3.6 min. The total duration of tremors was significantly reduced by the aqueous extract (254.1±2.1 and 207.2±7.4 min for 80 and 160 mg/kg, respectively) and ethanolic extracts (245.1±3.2 and, 198±3.6 min for 80 and 160 mg/kg, respectively) of saffron in the prophylactic study (Figure 2A and 2B). Treatment of mice with safranal at the doses of 0.1 and 0.3 ml/kg but not 0.5 ml/kg reduced the duration of harmaline-induced tremor (254.8±3.7 and 210.6±11.3 min, respectively; Figure 2C). Duration of tremor was significantly decreased after administration of propranolol (1, 2, and 5 mg/kg) as the reference drug.
Figure 2.
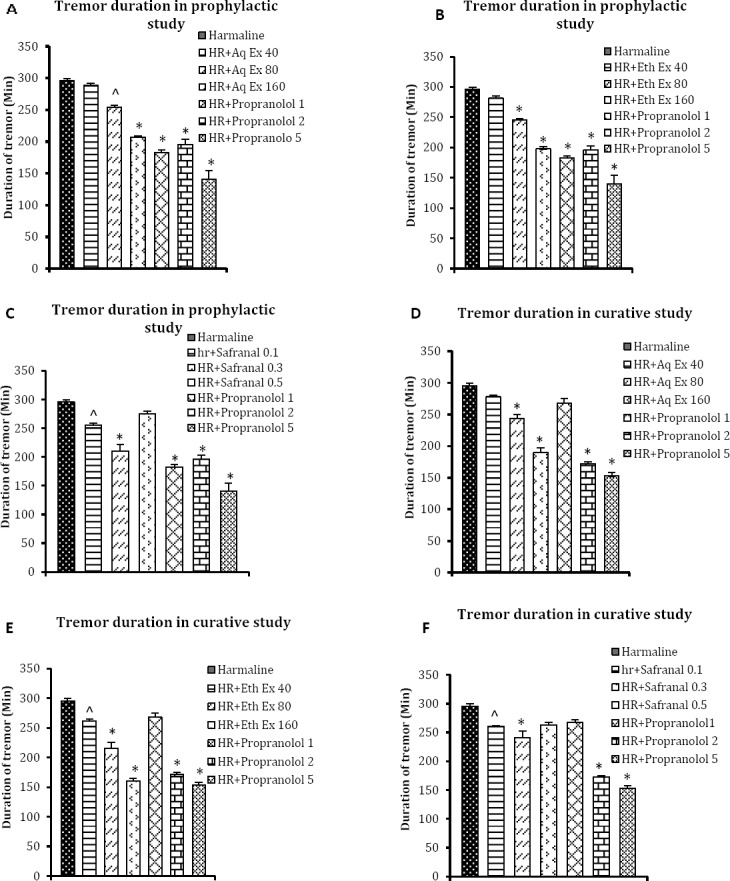
Effects of A: aqueous extract (Aq Ex, 40, 80, and 160 mg/kg), B: ethanolic extract (Eth Ex, 40, 80, and 160 mg/kg), and C: safranal (0.1, 0.3, and 0.5 ml/kg), on the duration of harmaline-induced tremor in prophylactic study. D, E, and F: effects of aqueous extract (Aq Ex, 40, 80, and 160 mg/kg), ethanolic extract (Eth Ex, 40, 80, and 160 mg/kg), and safranal (0.1, 0.3, and 0.5 ml/kg), respectively on the duration of harmaline-induced tremor in curative study. Values are mean±SEM of six mice, ^P<0.01 and *P<0.001 as compared with harmaline alone treated animals by ANOVA followed by post-hoc comparison using Tukey’s test. HR: harmaline. Times in x-line indicate time after harmaline treatment
In the curative study the aqueous extract (243.2±6.7 and 190±7.6 min for 80 and 160 mg/kg) and ethanolic extracts (215.5 ± 10.7 and 161 ± 3.4 min for 80 and 160 mg/kg) of saffron also significantly lowered the duration of harmaline-induced tremor (Figure 2D and 2E, respectively). Duration of harmaline-induced tremor was significantly attenuated by co-treatment of safranal at the doses of 0.1 and 0.3 ml/kg but not 0.5 ml/kg (Figure 2F). Propranolol at the doses of 2 and 5 mg/kg was able to decrease the duration of harmaline-induced tremor.
Effects of ethanolic and aqueous extracts of C. sativus on the intensity of tremor
Treatment of mice with harmaline resulted in an intensity of tremor, during the first 2 hr. Tremor intensity was 3.8±0.2 at 10 min, 3±0.1 at 30 min, 2.8±0.4 at 60 min, and 2.1±0.2 at 120 min following harmaline administration (Figure 3). The intensity of harmaline-induced tremor was significantly reduced during the all four recording periods by the prophylactic regimen of aqueous (Figure 3 A) and ethanolic extracts (Figure 3 B), only at the high dose of 160 mg/kg. This effect was less prominent with propranolol (2 and 5 mg/kg).
Figure 3.
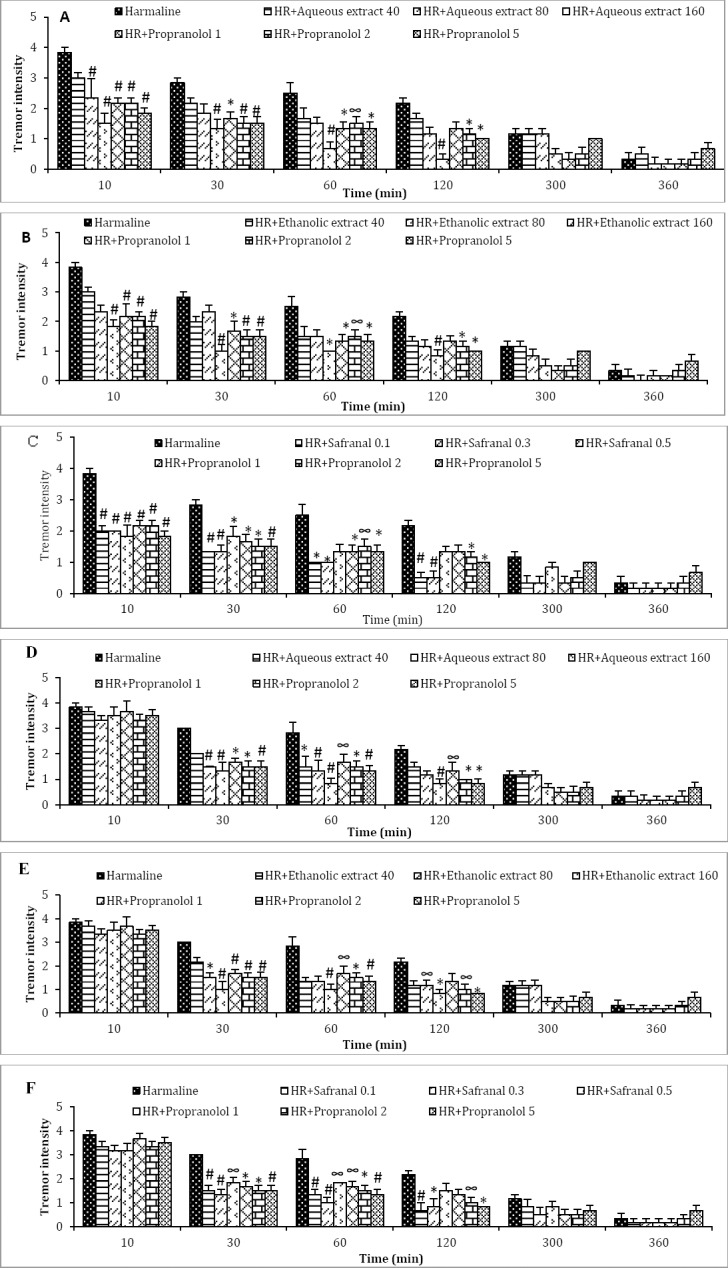
Effects of A: aqueous extract (Aq Ex, 40, 80, and 160 mg/kg), B: ethanolic extract (Eth Ex, 40, 80, and 160 mg/kg) of saffron, and C: safranal (0.1, 0.3, and 0.5 ml/kg), on the intensity of harmaline-induced tremor in prophylactic study. Effects of D: aqueous extract (Aq Ex, 40, 80, and 160 mg/kg), E: ethanolic extract (Eth Ex, 40, 80, and 160 mg/kg) of saffron, and F: safranal (0.1, 0.3, and 0.5 ml/kg), respectively on the intensity of harmaline-induced tremor in curative study. Values are mean±SEM of six mice. ∞P<0.05, *P<0.01, #P <0.001 compared with harmaline-alone–treated animals using two-way ANOVA followed by Bonferroni’s post hoc comparison at different time points. HR: harmaline. Times in x-line indicate time after harmaline treatment
A significant decrease in the intensity of the tremor was observed at 10, 30, 60, and 120 following harmaline treatment in the mice received safranal at 0.1 and 0.3 ml/kg body weight (Figure 3C).
In the therapeutic study, aqueous extract (160 mg/kg), ethanolic extracts (160 mg/kg), and safranal (0.1, 0.3 ml/kg) were able to attenuate tremor appeared after harmaline administration (Figure 3, B, D, and F respectively).
After 2 hr of harmaline treatment, there was a gradual decline in the tremor intensity in harmaline alone and harmaline plus saffron treated groups and complete cessation of tremors was observed within 3 hr. Consequently there was no statistically significant difference in the tremor intensity among the groups after 300 min as well as 360 min.
Discussion
β-carbolines exist in animal proteins, cereals, corn, tobacco, and beverages (wine, whiskey, beer, and sake). Endogenous forms of β-carbolines are also produced from the condensation of tryptophan-derived indole alkylamines with simple aldehydes or with pyruvic. However, the increased blood level concentration of β-carbolines harmaline and harmane are demonstrated in patients suffer from ET (29). The results of this study show that both extracts (80 and 160 mg/kg) delayed the onset of tremor which was similar to reference drug propranolol (5 mg/kg). In contrast, safranal at any administered doses did not affect such parameter. Hence other constituents of saffron may be responsible for the effect on the onset of tremor.
Treatment of animals with both aqueous and ethanolic extracts of saffron (80 and 160 mg/kg), attenuated duration of tremor-induce harmaline in prophylactic and curative regimens respectively. Safranal at the lower doses (0.1 and 0.3 ml/kg) decreased duration of harmaline-induced tremor. In contrast, mice receiving the highest dose (0.5 ml/kg), not only decreased tremor, but also they exhibited excitement and hyperactivity, which is consistent with a previous study who reported that animals became excited after approximately 5-7 min of treatment with safranal (30). Positive control drug, propranolol (1, 2, and 5 mg/kg) decreased duration of tremor as compared to harmaline treated animals.
Only the high dose of extracts (160 mg/kg) and low doses of safranal (0.1 and 0.3 ml/kg) were able to produce a statistically significant reduction in the intensity of harmaline-induced tremor. It seems that intensity of tremor induced by harmaline is more difficult to overcome with saffron.
Our results, is further supported by the previous studies on the anticonvulsant activity of the extracts of C. sativa stigma and its active constituents, crocin and safranal (31, 32).
Harmaline acts on inferior olive neurons, causing enhanced neuronal synchrony and rhythmicity in the olivocerebellar system (33). Several potential mechanisms have been attributed to tremorogenic effects of beta-carboline alkaloid, harmaline, which might be ameliorated by saffron. Harmaline was shown to have affinity to the 5-HT2c receptor (34). The serotonin uptake inhibitor, citalopram (10-40 mg/kg), augmented harmaline-induced tremor in rats (35). Saffron is rich in carotenoids such as crocin and crocetin, two main natural carotenoids, responsible for many pharmacological effects attributed to this spice. Georgiadou and co-workers, showed that crocins might alleviate the meta-chlorophenylpiperazine (mCPP)-induced excessive self-grooming by an antagonistic action at the 5-HT receptor site (36). MCPP is a 5-HT2c receptor agonist which is known to exacerbate self-grooming in rats and this is considered as an animal model of obsessive-compulsive disorder (OCD) (37). Hence, crocins might be responsible for antitremorogenic effects obsereved with the extracts. In contract, it has previously assumed that antidepressent effect of safranal could be through the reuptake of serotonin (13), which might explain the exciting effect observed with the high dose of safranal.
Another hypothesis is that harmaline induces tremor by acting as an N-methyl-D-aspartate (NMDA) receptor inverse agonist (38). Memantine, a non-competitive antagonist of NMDA receptors reduced intensity and duration of harmaline-induced tremor in rats (39). Riluzole (a positive GT activity regulator) reduced the harmaline-induced tremor, probably through its inhibitory effect on glutamatergic neurotransmission (25). In an in vitro study by Lechtenberg et al, hydro-ethanolic (80 vol.%) C. sativus extract and isolated trans-crocetin showed an affinity to the phencyclidine binding site of the NMDA receptor (40). Safranal caused a significant decrease in the concentration of glutamate and aspartate in the extracellular space of hippocampus following systemic administration of kainic acid in anesthetized rats (23). In another study, Nieber et al reported that post-synaptically located NMDA and non-NMDA (kainate) receptors were inhibited by a hydro-ethanolic C. sativus extract. Trans-crocetin decreased the NMDA induced membrane depolarization, but as compared to the extract, it did not inhibit the isolated non-NMDA component of the postsynaptic potentials. These mechanisms contribute to the neuroprotective effect of saffron (41). Saffron extracts and crocetin had a clear binding capacity at the PCP binding side of the NMDA receptor and at the sigma-1 receptor, while the crocins and picrocrocin were not effective. Moreover, in a recent work conducted on rats, the analgesic effect of saffron was attenuated by NMDA receptor antagonists (42). Results of Abe and Saito study showed that crocin, as an antagonist of NMDA receptor channel competes with the interaction of ethanol (8).
It has been demonstrated that harmaline caused neuronal cell loss and caspase-3 mediated apoptosis in cerebellar granular and purkinje cells as well as the inferior olivary neurons of rats (38). Crocin prevented cytotoxicity induced by a potent neurotoxin, acrylamide, as well as diazinon-induced hepatotoxicity in Wistar rats via decreasing the activation of caspases-9 and -3, Bax (an apoptosis promoter) to Bcl2 (an apoptosis inhibitor) ratio, as well as reactive oxygen species (ROS) generation (43, 44).
Another mechanism of harmaline in the induction of tremor is the reduction of gamma-aminobutyric acid (GABA) concentrations within the dentate nucleus of postmortem ET brains. The harmaline-induced tremor was suppressed by benzodiazepine, diazepam (1.5–5 mg/kg) in rodents (45). The GABAA receptor agonist, muscimolc, also depressed tremor induced by harmaline in mice (46). We previously showed that safranal protected against the clonic and tonic phases of experimental absence seizures induced by either γ-butyrolactone, baclofen or low doses of GABAA receptor antagonists including pentylenetetrazole, picrotoxin, and bicuculline; this effect could be attributed to some modification on the benzodiazepine binding sites of the GABAA receptor complex. However, this effect was not seen with the high dose of safranal. The authors in that study assumed that the exciting effects observed with the safranal at the high dose, might be through acting as a partial agonist of the GABAA receptor (47, 48).
Constituents of aqueous extract are more water hydrophilic/soluble ingredients such as crocins, with less permeability to CNS and ingredients of ethanolic extract are more oil soluble/hydrophobic including crocetin and safranal, with more permeability to CNS; however, there were no significant differences between the effects of ethanolic and aqueous extracts against harmaline-induced tremor. Neuroprotective effects of crocins have been reported in many studies (8, 49); for example, the protective effect of IP administration of crocin against spatial memory deficit induced by chronic cerebral hypoperfusion in rats (17). It has been indicated that the infarct volume in an ischemia-reperfusion brain model was reduced by the IV injection of crocin (21). Consequently, a plausible explanation is that crocin may be able to penetrate to the blood-brain barrier and responsible for anti-tremor effects observed with the extracts. For example, in a study by Xi et al after oral administration, crocin was found to converts to crocetin (50). It has been demonstrated that crocetin crosses the blood–brain barrier when saffron extract is administered IP (51).
One of tremorgenic mechanisms of harmaline has been said to triggering sodium action potential by acting on voltage-gated sodium channels and Ca++ conductance of inferior olive neurons. A potent inhibitory effect of C. sativus aqueous-ethanolic extracts on the calcium channel of guinea-pig heart has been reported (52).
Considering that no significant difference was found between administrations of saffron extracts, before or after the induction of tremor, different mechanisms and pathways may be involved in such protocols which are needed to be evaluated regarding cellular and molecular pathways, more carefully.
Conclusion
The results indicate that both aqueous and ethanolic extracts of saffron and with a lesser extent, safranal, could be a potential therapeutic or modulating agent in the treatment of ET. However, more studies are needed to clarify the detailed cellular and molecular anti-tremor mechanisms against harmaline.
Acknowledgment
We are thankful to the Pharmaceutical Research Center and the Vice Chancellor of Research, Mashhad University of Medical Sciences for their financial support. The results described in this paper were part of a Pharm D thesis.
Footnotes
Conflict of interests
The authors declare that there are no conflicts of interest.
References
- 1.Elble R, Deuschl G. Milestones in tremor research. Mov Disord. 2011;26:1096–1105. doi: 10.1002/mds.23579. [DOI] [PubMed] [Google Scholar]
- 2.Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB, Jr, et al. Evidence-based guideline update: treatment of essential tremor: report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2011;77:1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 4.Bernard JF, Horcholle-Bossavit G. Harmaline-induced rhythm in the lateral reticular nucleus. Arch Ital Biol. 1983;121:139–150. [PubMed] [Google Scholar]
- 5.Ahmed A, Taylor NR. The analysis of drug-induced tremor in mice. Br J Pharmacol Chemother. 1959;14:350–354. doi: 10.1111/j.1476-5381.1959.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zargari A. Tehran: University Press; 1990. Medicinal plants; pp. 574–578. [Google Scholar]
- 7.Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the canon of medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27:475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 8.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149–152. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari MR. Antitumor activity of PEGylated nanoliposomes containing crocin in mice bearing C26 colon carcinoma. Planta Med. 2013;79:447–451. doi: 10.1055/s-0032-1328363. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents, crocin and safranal. Pharmacogn Mag. 2009;5:419–424. [Google Scholar]
- 11.Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2013;16:64–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. International Symposium on Saffron Biology and Biotechnology (ISHS) 2003;650:435–445. [Google Scholar]
- 14.Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, et al. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med. 2010;64:24–30. doi: 10.1007/s11418-009-0360-6. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 17.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7–12. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinzadeh H, Shariaty VM. Anti-nociceptive effect of safranal, a constituent of Crocus sativus (saffron), in mice. Pharmacologyonline. 2007;2:498–503. [Google Scholar]
- 20.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83:888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770:578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54:8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Sadeghnia HR, Rahimi A. Effect of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic acid treatment in anesthetized rats. Planta Med. 2008;74:1441–1445. doi: 10.1055/s-2008-1081335. [DOI] [PubMed] [Google Scholar]
- 24.Washington: National Academy Press; 1996. National Research Council. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 25.Rahimi Shourmasti F, Goudarzi I, Lashkarbolouki T, Abrari K, Elahdadi Salmani M, Goudarzi A. Effects of riluzole on harmaline induced tremor and ataxia in rats: biochemical, histological and behavioral studies. Eur J Pharmacol. 2012;695:40–47. doi: 10.1016/j.ejphar.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20:298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- 27.Sharma PL. Mechanism of antitremorine-activity of adrenergic beta-receptor antagonists in the rat. Q J Exp Physiol Cogn Med Sci. 1970;55:202–206. doi: 10.1113/expphysiol.1970.sp002069. [DOI] [PubMed] [Google Scholar]
- 28.Biary N, Arshaduddin M, Al Deeb S, Al Moutaery K, Tariq M. Effect of lidocaine on harmaline-induced tremors in the rat. Pharmacol Biochem Behav. 2000;65:117–121. doi: 10.1016/s0091-3057(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 29.Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, et al. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002;59:1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosseinzadeh H, Sadeghi Shakib S, Khadem Sameni A, Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharm Res. 2013;12:93–99. [PMC free article] [PubMed] [Google Scholar]
- 31.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Im Irn Med. 2002;5:44–47. [Google Scholar]
- 33.Miwa H. Rodent models of tremor. Cerebellum. 2007;6:66–72. doi: 10.1080/14734220601016080. [DOI] [PubMed] [Google Scholar]
- 34.Grella B, Dukat M, Young R, Teitler M, Herrick-Davis K, Gauthier CB, et al. Investigation of hallucinogenic and related beta-carbolines. Drug Alcohol Depend. 1998;50:99–107. doi: 10.1016/s0376-8716(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 35.Arshaduddin M, Al Kadasah S, Biary N, Al Deeb S, Al Moutaery K, Tariq M. Citalopram, a selective serotonin reuptake inhibitor augments harmaline-induced tremor in rats. Behav Brain Res. 2004;153:15–20. doi: 10.1016/j.bbr.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Georgiadou G, Tarantilis P, Pitsikas N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of obsessive compulsive disorder. Neurosci Lett. 2012;528:27–30. doi: 10.1016/j.neulet.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 37.Graf M, Kantor S, Anheuer ZE, Modos EA, Bagdy G. m-CPP-induced self-grooming is mediated by 5-HT2C receptors. Behav Brain Res. 2003;142:175–179. doi: 10.1016/s0166-4328(02)00404-7. [DOI] [PubMed] [Google Scholar]
- 38.Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;770:26–29. doi: 10.1016/s0006-8993(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 39.Iseri PK, Karson A, Gullu KM, Akman O, Kokturk S, Yardymoglu M, et al. The effect of memantine in harmaline-induced tremor and neurodegeneration. Neuropharmacology. 2011;61:715–723. doi: 10.1016/j.neuropharm.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Lechtenberg M, Schepmann D, Niehues M, Hellenbrand N, Wunsch B, Hensel A. Quality and functionality of saffron: quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and sigma1 (sigma-1) receptors. Planta Med. 2008;74:764–772. doi: 10.1055/s-2008-1074535. [DOI] [PubMed] [Google Scholar]
- 41.Nieber K, Berger F, Hensel A. 6th European Congress of Pharmacology (EPHAR) Germany: 2012. Saffron extract and trans-crocetin inhibits excitotoxicity by inhibition of postsynaptically located glutamate receptors in rat brain neurons. [Google Scholar]
- 42.Tamaddonfard E, Hamzeh-Gooshchi N. Effects of intraperitoneal and intracerebroventricular injection of crocin on acute corneal pain in rats. Phytother Res. 2010;24:1463–1467. doi: 10.1002/ptr.3169. [DOI] [PubMed] [Google Scholar]
- 43.Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2015;31:367–376. doi: 10.1177/0748233713475519. [DOI] [PubMed] [Google Scholar]
- 45.Shinozaki H, Hirate K, Ishida M. Further studies on quantification of drug-induced tremor in mice: effects of antitremorgenic agents on tremor frequency. Exp Neurol. 1985;88:303–315. doi: 10.1016/0014-4886(85)90193-1. [DOI] [PubMed] [Google Scholar]
- 46.Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol. 2009;616:73–80. doi: 10.1016/j.ejphar.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Hosseinzadeh H, Sadeghnia HR. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Sadeghnia HR, Cortez MA, Liu D, Hosseinzadeh H, Snead OC. Antiabsence effects of safranal in acute experimental seizure models: EEG and autoradiography. J Pharm Pharm Sci. 2008;11:1–14. doi: 10.18433/j38g6j. [DOI] [PubMed] [Google Scholar]
- 49.Alavizadeh H, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem Toxicol. 2013;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Xi L, Qian Z, Du P, Fu J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine. 2007;14:633–636. doi: 10.1016/j.phymed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol. 2013;52:163–170. doi: 10.1016/j.fct.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Boskabady MH, Shafei MN, Shakiba A, Sefidi HS. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother. 2008;22:330–334. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]


