Abstract
Objective(s):
Multidrug resistance (MDR) of cancer cells is a major obstacle to successful chemotherapy. Overexpression of breast cancer resistance protein (BCRP) is one of the major causes of MDR. In addition, it has been shown that PI3K/Akt signaling pathway involves in drug resistance. Therefore, we evaluated the effects of novel approaches including siRNA directed against BCRP and targeted therapy against PI3K/Akt signaling pathway using LY294002 (LY) to re-sensitize breast cancer MCF7 cell line to mitoxantrone (MTX) chemotherapy.
Materials and Methods:
Anticancer effects of MTX, siRNA, and LY alone and in combination were evaluated in MCF7 cells using MTT cytotoxicity assay and flow cytometry analysis of cell cycle distribution and apoptosis induction.
Results:
MTT and apoptosis assays showed that both MTX and LY inhibited cell proliferation and induced apoptosis in MCF7 cells. Results indicated that inhibition of BCRP by siRNA or PI3K/Akt signaling pathway by LY significantly increased sensitivity of MCF7 cells to antiproliferation and apoptosis induction of MTX. Furthermore, MTX showed G2/M arrest, whereas LY induced G0/G1 arrest in cell cycle distribution of MCF7 cells. Combination of siRNA or LY with MTX chemotherapy significantly increased accumulation of MCF7 cells in the G2/M phase of cell cycle.
Conclusion:
Combination of MTX chemotherapy with BCRP siRNA and PI3K/Akt inhibition can overcome MDR in breast cancer cells. This study furthermore suggests that novel therapeutic approaches are needed to enhance anticancer effects of available drugs in breast cancer.
Keywords: Breast cancer, BCRP, Combination therapy, Mitoxantrone, PI3K/Akt, siRNA
Introduction
Breast cancer is one of the most common human malignancies and the second leading cause of cancer-related deaths in women (1, 2). Chemotherapy is still the most effective modality for the treatment of various types of cancers in a clinical setting (3). Successful chemotherapy is often hampered by the development of multidrug resistance (MDR) in tumor cells (4). MDR is characterized by cross-resistance to a broad spectrum of structurally and functionally unrelated cytotoxic agents (5). Various mechanisms are responsible for MDR in cancer cells that one of the main ones is associated with overexpression of ATP-binding cassette (ABC) transporters such as P-glycoprotein (P-gp), MDR-associated proteins (MRP) and breast cancer resistant protein (BCRP/ABCG2). These proteins actively pump out a broad spectrum of clinically relevant but structurally different compounds and decrease the intracellular drug accumulation (6). BCRP was first identified in a doxorubicin-resistant MCF-7 breast cancer cell line (7). It is a transporter consisting of a six transmembrane domain and one ATP-binding site. BCRP is widely expressed in a variety of normal tissues including placenta, breast, ovary, intestine, and liver, and it has been shown to play an important role against xenobiotics and their metabolites (4, 7). BCRP has been considered as one of the major transporters responsible for drug resistance in cancer cells (7). Some examples of BCRP substrates include topotecan, SN-38, mitoxantrone (MTX), and flavopiridol (4, 8). MTX is a topoisomerase II targeting drug that has been used in combination with other anti-cancer drugs for the clinical treatment of malignancy (9).
Overexpression of BCRP is associated with resistance to a wide range of different anticancer agents including MTX (6). There have been enormous efforts all over the world in the search for safe and effective inhibitors of the transporters as a novel strategy to overcome MDR. One specific approach to overcome MDR is application of the small interfering RNA (siRNA) that can direct sequence-specific degradation of target mRNA (i.e. BCRP/ABCG2), which in turn suppresses protein expression (10, 11). This is potentially an effective method in restoring drug sensitivity in cancer cells via MDR reversal (4). In addition, the PI3-kinase/Akt signaling pathway has been implicated as a key element in the regulation of a number of cellular functions such as cell cycle, cell proliferation and cell survival (1, 12). Also, in some of studies it has been shown that regulation of BCRP function and localization to the plasma membrane are mediated by Akt. This pathway plays a major role not only in tumor development but also in the tumor’s potential response to cancer treatment. Activation of this pathway has been shown to decrease sensitivity to chemotherapeutics resulting in diminished treatment success. The flavonoid derivative, LY294002 (LY), with the structure shown in Figure 1 is known as a specific inhibitor of the PI3K/Akt kinase pathway (13). It has been successfully used to enhance sensitivity of cancer cells to drug-induced apoptosis, and this effect has generally been mediated by its PI3K-Akt blocking activity (14). Therefore, inhibition of PI3K/Akt pathway has been considered as a strategy for targeted drug development. It has also been recently drawn considerable attention for combination therapy to achieve enhanced efficacy in drug-resistant cancer cells (1).
Figure 1.
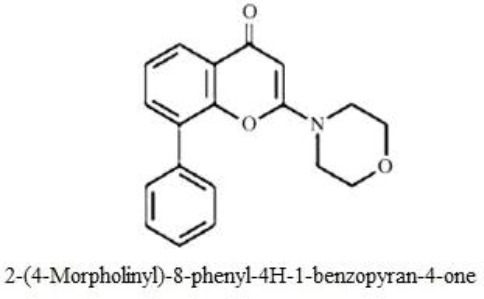
Chemical structure of LY294002
In this study, siRNA therapy was used to inhibit BCRP expression in breast cancer MCF7 cell line to enhance anticancer effects of MTX chemotherapy. Also, we investigated the effect of LY alone and in combination with MTX on proliferation, apoptosis induction and cell cycle distribution of breast cancer MCF7 cell line.
Materials and Methods
Materials
RPMI 1640 and fetal bovine serum (FBS) were purchased from Biosera (UK). Pen-strep and trypsin-EDTA were purchased from Gibco (UK). MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide), propidium iodide (PI), and Annexin V-FITC were purchased from Sigma (Germany). DAPI (4, 6-diamidine-2-phenylindole) and Nonidet P40 were purchased from Roche (Germany). Mitoxantrone was provided from Ebewe (Austria). LY294002 was purchased from Chemeda (USA).
Cell culture
The MCF7 cell line was purchased from cell bank of Pasteur Institute of Iran and cultured in RPMI1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin and incubated at 37 °C in a humidified 5% CO2 incubator.
Drug preparation
LY was initially solubilized in dimethylsulphoxide (DMSO) at a concentration of 10 mM, stored at 4 °C and protected from the light. Different concentrations of LY were then freshly prepared in complete culture medium before use and added to the cells in different experiments. In all experiments, DMSO concentration never exceeded 1% that has no effect on MCF7 cells. MTX (20 mg/ml) was diluted in complete culture medium freshly before use and added to the cells at different concentrations.
MTT cytotoxicity assay
Proliferation of MCF7 cells under different conditions was determined using the MTT assay. Briefly, 5000 MCF7 cells per well were seeded in 96-well plates. After 48 hr, culture media was removed and the cells were treated with MTX, LY, siRNA/polymer alone and in combination at varying concentrations and time points. Then MTT solution (4 mg/ml in phosphate-buffered saline (PBS)) was added to each well. After 3 hr incubation at 37 °C at 5% CO2, DMSO was added to each well to dissolve the formazan crystals, and the absorbance of each well was read at 540 nm using a microplate reader (Sunrise, Tecan, Switzerland). The results were presented as a percentage to the control RPMI. The growth inhibition rate was calculated using the following equation: inhibition rate (%) = ((OD)exp/(OD)con)× 100 in which ODexp and ODcon represent the optical densities of treated and untreated control cells, respectively. Drug concentrations that inhibited cell proliferation to 50% of the control RPMI (IC50) were determined from at least three independent experiments in quadruplicate format for each treatment.
siRNA preparation and transfection
The siRNA duplex targeting, BCRP mRNA, was purchased from Qiagen (UK) with the following sequences: Sense: (GGU CUA AUU UAU UAA UCU A) dTdT, Antisense: (UAG AUU AAU AAA UUA GAC C) dAdT. Briefly, 48 hr before transfection, cells were seeded in 96-well plates at a density of 5000 cells/well and were exposed to Chitosan + Alginate polymer alone, siRNA alone as well as siRNA-polymer (si+P) complex for 6 hr. Then transfection mixture was replaced with fresh culture medium or MTX. MCF7 cells were then incubated at 37 °C at 5% CO2 for 48 hr and cell proliferation was determined using the MTT assay.
Apoptosis assay
MCF7 cells were seeded into 6-well plates at a density of 2.5×105 cells/well. The cells were exposed to MTX, LY, siRNA-polymer (si+P) complex alone and in combination for 48 hr and then cells were harvested, washed twice with PBS, resuspended in binding buffer, and stained with Annexin V-FITC plus propidium iodide (PI) for 15 min at 4 °C in dark. Finally, stained cells were resuspended in binding buffer and assessed for apoptosis by Partec-PAS (Germany) flow cytometer, and data processed using Flowmax software.
Cell cycle distribution analysis
MCF7 cells were seeded into 6-well plates at a density of 2.5×105 cells/well. The cells were exposed to MTX, LY, siRNA-polymer (si+P) complex alone and in combination for 48 hr, and then cells were harvested, washed twice with PBS and stained with DAPI for 30 min at 4 °C in dark. Cell cycle distribution based on DNA content of cells was then determined by Partec-PAS flow cytometer, and data were processed using Flowmax software.
Statistical analysis
All experiments were repeated at least three times and data presented as mean±SD. One-way ANOVA with Tukey post hoc was used for multiple comparisons of data, and results were considered statistically significant at P<0.05.
Results
Cytotoxic effects of MTX or LY on MCF7 cells
MTT assay was used to determine the effect of different treatments on cell proliferation of MCF7 cells. Cell proliferation was significantly decreased following treatment of MCF7 cells with MTX or LY in a concentration- and time-dependent manner (Figure 2). The IC50 for MTX and LY alone was determined to be 600 nM and 15 µM after 48 hr treatment of MCF7 cells, respectively.
Figure 2.
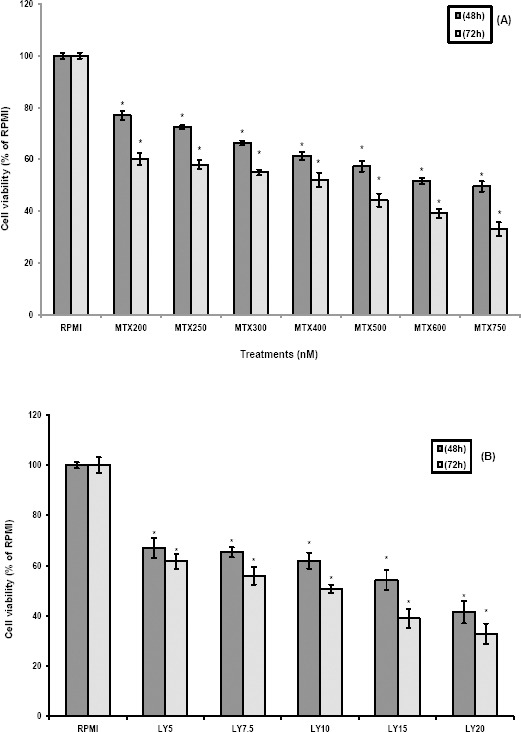
Cytotoxic effects of the MTX and LY on MCF7 cells
The MCF7 cells were treated with different concentrations of MTX (A) and LY (B). After 48 hr and 72 hr of exposure, the cell proliferation was determined using MTT assay. Each experiment was repeated at least three times in quadruplicates for each concentration and the results were expressed as mean±SD. *denotes P<0.001 for significant difference between treatments in comparison to control RPMI
Effect of down-regulation of BCRP by siRNA on sensitivity of MCF7 cells to MTX
Given the association of BCRP expression with chemoresistance, we determined whether down-regulation of BCRP using siRNA approach can re-sensitize MCF7 cells to MTX chemotherapy. Treatment of MCF7 cells with Chitosan+Alginate (Polymer), siRNA, and siRNA-Polymer (si+P) complex alone did not significantly decrease cell proliferation in comparison to control RPMI. Whereas, combination of BCRP siRNA-Polymer (si+P) complex with 250 nM concentration of MTX (about 1/3 of MTX IC50) significantly reduced cell proliferation to 50% of control RPMI (Figure 3). This indicates that BCRP down-regulation by siRNA significantly reduced MTX IC50 by increasing retention of drug within the cancer cells to exert anticancer effects. This in turn, reduces the MTX side effects on normal cells.
Figure 3.
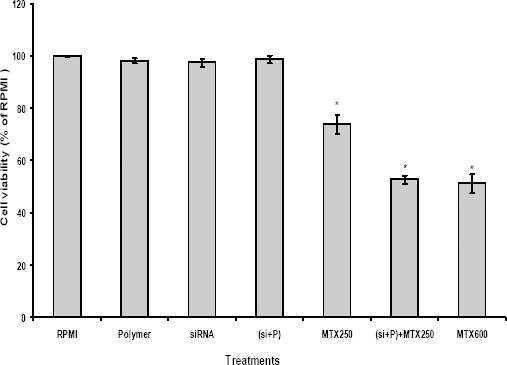
Effect of siRNA against BCRP on the sensitivity of MCF cells to mitoxantrone
The MCF7 cells were exposed to Chitosan + Alginate (Polymer), siRNA, siRNA-polymer (si+P) complex as well as combination of siRNA-polymer (si+P) complex with MTX 250 nM for 6 hr as described in the methods. Then MCF7 cells were incubated with RPMI or MTX for 48 hr and then cytotoxicity was determined by MTT assay. Each experiment was repeated at least three times in quadruplicates for each treatment and the results are expressed as mean±SD. *denotes P<0.001 for significant difference between treatments in comparison to control RPMI
Cytotoxicity of combination of LY and MTX on MCF7 cells
We also assessed the efficacy of MTX in combination with PI3K/Akt signaling inhibitor on MCF7 cells. Co-treatment of MCF7 cells with LY and MTX resulted in significant sensitization of these cells to MTX chemotherapy (Figure 4). Similar to BCRP siRNA, LY significantly reduced the IC50 of MTX from 600 nM to 250 nM. This indicates synergistic effects between MTX and LY that enhances anticancer effects as well as reduced side effects of MTX chemotherapy on normal cells.
Figure 4.
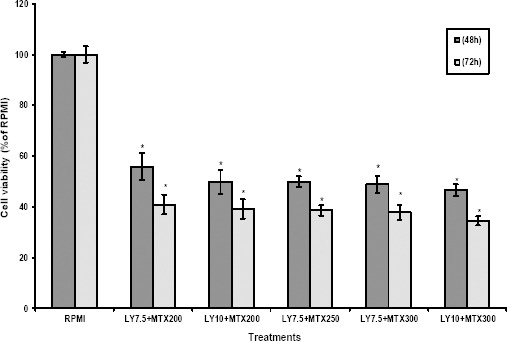
Effects of LY on mitoxantrone cytotoxicity in MCF7 cells
The MCF7 cells were co-treated with specified concentrations of LY + MTX. After 48 hr and 72 hr of exposure, the cell proliferation was determined using MTT assay. Each experiment was repeated at least three times in quadruplicates for each treatment and the results are expressed as mean±SD. *denotes P<0.001 for significant difference between treatments in comparison to control RPMI
Apoptosis
In order to study the induction of apoptosis, MCF7 cells were treated with MTX, LY, and siRNA-polymer (si+P) complex alone and in combination and analyzed by flow cytometry after double-staining with AnnexinV-FITC and PI. Results of flow cytometry analysis showed the percentage of necrotic (Q1: AnnexinV-FITC-/PI+), late apoptotic (Q2: AnnexinV-FITC+/PI+), viable (Q3: AnnexinV-FITC-/PI-), and early apoptotic (Q4: AnnexinV-FITC+/PI-) cells. The early and late apoptosis rates together for the control RPMI was 3.82%, while those of the MTX 250 nM, MTX 600 nM and LY 15 μM groups were 19.63%, 46.18%, and 42.50%, respectively (Figure 5). Interestingly, the percentage of early and late apoptotic cells in siRNA-polymer (si+P) complex+MTX 250 nM treated cells significantly increased to percentage similar to MTX 600 nM (43.56% versus 46.18%, respectively). Co-treatment of MTX 250 nM with half IC50 of LY 7.5 µM significantly increased apoptosis induced by MTX250 nM alone to percentage similar to MTX 600 nM (40.11% versus 46.18%, respectively). These results revealed that down-regulation of BCRP by siRNA or PI3K/Akt inhibition by LY significantly enhanced the sensitivity of MCF7 cells to apoptosis induction by MTX chemotherapy.
Figure 5.
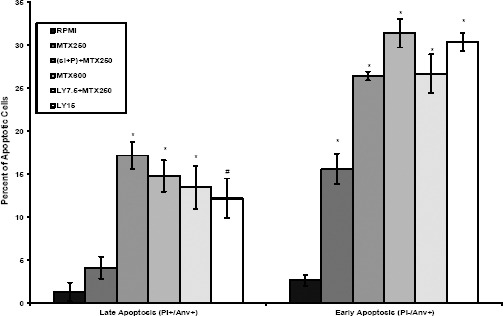
Apoptosis induction in MCF7 cells
The MCF7 cells were treated with mitoxantrone, LY, and siRNA-polymer (si+P) complex alone and in combination for 48 hr to evaluate induction of apoptosis using Annexin V-FITC/PI double-staining by flow cytometry. The percentage of early and late apoptotic cells were presented for each treatment group. Data are expressed as means ± SD of three independent experiments. *denotes P<0.001 and # P<0.01 for significant difference between treatments in comparison to control RPMI
Cell cycle distribution
The effect of MTX, LY, and siRNA-polymer (si+P) complex alone and in combination on cell cycle distribution of MCF7 cells was determined by flow cytometry. Approximately, 57.47% of control RPMI cells were in G0/G1 phase, 29.19% in S phase and 13.35% in G2/M phase (Figure 6). Compared with control RPMI cells, MTX induced cell accumulation in the G2/M phase while reducing the number of cells in G0/G1 and S phase. The G2/M cells accumulation in MCF7 cells increased from 13.35% in control RPMI to 44.50% for MTX 250 nM and 54.58% for MTX 600 nM. Further increase in G2/M cells accumulation (50.19%) was observed in MCF7 cells treated with siRNA-polymer (si+P) complex + MTX 250 nM in comparison to MTX 250 nM alone. Unlike MTX, LY 15 µM induced G0/G1 cells accumulation in comparison to control RPMI (76.02% versus 57.47%, respectively). Distribution pattern of cell cycle changed following treatment of MCF7 cells with MTX 250 nM + LY 7.5 µM in comparison to LY 15 µM alone.
Figure 6.
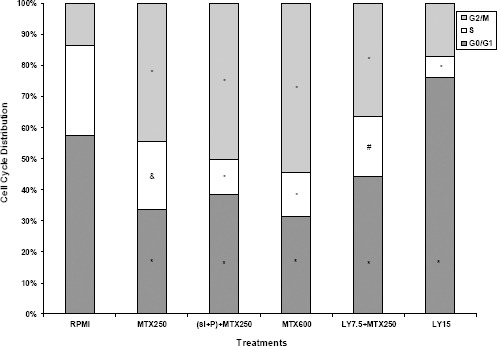
Cell cycle alterations in MCF7 cells
The MCF7 cells were treated with mitoxantrone, LY, and siRNA-polymer (si+P) complex alone and in combination for 48 hr and stained with DAPI and analyzed by flow cytometry to determine cell cycle distribution pattern. Data are presented as the mean±SD and each experiment was repeated at least three times in triplicate formats. *denotes P<0.001, # P<0.01 and & P<0.05 for significant difference between treatments in comparison to control RPMI
Discussion
Drug resistance is one of the clinical challenges in cancer treatment, which usually leads to the failure in cancer therapy or recurrence due to an incomplete elimination of the tumor cells (15). Acting as an efflux pump in tumor cells, overexpression of BCRP leads to reduced intracellular drug concentration and decreased cytotoxicity. In this study, MTX has been chosen to study drug resistance phenomenon due to its common use as anticancer chemotherapy agent (15). MTX resistance has been contributed to overexpression of various ABC transporters and in particular the BCRP. There are some chemical inhibitors of BCRP, such as Fumitremorgin C and GF120918, but their inhibitory effects are not clinically satisfactory. Therefore, developing less toxic and more efficient strategies are needed to overcome MDR mediated by BCRP (16). On the other hand, gene expression silencing approach using siRNA molecules has also been investigated in many studies. In this study, we evaluated effectiveness of siRNA to down-regulate BCRP expression as a strategy to re-sensitize MCF7 breast cancer cells to MTX. In order to confirm the effects of BCRP suppression on enhanced anticancer effects of chemotherapy, cytotoxicity, apoptosis induction and cell cycle changes were determined in MCF7 cells. MTX displayed a time- and concentration-dependent inhibition of cell proliferation. In the current study, we found that siRNA directed against BCRP significantly increased inhibitory effects of MTX on proliferation of MCF7 cells. Also, the rate of apoptosis induction by siRNA-polymer (si+P) + MTX 250nM was as high as that induced by MTX 600 nM. This is in agreement with published data that showed knockdown of BCRP by siRNAs increased the sensitivity of human choriocarcinoma BeWo cells to MTX (4). Additionally, in the present study we examined the effects of MTX alone or in combination with siRNA on cell cycle pattern of MCF7 cells. Consistent with the findings of Seifrtova M (17), in this study MCF7 cells were mostly arrested in G2/M phase when treated with MTX. Combination of siRNA-polymer (si+P) with MTX 250 nM further increased the percentage of cell accumulation in G2/M phase similar to MTX 600 nM. The induction of cell cycle arrest and apoptosis by chemotherapeutic agents could be an effective strategy to prevent the uncontrolled cell proliferation and the survival of cancerous cells.
Recent data have suggested the involvement of PI3K/Akt activity in controlling MDR (18). Since this pathway is important target for therapeutic interference, we sought to investigate whether blocking this pathway by LY294002, a PI3K-specific inhibitor, would result in a sensitization of cancer cells to chemotherapy. Consistent with the findings of Jiang et al (19) who showed that LY294002 inhibited cell proliferation and induced apoptosis in CNE-2Z cells, our results showed that LY markedly inhibited MCF7 cell proliferation and induced apoptosis. Furthermore, similar to findings of YangJiong et al (20), we found that co-treatment of MCF7 cells with MTX and LY significantly enhanced cytotoxicity of MTX on MCF7 cells. To test whether the anti-proliferative effects of LY was related to cell cycle arrest, the cycle progression was examined by the flow cytometry. Previous studies demonstrated that LY induces apoptosis and G0/G1 cell cycle arrest (21). Similarly in our study, LY showed cell cycle arrest in G0/G1 phase. Also, we observed that apoptosis rate induced by MTX 250nM was increased in the presence of the PI3/Akt signaling inhibitor (LY 7.5 µM) similar to the percentage of MTX 600 nM. In addition, MCF7 cells were arrested at G2/M phase following co-treatment of MTX 250 nM with LY 7.5 µM. The effect of LY on MTX cytotoxicity could be due to the suppression of BCRP via the inhibition of the PI3K/Akt signaling pathway that resulted in increase in the intracellular drug accumulation.
Conclusion
Taken together, this research further confirmed the role of BCRP in drug resistance of breast cancer cells and showed that siRNA against BCRP can re-sensitize human MCF7 breast cancer cell line against MTX chemotherapy. This suggests that siRNA therapy can be an effective strategy for prevention and reversion of MDR in cancer, and may potentially be of interest for future combination of chemotherapy and gene therapy approaches. In this study, our findings demonstrated that LY is also able to sensitize the MCF7 cells to MTX treatment, highlighting the importance of PI3K/AKT signaling pathway in the resistance of breast cancer cells to chemotherapy. However, further studies including in vivo evaluations are needed to verify clinical effectiveness of these combination therapies to enhance anticancer effects of available chemo-therapeutic agents in cancer cells.
Acknowledgment
The results described in this article are part of a PhD dissertation that was financially supported by Tehran University of Medical Sciences, Tehran, Iran.
References
- 1.Arafa el-SA, Zhu Q, Shah ZI, Wani G, Barakat BM, Racoma I, et al. Thymoquinone up-regulates PTEN expression and induces apoptosis indoxorubicin-resistant human breast cancer cells. Mutat Res. 2011;706:28–35. doi: 10.1016/j.mrfmmm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Momtazi-borojeni AA, Behbahani M, Sadeghi-aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia Marina. Iran J Basic Med Sci. 2013;16:1203–1208. [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Zhao G, Liu J, Ma N, Chivukula P, Perelman L, et al. Novel bio degradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release. 2009;140:277–283. doi: 10.1016/j.jconrel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Ee PL, He X, Ross DD, Beck WT. Modulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interference. Mol Cancer Ther. 2004;3:1577–1584. [PubMed] [Google Scholar]
- 5.Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down-regulates the multidrug resistance mdr1b gene by inhibiting the PI3K/Akt/NFjB pathway. Cancer Lett. 2008;259:111–118. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Dai CL, Liang YJ, Wang YS, Tiwari AK, Yan YY, Wang F, et al. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer lett. 2009;279:74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S, et al. Suppression of ABCG2 inhibits cancer cell proliferation. Int J Cancer. 2010;126:841–851. doi: 10.1002/ijc.24796. [DOI] [PubMed] [Google Scholar]
- 8.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16:1443–1447. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- 9.Su Y, Lee SH, Sinko PJ. Inhibition of efflux transporter ABCG2/BCRP does not restore mitoxantrone sensitivity in irinotecan-selected human leukemia CPT-K5 cells: Evidence for multifactorial multidrug resistance. Eur J Pharm Sci. 2006;29:102–110. doi: 10.1016/j.ejps.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Aliabadi HM, Landry B, Mahdipoor P, Hsu CY, Uludag H. Effective down-regulation of breast cancer resistance protein (BCRP) by siRNA delivery using lipid-substituted aliphatic polymers. Eur J Pharm Biopharm. 2012;81:33–42. doi: 10.1016/j.ejpb.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Ganjalikhani hakemi M, Hashemi M. siRNA delivery improvement by co-formulation of different modified polymers in erythroleukemic cell line K562. Iran J Basic Med Sci. 2013;16:973–978. [PMC free article] [PubMed] [Google Scholar]
- 12.Hegedus C, Truta-Feles K, Antalffy G, Brozik A, Kasza I, Nemet K, et al. PI3-kinase and mTOR inhibitors differently modulate the function of the ABCG2 multidrug transporter. Biochem Biophys Res Commun. 2012;420:869–874. doi: 10.1016/j.bbrc.2012.03.090. [DOI] [PubMed] [Google Scholar]
- 13.Barancik M1, Bohacova V, Sedlak J, Sulova Z, Breier A. LY294002, a specific inhibitor of PI3K/Akt kinase pathway, antagonizes P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci. 2006;29:426–434. doi: 10.1016/j.ejps.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson KM, Quinn DM, Kellett GL, Warr JR. LY294002, an inhibitor of phosphatidylinositol-3-kinase, causes preferential induction of apoptosis in human multidrug resistant cells. Cancer Lett. 2003;190:31–36. doi: 10.1016/s0304-3835(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 15.Song N, Wu X, Gao Z, Zhou G, Zhang WJ, Liu W. Enhanced expression of membrane transporter and drug resistance in keloid fibroblasts. Hum Pathol. 2012;43:2024–2032. doi: 10.1016/j.humpath.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Lv H, He Z, Liu X, Yuan J, Yu Y, Chen Z. Reversal of BCRP-mediated multidrug resistance by stable expression of small interfering RNAs. J Cell Biochem. 2007;102:75–81. doi: 10.1002/jcb.21276. [DOI] [PubMed] [Google Scholar]
- 17.Seifrtova M, Havelek R, Chmelarova M, Cmielova J, Muthna D, Stoklasova A, Zemankova S, Rezacova M. The effect of ATM and ERK1/2 inhibition on mitoxantrone-induced cell death of leukaemic cells. Folia Biol (Praha) 2011;57:74–81. [PubMed] [Google Scholar]
- 18.Abdul-Ghani R, Serra V, Gyorffy B, Jurchott K, Solf A, Dietel M, et al. The PI3K inhibitor LY294002 blocks drug export from resistant colon carcinoma cells overexpressing MRP1. Oncogene. 2006;25:1743–1752. doi: 10.1038/sj.onc.1209201. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Fan D, Zhou G, Li X, Deng H. Phosphatidylinositol 3-kinase inhibitor (LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:34. doi: 10.1186/1756-9966-29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.YangJiong X, Ping Z, MingHui H, Fei C, JiWu C, WenXin Q. Diverse effects of two kinds of PI3K inhibitors on drug-resistant human breast cancer MCF-7/MIT cells. Tumor. 2009;29:620–625. [Google Scholar]
- 21.Gong C, Liao H, Wang J, Lin Y, Qi J, Qin L, et al. LY294002 induces G0/G1 cell cycle arrest and apoptosis of cancer stem-like cells from human osteosarcoma Via Down-regulation of PI3K activity. Asian Pac J Cancer Prev. 2012;13:3103–3107. doi: 10.7314/apjcp.2012.13.7.3103. [DOI] [PubMed] [Google Scholar]


