Abstract
Objective(s):
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disorder. One of the hallmarks of AD is an abnormal accumulation of fibril forms of tau protein which is known as a microtubule associated protein. In this regard, inhibition of tau aggregation has been documented to be a potent therapeutic approach in AD and tauopathies. Unfortunately, the available synthetic drugs have modest beneficial efficacy with several side effects. Therefore, pipeline drugs from natural sources with anti-aggregation properties can be useful in the prevention and treatment of AD. Among medicinal plants, saffron (Crocus sativus, L.), as a traditional herbal medicine has different pharmacological properties and can be used as treatment for several nervous system impairment including depression and dementia. Crocin as a major constituent of saffron is the glycosylated form of crocetin.
Materials and Methods:
In this study, we investigated the inhibitory effect of crocin on aggregation of recombinant human tau protein 1N/4R isoform using biochemical methods and cell culture.
Results:
Results revealed that tau protein under the fibrillation condition and in the presence of crocin had enough stability with low tendency for aggregation. Crocin inhibited tau aggregation with IC50 of 100 µg/ml. Furthermore, transmission electron microscopy images confirmed that crocin could suppress the formation of tau protein filaments.
Conclusion:
Inhibitory effect of crocin could be related to its interference with nucleation phase that led to increases in monomer species of tau protein. Based on our results, crocin is recommended as a proper candidate to be used in AD treatment.
Keywords: Alzheimer’s disease, Anti-aggregation, Crocin, Tau protein
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder among the elderly. It has a multifactorial etiology and a complex pathogenesis (1, 2). The prevalence of this chronic disease is rising more quickly in western countries and lack of effective treatment for AD is becoming a global health care concern (3). Two hallmarks of AD in the brain are accumulation of the amyloid-β-peptide (Aβ) as senile plaque and intracellular aggregation of the microtubule associated tau protein as neurofibrillary tangles (4). Consequently, abnormal protein accumulations lead to neuronal loss and episodic memory impairment in AD patients (4). Current therapeutic approaches such as acetyl cholinesterase (AchE) inhibition and N-methyl D-aspartate (NMDA) glutamate receptor blockers have limited beneficial efficacy with several side effects (5).
Recent evidence indicates that abnormal hyper-phosphorylated tau protein is a critical aspect in the pathology of AD. Surprisingly, there is a growing interest in focusing on the structure and function of tau protein to develop new drugs (6, 7). In this regard, tau-directed drug discovery is divided into three major categories, namely anti-aggregation strategy context (methylene blue chloride-Rember ™), inhibitors of tau hyperphosphorylation (lithium) and microtubule stabilizing agents such as Paclitaxle (8-10). Combinatorial library screen methods show inhibitory activity of small molecules with several chemical properties against tau protein aggregation, but most of these compounds have toxic side effects and low permeability across the blood-brain barrier (BBB) (11-14). Thus, more attention has been attracted to natural phytochemicals such as polyphenols including curcumin (15, 16), fulvic acid (17), cinnamon (18), oleocanthal (19, 20) and oleuropein (21) which have anti-aggregation effects. Saffron (Crocus sativus, L.) has been frequently used in traditional herbal medicine for its sedative, anti-spasmodic, eupeptic, anti-depressant, stoma-chic, and anti-catarrhal features as well as protect-ting characteristics against age-dependent neuro-degeneration and dementia (22-24). In addition, recent pharmaceutical studies in human and animal models show that saffron extract reveals therapeutic effects such as anti-tumor cell proliferation, insulin resistance prevention and neuronal injury protection (22, 25). Carotenoids are the major secondary metabolites of saffron and crocin (di-glucosyl esters of crocetin) is the main glycosylated carotenoids constituent (Figure 1). This compound is water soluble carotenoid which is responsible for saffron’s color (26).
Figure 1.
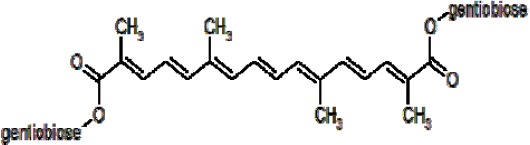
Chemical structure of crocin
The cumulative basic and clinical evidences support the memory-enhancing and anti-Alzheimer’s disease properties of saffron (27-29). Experimental studies have indicated acetylcholinesterase inhibi-tion, pro-inflammatory markers reduction, as well as impaired memory retention inhibition effects of saffron (29). More importantly, recent clinical trials demonstrated that saffron has preventive properties against mild to moderate AD (29). Moreover, Ghahghaei et al (30) and Papandreou et al (31) showed anti-aggregation activity of crocin on amyloid-β1-40/1-42 peptides in different in vitro and in vivo experimental models. To the best of our knowledge, there is no study on the inhibitory effects of crocin on tau aggregation process. According to the similarity of structural fibril formation in both amyloid β and tau protein (32), in the present study, we investigated the inhibitory effect of crocin on the aggregation of recombinant human tau protein (1N/4R) isoform, in vitro.
Materials and Methods
Materials
All chemicals were of analytical grade and purchased from Merck, GmbH, Germany.
Purification of crocin
Crocin was purified from Crocus sativus L. extract as described previously (33). In all steps, crocin stock (2 mg/ml) was prepared from its powder that was dissolved in piperazine-N, N′-bis 2-ethanesulfonic acid (PIPES) buffer (pH 6.8).
Recombinant tau protein expression and purification
Expression and purification of tau protein were done based on our previous work with minor modification (34). Briefly, Escherichia coli strain BL21 (DE3) was infected with pET-21a vector including human tau 1N/4R gene (htau34). Recombinant tau was purified via a succession of Ni-NTA-Agarose precipi- tation (equilibrated with 10 mM HEPES, 100 mM NaCl, and 15 mM imidazole, pH 7.4) and eluted with 80 mM imidazole. The concentration of purified tau was determined using OD 280 nm with extinction coefficient 7700 M−1cm−1 and the purity of the protein was verified with SDS-PAGE gel electrophoresis. The protein was stored at –80 °C until use.
Tau aggregation via Thioflavin T (ThT) fluorescence
The tau fibrillation was assayed using Thioflavin T emission fluorescence based on Monti et al methods with minor modification (20). In brief, solutions of tau (20 μM) were prepared using an assembly buffer (10 mM HEPES, 100 mM NaCl, 3 mM dithiothreitol (DTT), and 800 μM arachidonic acid as inducer of fibrillation) into a Grenier solid black 96-well plate. After 1 hr incubation at 37 °C, ThT (50 μM) was added to assay the fibrillation reaction. The plate was covered with self-adhesive aluminum foil to avoid exposure to light and incubated with shaking at 250 rpm for 120 hr at 37 °C. Finally, fluorescence was measured every 24 hr by a multimode microplate reader Synergy H4 (Biotek Instruments, Winooski, VT) at excitation 440 nm and emission 490 nm. The background fluorescence of tau, crocin, arachidonic acid and ThT was subtracted. To study the inhibitory effect of crocin on tau protein fibrillation, tau was incubated in the absence and presence of crocin at different concentrations ranging from 0.2 μg/ml to 600 μg/ml. Briefly, aggregation procedure for 20 μM tau protein in the presence of 800 μM arachidonic acid was performed at different concentrations of crocin (0.2, 2, 20, 50, 100, 200, 400 and 600 μg/ml). The amount of filament formation was determined by ThT fluorescence spectrometry assay. The percentage of inhibition of tau aggregation in the presence of crocin was compared with tau aggregation in the absence of crocin (100%). The normalized data was plotted against the logarithm of crocin concentrations and fitted to dose-response curve. In essence, 100 μM methylthioninium chloride (Methylene blue) was used as the reference of tau inhibition. All measurements were carried out in triplicate separate assays with at least two preparations of purified proteins.
Circular dichroism (CD) spectroscopy
Far-UV CD spectra were documented in the presence and absence of crocin to monitor changes in secondary structure of tau protein during aggregation. At the end of the experiment after 120 hr incubation, samples were diluted 1:3 in buffer containing 10 mM HEPES. The measurements were done in a 0.1 cm path length cuvette, using an Aviv model 215 Spectropolarimeter (Lakewood, NJ, USA). Spectra were recorded in the range of 195-260 nm with a data interval of 1 nm. Each spectrum was an average of two scans with a subtraction of buffer baseline.
Dynamic light scattering (DLS)
Next, samples were diluted 1:3 again in 10 mM HEPES buffer and DLS measurements were performed by a ZetaPlus (Zeta Potential Analyzer-Brookhaven, USA) using the particle sizing software (Version 5.2). Samples were thermally equilibrated at 25 °C for 2 min before data collection. Particle size was recorded as the average of five measurements and expressed as percentage of mass and mean radius (nm).
Transmission electron microscopy (TEM)
Aliquots of samples (2 μl) were diluted 1:3 again in 10 mM HEPES buffer and absorbed into carbon-coated gold TEM grids (SPI Supplies, Westchester, USA). The grids were dried with filter paper and were negatively stained with 2% uranyl acetate. The observations were performed with a H600 transmission electron microscope (Hitachi Co.) operating at 50,000× at 75 kV excitation voltages.
Cell culture
For detection of suspected toxicity of producing aggregates, cell viability was evaluated with conventional MTT reduction assay in the presence and absence of crocin in PC12 cell line (35). PC12 cell line was obtained from Pasture Institute of IRAN, Tehran, Iran. All cells were cultured in sterile flasks with DMEM medium and 10% fetal bovine serum (FBS). In order to evaluate cell viability, cells were incubated with 10 µl of crocin (after 120 hr) for 24 hr at 37 °C.
Statistical analysis
Aggregation data were adjusted to a sigmoidal model and graphed by SigmaPlot version 12.0 Ink. Data are expressed as mean±standard deviation (SD). Cell viability was compared by t-test and P<0.05 was considered as statistically significant. Statistical analyses were performed using SPSS software version 15.
Results
Tau expression and purification
Our previous study showed that tau protein (1N/4R) can be expressed in E. coli strain BL21 (DE3) with the pET-21a vector in high quantity (34). As shown in Figure 2, the tau protein 412 amino acid (htau 34) was the major protein expressed. Two samples of induced and none induced by IPTG were compared in lane A and B which showed a considerable concentrated band at 48-63 kDa. Next, the purification of htau34 monomeric with a purity of >98% was achieved following Ni-NTA-Agarose precipitation step as described above with a final volume of 5 ml containing 1 to 2 mg/ml of protein. The eluted fractions containing htau34 showed a single dense band at 48-63 kDa (lane E).
Figure 2.
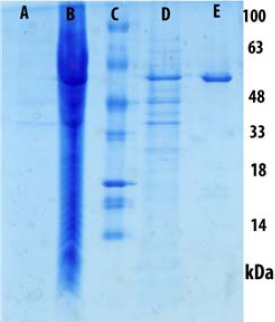
Expression and purification of Tau 412 AA (htau 34); The samples were mixed with SDS- sample buffer, separated on 12% SDS-PEGE gel and stained with Coomassie Brilliant Blue R250. The tau monomer band is in the range of 48-63 kDa. A: Before IPTG- induction, B: After IPTG- induction, C: Mr. Marker D: Tau 412 AA (htau 34), E: tau after purification with Ni-NTA-Agarose precipitation
Evaluation of tau aggregation in the presence of crocin using ThT fluorescence assay
The ThT fluorescence assay can be used in order to show the polymerization of tau to filaments. The ThT binds to beta–sheet structures and changes the fluorescence emission spectra. This can be used in order to confirm the polymerization of tau to form an aggregate. In our experiment, the time–course of tau aggregation was nucleation-elongation reaction model that involves the formation of the beta-structure followed by a sigmoid curve that reached plateau after 120 hr of incubation (Figure 3). The amyloid aggregation systems characterized by sigmoidal curve with three steps, nucleation, elongation and steady state phase. As shown in Figure 3, the nucleation phase occurred within 5 hr of incubation; however, the elongation step happened between 5 and 96 hr. Its kinetic reached a steady state phase after 120 hr of incubation followed by slow drop in ThT fluorescence.
Figure 3.
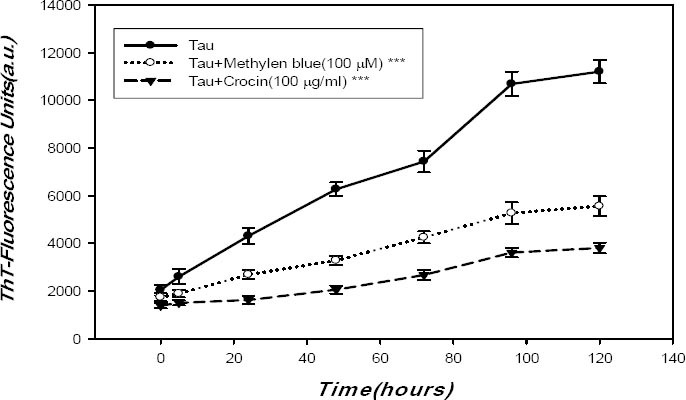
Tau 412 AA (htau 34) was aggregated by arachidonic acid as an inducer of fibrillation for different time (0, 5, 24, 48, 72, 96 and 120 hr) which was measured by ThT emission spectrometry. Tau protein concentration was 20 μM, with 800 μM arachidonic acid in assembly buffer pH 7.4, and 3 mM DTT. The plateau was reached after 120 hr of incubation. A shift in the fluorescence emission spectra and enhancement of intensity were seen. Tau aggregation in the absence and presence of 100 μg/ml crocin (▲) and 100 μM methylene blue (○) was measured by ThT fluorescence spectrometry. Data are expressed as mean±SD, n=3. *** P<0.001 compared with tau group. MB and crocin are significantly different from tau (*** P<0.001)
For determination of IC50, we used crocin at various concentrations ranging from 0.2 to 600 μg/ml. Results showed that 100 μg/ml of crocin was required for inhibition of 50% of tau aggregates in a dose-dependent manner (Data not shown). As indicated in Figure 3, under fibril condition, when crocin at final concentration of 100 μg/ml was added to tau protein, incubated for 120 hr, the intensity of ThT fluorescence was significantly changed (P<0.001). This implied that crocin can bind to intermediate structures of tau protein and inhibit its conversion to more aggregated conformations during the fibrillation process.
Circular dichroism (CD) spectroscopy
CD spectroscopy is widely used for testing protein structures in solutions. Figure 4 shows the CD spectra of monomer, aggregated and incubated in the presence of crocin (black, green and blue lines, respectively). As shown in Figure 4, the CD spectrum of tau protein monomer (black line) before applying the fibrillation process spectacles very small positive transition near [θ] 220 and a single large negative peak at [θ] 200. This spectrum typically shows the random coil structures of tau protein. Moreover, the CD spectrum after 120 hr of incubation converted into strong negative ellipticities at near [θ] 217 which is the expected spectrum of beta-sheet structures of tau fibrils the transition from the random coil to beta-sheet structure was clearly observed after 120 hr. In addition, after adding crocin (100 μg/ml) to tau protein under fibril condition, the intensity of ellipticity of [θ] 217 was significantly reduced and crocin impeded the formation of beta-sheet structures of tau protein. These results reflect an increase in the stability of the random coil structure of tau protein and a decline in the amount of beta-sheet structure of tau fibrils.
Figure 4.
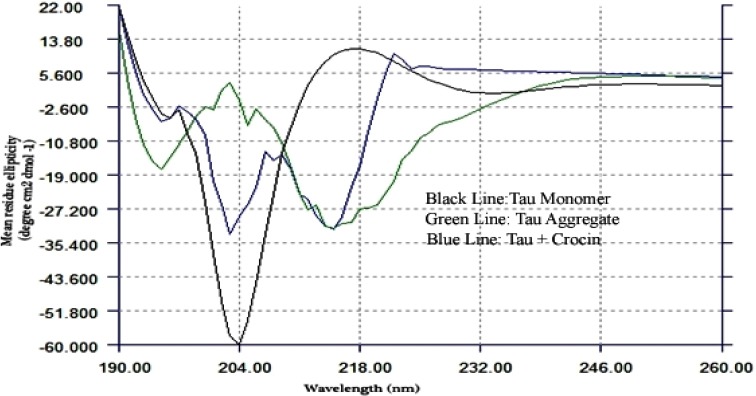
Far-UV CD spectra were documented in the presence and absence of crocin to monitor changes in secondary structure of tau protein aggregation. At the end of experiment (120 hr), the samples were diluted 1:3 in composite buffer containing 10 mM HEPES buffer (pH 7.4) for CD measurements. CD spectrum after 120 hr of incubation, converted into strong negative ellipticities at [θ] 217 which is the expected spectrum of beta-structures of tau fibrils. Tau monomer (black line) - Tau Aggregate (green line)- after incubation with 100 μg/ml crocin (blue line)
Dynamic light scattering (DLS)
In our study, DLS was performed to observe the size distribution of population of tau protein aggregate species using the particle sizing software (Version 5.2). As shown in Table 1, at the end of the fibrillation process of tau protein without crocin, the diameter of particles was significantly increased. Filaments represented a heterogeneous mixture of aggregates of different size with an average diameter of approximately 1745 nm (95% of total mass). Additionally, regarding tau protein with crocin (100 μg/ml) under fibril condition, the diameter of aggregate forms was remarkably decreased with a mean size of approximately 111 nm (87% of total mass).
Table 1.
Size distribution was obtained by analysis of dynamic light scattering data. DLS measurements were performed with a ZetaPlus (Zeta Potential Analyzer-Brookhaven, USA) using the particle sizing software (Version 5.2). At the end of fibrillation process for tau protein without crocin, the diameter of particles was significantly increased. In tau protein with crocin (100 μg/ml), the diameter of aggregate forms was remarkably decreased
| Mass (%) | Mean radius±SD (nm) | |
|---|---|---|
| Tau | 95 | 1745 ± 145 |
| 5 | 231 ± 55 | |
| Tau + Methylene blue | 95 | 170 ± 39 |
| 5 | 200 ± 21 | |
| Tau +Crocin | 87 | 111 ± 25 |
| 13 | 600 ± 171 |
Transmission electron microscopy (TEM)
Morphological forms of the aggregates in the absence and presence of crocin were observed by TEM (Figure 5). After incubation for 120 hr under fibril condition, tau protein without crocin was distinct mature fibers as well as amorphous aggregates with dimensions of approximately 10-22 nm in width and up to 1 μm in length (Figure 5A). These structures are characterized by paired helical filaments (PHFs) and contained extended beta-sheet and hydrophobic structures. On the other hand, in the presence of crocin, the majority of tau proteins were in amorphous form (Figure 5B).
Figure 5.
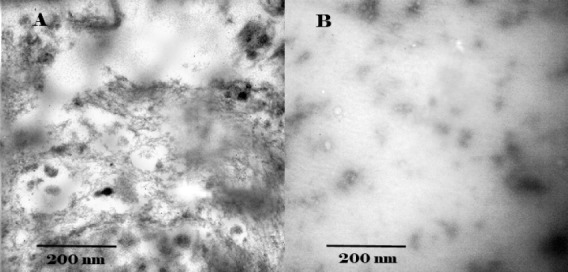
Electron micrographs of A: tau protein without crocin; B: tau protein with crocin. Samples were applied to carbon-coated gold grids, negatively stained with 2% uranyl acetate and analyzed by H600 transmission electron microscope (Hitachi Co.) operating at 50,000× at 75 kV excitation voltages. After incubation for 120 hr tau protein without crocin sample showed mature fibers as well as amorphous aggregates. In the presence of crocin, the majority of tau proteins was amorphous form. The Scale bar represents 200 nm
PC12 cell culture
To investigate the toxicity of structures that were produced in the presence of crocin, MTT assay was performed. After incubation for 120 hr under fibril condition, the result of MTT assay showed less toxicity (about 20%) in samples containing tau protein and crocin compared to samples that contained tau protein only which proposed that the mixture of tau protein and crocin was not toxic for the cells (Figure 6).
Figure 6.
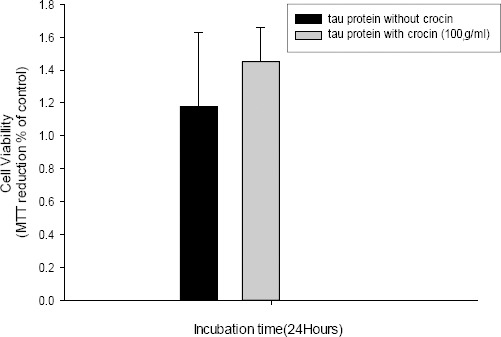
Cell viability after 24 hr incubation was determined by MTT assay. The results of MTT assays show less toxicity (about 20%) of tau protein with crocin as compared to tau protein without crocin which proposes that products resulting from inhibition of tau aggregation are not toxic to PC12 cells. Data are expressed as mean± standard deviation (SD)
Discussion
Considering the complexity of pathogenesis and multi-step process of AD development, current therapeutic approaches involve Multi-Target-Directed Ligands (MTDLs) (36, 37). According to MTDLs, natural products such as phytochemicals including alkaloids, polyphenols and terpenoids are important therapeutic substances (38). The major advantages of herbal compounds are their multiple actions and multi–target mechanisms (39). Therefore, AD therapeutic candidates should have several effects such as anti-oxidation, anti-inflammation, inhibition of amyloid-β and tau protein aggregation and Acetylcholine esterase inhibitory activity (39-41).
A previous study showed that crocin acts as a potent radical scavenger (42) and Nam et al suggested that crocin represses microglial activation in rat brain and could be effective in the inhibition of LPS-induced nitric oxide (NO) release (43). Moreover, an in vitro study has shown that saffron extract has an acetylcholinesterase (AchE) inhibitory activity (44). In addition, Ghahghaei et al (30) and Papandreou et al (31) showed that crocin effectively reduces the amount of amyloid-β fibrils.
Herein, we showed that crocin can inhibit the aggregation of human tau protein. Our results revealed that in the presence of crocin, the beta- structure/random coil ratio of tau protein under fibril condition decreased significantly (Figure 4). The probable mechanism of anti-tau-aggregation of crocin could be related to its chemical structure that consists of three parts containing a polyene hydrocarbon chain, carbonyl groups and β-D-gentiobiosyl at both ends (Figure 1) (26, 30). The partial negative charge of carbonyl groups can likely interact with positive residues such as Lysine and Argenin. On the other hand, positive residue especially lysine exists in hexapeptide aggregation cores of protein (275VQIINK280 and 306VQIVYK311) and it plays a critical role in the self-assembly of tau protein into abnormal fibrils (45). According to the above mentioned facts, carbonyl groups of crocin could interact with lysine residues and impair the self-assembly procedure of nucleation and elongation of fibril formation.
Additionally, previous evidence has identified that adding sugar moieties to curcumin for making sugar-curcumin has led to disruption of tau aggregation fibrils at low IC50 and caused potent neuroprotective effects (46). Meanwhile, the permeability of sugar-curcumin across the blood brain-barrier was improved (46). In comparison with water-soluble sugar derivatives of curcumin, crocin naturally has two sugar moieties attached to the end of the polyene hydrocarbon chain and these sugar groups probably have similar actions. After attaching crocins to tau protein, gentiobiose units could increase the total hydrodynamic radius of tau protein and consequently inhibit the required hydrophobicity for beta-sheet formation of the aggregates. Finally, soluble species of tau protein were non-toxic in the presence of crocin which suggest it as a safe candidate.
From the above data, it is evident that crocin appears to have multiple neuro- protective mechanisms and it also has a safety profile in human (28, 30, 31, 43, 47-50). Importantly, a randomized controlled trial illustrated the efficacy of saffron in the treatment of patients with mild to moderate AD (50). As mentioned above, anti-tau-aggregation property of crocin makes this molecule a multifunctional drug.
Conclusion
We conclude that corcin has a multi structural characteristics which leads to a variable neuroprotective properties. We suggest that the effect of gentiobiose on tau aggregation process. Would be a good target for future investigations.
Acknowledgment
This article was a part of a PhD thesis that was done in Institute of Biochemistry and biophysics (IBB), University of Tehran, Tehran, Iran.
References
- 1.Abbott A. Dementia: a problem for our age. Nature. 2011;475:S2–S4. doi: 10.1038/475S2a. [DOI] [PubMed] [Google Scholar]
- 2.Minati L, Edginton T, Bruzzone MG, Giaccone G. Current concepts in Alzheimer's disease: a multidisciplinary review. Am J Alzheimers Dis Other Demen. 2009;24:95–121. doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reitz C, Mayeux R. Alzheimer disease: epidemio-logy, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. Alzheimer's disease. Subcell Biochem. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard HB. Current and emerging therapies for Alzheimer's disease. Clin Ther. 2013;35:1480–1489. doi: 10.1016/j.clinthera.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Brunden KR, Ballatore C, Crowe A, Smith AB, 3rd, Lee VM, Trojanowski JQ. Tau-directed drug discovery for Alzheimer's disease and related tauopathies: a focus on tau assembly inhibitors. Exp Neurol. 2010;223:304–310. doi: 10.1016/j.expneurol.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal K, Gong CX, Liu F. Microtubule-associated protein tau as a therapeutic target in Alzheimer's disease. Expert Opin Ther Targets. 2014;18:307–318. doi: 10.1517/14728222.2014.870156. [DOI] [PubMed] [Google Scholar]
- 8.Bulic B, Pickhardt M, Mandelkow EM, Mandelkow E. Tau protein and tau aggregation inhibitors. Neuropharmacology. 2010;59:276–289. doi: 10.1016/j.neuropharm.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Bulic B, Pickhardt M, Mandelkow E. Progress and developments in tau aggregation inhibitors for Alzheimer disease. J Med Chem. 2013;56:4135–4155. doi: 10.1021/jm3017317. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe A, Ballatore C, Hyde E, Trojanowski JQ, Lee VM. High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation. Biochem Biophys Res Commun. 2007;358:1–6. doi: 10.1016/j.bbrc.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honson NS, Johnson RL, Huang W, Inglese J, Austin CP, Kuret J. Differentiating Alzheimer disease-associated aggregates with small molecules. Neurobiol Dis. 2007;28:251–260. doi: 10.1016/j.nbd.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paranjape SR, Chiang YM, Sanchez JF, Entwistle R, Wang CC, Oakley BR, Gamblin TC. Inhibition of Tau aggregation by three Aspergillus nidulans secondary metabolites:2,ω-dihydroxyemodin, asperthecin, and asperbenzaldehyde. Planta Med. 2014;80:77–85. doi: 10.1055/s-0033-1360180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer KN, Cisek K, Huseby CJ, Chang E, Kuret J. Structural determinants of Tau aggregation inhibitor potency. J Biol Chem. 2013;288:32599–32611. doi: 10.1074/jbc.M113.503474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monroy A, Lithgow GJ, Alavez S. Curcumin and neurodegenerative diseases. Biofactors. 2013;39:122–132. doi: 10.1002/biof.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi T, Ono K, Yamada M. REVIEW: Curcumin and Alzheimer's disease. CNS Neurosci Ther. 2010;16:285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornejo A, Jiménez JM, Caballero L, Melo F, Maccioni RB. Fulvic acid inhibits aggregation and promotes disassembly of tau fibrils associated with Alzheimer's disease. J Alzheimers Dis. 2011;27:143–153. doi: 10.3233/JAD-2011-110623. [DOI] [PubMed] [Google Scholar]
- 18.Peterson DW, George RC, Scaramozzino F, LaPointe NE, Anderson RA, Graves DJ, et al. Cinnamon extract inhibits tau aggregation associated with Alzheimer's disease in vitro. J Alzheimers Dis. 2009;17:585–597. doi: 10.3233/JAD-2009-1083. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB, 3rd, Lee VM. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J Neurochem. 2009;110:1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monti MC, Margarucci L, Riccio R, Casapullo A. Modulation of tau protein fibrillization by oleocanthal. J Nat Prod. 2012;75:1584–1588. doi: 10.1021/np300384h. [DOI] [PubMed] [Google Scholar]
- 21.Daccache A, Lion C, Sibille N, Gerard M, Slomianny C, Lippens G, et al. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem Int. 2011;58:700–707. doi: 10.1016/j.neuint.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50:761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27:475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H. Saffron: a herbal medicine of third millennium. Jundishapur J Nat Pharm Prod. 2014;9:1–2. doi: 10.17795/jjnpp-16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Gohari AR, Saeidnia S, Mahmoodabadi MK. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn Rev. 2013;7:61–66. doi: 10.4103/0973-7847.112850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- 29.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Ghahghaei A, Bathaie SZ, Kheirkhah H, Bahraminejad E. The protective effect of crocin on the amyloid fibril formation of Aβ42 peptide in vitro. Cell Mol Biol Lett. 2013;18:328–339. doi: 10.2478/s11658-013-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54:8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 32.Uversky VN. Amyloidogenesis of natively unfolded proteins. Curr Alzheimer Res. 2008;5:260–287. doi: 10.2174/156720508784533312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolhasani A, Bathaie S, Yavari I, Moosavi-Movahedi A, Ghaffari M. Separation and purification of some components of Iranian saffron. Asian J Chem. 2005;17:725–729. [Google Scholar]
- 34.Khalili MA, Riazi G, Ahmadian S, Khodarahmi R, Khodadadi S, Afrasiabi A, et al. The role of anionic peptide fragments in 1N4R human tau protein aggregation. Protein Pept Lett. 2014;21:511–516. doi: 10.2174/0929866521666131223120713. [DOI] [PubMed] [Google Scholar]
- 35.Riazi G, Kaghazian H, RezaLornejad M, Mokhtari F, Farhadinejad S, Shahriari L, et al. Dissimilar response of 2D and 3D astrocyte cell cultures to protective effect of recombinant human erythropoietin against amyloid-β25-35 toxicity. Int J Pharm Sci Rev Res. 2014;24:219–226. [Google Scholar]
- 36.Agis-Torres A, Sölhuber M, Fernandez M, Sanchez-Montero JM. Multi-target-directed ligands and other therapeutic strategies in the search of a real solution for alzheimer's disease. Curr Neuropharmacol. 2014;12:2–36. doi: 10.2174/1570159X113116660047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolognesi ML, Simoni E, Rosini M, Minarini A, Tumiatti V, Melchiorre C. Multitarget-directed ligands: innovative chemical probes and therapeutic tools against Alzheimer's disease. Curr Top Med Chem. 2011;11:2797–2806. doi: 10.2174/156802611798184373. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Decker M. Multi-target compounds acting in the central nervous system designed from natural products. Curr Med Chem. 2013;20:1673–1685. doi: 10.2174/0929867311320130007. [DOI] [PubMed] [Google Scholar]
- 39.Howes MJ, Perry E. The role of phytochemicals in the treatment and prevention of dementia. Drugs Aging. 2011;28:439–468. doi: 10.2165/11591310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Calcul L, Zhang B, Jinwal UK, Dickey CA, Baker BJ. Natural products as a rich source of tau-targeting drugs for Alzheimer's disease. Future Med Chem. 2012;4:1751–1761. doi: 10.4155/fmc.12.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howes MJ, Houghton PJ. Ethnobotanical treatment strategies against Alzheimer's disease. Curr Alzheimer Res. 2012;9:67–85. doi: 10.2174/156720512799015046. [DOI] [PubMed] [Google Scholar]
- 42.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 43.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, et al. Saffron as a source of novel acetylcholinesterase inhibitors: molecular docking and in vitro enzymatic studies. J Agric Food Chem. 2012;60:6131–6138. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]
- 45.Meraz-Ríos MA, Lira-De León KI, Campos-Peña V, De Anda-Hernández MA, Mena-López R. Tau oligomers and aggregation in Alzheimer's disease. J Neurochem. 2010;112:1353–1367. doi: 10.1111/j.1471-4159.2009.06511.x. [DOI] [PubMed] [Google Scholar]
- 46.Dolai S, Shi W, Corbo C, Sun C, Averick S, Obeysekera D, et al. Clicked” sugar-curcumin conjugate: modulator of amyloid-β and tau peptide aggregation at ultralow concentrations. ACS Chem Neurosci. 2011;2:694–699. doi: 10.1021/cn200088r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30:185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770:578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Med Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 50.Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. A 22-week, multicenter, randomized,double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psycho-pharmacology (Berl) 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]


