Abstract
Objective(s):
Intracerebral injection of bone marrow stromal cells (BMSCs) is being investigated as a therapeutic tool to prevent Alzheimer’s disease (AD). Our aim was to investigate the effects of BMSCs by intrathecal injection in AD rat model.
Materials and Methods:
BMSCs were obtained from the bone marrow of Wistar rat and transplanted into AD rat model via intrathecal injection. The rat model had received an injection of β amyloid into the hippocampus for histological and immunohistochemical studies.
Results:
Histological examination of the brains in transplanted rats compared to controls demonstrated the migration of BrdU-labeled BMSCs from the site of delivery, confirmed the differentiation of BMSCs transplanted cells into the cholinergic neurons, and increased number of healthy and decreased number of dark neurons.
Conclusion:
Our results showed that BMSCs intratechal administration could be a promising method for treatment of Alzheimer’s disease in rat model.
Keywords: Alzheimer’s disease, Bone marrow stromal cells, Intrathecal delivery, Rat model
Introduction
Alzheimer Disease (AD) is a complex, destroying, and an age-related brain disorder. AD patients endure memory loss and cognitive dysfunction. The symptoms of AD gradually lead to changes in behavior and personality, decrease in cognitive abilities such as decision-making and language skills, problems recognizing family and friends, and finally loss of the ability to perform basic activities of daily living including eating, walking, dressing and grooming.
Currently, there is no effective treatment that can significantly delay or stop the progressive brain damage in AD. The drugs currently in use treat only the symptoms and slow the progression of cognitive decline. Stem cells transplantation seems to be a promising strategy for treatment of several CNS degenerative diseases such as AD.
There are many types of stem cells in the body, which can broadly be divided into embryonic and adult (somatic) stem cells. Embryonic stem (ES) cells develop into multipotent adult stem cells such as neural stem cells (NSCs) and mesenchymal stem cells (MSCs). NSCs are very difficult to obtain from adult’s brain and so current studies mainly use fetal NSCs, which could also generate ethical problems. MSCs have self-renewal and a high proliferative capacity and have the ability to migrate into damaged organs.
These cells seem to be able to differentiate into multiple cells such as hepatocytes (1), skeletal muscle (2) and cardiomyocytes (3). It was found that MSCs derived from bone marrow, adipose tissue and umbilical cord blood could be transdifferentiated into neuronal cells (4). As embryonic stem cells have the potential to generate any cell kind, they are considered as an attractive cell source for tissue repair applications. Meanwhile, the presence of undifferentiated cells in a transplant could result in the formation of cardiac teratomas rather than new myocardium. Other previous study indicated the effect of bone marrow stromal cells on neuronal cells in animal model of Alzheimer’s disease (5). In addition, BMSCs avoid allergenic rejection, and therefore they serve as good choices to treat damaged tissues because of such capacities.
Intrathecal injection is a safe and effective strategy for cell transplantation. Jung Yeon Lim et al reported migration of hUCB-MSCs to brain and differentiation of MSCs into cells by intrathecal injection in rat model of cerebral ischemia (6). There is little data available on the intrathecal injection of stem cells.
We have selected intrathecal injection of the BMSC by lumbar puncture (LP) for treating AD rat model. A previous study used directed injection of the BMSCs to brain for treating AD mouse model (7). To our knowledge, this is the first study to report the effects of intrathecal injection of BMSCs in AD rat model. The route of BMSC delivery is the main difference between the present study and the previous studies. Our aim was to examine the effects of BMSCs by intrathecal injection in AD rat model.
Materials and Methods
In this study, a rat model of AD was created by stereotaxic injection of amyloid-β (Aβ) into hippocampus via needle of Hamilton syringe (26 gauge; Hamilton Company, UK). Then, the rats received bone marrow stromal cells intrathecally and underwent histological examination. Animals were housed with free access to food and water in a 12 hr light/dark cycle and constant temperature of 22 °C and received human care, as outlined in the guideline for the care and use of laboratory animals. This study was confirmed by the Ethical Committee of Iran University of Medical Sciences. For Histological and immunostaining study, nine adult male Wistar rats (250-320 g) were randomly divided into 3 groups, with 3 animals in each group. In the sham group, a needle of Hamilton syringe was stereotaxically inserted into the hippocampus and removed without any injection. After 2 weeks, the experimental group of AD rat model received the rat bone marrow stromal cells via intrathecal injection.
To create the model of Alzheimer’s disease, synthetic amyloid-β protein fragment 1-40 (Sigma-Aldrich, Germany) was dissolved in deionized water, aliquoted and stored at −70 °C until use. At the time of application, the frozen aliquots were thawed out by incubation at 37 °C. On the day of injection, rats were anesthetized with intraperitoneal (IP) injection of a cocktail of ketamine hydrochloride (80 mg/kg) and xylazine (8 mg/kg) (Sigma-Aldrich, Germany), and placed in the stereotaxic frame (Stoelting Co, USA). The hippocampus was localized at 3.8 mm posterior and ±2.4 mm lateral to the Bregma and 2.9 mm below the top of the skull according to the Paxinos Atlas. Each rat received 5 μl of amyloid-β solution in left side and 5 μl in right side over 10 minutes (8).
BMSCs were isolated, from the male Wistar rats and expanded, as previously described. They were previously characterized in our laboratory (9). Briefly, both ends of rat femoral bones were cut under sterile conditions, and the marrow cavities were flushed with phosphate buffered saline (PBS). The recovered cells were cultured in low-glucose DMEM (Sigma-Aldrich, Germany) supplemented with 20% fetal bovine serum and penicillin/strepto-mycin (100 U/ml and 100 μg/ml, respectively) (both from Biosera, France). The cells were subcultured or harvested using Accutase (Sigma-Aldrich, Germany) and were used freshly without cryopreservation. All cultures were incubated at 37 °C in a humidified chamber of 5% CO2-95% air.
In order to enable cell tracing in vivo, BMSCs were incubated with 5 μmol/ml of BrdU (Sigma-Aldrich, Germany) for 72 hr before transplantation. To confirm incorporation of BrdU into the cells, they were fixed in 4% formaldehyde for 20 min and immunostained with application of 1:500 dilution of primary anti-BrdU mouse monoclonal antibody (Sigma-Aldrich, Germany) followed by goat anti-mouse FITC-conjugated secondary antibody (1:400 dilution factor) (Abcam, Cambridge, UK) as described earlier.
In established models of Alzheimer Disease, the cells were intrathecally delivered 14 days after injection of amyloid-β using the method described by Lim et al (6). Briefly, the rats were anesthetized by ketamine/xylazine cocktail (as above) and a small longitudinal incision was made over the L3-L4 or L4-L5 spinous processes. Then, a human neonatal lumbar puncture needle (25-gauge) was advanced into the spinal canal at the L3-L4 or L4-L5 level, and 1 × 106 BMS cells (in 20 μl PBS) were injected into the cerebrospinal fluid (CSF) over 30 sec. Eight weeks after the stereotaxic surgery, all rats were euthanized by 150 mg/kg of ketamine and perfused through the ascending aorta with 50 to 100 ml of 0.9% saline followed by 100 to 150 ml of fixative solution containing 4% formaldehyde in 0.1 M phosphate buffer solution (PBS, pH 7.4) and 100 ml of 0.1 M PBS, respectively. The brains were removed from the skulls and fixed in 4% formaldehyde for 24 hr. Following routine paraffin processing, serial sections of the brain were cut coronally at 5 μm thickness in a rotary microtome.
Amyloid-β was stained with Congo red. On the other hand, Nissl staining with 1% cresyl violet was used for staining of neurons. Three periodic Nissl-stained coronal brain sections were used for cell counting under the magnification of ×40 using a light microscope (Olympus Optical Co, Ltd, Japan). Non-basophilic neurons with pale nuclei were counted as normal neurons while those with massive shrunken, hyperbasophilic features were counted as injured and abnormal neurons (so-called dark neurons). In each section, 3 fields in CA1, 1 field in CA2, 2 fields in CA3 and 2 fields in CA4 were counted.
Three periodic coronal brain sections (from ever animal) were used for immunostaining methods as well. But for these methods, our aim was the tracing of Brdu and CHAT markers in the brain of treated AD group. (No Statistical analysis).
EnVison system’s HRP-based immunostaining method (Dako, Denmark) was used for tracing of BrdU-labeled BMSCs in the histological sections. The coronal brain sections were used for this method, and observed under the magnification of ×40 using a light microscope (Olympus Optical Co, Ltd, Japan). After antigen retrieval at 60 °C for 60 min and blocking of endogenous peroxidase activity, the sections were incubated with anti-BrdU mouse monoclonal antibody (1:400) (Sigma-Aldrich, Germany). The sections were stained with hematoxylin.
To evaluate the differentiation of labeled BMSCs into the cholinergic neurons, a double immune-fluorescence staining for BrdU and choline acetyltransferase (ChAT) has been performed after antigen retrieval as above. Primary antibodies were diluted at 1:200 for anti-BrdU mouse monoclonal antibody (Sigma-Aldrich, Germany) and at 1:500 for anti-ChAT rabbit polyclonal antibody (Abcam, Cambridge, UK). The secondary antibody for detection of anti-BrdU was conjugated with Texas Red, and that for detection of anti-ChAT was conjugated with FITC (both from Santa Cruz, Germany). DAPI (Sigma, Germany) was used for counterstaining the nuclei. The coronal brain sections were used for this method, and observed under the magnification of ×40 using a fluorescence microscope.
One way ANOVA with Bonferroni Post hoc test was used for comparison of the counts of normal and dark neurons in sections of hippocampus in the sham, untreated and treated Alzheimer’s disease groups. Significant level was set at P< 0.05.
Results
Histological examination of the brains demonstrated migration of BrdU-labeled BMS cells from the site of delivery, increased the number of healthy and decreased the number of dark neurons following BMS cells transplantation, and showed differentiation of transplanted cells into the cholinergic neurons.
Immunocytochemical staining of BMS cells before transplantation confirmed incorporation of BrdU into these cells. Congo red staining showed amyloidal-β deposits in the CA1 region of hippocampus, dentate gyrus, thalamus and cortex of untreated rat models of established AD. No amyloid deposition could be detected in the brains of rats in other groups.
Nissl staining of coronal brain sections showed numerous normal and dark neurons in the hippocampus of the rats under the experiment. Statistical analysis (Table 1 and 2) indicated that in CA1, the counts of normal neurons were significantly higher in sham group compared with untreated and treated AD groups. The number of normal neurons in treated AD group with the BMSC was also significantly higher compared with the untreated AD group (P<0.05). In CA2 however, the number of normal neurons did not reveal any significant difference between the compared groups. In CA3 and CA4, the counts of normal neurons did not show any significant difference between sham and untreated AD group. However, the number of normal neurons in CA3 and CA4 were significantly higher in treated AD group (with BMSC) compared with both sham and untreated AD groups. On the other hand, the number of dark neurons was significantly higher in untreated AD group in CA1 compared with sham and treated AD groups (P<0.05). The number of dark neurons showed no significant change in other comparisons.
Table 1.
Comparison of the counts of normal and dark neurons in sections of hippocampus in three experimental groups of sham, and untreated and treated rats with Alzheimer disease
| Sections | Groups | Normal neurons | Dark neurons | ||||
|---|---|---|---|---|---|---|---|
| Counts | 95% CI | P-value* | Counts | 95% CI | P- value* | ||
| CA1 | Sham vs U-AD | 28.00±14.86 vs 15.25±14.96 | 1.66 to 23.84 | < 0.05 | 8.58±13.31 vs 27.83±17.50 | -30.70 to -7.80 | < 0.05 |
| Sham vs T-AD | 28.00±14.86 vs 46.29±17.29 | -29.38 to -7.20 | < 0.05 | 8.58±13.31 vs 8.42±17.32 | -11.28 to 11.62 | ns | |
| U-AD vs T-AD | 15.25±14.96 vs 46.29±17.29 | -42.13 to -19.95 | < 0.05 | 27.83±17.50 vs 8.42±17.32 | 7.97 to 30.87 | < 0.05 | |
| CA2 | Sham vs U-AD | 22.38±13.22 vs 25.88±20.36 | -23.14 to 16.14 | ns | 7.00±9.17 vs 26.13±26.31 | -44.93 to 6.68 | ns |
| Sham vs T-AD | 22.38±13.22 vs 25.88±9.74 | -23.14 to 16.14 | ns | 7.00±9.17 vs 16.00±19.94 | -34.80 to 16.80 | ns | |
| U-AD vs T-AD | 22.88±20.36 vs 25.88±9.74 | -19.64 to 19.64 | ns | 26.13±26.31 vs 16.00±19.94 | -15.68 to 35.93 | ns | |
| CA3 | Sham vs U-AD | 10.81±12.24 vs 5.19±5.44 | -5.03 to 16.28 | ns | 18.56±16.79 vs 29.13±15.75 | -25.82 to 4.70 | ns |
| Sham vs T-AD | 10.81±12.24 vs 21.75±6.17 | -21.60 to -0.28 | < 0.05 | 18.56±16.79 vs 20.75±19.34 | -17.45 to 13.07 | ns | |
| U-AD vs T-AD | 5.19±5.44 vs 21.75±6.17 | -27.22 to -5.90 | < 0.05 | 29.13±15.75 vs 20.75±19.34 | -6.89 to 23.64 | ns | |
| CA4 | Sham vs U-AD | 4.31±9.94 vs 3.94±6.42 | -9.24 to 9.99 | ns | 19.75±13.60 vs 28.81±21.94 | -23.91 to 5.788 | ns |
| Sham vs T-AD | 4.31±9.94 vs 19.44±14.78 | -24.74 to -5.51 | < 0.05 | 19.75±13.60 vs 16.56±13.74 | -13.01 to 17.18 | ns | |
| U-AD vs T-AD | 3.94±6.42 vs 19.44±14.78 | -25.11 to -5.89 | < 0.05 | 28.81±21.94 vs 16.56±13.74 | -3.95 to 26.24 | ns | |
One way ANOVA with Bonferroni post-hoc test
Abbreviations: U-AD untreated Alzheimer disease group, T-AD treated Alzheimer disease group, ns not significant
Table 2.
Mean and standard deviation of healthy cells
| Rat CA1 | Groups | Mean | SD |
|---|---|---|---|
| Sham | 28 | 14.86 | |
| Alzheimer | 15.25 | 14.69 | |
| Treatment | 46.29 | 17.29 | |
| Rat CA2 | |||
| Sham | 22.38 | 13.22 | |
| Alzheimer | 25.88 | 20.36 | |
| Treatment | 25.88 | 9.74 | |
| Rat CA3 | |||
| Sham | 10.81 | 12.24 | |
| Alzheimer | 5.19 | 5.44 | |
| Treatment | 21.75 | 16.17 | |
| Rat CA4 | |||
| Sham | 4.31 | 9.94 | |
| Alzheimer | 3.94 | 6.42 | |
| Treatment | 19.44 | 14.78 |
Tracing of BrdU-labeled cells in the treated group showed that intrathecally delivered BMS cells accommodated in the brain regions including dentate gyrus, subiculum and thalamus cortex (Figure 1). On the other hand, double immunofluorescent staining for BrdU and ChAT showed that intrathecally administered BMS cells were differentiated into cholinergic neurons (Figure 2).
Figure 1.
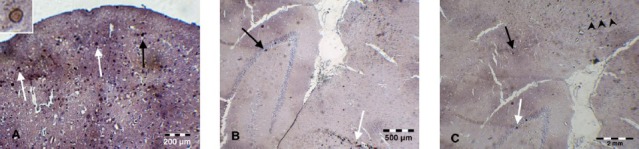
Immunohistochemical staining for tracing of BrdU-labeled cells; (A) the brown stain of the nuclei of BrdU+ cells are indicated by black and white arrows in cortex (scale bar 200 μm), a BrdU+ nucleus is shown by a higher magnification at the left upper corner (B) BrdU+ BMS cells in thalamus (white arrow) and dentate gyrus (Black arrow) (scale bar 500 μm) (C) BrdU-labeled BMS cells are observed in subiculum (black arrow), cortex (black arrow heads) and dentate gyrus (white arrow) (scale bar 2 mm)
Figure 2.
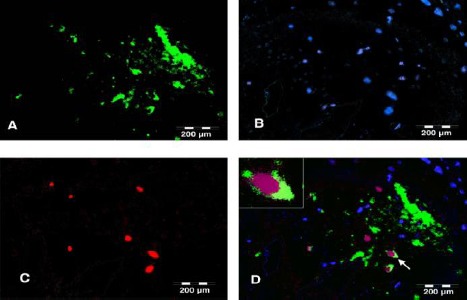
In vivo differentiation of BMS cells into cholinergic neurons; fluorescent staining for BrdU (red), ChAT (green) and nuclei (DAPI – blue) in a CA1 section of hippocampus. (A) green fluorescence represents ChAT+ cholinergic neurons, (B) DAPI staining of cell nuclei, (C) BrdU+ cells representing BMS cells migrated into the brain and (D) Merged image of the double staining for ChAT, nuclei and BrdU confirming differentiation of some BMS cells into cholinergic neurons (white arrow). The cell indicated by white arrow is shown with higher magnification in the inset. The purple color of the nucleus is the result of blue and red merger. All the scale bars are 200 μm
Discussion
Our study showed that the BMSC implantation increase the number of healthy cells and eliminate β amyloid deposition in treated group. Localization of BrdU+ BM-MSCs in the brain by immune-histochemical and immune fluorescent staining in this study, confirmed their migration into brain. In addition, BM-MSCs differentiation into cells with positive staining for ChAT neural marker (in immune fluorescent staining) indicated the survival of the implanted cells. BMSCs may be differentiated to neuronal cells or induced neurogenesis of endogenous neural stem cells (5). BMSCs may improve cognitive function of learning in AD by expressing ChAT. This study also confirmed the differentiation of the BMSCs to ChAT neural cells in AD rat model after directed injection of BM-MSCs (8). Another study reported that human NSCs (expressing ChAT) have lesion-tropic property and improve cognitive function of learning by increasing acetylcholine level in deficit model rats with hippocampal injury (10).
Direct injection of stem cell into brain in AD and other neurodegenerative animal models has been reported before (11). However, this method (directed injection) is often impractical for patients with clinically severe conditions (6). Jung Yeon Lim et al reported that both intravenous and intrathecal administration of cells demonstrated similar effects for neurological recovery in ischemic stroke regardless of migration and grafting difference within the ischemic brain.
They reported that many more grafted cells survived in animals after intrathecal administration when compared with animals after intravenous administration, due to the comparative insufficiency of the stem cell dosage in intravenous method. On the other hand, applying a large number of cells has some restrictions in human clinical trials.
Generally, the number of neurons determines the functional capacity of the brain or any particular neural structure (12). Our statistical analysis also shows the neuronal loss in the hippocampus of AD compared to sham group. This was mainly expressed by a significant decrease of neuronal density, especially in the CA1 area as the major site of neuronal loss in AD hippocampus, which is reported in reference to the degeneration of neurons in AD models. A significant reduction of neuronal density, especially in the CA1 and CA3 areas was also shown in AD with the most prominent decrease in CA1 area in other studies. Besides, the neuronal loss was detected in the CA2 and CA4 areas of AD group, although it was not statistically significant.
According to our findings, the treatment group shows an increase of healthy neuron in CA1, CA3 and CA4 (but not in CA2) compared to AD group (P<0.05).
To date, many experiments confirm the interconnected networks corresponding to the anatomical sub-fields of dentate gyrus (DG), CA3 and CA1 (the hippocampal circuit diagram). This is perhaps in conformity to this pattern. More experimental studies predicted the important announcement of the CA2 in neurodegenerative diseases such as AD (13). In agreement to previous studies, the findings of this study indicate consistent results in the AD model.
Here we show in the treatment group that beta amyloidal plaques might be eliminated, which could be confirmed via a meta-analysis.
Previous studies confirmed that BMSCs migration by directed method into brain, reduces β amyloid deposition (4, 14). It promotes neurogenesis of endogenous neural stem cells in the hippocampus of AD rat, and shows some therapeutic effects in neurodegenerative diseases. Shin JY et al reported that MSCs significantly enhance autolysosome formation and clearance of Aβ in AD models, which may increase neuronal survival against Aβ toxicity (15).
Generally addressing to the results of this study, further experiments are necessary for confirmation of neurogenesis and of ChAT influences on learning by intrathecal injection of BMS cells in AD.
Conclusion
Our finding led us to hypothesize that BMSCs injection via intrathecal method in AD rat model migrated into brain, survived and differentiated into ChAT-like neuron cells, decreased neurotoxicity of beta amyloid, increased the number of neuron cells and it may be a clinically feasible route of providing less invasive transplantation trophy in Alzheimer’s disease.
Acknowledgment
This work was supported by a research grant from the Iran University of Medical Sciences. The results of this study were part of student dissertation.
References
- 1.Nikoozad Z, Ghorbanian MT, Rezaei A. Comparison of the liver function and hepatic specific genes expression in cultured mesenchymal stem cells and hepatocytes. Iran J Basic Med Sci. 2014;17:27–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Dreyfus PA, Chretien F, Chazaud B, Kirova Y, Caramelle P, Garcia L, et al. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 2009;450:136–141. doi: 10.1016/j.neulet.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, et al. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer's disease. Neurosci Lett. 2010;481:30–35. doi: 10.1016/j.neulet.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Lim JY, Jeong CH, Jun JA, Kim SM, Ryu CH, Hou Y, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38. doi: 10.1186/scrt79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu AKL. Stem cell therapy for Alzheimer's disease: hype or hope? Biosci Horiz. 2013;6:hzt011.6–15. [Google Scholar]
- 8.Wu QW, Li J, Feng ZT, Wang TH. Bone marrow stromal cells of transgenic mice can improve the cognitive ability of an Alzheimer's disease rat model. Neurosci Lett. 2007;417:281–285. doi: 10.1016/j.neulet.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 9.Bakhtiary M, Marzban M, Mehdizadeh M, Ghataei MT, Khoei K, Pirhajati Mahabadi M, et al. Comparison of transplantation of bone marrow stromal cells BMSC) and stem cell mobilization by granulocyte colony stimulating factor after traumatic brain injury in rat. Iran Biomed J. 2010;14:142–149. [PMC free article] [PubMed] [Google Scholar]
- 10.Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012;21:365–371. doi: 10.3727/096368911X586765. [DOI] [PubMed] [Google Scholar]
- 11.Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 2009;450:136–141. doi: 10.1016/j.neulet.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Padurariu M, Ciobica A, Mavroudis J, Fotiou D, Baloyannis S. 4.Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer's disease patients. Psychiatr Danub. 2012;24:152–158. [PubMed] [Google Scholar]
- 13.Jones MW, McHugh TJ. Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 2011;34:526–535. doi: 10.1016/j.tins.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, et al. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10:32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]


