Abstract
Inflammatory caspases, including caspase-11, are upregulated in CD8+ T cells after antigen-specific activation, but little is known about their function in T cells. We report that caspase-11-deficient (Casp11−/−) T cells proliferated more readily in response to low affinity and low abundance ligands both in vitro and in vivo due to an increased ability to signal through the TCR. In addition to increased numbers, Casp11−/− T cells had enhanced effector function compared to wild-type cells including increased production of IL-2 and reduced expression of CD62L. Casp11−/− T cells specific for endogenous antigens were more readily deleted than wild-type cells. These data indicate caspase-11 negatively regulates TCR signaling possibly through its ability to regulate actin polymerization, and inhibiting its activity could enhance the expansion and function of low affinity T cells.
Introduction
Caspase-11 in mice and caspases-4 and -5 in humans are members of the family of inflammatory caspases, that also includes caspases-1 and -12(1). Unlike other caspases, caspase-11 is expressed at low levels in resting cells and upregulated upon activation via a type I interferon-dependent process(2). Caspase-11 can bind directly to intracellular LPS resulting in caspase-11 processing and activation(3), leading to downstream events that can include caspase-1 activation, cell death, and inflammatory cytokine processing and release(1). Caspase-11 can also act in its proform to regulate actin dynamics; caspase-11 promotes actin depolymerization by facilitiating interactions between actin interacting protein 1 (Aip1), cofilin, and F-actin(4). Accordingly, caspase-11-deficient (Casp11−/−) cells have altered migration and display reduced fusion of lysosomes to pathogen containing vacuoles(4, 5).
Surprisingly, activation-dependent expression of caspase-11 is also observed in CD4+ and CD8+ T cells; both effector and memory T cells have increased expression of caspase-11 when compared to naïve cells(6–8). Caspase-11 processing was not observed in activated T cells, but T cell migration was affected, with Casp11−/− cells migrating less efficiently into lymphoid tissues(4). Modulation of actin polymerization by caspase-11 could regulate additional aspects of T cell biology, including TCR signaling(9). The strength of signals received through the TCR can have affects on the phenotype and function of T cells after activation. Activation of CD8+ T cells with high affinity peptides results in increased expansion and effector function compared to stimulation with lower affinity peptides(10). The strength of TCR stimulation can also affect the phenotype of CD4+ T cells, with CD4+ T cells receiving higher levels of stimulation preferentially developing into TFH cells(11, 12) and low concentrations of high affinity peptide favoring FoxP3 expression(13).
We have addressed the role of caspase-11 in the activation and function of CD8+ T cells and found that Casp11−/− cells proliferate more readily in response to suboptimal levels of TCR stimulation, leading to a larger effector and memory pool and increased effector function in response to both low abundance and low affinity ligands in vivo. However, in the presence of low concentrations of self antigen, the increased sensitivity of Casp11−/− cells results in more rapid deletion in tissues in which the antigen is expressed. These data indicate that in addition to promoting cell death and inflammatory cytokine production, caspase-11 acts as a negative regulator of TCR signaling and limits the expansion and function of T cells in response to low abundance or low affinity TCR ligands.
Materials and Methods
Mice and immunizations
C57BL/6 and Nur77-GFP mice were purchased from Jackson Laboratory. Nur77-GFP, OT-I, iFABP-tOVA 232-6(14), and Caspase-11−/−(15) mice were maintained at the University of Washington and used in accordance with Institutional Animal Care and Use Committee guidelines.
Mice received 1–2x104 naïve OT-I T cells and were immunized with 2x103 LM-OVA, 150μg ovalbumin, or 1–2x106 DCs pulsed with LPS and 1μM N4, Q4, or T4 peptide as previously described(16). Mice were pulsed with 1mg BrdU i.p. 2.5 hours before sacrifice. OT-I T cells were stimulated ex vivo with peptide for 4 hours in the presence of Brefeldin A prior to intracellular cytokine staining.
Flow cytometry and cell sorting
Cells were stained with the indicated antibodies (BD Biosciences and eBioscience) or Alexa Fluor 647 phalloidin (Life Technologies), flow cytometry was performed on a FACSCanto (BD), and data were analyzed using FlowJo software (TreeStar). For cell sorting, thymocytes were stained with CD4, CD8α, and TCRβ, and effector OT-I T cells were stained using CD8 and CD45.1. Cells were sorted on a FACS Aria.
RT-PCR
RNA was isolated using a Qiagen RNeasy kit per manufacturers instructions. Quantatative RT-PCR was performed using the Quantitect SYBR green RT-PCR kit (Qiagen) and the following primers: casp11F: 5′-CCTGAAGAGTTCACAAGGCTT-3′; casp11R: 5′-CCTTTCGTGTACGGCCATTG-3′; actbF: 5′-GGCTGTATTCCCCTCCATCG-3′, actbR: 5′-CCAGTTGGTAACAATGCCATGT-3′.
In vitro stimulations
Splenocytes were pulsed with 1nM-1μM N4, Q4, or T4 peptide or no peptide at 37°C for 40 minutes, then washed thoroughly with media. 5x104 splenocytes were mixed with 1–2x104 naïve OT-I T cells. T cells were labeled with 2μM CFSE (Invitrogen) where indicated.
Statistical analysis
All graphs depict mean ± SD. Two-tailed Student’s t test was used to determine statistical significance.
Results
Caspase-11 limits CD8+ T cell expansion after protein immunization
Caspase-11 is upregulated in a variety of cell types undergoing activation, including CD8+ T cells(6). We examined the levels of caspase-11 mRNA during development of CD8+ T cells by sorting double negative, double positive, and CD8 single positive thymocytes as well as naïve CD8+ peripheral T cells. Casp11 was upregulated as thymocytes matured and further increased after cells entered the periphery (Fig. 1A). We also examined whether casp11 expression was upregulated after antigen-dependent activation in the periphery. Naïve OT-I T cells, which express a TCR specific for the ovalbumin peptide SIINFEKL, were transferred into mice which were then infected with Listeria monocytogenes expressing ovalbumin (LM-OVA) or immunized with ovalbumin protein. Activated OT-I T cells were sorted and casp11 expression analyzed. Casp11 was upregulated an additonal 5-fold in activated OT-I T cells in response to both protein immunization and infection (Fig. 1B) compared to naïve cells.
Figure 1. Caspase-11 expression is induced in CD8+ T cells during development and priming and limits the size of the CD8+ T cell pool after protein immunization.
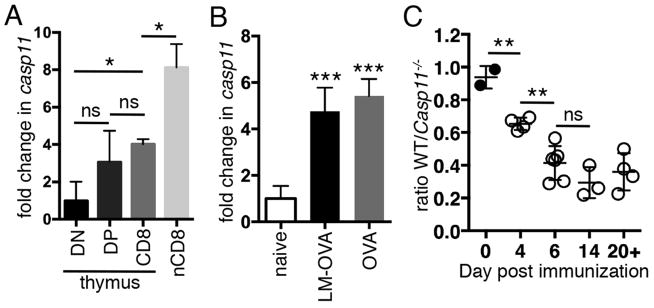
(A) Cells were sorted from C57BL/6 mice and casp11 expression was examined by qRT-PCR and normalized to actB. Data are pooled from 2 mice, representative of 2 experiments. (B) 104 naïve OT-I T cells were transferred into mice followed by LM-OVA infection or ovalbumin immunization (OVA). OT-I T cells were sorted at day 7 for LM-OVA or day 6 for OVA immunization and casp11 expression was analyzed as in (A). Data pooled from 3 independent experiments with 3 mice/grp. (C) 104 WT and Casp11−/− OT-I T cells were cotransferred into mice followed by i.p. immunization with 150μg OVA and the ratio of WT/Casp11−/− cells was determined at the indicated timepoints. Data in (C) are pooled from 2 experiments. ns, not significant, *p<0.05, **p<0.005, ***p<0.0005
To analyze the function of caspase-11 during the activation of CD8+ T cells, caspase-11-sufficient (WT) and Casp11−/− OT-I T cells were transferred into mice followed by challenge with LM-OVA or ovalbumin protein. There were equal numbers of WT and Casp11−/− OT-I T cells 7 days after LM-OVA challenge (data not shown). However, after ovalbumin immunization, Casp11−/− cells were present at higher numbers than WT OT-I T cells as early as 4 days after immunization and this difference became more pronounced at 6 days (Fig. 1C). The increased number of Casp11−/− compared to WT cells was maintained at memory timepoints (Fig. 1C). Previous reports indicated that caspase-11 is able to regulate the migration of T cells(4), and we hypothesized that failure of Casp11−/− T cells to migrate out of the spleen might explain their increase relative to WT cells. However, we saw no increase in the ratio of WT to Casp11−/− CD8+ T cells in the blood or inguinal LN suggesting WT cells were not exiting the spleen more readily than Casp11−/− cells (data not shown). These data suggest that Caspase-11 limits the accumulation of CD8+ T cells in response to protein immunization but has little affect on their migration out of central lymphoid organs.
Caspase-11 limits CD8+ T cell accumulation in response to low abundance and low affinity ligands
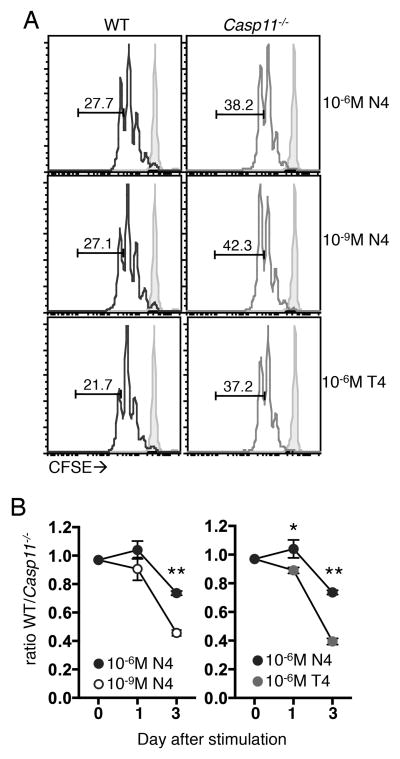
We hypothesized the increased numbers of Casp11−/− OT-I T cells after protein immunization and not LM-OVA infection could be due to their enhanced response to ligands of low abundance. This was addressed in vitro by examining the expansion of WT and Casp11−/− OT-I T cells in response to splenocytes pulsed with varying concentrations of a high affinity ligand (SIINFEKL, N4). We found that in response to high concentrations of N4, WT and Casp11−/− OT-I T cells diluted CFSE similarly and WT cells showed a mild defect in accumulation (Fig. 2A, Fig. 2B). In response to low concentrations of N4, we saw an increased percentage of CFSElo cells in the Casp11−/− population compared to WT (Fig. 2A) and increased accumulation of Casp11−/− cells (Fig. 2B). When a lower affinity ligand (SIITFEKL, T4) was used, we also observed an increase in proliferation and outgrowth of Casp11−/− compared to WT cells (Fig. 2A, Fig. 2B). These data indicate that there is a cell intrinsic enhancement in the ability of Casp11−/− T cells to proliferate in response to low levels of antigenic stimulation, as WT and Casp11−/− cells have equal access to antigen, cytokines, and other factors in this in vitro setting.
Figure 2. Caspase-11 limits CD8+ T cell accumulation in response to low abundance and low affinity ligands in vitro.
WT and Casp11−/− OT-I T cells were CFSE labeled and stimulated with splenocytes pulsed with the indicated concentrations of N4 or T4 peptides. (A) CFSE dilution 2 days after peptide stimulation (open histograms) or in unstimulated controls (shaded histograms), values indicate the percentage of OT-I T cells that have undergone 4 or more divisions. (B) Ratio of WT to Casp11−/− OT-I T cells. Data are from a single experiment and representative of 3 experiments. *p<0.05, **p<0.0005
Casp11−/− CD8+ T cells primed with low affinity ligands in vivo display enhanced proliferation and effector function
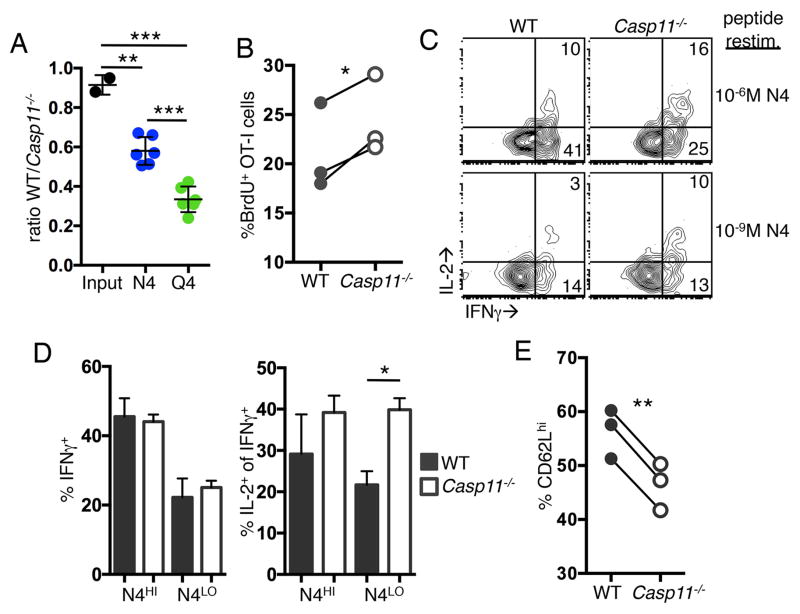
Higher levels of TCR signaling can lead to differences in effector function, and we hypothesized the increased TCR sensitivity of Casp11−/− cells may result in enhanced effector function. To address this, mice were primed with DCs pulsed with N4 or T4 and the expansion of WT and Casp11−/− OT-I T cells was examined. T4-DC immunization was not sufficient to activate all transferred cells (data not shown); therefore, we used the intermediate affinity peptide SIIQFEKL (Q4) to pulse DCs. Consistent with what we observed in vitro, we saw increased accumulation of Casp11−/− cells compared to WT cells after both N4-DC and Q4-DC immunization and the difference was enhanced with lower affinity antigen (Fig. 3A). BrdU incorporation at 3 days after Q4-DC immunization revealed that Casp11−/− OT-I T cells proliferated more than WT cells (Fig. 3B). No difference was observed in Annexin V staining (data not shown), indicating accumulation of Casp11−/− cells was due to enhanced proliferation and not decreased sensitivity to cell death.
Figure 3. Casp11−/− CD8 T cells primed with low affinity ligands in vivo display enhanced proliferation and effector function.
C57BL/6 mice received 1x104 WT and Casp11−/− OT-I T cells and 1–2x106 N4 or Q4 peptide pulsed DCs. (A) The ratio of WT:Casp11−/− OT-I T cells in the spleen on day 4 after immunization. Data are pooled from 2 experiments. (B–E) Mice were immunized with Q4-DCs and BrdU incorporation was measured on day 3 (B) and cytokine production after restimulation with the indicated concentration of N4 peptide on day 4 (C–D). CD62L expression on day 4 (E). Data are from a single experiment and representative of 2–3 experiments. *p<0.05, **p<0.005, ***p<0.0005
Cytokine production by Casp11−/− OT-I T cells relative to controls was examined 4 days after Q4-DC immunization. OT-I T cells were restimulated in vitro with high and low concentrations of N4 peptide, and the production of IFNγ and IL-2 was measured. A similar percentage WT and Casp11−/− cells produced IFNγ after stimulation with both high and low concentrations of N4 (Fig. 3C, 3D). However, the percentage of IFNγ+ cells producing IL-2 was increased in Casp11−/− OT-I T cells in response to low concentrations of N4 (Fig. 3C, 3D). We also examined the expression of CD62L after Q4-DC immunization and found that Casp11−/− OT-I T cells had reduced expression compared to WT cells (Fig. 3E). These data indicate that Casp11−/− T cells expand more readily to in vivo peptide-DC stimulation and have enhanced effector function including an increased ability to produce IL-2.
Enhanced TCR signaling in Casp11−/− CD8+ T cells
We hypothesized that caspase-11 modulated the strength of the TCR signal received during priming. This was measured in WT and Casp11−/− OT-I T cells using a Nur77-GFP reporter(17). Casp11−/− and WT cells had similar GFP expression in the absence of peptide stimulation (data not shown). WT and Casp11−/− Nur77-GFP OT-I T cells were stimulated with N4, Q4, or T4 peptide for 12 hours, and the fold change in GFP expression in the GFPhi population over unstimulated WT OT-I T cells was compared. We found Casp11−/− T cells had significantly increased GFP expression over a range of peptide affinities (Fig. 4A) and concentrations (data not shown). Additionally, more cells in the Casp11−/− population responded to low affinity ligands; a higher percentage of Casp11−/− became Nur77-GFPhi within 12 hours of stimulation (Fig. 4B). By 24 hours post activation, this phenotype was less apparent (data not shown), suggesting a difference in the rate of activation rather than the inability of a subset of WT cells to respond. This data could be explained by increased expression of the TCR and associated molecules in Casp11−/− T cells; however, naïve WT and Casp11−/− OT-I T cells had similar surface expression of Vα2, Vβ5, CD3, and CD8α (data not shown). These data indicate TCR signaling in response to low abundance/affinity ligands is inhibited by caspase-11, resulting in weaker TCR signaling overall and delayed activation.
Figure 4. Enhanced TCR signaling in Casp11−/− T cells after in vitro stimulation.
WT Nur77-GFP and Casp11−/− Nur77-GFP OT-I T cells were stimulated for 12 hours with unpulsed splenocytes or splenocytes pulsed with 1μM N4, Q4, or T4. The GFP MFI of unstimulated WT cells was set to 1.0. (A) The fold change in the MFI of GFPhi peptide pulsed WT and Casp11−/− cells relative to unstimulated cells. (B) The percentage of cells that are Nur77-GFPhi after stimulation. Data are from one experiment, representative of 2 experiments. ns, not significant, *p<0.05, **p<0.005
Caspase-11 has been shown to positively regulate actin depolymerization, leading to increased levels of F-actin in Casp11−/− cells(4,5), and we speculated this could lead to enhanced TCR signaling in Casp11−/− T cells. Using phalloidin staining, we verified increased F-actin levels in Casp11−/− compared to WT OT-I T cells after stimulation with low affinity ligands (Supplemental Fig. 1A, 1B). In addition, onset of Nur77-GFP expression in WT OT-I T cells after T4 stimulation correlated with higher levels of F-actin when compared to Nur77-GFPlo cells (Supplemental Fig. 1C).
More efficient deletion of self-reactive Casp11−/− T cells
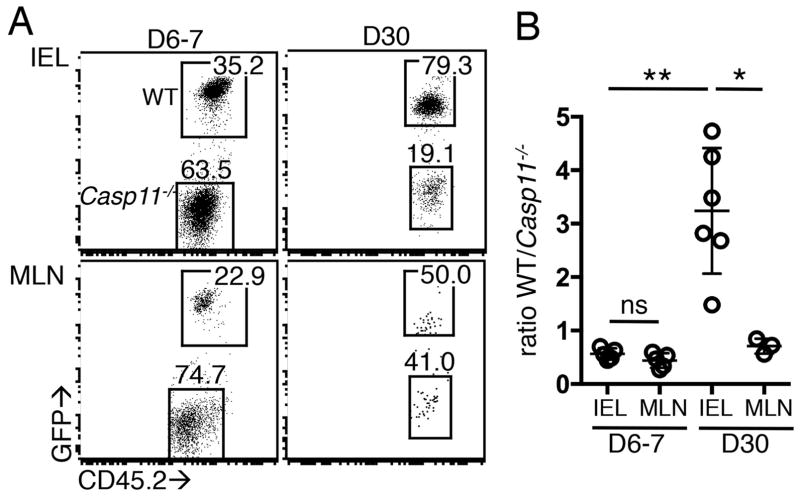
To determine if self-reactive Casp11−/− T cells respond more strongly to endogenous ligands, we co-transferred WT and Casp11−/− OT-I T cells into mice expressing ovalbumin under the control of the fatty acid binding promoter (iFABP-tOVA), which leads to ovalbumin production by mature enterocytes of the small intestine(14). At 6–7 days post-transfer, Casp11−/− OT-I T cells outnumbered WT cells in the intestinal epithelium (IEL) and mesenteric lymph node (MLN) (Fig. 5A, 5B). Thirty days after transfer, OT-I cell numbers were significantly lower in the MLN as has been reported(14, 18). At this time, 50% of mice had no OT-I T cells of either genotype in the MLN, and the remaining mice had equal numbers of WT and Casp11−/− cells (Fig. 5A, 5B). Notably, in the IEL compartment at this late timepoint, WT OT-I T cells outnumbered Casp11−/− cells (Fig. 5A, 5B). These data suggest the sensitivity of Casp11−/− OT-I T cells to low abundance endogenous ligands allowed preferential early expansion and increased numbers in the intestinal tissue relative to WT cells; however, Casp11−/− OT-I T cells were more efficiently deleted in the intestine over time.
Figure 5. Self-reactive Casp11−/− T cells do not persist after transfer.
CD45.1+ iFABP-OVA mice received 0.5–1x106 GFP+ WT and CD45.2+ Casp11−/− OT-I T cells IEL and MLN were examined 6–7 and 30 days post transfer. (A) Representative plots showing WT and Casp11−/− OT-I cells of total transferred cells. (B) Ratio of WT to Casp11−/− OT-I T cells. Data are pooled from 3 independent experiments. *p<0.05, **p<0.005
Discussion
Caspase-11 expression is upregulated in CD8+ T cells as they mature and functions to negatively regulate TCR signaling following antigenic stimulation, resulting in decreased expansion and effector function, especially in response to low level antigen stimulation. The stimulus for caspase-11 upregulation in this setting is still unclear. Our data suggests it is not type I interferon-dependent process, which is observed in other cell types. Upregulation of caspase-11 is equivalent during priming with Listeria, which results in robust type I interferon production, and protein immunization. Reactive oxygen species (ROS) have also been demonstrated to stimulate caspase-11 upregulation(19), and TCR stimulation results in a metabolic shift that generates ROS that could account for the upregulation of Casp11 during T cell activation(20).
The stepwise increase in the expression of caspase-11 by CD8+ T cells correlates with the reduced sensitivity of the TCR. Naïve CD8+ T cells that have recently left the thymus are more sensitive to TCR signals than those that have undergone maturation in the periphery(21), and antigen sensitivity leading to cell division is further reduced in CD8+ memory cells(22). Reduced TCR signaling in maturing T cells is more readily apparent when low abundance and low affinity antigens are used(21, 22). Lower expression of the TCR, CD3, and other surface molecules associated with robust TCR activation have been implicated reduced signaling (21–23). In addition, protein tyrosine phosphatases that negatively regulate TCR signaling are also upregulated in CD8+ memory T cells(22). Our data suggests caspase-11 represents an additional regulatory mechanism for tuning the sensitivity of maturing T cells to both foreign and endogenous antigens.
Overall, these data indicate caspase-11 is upregulated as T cells become more antigen experienced and acts as a negative regulator of TCR signaling particularly in response to low abundance and low affinity TCR ligands. Caspase-11 can regulate actin polymerization which is known to play a critical role in regulating TCR signaling(9). These data suggest that modulating the function of caspase-11 in T cells could be used in the context of immunization to regulate their sensitivity to low affinity antigens, allowing increased expansion and enhanced effector function without the risk of developing autoimmunity.
Supplementary Material
Acknowledgments
TB is supported by the Irvington Postdoctoral Fellowship from the Cancer Research Institute. This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grants AI-19335 (to MJB).
We thank Vishva Dixit and Timothy Hla for providing Caspase-11−/− mice.
Abbreviations
- IEL
intestinal epithelium
- MLN
mesenteric lymph node
- WT
wild-type
References
- 1.Vanaja SK, Rathinam VAK, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rathinam VAK, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Brieher WM, Scimone ML, Kang SJ, Zhu H, Yin H, Von Andrian UH, Mitchison T, Yuan J. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol. 2007;9:276–286. doi: 10.1038/ncb1541. [DOI] [PubMed] [Google Scholar]
- 5.Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DHA, Voss OH, Doseff AI, Hassan H, Azad AK, Schlesinger LS, Wewers MD, Gavrilin MA, Amer AO. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haring JS, Harty JT. Interleukin-18-Related Genes Are Induced during the Contraction Phase but Do Not Play Major Roles in Regulating the Dynamics or Function of the T-Cell Response to Listeria monocytogenes Infection. Infect Immun. 2009;77:1894–1903. doi: 10.1128/IAI.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth TC, Xue H-H, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritter AT, Angus KL, Griffiths GM. The role of the cytoskeleton at the immunological synapse. Immunol Rev. 2013;256:107–117. doi: 10.1111/imr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single Naive CD4(+) T Cells from a Diverse Repertoire Produce Different Effector Cell Types during Infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 16.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J-W, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura A-R, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 19.Lupfer CR, Anand PK, Liu Z, Stokes KL, Vogel P, Lamkanfi M, Kanneganti T-D. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014;10:e1004410. doi: 10.1371/journal.ppat.1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkley AM, Fink PJ. Cutting edge: CD8+ recent thymic emigrants exhibit increased responses to low-affinity ligands and improved access to peripheral sites of inflammation. J Immunol. 2014;193:3262–3266. doi: 10.4049/jimmunol.1401870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehlhop-Williams ER, Bevan MJ. Memory CD8+ T cells exhibit increased antigen threshold requirements for recall proliferation. J Exp Med. 2014;211:345–356. doi: 10.1084/jem.20131271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat Rev Immunol. 2011;11:544–549. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.