Abstract
Background & Aims
The benefit of continuing immunomodulators when “stepping up” to anti-tumor necrosis factor (anti-TNF) therapy for Crohn's disease (CD) is uncertain. This study assessed the effectiveness and safety of immunomodulators with anti-TNF therapy in CD.
Methods
We conducted a retrospective cohort study of new users of anti-TNF therapy for CD in Medicare. Users of anti-TNF combination therapy with immunomodulators were matched to up to 3 users of anti-TNF monotherapy via propensity score and compared using 3 metrics of effectiveness – surgery, hospitalization, and discontinuation of anti-TNF therapy or surgery – and 2 metrics of safety – serious infection and non-Candida opportunistic infection. Cox regression was used for all analyses.
Results
Among new users of infliximab, we matched 381 users of combination therapy to 912 users of monotherapy; among new users of adalimumab, we matched 196 users of combination therapy to 505 users of monotherapy. Combination therapy occurred predominantly as “step up” after thiopurine therapy. The rates of surgery (hazard ratio [HR] 1.20, 95% CI 0.73-1.96), hospitalization (HR 0.82 [0.57-1.19]), discontinuation of anti-TNF therapy or surgery (HR 1.09, [0.88-1.34]), and serious infection (HR 0.93 [0.88-1.34]) did not differ between users of anti-TNF combination therapy and monotherapy. However, the risk of opportunistic infection (HR 2.64 [1.21-5.73]) and herpes zoster (HR 3.16 [1.25-7.97]) were increased with combination therapy.
Conclusions
We found that continuation of immunomodulators after “stepping up” to anti-TNF therapy did not improve outcomes but was associated with an increased risk of opportunistic infection.
Keywords: thiopurines, infliximab, adalimumab, surgery, hospitalization, infection
Introduction
Combination anti-TNF and thiopurine therapy is currently the most efficacious therapy for moderate-to-severe CD in patients naïve to treatment with either drug.1 However, the benefit of continuing immunomodulators when “stepping-up” to anti-TNF therapy is less clear.2-5 The primary reasons to continue immunomodulators in this setting are to achieve higher drug levels of2,6,7 and lower levels of antibodies against the anti-TNF agent.8,9 However, it has been shown that scheduled (as opposed to episodic) anti-TNF treatment has the greatest impact on reduction of anti-drug antibody formation.10,11 The incremental benefit on clinical outcomes with continued immunomodulator use when “stepping-up” to scheduled anti-TNF therapy is unknown.
The use of immunosuppressive agents is not without risk, most notably serious infection and malignancy. While most studies have found that thiopurines increase the risk of malignancy whether used as monotherapy or in combination with anti-TNF medications,12-14 it is less clear whether addition of thiopurines to anti-TNF treatment increases the risk of serious or opportunistic infections. Thiopurines have been shown to increase the risk of infections, particularly viral infections such as herpes zoster.15 Anti-TNF therapy has also been associated with susceptibility to infections.16 Limited data with combination therapy suggest that while the risk of serious infections may not be increased compared to monotherapy,1 there may be an increased risk of opportunistic infections.17
The aims of our study were to assess the effectiveness and safety of immunomodulators when combined with anti-TNF therapy, compared to anti-TNF monotherapy, in patients with CD.
Methods
Study Design
We conducted a retrospective cohort study of new users of anti-TNF therapy for the treatment of CD among patients with Medicare drug benefits. This cohort has been described in detail in a previous study.18 As this dataset included only a small number of users of certolizumab pegol, we restricted our study to patients treated with infliximab or adalimumab. We used medical and pharmacy claims data from February 1, 2007 (when adalimumab became FDA-approved) through December 31, 2010; however, data from January 1, 2006 were used for collection of covariates.
Exposure Definitions
We identified all patients who newly initiated treatment with infliximab or adalimumab who had at least 1 diagnostic code for CD in the 12 months prior to starting anti-TNF therapy. To be classified as a new user of anti-TNF therapy, patients could not have received a dispensing of any anti-TNF agent during the 12 months preceding the date of their first anti-TNF prescription. If there were less than 12 months of available data prior to the date of first anti-TNF prescription (16% of overall cohort), we required a minimum of 6 months of available data with no prescription for anti-TNF therapy. We excluded patients who were hospitalized with a diagnosis of inflammatory bowel disease during the 8 weeks prior to the date of first anti-TNF prescription to assure that we did not misclassify the start date of anti-TNF therapy, since anti-TNF therapy could be initiated in the hospital. Additional exclusion criteria are listed in Supplementary Methods.
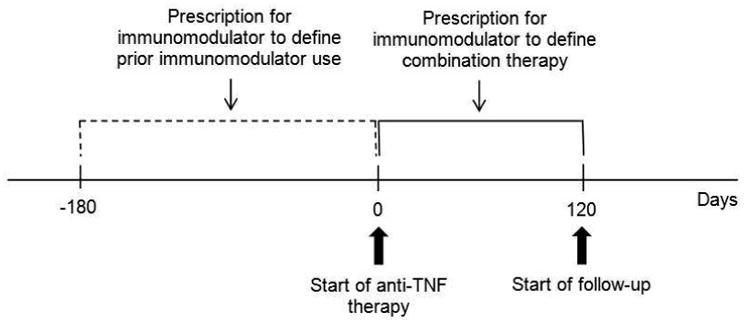
We defined anti-TNF monotherapy and combination therapy as follows (Figure 1): 1) monotherapy without prior immunomodulators (no use of immunomodulators [thiopurines or methotrexate] in the 180 days before or the 120 days after initiating anti-TNF therapy); 2) monotherapy with prior immunomodulators (use of immunomodulators in the 180 days before but not the 120 days after initiating anti-TNF therapy); 3) combination therapy without prior immunomodulators (use of immunomodulators in the 120 days after but not 180 days before initiating anti-TNF therapy); and 4) combination therapy with prior immunomodulators (use of immunomodulators in the 120 days after and 180 days before initiating anti-TNF therapy). A preliminary analysis of the data revealed that only 75 patients were new users of combination therapy (i.e. most combination therapy occurred as “step-up”). For this reason, we combined the 2 combination therapy groups and combined the 2 monotherapy groups and then adjusted all analyses for prior use of immunomodulators. Start of follow-up was defined as 120 days after the date of first prescription for anti-TNF therapy, such that combination therapy and monotherapy status could be classified as accurately as possible.
Figure 1. Classification of monotherapy vs. combination therapy and start of follow-up.
Outcome Measures
To measure comparative effectiveness and safety across multiple relevant domains, we employed 3 effectiveness primary outcome measures – surgery, hospitalization, and discontinuation of anti-TNF therapy or surgery – and 2 safety primary outcome measures – serious infection and opportunistic infection. We elected not to include malignancy as a safety outcome in this study as preliminary analysis revealed a median follow-up time of <2 years.
Surgery was defined as any CD-related bowel resection, ostomy creation, or surgical treatment of a perforation or abscess, based on manual review of the claims data for patients undergoing surgery.18 Our primary definition of hospitalization required that CD be the primary discharge diagnosis. A preliminary analysis of the data identified that a gap of 60 days after the expected next dose of anti-TNF therapy was the most appropriate to define discontinuation.
Serious infections were defined as hospitalized bacterial infections identified using ICD-9 codes found on principal discharge diagnosis, as in a similar prior study in Medicare.19 Opportunistic infections were defined as in prior similar studies in Medicare and included infection with the following organisms: Aspergillus, Blastomyces, Coccidiodes, Cryptococcus, Histoplasma, Pneumocystis, Actinomyces, Legionella, Listeria, Norcardia, Salmonella, tuberculous and non-tuberculous mycobacteria, Toxoplasma, herpes zoster, and JC virus.20,21
Of note, Candida species were not included, and herpes zoster and tuberculosis were also analyzed separately. Opportunistic infections were identified using ICD-9 codes from primary or non-primary diagnoses from inpatient and outpatient records as well as antimicrobial therapy for certain organisms (see Supplementary Methods). For the outcomes of serious and opportunistic infection, we excluded patients with any of these infections in the 183 days prior to start of anti-TNF therapy.
Covariates
Covariate selection was based on 2 requirements: potential for confounding and adequate data in Medicare. Covariates were measured either in the 12 months (for demographic and non-CD-related information) or 90 days (for CD severity- and medication-related variables) prior to start of anti-TNF therapy. The covariates included: calendar year, comorbidities included in the Schneeweiss comorbidity index,22 age, sex, race/ethnicity, eligibility for Medicaid benefits (as a proxy for socioeconomic status), initial eligibility for Medicare due to disability, nursing home residence, bowel resection surgery, ostomy, perianal disease, bowel obstruction, endoscopy with stricture dilation, computed tomography of the abdomen and/or pelvis, colonoscopy or sigmoidoscopy, upper endoscopy, small bowel series, magnetic resonance imaging of the abdomen and/or pelvis, number of emergency department visits with a CD or related diagnosis as the primary diagnosis, other medical therapies for CD (oral mesalamine, rectal mesalamine, oral steroids, rectal steroids, narcotics), and the number of additional medications prescribed for other conditions. Of note, in Medicare there are no data regarding duration of CD, and smoking data are not reliably captured; thus, these were not included as covariates. See Supplementary Methods for other details regarding covariates.
Statistical analysis
The statistical analyses compared the effectiveness and safety of anti-TNF monotherapy and combination therapy, separately for users of infliximab and adalimumab, as efficacy data on combination therapy with adalimumab and immunomodulators are lacking and there are data suggesting that adalimumab levels may not increase to the same degree as infliximab levels when combined with immunomodulators.23 Combined anti-TNF cohort analyses were also performed. Because of the large number of potential covariates relative to the number of outcomes, we combined the covariates into a single propensity score, which was estimated from a logistic regression model with monotherapy relative to combination therapy as the dependent variable. See Supplementary Methods for further details regarding the propensity score.
Propensity score matched Cox regression was used for all outcomes, which were analyzed as time-to-event. End of follow-up was defined as the earliest of the following: outcome of interest, initiation of another anti-TNF agent, end of available data, death, or loss of Medicare medical or prescription benefits. Patients who experienced any of these events prior to the first 120 days after start of anti-TNF therapy (start of follow-up) were excluded. We also performed a number of pre-specified secondary and sensitivity analyses as well as 1 post-hoc analysis (see Supplementary Methods). Given the sample sizes and numbers of outcomes observed, we had 80% power at the 0.05 significance level to detect HRs for surgery, hospitalization, discontinuation of anti-TNF therapy or surgery, serious infection, or opportunistic infection of 2.2, 1.7, 1.5, 1.8, or 3.2, respectively, for the infliximab cohort, and 2.3, 1.9, 1.9, 2.2, or 4.7, respectively, for the adalimumab cohort.
The study was approved by the institutional review boards at the University of Pennsylvania and University of Alabama Birmingham.
Results
Our original cohort included 1,459 new users of infliximab and 871 new users of adalimumab.18 Among patients treated with infliximab, 381 users of combination therapy were matched to 912 users of monotherapy. Of the 381 patients receiving infliximab combination therapy, 54 (14%) were new users of combination therapy and 327 (86%) had received prior immunomodulators (“step-up” therapy). Among patients treated with adalimumab, 196 users of combination therapy were matched to 505 users of monotherapy. Of the 196 patients receiving adalimumab combination therapy, 21 (11%) were new users of combination therapy and 175 (89%) had received prior immunomodulators. Notably, thiopurines constituted 92% and 89% of the immunomodulator use among the combination infliximab and adalimumab cohorts, respectively. The median time from the first documented immunomodulator prescription to start of anti-TNF therapy among patients treated with “step-up” combination therapy was 423 days (interquartile range [IQR] 153-960 days) in the infliximab cohort and 750 days (IQR 286-1712 days) in the adalimumab cohort; 84% and 87% of these patients started immunomodulators ≥90 days prior to starting inflximab and adalimumab, respectively. More women than men were included, most patients were white, and nearly half were treated with oral steroids in the 90 days prior to start of anti-TNF therapy (Table 1). The matching produced excellent balance with respect to all covariates.
Table 1. Baseline characteristics of the study cohorts*.
| Infliximab | Adalimumab | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Group | Combination Therapy (n=381) | Monotherapy (n=912) | SMD (wt by # matches) | Combination Therapy (n=196) | Monotherapy (n=505) | SMD (wt by # matches) |
| Age | 20-29 | 16 (4.2%) | 44 (4.8%) | 0.02 | 17 (8.7%) | 44 (8.7%) | 0.06 |
| 30-39 | 51 (13.4%) | 126 (13.8%) | 0.01 | 42 (21.4%) | 105 (20.8%) | 0.01 | |
| 40-49 | 56 (14.7%) | 136 (14.9%) | 0.01 | 53 (27.0%) | 133 (26.3%) | 0.03 | |
| 50-59 | 59 (15.5%) | 144 (15.8%) | 0.01 | 32 (16.3%) | 86 (17.0%) | 0.01 | |
| 60-69: disabled | 48 (12.6%) | 93 (10.2%) | 0.01 | <11 (<5.6%) | 26 (5.1%) | 0.02 | |
| 60-69: not disabled | 73 (19.2%) | 165 (18.1%) | 0.00 | 17 (8.7%) | 44 (8.7%) | 0.03 | |
| ≥70 | 78 (20.5%) | 204 (22.4%) | 0.01 | 25 (12.8%) | 67 (13.3%) | 0.04 | |
| Sex | Female | 236 (61.9%) | 571 (62.6%) | 0.00 | 124 (63.3%) | 330 (65.3%) | 0.03 |
| Race | White | 334 (87.7%) | 789 (86.5%) | 0.01 | 166 (84.7%) | 430 (85.1%) | 0.00 |
| Black | 35 (9.2%) | 91 (10.0%) | 0.00 | 21 (10.7%) | 55 (10.9%) | 0.00 | |
| Other | 12 (3.1%) | 32 (3.5%) | 0.01 | <11 (<5.6%) | 20 (4.0%) | 0.00 | |
| Medicaid eligible | 170 (44.6%) | 405 (44.4%) | 0.00 | 118 (60.2%) | 308 (61.0%) | 0.00 | |
| Skilled nursing facility | 16 (4.2%) | 39 (4.3%) | 0.00 | <11 (<5.6%) | 22 (4.4%) | 0.03 | |
| Emergency department visit | 36 (9.4%) | 98 (10.7%) | 0.00 | 22 (11.2%) | 61 (12.1%) | 0.06 | |
| Bowel resection | <11 (<2.9%) | <11 (<1.2%) | 0.00 | <11 (<5.6%) | <11 (<2.2%) | 0.03 | |
| Exam under anesthesia | 43 (11.3%) | 109 (12.0%) | 0.01 | 23 (11.7%) | 57 (11.3%) | 0.05 | |
| MRI or CT scan | 93 (24.4%) | 209 (22.9%) | 0.05 | 39 (19.9%) | 109 (21.6%) | 0.02 | |
| Colonoscopy or sigmoidoscopy | 121 (31.8%) | 296 (32.5%) | 0.00 | 53 (27.0%) | 139 (27.5%) | 0.04 | |
| Upper endoscopy | 33 (8.7%) | 83 (9.1%) | 0.00 | 20 (10.2%) | 57 (11.3%) | 0.08 | |
| Small bowel series | 41 (10.8%) | 93 (10.2%) | 0.01 | 15 (7.7%) | 36 (7.1%) | 0.01 | |
| Small bowel obstruction | 21 (5.5%) | 51 (5.6%) | 0.00 | <11 (<5.6%) | 22 (4.4%) | 0.06 | |
| Narcotics | 162 (42.5%) | 386 (42.3%) | 0.00 | 106 (54.1%) | 277 (54.9%) | 0.01 | |
| Oral steroids | No steroids w/in 90d | 192 (50.4%) | 471 (51.6%) | 0.01 | 99 (50.5%) | 267 (52.9%) | 0.02 |
| Steroids started ≤28 prior | 36 (9.4%) | 83 (9.1%) | 0.01 | 19 (9.7%) | 48 (9.5%) | 0.04 | |
| Steroids started >28d prior | 153 (40.2%) | 358 (39.3%) | 0.00 | 78 (39.8%) | 190 (37.6%) | 0.04 | |
| Oral mesalamine | 123 (32.3%) | 301 (33.0%) | 0.03 | 68 (34.7%) | 162 (32.1%) | 0.01 | |
| Rectal mesalamine | 17 (4.5%) | 32 (3.5%) | 0.01 | <11 (<5.6%) | 11 (2.2%) | 0.01 | |
| Rectal steroids | <11 (<2.9%) | 22 (2.4%) | 0.04 | <11 (<5.6%) | <11 (<2.2%) | 0.03 | |
| Number of non-CD medications | 0 | 63 (16.5%) | 178 (19.5%) | 0.07 | 36 (18.4%) | 83 (16.4%) | 0.08 |
| 1 | 82 (21.5%) | 204 (22.4%) | 0.01 | 36 (18.4%) | 104 (20.6%) | 0.03 | |
| 2 | 78 (20.5%) | 190 (20.8%) | 0.01 | 41 (20.9%) | 106 (21.0%) | 0.00 | |
| 3 | 67 (17.6%) | 124 (13.6%) | 0.11 | 28 (14.3%) | 87 (17.2%) | 0.09 | |
| ≥4 | 91 (23.9%) | 216 (23.7%) | 0.03 | 55 (28.1%) | 125 (24.8%) | 0.04 | |
| Schneeweiss comorbidity index | 0-1 | 230 (60.4%) | 555 (60.9%) | 0.00 | 124 (63.3%) | 328 (65.0%) | 0.06 |
| ≥2 | 151 (39.6%) | 357 (39.1%) | 0.00 | 72 (36.7%) | 177 (35.0%) | 0.06 | |
For cell sizes <11, actual numbers/percentages are not displayed in order to protect patient privacy in accordance with our data use agreement with Centers for Medicare and Medicaid Services (CMS).
Abbreviations: SMD, standardized mean difference; wt, weight
For all primary outcomes, patients in the study cohorts were followed for a median of 1.4-1.7 years. Among users of infliximab or adalimumab, combination therapy with immunomodulators did not alter the risk of surgery (combined anti-TNF adjusted hazard ratio [HR] 1.20, 95% CI 0.73-1.96), hospitalization (HR 0.82 [0.57-1.19]), or discontinuation of anti-TNF therapy or surgery (HR 1.09 [0.88-1.34]) (Table 2). Overall rates of dose escalation were 16% for infliximab users (11% in the first year) and 13% for adalimumab users (8.4% in the first year). There was also no difference in time to the composite outcome of discontinuation, surgery, or dose escalation between users of combination therapy and anti-TNF monotherapy (HR 1.09 [0.83-1.44]). Moreover, effectiveness did not seem to vary by past immunomodulator use, as tests for interaction between prior immunomodulator use and therapy group for the 3 primary effectiveness outcomes were not significant (p >0.09 for all outcomes).
Table 2. Rates and risk of surgery, hospitalization, or discontinuation of anti-TNF therapy or surgery in patients receiving anti-TNF combination therapy or monotherapy.
| Infliximab | Adalimumab | Combined Anti-TNF | |||||
|---|---|---|---|---|---|---|---|
| Outcome | Rate in Combination Therapy (E/100 PY) | Rate in Mono-therapy (E/100 PY) | Adjusted HR* (95% CI) | Rate in Combination Therapy (E/100 PY) | Rate in Mono-therapy (E/100 PY) | Adjusted HR* (95% CI) | Adjusted HR* (95% CI) |
| Surgery | |||||||
| With manual review | 6.1 | 3.9 | 1.40 (0.75-2.62) | 4.9 | 6.1 | 0.90 (0.39-2.04) | 1.20 (0.73-1.96) |
| Without manual review | 6.3 | 4.7 | 1.08 (0.60-1.94) | 4.9 | 6.5 | 0.78 (0.35-1.73) | 0.97 (0.60-1.55) |
| Hospitalization | |||||||
| CD as 1° diagnosis | 8.9 | 9.8 | 0.74 (0.46-1.20) | 11.2 | 14.4 | 0.91 (0.52-1.61) | 0.82 (0.57-1.19) |
| CD as 1° or 2° diagnosis | 13.2 | 13.1 | 0.85 (0.56-1.31) | 15.3 | 21.6 | 0.73 (0.45-1.19) | 0.81 (0.59-1.11) |
| CD or UC as 1° or 2° diagnosis | 13.8 | 13.5 | 0.89 (0.58-1.36) | 15.3 | 22.0 | 0.73 (0.45-1.19) | 0.83 (0.60-1.14) |
| Discontinuation or surgery | 53.1 | 55.9 | 1.22 (0.94-1.59) | 51.3 | 70.8 | 0.90 (0.65-1.26) | 1.09 (0.88-1.34) |
| Discontinuation, surgery, or dose escalation | 83.0 | 82.4 | 1.15 (0.83-1.58) | 87.0 | 83.3 | 0.97 (0.58-1.62) | 1.09 (0.83-1.44) |
Adjusted for prior immunomodulator use
Abbrevations: E, events; PY, person-years; HR, hazard ratio; CI, confidence interval
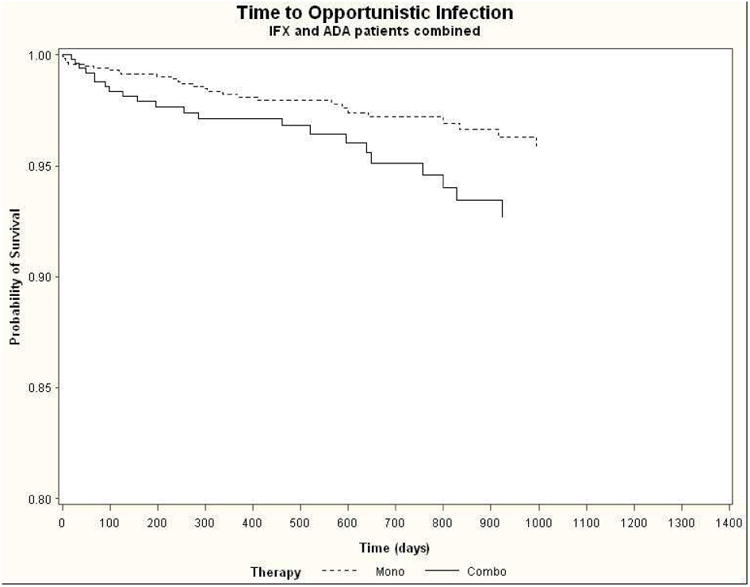
The rates of serious infection did not differ between patients treated with combination therapy or anti-TNF monotherapy (HR 0.93 [0.88-1.34]), with nearly identical results when adjusting for oral steroid use in a time-updating manner (Table 3). In contrast, the rates of non-Candida opportunistic infection overall and herpes zoster in particular were increased by more than 2.5-fold with combination therapy in the infliximab (HR 2.65 [1.05-6.66] and 3.38 [1.15-9.94], respectively) and combined anti-TNF (HR 2.64 [1.21-5.73] and 3.16 [1.25-7.97], respectively) cohorts. Kaplan-Meier survival curves for time to opportunistic infection for the combined anti-TNF cohorts are shown in Figure 2. Of the 50 cases of opportunistic infection among the anti-TNF cohorts, 34 (68%) were due to herpes zoster; there were no cases of tuberculosis.
Table 3. Rates and risk of serious or opportunistic infection in patients receiving anti-TNF combination therapy or monotherapy.
| Infliximab | Adalimumab | Combined Anti-TNF | |||||
|---|---|---|---|---|---|---|---|
| Outcome | Rate in Combination Therapy (E/100 PY) | Rate in Mono-therapy (E/100 PY) | Adjusted HR* (95% CI) | Rate in Combination Therapy (E/100 PY) | Rate in Mono-therapy (E/100 PY) | Adjusted HR* (95% CI) | Adjusted HR* (95% CI) |
| Serious infection | |||||||
| Without time-varying steroid adjustment | 6.8 | 8.0 | 0.80 (0.48-1.34) | 9.0 | 7.4 | 1.22 (0.61-2.45) | 0.93 (0.88-1.34) |
| With time-varying steroid adjustment | 6.8 | 8.0 | 0.80 (0.48-1.34) | 9.0 | 7.4 | 1.14 (0.57-2.30) | 0.91 (0.60-1.38) |
| Opportunistic infection | |||||||
| Without time-varying steroid adjustment | 2.7 | 1.6 | 2.65 (1.05-6.66) | 2.6 | 1.3 | 2.61 (0.63-10.8) | 2.64 (1.21-5.73) |
| With time-varying steroid adjustment | 2.7 | 1.6 | 2.60 (1.03-6.58) | 2.6 | 1.3 | 2.19 (0.54-8.89) | 2.51 (1.15-5.46) |
| Herpes zoster | 2.2 | 1.0 | 3.38 (1.15-9.94) | 1.8 | 0.7 | 2.64 (0.47-15.0) | 3.16 (1.25-7.97) |
Adjusted for prior immunomodulator use
Abbrevations: E, events; PY, person-years; HR, hazard ratio; CI, confidence interval
Figure 2. Kaplan-Meier survival curves for time to opportunistic infection for combined infliximab and adalimumab cohorts.
None of the results of the primary or secondary analyses changed appreciably when follow-up was censored at 60 days after last dispensing of the anti-TNF agent or when patients changed from combination therapy to anti-TNF monotherapy or vice versa, except the risk of overall opportunistic infection and herpes zoster in the infliximab and combined anti-TNF cohorts which increased even further with combination therapy (Supplementary Table 1). There were also no appreciable changes when the definition of start of follow-up was changed to date of first prescription for anti-TNF therapy (Supplementary Table 2) or when excluding patients who initiated immunomodulators in the period 89 days before to any time after initiating anti-TNF therapy (Supplementary Table 3). When restricting the analyses to the 824 patients with at least 2 years of data prior to first prescription for an anti-TNF agent, there was no longer an increased risk of opportunistic infection or herpes zoster with combination therapy in the infliximab cohort, and combination therapy was associated with a decreased risk of hospitalization in the adalimumab cohort (Supplementary Table 4); however, given the small number of events in this restriction analysis, we urge caution when interpreting these results.
Because our cohort was relatively old, we tested for interaction by age (<65 vs. ≥65 years) for all primary analyses and found no evidence of interaction for any of the outcomes (p >0.05 for all outcomes). For the post-hoc analysis of impact of thiopurine dose or use of methotrexate, we found that the risk of all outcomes, compared to anti-TNF monotherapy, was comparable among users of low-dose thiopurines, high-dose thiopurines, and methotrexate, given mostly overlapping 95% CIs (Supplementary Table 5); however, given the small number of events in each of these substrata, we urge caution when interpreting these results.
Discussion
In this large cohort of patients with CD, in which combination therapy occurred predominantly in the setting of “step-up” therapy after thiopurines, we found that combination therapy did not increase the frequency of beneficial outcomes or serious infection but was associated with an increased risk of non-Candida opportunistic infections, including herpes zoster. Our results were robust to changes in definitions of outcomes and follow-up time and also did not differ by age (<65 vs. ≥65 years). Propensity score matching succeeded in balancing the therapy groups with respect to multiple potential confounders.
In contrast to the indisputable superiority of de novo combination therapy with infliximab and azathioprine over monotherapy in patients with CD naïve to both therapies1 (“top-down” therapy), it is unclear whether immunomodulators should be continued when “stepping-up” to anti-TNF therapy.2-5 While immunomodulators may inhibit the formation of anti-TNF drug antibodies, even in the setting of scheduled anti-TNF therapy, it appears that the amount of anti-TNF drug given may be more important.3 Whether dosing anti-TNF drugs by level can obviate the need for concomitant immunomodulators in this setting (albeit at potentially greater cost) is unknown. It is possible that over time (likely >2 years2), incremental increases in anti-TNF drug levels and/or decreases in anti-TNF drug antibody levels affected by continuing immunomodulator therapy may translate into increased clinical efficacy.
However, the risks of such a strategy must be considered. Thiopurines increase the risk for infections, especially viral, such as herpes zoster (3-fold increased risk)15 and Epstein-Barr virus,26 which have the possible long-term consequences of post-herpetic neuralgia and lymphoma, respectively. Thiopurine use is also associated with other malignancies, including non-melanoma skin cancer (2-6-fold increased risk)13 and potentially overall cancers (1.4-1.8-fold increased risk).27,28 These risks may be further increased with combination therapy.14,17 Thus, to justify continuing thiopurines when “stepping-up” to anti-TNF therapy, the benefits must outweigh the risks, and currently there are no prospective randomized data to guide us in this regard. An alternative strategy, other than discontinuation of thiopurines, is to consider using methotrexate instead of thiopurines to help increase anti-TNF drug levels and decrease anti-TNF drug antibody levels, as methotrexate has been shown to be as effective as thiopurines in these capacities.6 Pooled analyses of anti-TNF use, often in combination with methotrexate, in various autoimmune diseases have demonstrated no increased risk of malignancies, other than a 2-fold increased risk of non-melanoma skin cancer.29 Additionally, although unknown for CD, low-dose oral methotrexate may be sufficient to increase anti-TNF drugs levels and suppress antibodies, given that this is the way that methotrexate is used for many patients receiving combination therapy for rheumatoid arthritis.
An important issue implicit in our results is the need for vaccination in the immunosuppressed. Both thiopurines and anti-TNF agents have been shown to increase the risk of herpes zoster infection.15,16 Thus, we were not surprised to find that patients receiving combination infliximab and immunomodulators had an increased risk of herpes zoster compared to those receiving infliximab alone. Vaccination against this and other organisms should be considered, ideally prior to commencement with immunosuppressive therapy.30
Our study has several notable strengths. By using 3 different clinically relevant metrics of effectiveness and 2 metrics of safety, we were able to rigorously compare anti-TNF combination therapy and monotherapy. With respect to opportunistic infections, we excluded infections by Candida species and focused instead on more virulent organisms, which we felt were more clinically meaningful. Additionally, our sample sizes were relatively large, which enabled us to detect relatively small differences in effect.
Our study also has several limitations. As with all observational studies, there is potential for residual confounding from unmeasured variables. However, we included a large number of covariates in our propensity score and the matching produced excellent balance with respect to all covariates. Nondifferential misclassification of exposure or outcome is also possible in any large administrative dataset. However, with respect to exposure, we limited our study to patients with Part D prescription benefits and excluded those with a hospitalization within 8 weeks of starting anti-TNF therapy in order to capture all anti-TNF prescriptions. Misclassification of immunomodulator use is possible if patients filled a prescription but did not take the medication. We required patients to have filled at least 1 prescription in the 120 days following initiation of anti-TNF therapy to be considered exposed. Most of these patients had filled a prescription prior to starting anti-TNF therapy, so it is unlikely that they would fill another prescription if they were not taking the medication. With respect to outcomes, the effectiveness and safety outcomes we selected have all been used in multiple prior studies in Medicare.18-21 Missing data is also a possibility but not captured in Medicare in that if an event or diagnosis is not recorded, it may have not occurred or be missing. Although our study was conducted among Medicare beneficiaries, a substantial proportion of our study population was <65 years old and received Medicare benefits because of disability. As we found no significant interaction by age for any of the outcomes, we suspect that our results may be generalizable to the broader population of patients with CD. Lastly, we studied predominantly “step-up” combination therapy. Similar studies in populations treated with de novo combination therapy are needed to determine whether our results are generalizable to that population.
In conclusion, in our cohort of patients with CD, in which combination therapy was largely “step-up” therapy after thiopurines, we found that combination therapy did not improve outcomes but was associated with an increased risk of opportunistic infection. These data highlight the need for large comparative effectiveness trials of alternative treatment strategies. Until such trials are completed, for patients “stepping-up” from thiopurine to anti-TNF therapy, consideration should be given for discontinuation of the thiopurine or changing to another immunomodulator, such as methotrexate, once remission is achieved with anti-TNF therapy. Our results also highlight the importance of herpes zoster vaccination and potentially other vaccinations prior to initiation of immunosuppressive, and especially combination, therapy.
Supplementary Material
Supplementary Table 1. Primary and secondary analyses with follow-up censored after discontinuation of anti-TNF therapy or also after change from combination to monotherapy or vice versa.
Supplementary Table 2. Primary analyses with change in start of follow-up to date of first prescription for anti-TNF therapy.
Supplementary Table 3. Primary analyses excluding patients who initiated immunomodulators in the period 89 days before to any time after initiating anti-TNF therapy.
Supplementary Table 4. Primary analyses restricted to patients with at least 2 years of data prior to first prescription for anti-TNF therapy.
Supplementary Table 5. Primary analyses stratified by type/dose of immunomodulator.
Acknowledgments
Dr. Osterman has served on advisory boards for Janssen, Abbott, and UCB. He has received research funding from UCB.
Dr. Haynes has received research support from AstraZeneca/BristolMeyersSquibb.
Dr. Delzell has received research support from Amgen.
Dr. Zhang has received research support from Genentech and Amgen.
Dr. Bewtra has received research support from Janssen.
Dr. Curtis has received honoraria for consulting from Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo and AbbVie. He has received research support from Research: Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, AbbVie.
Dr. Lewis has served as a consultant to Takeda, Rebiotix, Amgen, Millennium Pharmaceuticals, Prometheus, Lilly, Shire, AstraZeneca, Janssen Pharmaceuticals, Merck, MedImmune, and AbbVie. He has served on a Data and Safety Monitoring Board for clinical trials sponsored by Pfizer. He has received research support from Bayer, Shire, Centocor, Nestle, and Takeda.
Financial support: The work was supported with funding from grants from AHRQ R01-HS018517 and the NIHK24-DK078228, K08 DK084347.
Footnotes
| Study concept and design | Acquisition of data | Analysis and interpretation of data | Drafting of the manuscript | Critical revision of the manuscript for important intellectual content | Statistical analysis | Obtained funding | |
| Osterman MT | X | X | X | X | |||
| Haynes K | X | X | X | ||||
| Delzell E | X | X | X | ||||
| Zhang J | X | X | X | ||||
| Bewtra M | X | X | X | ||||
| Brensinger C | X | X | X | X | |||
| Chen L | X | X | X | X | |||
| Xie F | X | X | X | X | |||
| Curtis JR | X | X | X | X | X | X | |
| Lewis JD | X | X | X | X |
Conflicts of interest: The following authors report no potential conflict of interest: Dr. Chen, Ms. Brensinger, Mr. Xie.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 2.Van Assche G, Magdelaine-Beuzelin M, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861–8. doi: 10.1053/j.gastro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210–26. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 4.Sokol H, Seksik P, Carrat F, et al. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2011;59:1363–8. doi: 10.1136/gut.2010.212712. [DOI] [PubMed] [Google Scholar]
- 5.Reenaers C, Louis E, Belaiche J, et al. Does co-treatment with immunosuppressors improve outcome in patients with Crohn's disease treated with adalimumab? Aliment Pharmacol Ther. 2012;36:1040–8. doi: 10.1111/apt.12076. [DOI] [PubMed] [Google Scholar]
- 6.Vermeire S, Noman M, Van Assche, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007;56:1226–31. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis. 2013;7:736–43. doi: 10.1016/j.crohns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 9.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 10.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:542–53. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 11.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248–54. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–5. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–8. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 14.Osterman MT, Sandborn WJ, Colombel JF, et al. Increased risk of malignancy with adalimumab combination therapy, compared to monotherapy, in Crohn's disease. Gastroenterology. 2014;146:941–9. doi: 10.1053/j.gastro.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–90. doi: 10.1016/j.cgh.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Osterman MT, Lichtenstein GR. Current and future anti-TNF therapy for inflammatory bowel disease. Curr Treat Options Gastroenterol. 2007;10:195–207. doi: 10.1007/s11938-007-0013-3. [DOI] [PubMed] [Google Scholar]
- 17.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn's disease. Clin Gastroenterol Hepatol. 2014;12:811–7. doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune disease. JAMA. 2011;306:2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beukelman T, Xie F, Baddley JW, et al. Incidence of selected opportunistic infections among children with juvenile idiopathic arthritis. Arthritis Rheum. 2013;65:1384–9. doi: 10.1002/art.37866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baddley JW, Winthrop KL, Chen L, et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety assessment of Biologic thERapy (SABER) study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203407. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Wang PS, Avorn J, et al. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38:1103–20. doi: 10.1111/1475-6773.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awni WM, Eckert D, Sharma S, et al. Pharmacokinetics of adalimumab in adult patients with moderately to severely active ulcerative colitis. Gastroenterology. 2013;144(Suppl 1):S–229. [Google Scholar]
- 24.Hanauer SB, Feagan G, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 25.Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol. 2011;106:674–84. doi: 10.1038/ajg.2011.60. [DOI] [PubMed] [Google Scholar]
- 26.Linton MS, Kroeker K, Fedorak D, et al. Prevalence of Epstein-Barr virus in a population of patients with inflammatory bowel disease: a prospective cohort study. Aliment Pharmacol Ther. 2013;38:1248–54. doi: 10.1111/apt.12503. [DOI] [PubMed] [Google Scholar]
- 27.Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn's disease who respond to azathioprine. Clin Gastroenterol Hepatol. 2013;11:389–94. doi: 10.1016/j.cgh.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Pasternak B, Svanstrom H, Schmiegelow K, et al. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177:1296–305. doi: 10.1093/aje/kws375. [DOI] [PubMed] [Google Scholar]
- 29.Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119–30. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 30.Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis. 2009;15:1410–6. doi: 10.1002/ibd.20943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Primary and secondary analyses with follow-up censored after discontinuation of anti-TNF therapy or also after change from combination to monotherapy or vice versa.
Supplementary Table 2. Primary analyses with change in start of follow-up to date of first prescription for anti-TNF therapy.
Supplementary Table 3. Primary analyses excluding patients who initiated immunomodulators in the period 89 days before to any time after initiating anti-TNF therapy.
Supplementary Table 4. Primary analyses restricted to patients with at least 2 years of data prior to first prescription for anti-TNF therapy.
Supplementary Table 5. Primary analyses stratified by type/dose of immunomodulator.


