Abstract
Pneumococcal infections are a major cause of morbidity and mortality in developing countries. The introduction of pneumococcal conjugate vaccines (PCVs) has dramatically reduced the incidence of pneumococcal diseases. PCVs are not currently being used in Algeria. We conducted a prospective study from 2005 to 2012 in Algeria to determine antimicrobial drug resistance and serotype distribution of Streptococcus pneumoniae from children with pneumococcal disease. Among 270 isolated strains from children, 97 (36%) were invasive disease; of these, 48% were not susceptible to penicillin and 53% not susceptible to erythromycin. A high rate of antimicrobial nonsusceptibility was observed in strains isolated from children with meningitis. The serotype distribution from pneumococci isolated from children with invasive infections was (by order of prevalence): 14, 1, 19F, 19A, 6B, 5, 3, 6A and 23F. Multidrug resistance was observed in serotypes 14, 19F, 19A and 6B. The vaccine coverage of serotypes isolated from children aged <5 years was 55.3% for PCV7, 71.1% for PCV10 and 86.8% for PCV13. Our results highlight the burden of pneumococcal disease in Algeria and the increasing S. pneumoniae antibiotic resistance. The current pneumococcal vaccines cover a high percentage of the circulating strains. Therefore, vaccination would reduce the incidence of pneumococcal disease in Algeria.
Keywords: Algeria, antibiotics, burden of disease, children, pneumococcal conjugate vaccines, pneumococcal diseases, serotype distribution, Streptococcus pneumoniae
Introduction
Streptococcus pneumoniae is a leading respiratory pathogen that is responsible for infections such as pneumonia, meningitis, bacteremia and otitis media. In 2003, the World Health Organization (WHO) estimated that pneumococcal disease is responsible for 1 million deaths annually, most of which occur in children <5 years of age in the developing world [1]. Although pneumococcal meningitis is relatively rare, it is strongly associated with mortality or subsequent neurologic damage [2].
Pneumococcal resistance to penicillin was first described in 1967, and since the 1990s an increasing rate of resistance has been reported worldwide [3,4]. This resistance makes the treatment of serious pneumococcal infections difficult, and many antibiotic treatment failures have been reported [5].
In Algeria, several reports have shown an increase in antibiotic resistance from 1996 to 2010, especially among children [6–9]. Although epidemiologic surveillance data for invasive pneumococcal infections are available, clinical data are lacking. Moreover, since the introduction of Haemophilus influenzae type b vaccination in 2008 in Algeria, S. pneumoniae has become the predominant pathogen in bacterial meningitis (unpublished data).
The S. pneumoniae polysaccharide capsule is a major virulence factor; more than 90 serotypes have been identified, and their distribution differs in different regions and between developing and developed countries [10]. This highlights the importance of having national data before implementation of a pneumococcal vaccine. The aim of the study was to investigate the evolution of antibiotic resistance and serotype distribution of S. pneumoniae in infections in children in Algeria.
Material and methods
From January 2005 to June 2012, a total of 270 unique S. pneumoniae isolates were collected from children aged 0 to 16 years with invasive and noninvasive infections; about 89% were from Algiers and 11% from Oran, a town located in the western part of the country. The hospital clinical laboratories were asked to send all their viable isolates. Every year, the centers isolate between 15 and 50 S. pneumoniae strains from children who are diagnosed by physicians with invasive pneumococcal disease (IPD) and non-IPD (NIPD). S. pneumoniae isolates were identified by colony morphology, Gram staining, catalase reaction, optochin susceptibility and bile lysis. Antibiotic susceptibilities for oxacillin, erythromycin, clindamycin, tetracyclin, chloramphenicol and cotrimoxazole were determined following the Clinical and Laboratory Standards Institute (CLSI) recommendations [11,12]. Minimum inhibitory concentrations (MICs) for penicillin, amoxicillin and cefotaxim were determined using the E-test following the manufacturer's instructions (Solna, Sweden) for all strains. The S. pneumoniae ATCC 49619 strain was used for quality control.
Serotyping was performed by latex agglutination for determining pools, and serotypes were identified using the Neufeld test (Pneumo test latex; Statens Serum Institute, Copenhagen, Denmark). A total of 127 isolates were serotyped, 85 from invasive samples and 42 from noninvasive samples. The isolates that were serotyped were selected on the basis of the clinical data, with priority given to IPD and the number of viable isolates. Isolates were stored in 10% glycerol broth at −80°C after primary isolation.
Results
From the 270 isolates collected (IPD n = 97, NIPD n = 173), 197 (73.0%) were from children <5 years, of whom 151 (76.7%) were <2 years. Among the isolates from children with IPD, 78.4% were collected from children <5 years of age, of whom 76.3% were <2 years old. The IPD isolates were collected from children with meningitis (n = 53), pneumonia and pleuropneumonia (n = 25), bacteremia (n = 11), arthritis or peritonitis infections (n = 8). The non-IPD isolates were from ear, nose and throat infections (n = 91), bronchopulmonary infections (n = 77) and other suppurative infections (n = 5). Among the isolates from children with NIPD, 69.9% were from children <5 years of age; 53.8% were <2 years old (Table 1).
Table 1.
Number of Streptococcus pneumoniae isolates by sample origin and patient age
| Sample | Patient age |
Total | ||
|---|---|---|---|---|
| <2 years | 2–5 years | >5–16 years | ||
| Invasive samples | ||||
| CSF | 30 | 10 | 13 | 53 |
| Blood culture1 | 15 | 4 | 2 | 21 |
| Puncture fluids2 | 13 | 4 | 6 | 23 |
| Total | 58 | 18 | 21 | 97 |
| Noninvasive samples | ||||
| Lower respiratory tract samples | 57 | 8 | 12 | 77 |
| Auricular swabs | 31 | 7 | 9 | 47 |
| Sinus and nasal aspirates | 5 | 10 | 29 | 44 |
| Other samples3 | 0 | 3 | 2 | 5 |
| Total | 93 | 28 | 52 | 173 |
| Overall total | 151 | 46 | 73 | 270 |
Pneumonia (n = 10), bacteremia (n = 11).
Pleural (n = 15), joint (n = 5), peritoneal (n = 3).
Conjunctive (n = 4), genital sample (n = 1).
Nonsusceptibility to penicillin was detected in 48% of the S. pneumoniae isolates (MICs ranged from 0.016 μg/mL to 4 μg/mL); 2.6% of isolates had intermediate resistance to amoxicillin; 7% and 1.7% of isolates had intermediate and full resistance to cefotaxime, respectively. For the IPD isolates, the MIC90s were 2 μg/mL for penicillin and amoxicillin and 1.5 μg/mL for cefotaxime. The highest rate of cefotaxim resistance was observed in isolates from meningitis: 20.8% intermediate and 3.8% resistant (Table 2).
Table 2.
MICs for penicillin, amoxicillin and cefotaxime in isolates from IPD and NIPD
| Disease | Penicillin |
Amoxicillin |
Cefotaxime |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I (%) | R (%) | MIC50 (μg/mL) | MIC90 (μg/mL) | I (%) | R (%) | MIC50 (μg/mL) | MIC90 (μg/mL) | I (%) | R (%) | MIC50 (μg/mL) | MIC90 (μg/mL) | |
| Meningitis (n = 53) | 49 | 0.3 | 2 | 20.8 | 1.5 | 3.8 | 0.4 | |||||
| Other IPD (n = 44) | 20.4 | 27.2 | 0.5 | 2 | 6.8 | 2.3 | 0.5 | 2 | 4.5 | 0 | 0.5 | 1.5 |
| Total IPD (n = 97) | 10 | 39.2 | 0.38 | 2 | 3.1 | 1 | 0.5 | 2 | 13.4 | 2 | 0.5 | 1.5 |
| Otitis (n = 47) | 25.5 | 10.6 | 0.8 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 0.5 | 1 |
| LRTI (n = 77) | 34.6 | 32.7 | 1 | 2 | 4.1 | 0 | 1.5 | 4 | 0 | 2 | 0.5 | 2 |
| Other NIPD (n = 49) | 34.2 | 2.5 | 0.2 | 1.5 | 2.5 | 0 | 0.3 | 2 | 2.5 | 0 | 0.2 | 1.5 |
| Total NIPD (n = 173) | 31.4 | 16 | 0.5 | 2 | 2.2 | 0 | 0.8 | 4 | 0.8 | 0.8 | 0.5 | 1.5 |
Global MICs were as follows: penicillin, I = 20.6%, R = 27.5%; amoxicillin, I = 2.56%, R = 0.42%; cefotaxime, I = 7%, R = 1.68%.
MIC, minimum inhibitory concentration; IPD, invasive pneumococcal disease; I, intermediate resistance; R, resistant; LRTI, lower respiratory tract infection; NIPD, noninvasive pneumococcal disease.
The percentages of isolates that were resistant to non-β-lactam antibiotics were 53.0% for erythromycin and cotrimoxazole; 43.7% for clindamycin; 42.0% for tetracycline; and 5.3% for chloramphenicol. According to the new breakpoints suggested by CLSI, whereby meningitis and nonmeningitis isolates have different breakpoints, 49.0% of the meningitis isolates were resistant to penicillin (MIC ≥0.12 μg/mL), among which 26.9% had a MIC of ≥2 μg/mL.
After meningitis isolates, the next highest rate of penicillin-nonsusceptible S. pneumoniae (PNSP) was observed in the isolates from NIPD. From all the invasive isolates (n = 97), 40.3% were PNSP, with 81.5% of these from children <5 years and 72.7% from children <2 years.
Among the 85 IPD samples that were serotyped, meningitis was the most common diagnosis, at 50 (58.8%), followed by pneumonia and pleuropneumonia, at 21 (24.7%), and bacteremia, at 9 (10.6%) (Table 3). The prevalence of IPD in children aged <5 years and <2 years was 78.3% (n = 76) and 59.8% (n = 58), respectively (Table 1). The most frequent serotypes for invasive isolates were 14 (29.4%), 1 (10.6%), 19F (10.6%), 19A (7%), 6B (7%), 5 (4.7%), 3 (4.7%), 6A (3.5%) and 23F (3.5%). Serotype 14 was the most prevalent in meningitis (34%) and pleuropneumonia (28.6%), while serotypes 19F (38%) and 14 (23.8%) were the most frequent in noninvasive samples (Table 3, Fig. I). In order of prevalence, serotypes 14, 19F, 6B, 19A, 1, 5, 23F, 6A, 3, 7F and 18C accounted for 91.0% of IPD in children <5 years old. Serotypes 5, 7F, 6B, 18C and 19A were found exclusively in children <2 years of age.
Table 3.
Serotype distribution among isolates from children with IPD and NIPD
| Serotype | Age <5 years |
Age 5–16 years |
Overall total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPD (n = 67) |
NIPD (n = 35) |
IPD (n = 18) |
NIPD (n = 7) |
||||||||||||
| Bacteremia | Meningitis | Pleuropneumonia | Other IPD | Total IPD for age <5 years | Otitis | LRTI | Total for age <5 years | Meningitis | Pleuropneumonia | Bone and joint infection | Otitis | LRTI | Total for age 5–16 years | ||
| 14 | 2 | 15 | 6 | 23 | 5 | 3 | 31 | 2 | 1 | 1 | 4 | 35 | |||
| 19F | 2 | 3 | 2 | 7 | 10 | 6 | 23 | 2 | 2 | 25 | |||||
| 6B | 1 | 3 | 2 | 6 | 2 | 8 | 0 | 8 | |||||||
| 23F | 1 | 0 | 1 | 2 | 2 | 4 | 1 | 1 | 5 | ||||||
| 18C | 1 | 1 | 1 | 2 | 0 | 2 | |||||||||
| 4 | 0 | 1 | 1 | 1 | |||||||||||
| 1 | 1 | 4 | 1 | 6 | 6 | 1 | 2 | 3 | 9 | ||||||
| 5 | 2 | 2 | 4 | 4 | 0 | 4 | |||||||||
| 7F | 1 | 1 | 2 | 2 | 0 | 2 | |||||||||
| 3 | 2 | 2 | 1 | 3 | 2 | 1 | 3 | 6 | |||||||
| 6A | 1 | 1 | 2 | 2 | 1 | 1 | 3 | ||||||||
| 19A | 2 | 2 | 2 | 6 | 4 | 10 | 0 | 10 | |||||||
| 35B | 1 | 1 | 1 | 2 | 2 | 3 | |||||||||
| 9A | 1 | 1 | 1 | 1 | 1 | 2 | |||||||||
| 16F | 0 | 1 | 1 | 2 | 2 | ||||||||||
| 23B | 1 | 1 | 1 | 1 | 2 | ||||||||||
| 6C | 1 | 1 | 1 | 0 | 1 | ||||||||||
| 8 | 0 | 1 | 1 | 1 | |||||||||||
| 12 | 1 | 1 | 1 | 0 | 1 | ||||||||||
| 17F | 0 | 1 | 1 | 1 | |||||||||||
| 23A | 0 | 1 | 1 | 1 | |||||||||||
| 24A | 0 | 1 | 1 | 1 | |||||||||||
| 28F | 1 | 1 | 1 | 0 | 1 | ||||||||||
| 35F | 1 | 1 | 1 | 0 | 1 | ||||||||||
| Total | 9 | 37 | 17 | 4 | 67 | 26 | 9 | 102 | 13 | 4 | 1 | 5 | 2 | 25 | 127 |
IPD, invasive pneumococcal disease; NIPD, noninvasive pneumococcal disease.
Fig. 1.
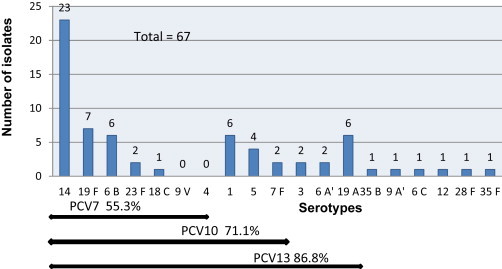
Serotype prevalence and pneumococcal vaccine coverage of serotypes isolated from children ≤5 years with invasive pneumococcal disease. PCV, pneumococcal conjugate vaccine.
The serotypes that were most often antibiotic resistant were 14, 19F, 19A and 6B; they were mainly highly resistant to penicillin, and only serotypes 14 and 19A were resistance to cefotaxime (Fig. 2, Table 4).
Fig. 2.
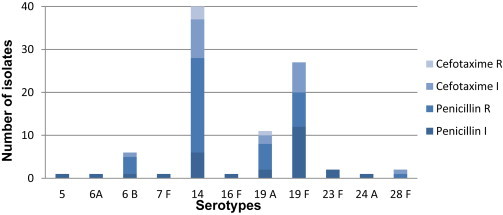
Resistance to penicillin and cefotaxime of Streptococcus pneumoniae serotypes isolated from children ≤5 years. I, intermediate resistance; R, resistant.
Table 4.
Resistance profiles by isolated S. pneumoniae serotypes
| Antimicrobial | 14 | 19F | 6B | 23F | 5 | 7F | 6A | 19A | 16F | 24A | 28F | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| oxa | 2 | 2 | 4 | |||||||||
| oxa, sxt | 10 | 1 | 11 | |||||||||
| oxa, sxt, tet | 1 | 1 | ||||||||||
| oxa, ery, clin | 4 | 4 | ||||||||||
| oxa, ery, clin, sxt | 4 | 6 | 1 | 1 | 1 | 13 | ||||||
| oxa, ery, clin, tet | 1 | 7 | 2 | 1 | 11 | |||||||
| oxa, ery, sxt, tet | 1 | 1 | 1 | 3 | ||||||||
| oxa, ery, clin, sxt, tet | 5 | 5 | 3 | 1 | 6 | 1 | 21 | |||||
| oxa, ery, clin, chl, sxt, tet | 1 | 1 | ||||||||||
| Total | 28 | 20 | 5 | 2 | 1 | 1 | 1 | 8 | 1 | 1 | 1 | 69 |
oxa = oxacillin; sxt = cotrimoxazole; tet = tetracyclin; ery = erythromycin; clin = clindamycin; chl = chloramphenicol.
Discussion
The prevalence of penicillin resistance among pneumococcal disease isolates in Algeria, which was measured by the oxacillin disc diffusion method, appears to have increased over the years, from 34.6% in 1995–2000 to 48.1% in 2005–2012 (current study) [6–9]. The percentage of PNSP among IPD isolates in children was reported to be approximately 11% in Western European countries, with the highest percentage (49%) in Spain [1].
Our results are more consistent with the increasing antibiotic resistance rates reported for North African countries. For instance, in Tunisia and Morocco, the rates of PNSP were, respectively, 52.8% and 43.3% [6–9,13–15]. The dramatic increase in the antibiotic resistance rate since our previous study (PNSP of 48.1% in this study compared with 34.6% in 2001) could be explained by overuse of antibiotics for acute respiratory tract infections in Algeria [8].
We also observed an evolution in the serotype distribution in Algeria. Although the distribution is similar to that reported for Morocco and Tunisia, as well as other developing countries, it differs from that reported in our previous study, where we found that serotypes/serogroups 1, 5, 14 and 6 were the most frequent [8,9,15,16].
Some pneumococcal serotypes, such as serotypes 1, 5 and 7F, have more invasive potential than others; serotype 7F has been reported to be associated with a higher risk of severe and fatal outcomes [17]. In Africa, serotypes 1 and 5 are commonly associated with invasive diseases. In Algeria, the frequency of serotype 1 was low during the 2002–2004 and 2005–2007 periods but increased in 2008–2009 (unpublished data). Similar observations of cyclical peaks in serotype 1 incidence have been reported in many countries. Although serotypes 1 and 5 are designated as ‘developing country serotypes,’ they have also been reported to be frequently responsible for pediatric IPD in industrialized countries such as Germany, Sweden and England [18,19]. Serotype 1 has remained one of the most prevalent invasive serotypes and is usually associated with meningitis outbreaks in Africa and in crowded communities [16,20]. It is ranked as one of the four serotypes with the highest IPD burden in Africa, Asia and Latin American, while serotype 5 is ranked third in Africa and Latin America and fourth in Asia [10]. In Thailand, the seven most frequent serotypes associated with IPD in patients <5 years old were 6B, 23F, 14, 19F, 19A, 6A, and 4 or 9V [21].
Two studies in Oxford and Stockholm that characterized the genotypes and serotypes of nasopharyngeal and invasive pneumococcal isolates from children and adults identified serotypes 1, 4 and 7F as having a high level of invasiveness, and serotypes 3 and 7F as having a higher case fatality rate compared with other serotypes [22].
The predominant serotypes of PNSP in our study are similar to those reported in other studies and previous studies in Algeria, with the emergence of serotype 19A in the absence of routine pneumococcal vaccination in our country [2,8,9,14,15]. The most commonly multidrug-resistant serotypes among the PNSP were 19A, 19F, 14 and 6B, which were resistant to erythromycin, cotrimoxazole, clindamycin and tetracycline.
Serotype 14 is one of the most invasive serotypes that can cause life-threatening IPDs. Moreover, it can harbour multiple resistance determinants, conferring resistance to penicillin, erythromycin and cefotaxime [23]. In many countries the multidrug-resistant serotype 19A has become the most predominant nonvaccine serotype isolated after PCV7 vaccination [24].
On the basis of the serotype distribution observed in our study, the PCV7 coverage in children <5 years of age with IPD in Algeria is 55.3%, the PCV10 coverage is 71.1% and the PCV13 coverage is 86.8%. This vaccine coverage among children <2 years is 51.7% for PCV7, 69% for PCV10 and 87.9% for PCV13. These data may not reflect the situation in all cases of IPD in the studied regions because many children with IPD are treated empirically. In addition, the limitations of existing diagnostic tests affect the ability to obtain accurate IPD burden data: only 10% of blood culture results are positive, so most patients with pneumonia are not bacteriemic [25]. In Algeria, the lack of laboratory facilities, including no facilities for storing samples frozen at −80°C in different regions of the country, is another limiting factor for conducting a multicenter study in order to obtain accurate IPD burden data. Despite limited financial resources dedicated to IPD surveillance in Algeria, we have conducted a nasopharyngeal carriage in healthy children and the most prevalent serogroups were 6, 14 and 19 (23rd European Congress of Clinical Microbiology and Infectious Diseases, abstract R2717).
The pneumococcal conjugate vaccines (PCVs) currently available have been shown to protect children. The first licensed 7-valent vaccine, which was widely used, resulted in dramatic reductions in pneumococcal disease mortality and morbidity. The emergence of the multidrug-resistant serotype 19A and the absence of the so-called developing country serotypes, 1 and 5, has led manufacturers to develop higher valency PCVs, such as PCV9, PCV10, PCV11 and PCV13 [26]. The WHO recommends the inclusion of PCVs in childhood immunization programs worldwide, especially in countries with childhood mortality that exceeds 50 deaths per 1000 births [27]. Although there are some limitations with our study, the results show that the burden of IPD is high in our country. The good vaccine serotype coverage suggests that the introduction of childhood vaccination with PCVs could be expected to have a dramatic effect on the burden of IPD in our country.
Continuous national monitoring of IPD and nasopharyngeal carriage as well as judicious use of antibiotics are crucial before and after PCV implementation in order to evaluate the effect of vaccination and to prevent the selection of multidrug-resistant clones.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank the following people for sending strains and for technical support: Mohamed Bachtarzi, Fazia Djennane (Centre Hospitalier Universitaire Mustapha Bacha, Algiers, Algeria); Souad Zouagui (Centre Hospitalier Universitaire d’Oran, Oran, Algeria); Sadjia Mahrane, Radia Boushaki (Centre Hospitalier Universitaire Nafissa Hamoud, Algiers, Algeria); Dalila Haouchine (Centre Hospitalier Universitaire Nedir Mohamed, Tizi Ouzou, Algeria); Azzoug Saloua (Centre Hospitalier Universitaire Ibrahim Hadjrass, Algiers, Algeria) Nadjet Aggoune, Fatma Zohra Henniche (Hôpital Central de l’Armée Mohamed, Algiers, Algeria); Karima Lassas (Hôpital de Boufarik, Blida, Algeria); Farida Sahli (Centre Hospitalier Universitaire Saadna Abdennour, Sétif, Algeria); Farah Lalaoui (Hôpital Fares Yahia, Tipaza, Algeria); Radia Abiayad, Ilhem Boubekri (Etalissement Hospitalier Universitaire 1er Novembre, Oran, Algeria).
The authors would like to thank Pfizer for funding the sera used for serotyping, and the French National Reference Center for Pneumococci (CNR pneumo) for performing confirmatory serotyping. The authors acknowledge editorial assistance from Margaret Haugh, MediCom Consult, funded by Pfizer.
References
- 1.O’Brien K.L., Wolfson L.J., Watt J.P., Henkle E., Deloria-Knoll M., McCall N. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Koedel U., Scheld W.M., Pfister H.W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2:721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 3.Hansman D., Bullen M.M. A resistant pneumococcus. Lancet. 1967;290:264–265. doi: 10.1016/s0140-6736(75)91547-0. [DOI] [PubMed] [Google Scholar]
- 4.Lynch J.P., 3rd, Zhanel G.G. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010;16:217–225. doi: 10.1097/MCP.0b013e3283385653. [DOI] [PubMed] [Google Scholar]
- 5.Klein M., Pfister H.W., Leib S.L., Koedel U. Therapy of community-acquired acute bacterial meningitis: the clock is running. Expert Opin Pharmacother. 2009;10:2609–2623. doi: 10.1517/14656560903277210. [DOI] [PubMed] [Google Scholar]
- 6.Benouda A., Ben Redjeb S., Hammami A., Sibille S., Tazir M., Ramdani-Bouguessa N. Antimicrobial resistance of respiratory pathogens in North African countries. J Chemother. 2009;21:627–632. doi: 10.1179/joc.2009.21.6.627. [DOI] [PubMed] [Google Scholar]
- 7.Borg M.A., Cookson B.D., Zarb P., Scicluna E.A., ARMed Steering Group & Collaborators Prevalence of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae isolates reported by laboratories in the southern and eastern Mediterranean region. Clin Microbiol Infect. 2009;15:232–237. doi: 10.1111/j.1469-0691.2008.02651.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramdani-Bouguessa N., Rahal K. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolated in Algiers, Algeria. Antimicrob Agents Chemother. 2003;47:824–826. doi: 10.1128/AAC.47.2.824-826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tali-Maamar H., Laliam R., Bentchouala C. Serotyping and antibiotic susceptibility of Streptococcus pneumoniae strains isolated in Algeria from 2001 to 2010. Med Mal Infect. 2012;42:59–65. doi: 10.1016/j.medmal.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Johnson H.L., Deloria-Knoll M., Levine O.S., Stoszek S.K., Freimanis Hance L., Reithinger R. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA: 2011. Performance standards for antimicrobial susceptibility testing. 21st informational supplement. CLSI document M100–S21. [Google Scholar]
- 12.Versalovic J., Carroll K.C., Funke G., Jorgensen J.H., Landry M.L., Warnock D.W. 10th ed. American Society for Microbiology; Washington, DC: 2011. Manual of clinical microbiology. [Google Scholar]
- 13.Benbachir M., Elmdaghri N., Belabbes H., Haddioui G., Benzaid H., Zaki B. Eleven-year surveillance of antibiotic resistance in Streptococcus pneumoniae in Casablanca (Morocco) Microb Drug Resist. 2012;18:157–160. doi: 10.1089/mdr.2011.0130. [DOI] [PubMed] [Google Scholar]
- 14.Rachdi M., Boutiba-Ben Boubaker I., Mahjoubi-Rhimi F., Smaoui H., Hammami A., Kéchrid A. Serotype distribution and antimicrobial resistance patterns of Streptococcus pneumoniae isolated in Tunisia. J Med Microbiol. 2011;60:391–393. doi: 10.1099/jmm.0.024901-0. [DOI] [PubMed] [Google Scholar]
- 15.Smaoui H., Amri J., Hajji N., Kechrid A. Antimicrobial susceptibility and serotype distribution of Streptococcus pneumoniae isolates in children in Tunis. Arch Pediatr. 2009;16:220–226. doi: 10.1016/j.arcped.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Dagan R. Serotype replacement in perspective. Vaccine. 2009;27(Suppl. 3):C22–C24. doi: 10.1016/j.vaccine.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Ruckinger S., von Kries R., Siedler A., van der Linden M. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr Infect Dis J. 2009;28:118–122. doi: 10.1097/INF.0b013e318187e215. [DOI] [PubMed] [Google Scholar]
- 18.Hedlund J., Sorberg M., Henriques Normark B., Kronvall G. Capsular types and antibiotic susceptibility of invasive Streptococcus pneumoniae among children in Sweden. Scand J Infect Dis. 2003;35:452–458. doi: 10.1080/00365540310013315. [DOI] [PubMed] [Google Scholar]
- 19.Kyaw M.H., Lynfield R., Schaffner W., Craig A.S., Hadler J., Reingold A. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 20.Le Hello S., Watson M., Levy M., Marcon S., Brown M., Yvon J.F. Invasive serotype 1 Streptococcus pneumoniae outbreaks in the South Pacific from 2000 to 2007. J Clin Microbiol. 2010;48:2968–2971. doi: 10.1128/JCM.01615-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srifeungfung S., Tribuddharat C., Comerungsee S., Chatsuwan T., Treerauthanaweeraphong V., Rungnobhakhun P. Serotype coverage of pneumococcal conjugate vaccine and drug susceptibility of Streptococcus pneumoniae isolated from invasive or non-invasive diseases in central Thailand, 2006–2009. Vaccine. 2010;28:3440–3444. doi: 10.1016/j.vaccine.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 22.Hausdorff W.P., Feikin D.R., Klugman K.P. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 23.Ding F., Tang P., Hsu M.H., Cui P., Hu S., Yu J. Genome evolution driven by host adaptations results in a more virulent and antimicrobial-resistant Streptococcus pneumoniae serotype 14. BMC Genomics. 2009;10:158. doi: 10.1186/1471-2164-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linares J., Ardanuy C., Pallares R., Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010;16:402–410. doi: 10.1111/j.1469-0691.2010.03182.x. [DOI] [PubMed] [Google Scholar]
- 25.Werno A.M., Murdoch D.R. Medical microbiology: laboratory diagnosis of invasive pneumococcal disease. Clin Infect Dis. 2008;46:926–932. doi: 10.1086/528798. [DOI] [PubMed] [Google Scholar]
- 26.Pittet L.F., Posfay-Barbe K.M. Pneumococcal vaccines for children: a global public health priority. Clin Microbiol Infect. 2012;18(Suppl. 5):25–36. doi: 10.1111/j.1469-0691.2012.03938.x. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization Pneumococcal vaccines WHO position paper—2012 recommendations. Vaccine. 2012;30:4717–4718. doi: 10.1016/j.vaccine.2012.04.093. [DOI] [PubMed] [Google Scholar]


