Abstract
Throughout most of the ovulatory cycle, estrogen negative feedback restrains the GnRH neuronal system. Just before ovulation, however, estrogen negative feedback is removed to permit stimulation of the preovulatory GnRH/LH surge (positive feedback) by the circadian clock in the suprachiasmatic nucleus (SCN). The mammalian ortholog of avian gonadotropin-inhibitory hormone, RFamide-related peptide 3 (RFRP-3), participates in the circadian-timed removal of estrogen negative feedback to permit the LH surge. The present study examined the specific neurochemical means by which the SCN controls RFRP-3 activity and explored whether the RFRP-3 system exhibits time-dependent responsiveness to SCN signaling to precisely time the LH surge. We found that RFRP-3 cells in female Syrian hamsters (Mesocricetus auratus) receive close appositions from SCN-derived vasopressin-ergic and vasoactive intestinal peptide (VIP)-ergic terminal fibers. Central VIP administration markedly suppressed RFRP-3 cellular activity in the evening, but not the morning, relative to saline controls, whereas vasopressin was without effect at either time point. Double-label in situ hybridization for Rfrp-3 and the VIP receptors VPAC1 and VPAC2 revealed that the majority of RFRP-3 cells do not coexpress either receptor in Syrian hamsters or mice, suggesting that SCN VIP-ergic signaling inhibits RFRP-3 cells indirectly. The timing of this VIP-mediated disinhibition is further coordinated via temporally gated responsiveness of RFRP-3 cells to circadian signaling. Together, these findings reveal a novel circadian hierarchy of control coordinating the preovulatory LH surge and ovulation.
Circadian timing is critical for successful female reproduction across mammalian species, including humans. Women with irregular work or sleep cycles, for example, exhibit reduced fertility (1, 2) and increased spontaneous abortion rates (2–4). Analogously, in rodents, disruptions to circadian functioning lead to pronounced abnormalities in sexual motivation, ovulation, and fecundity (5–12). The circadian system coordinates the timing of ovulation and sexual behavior to coincide with an individual's species-specific temporal niche, with the preovulatory LH surge occurring in early morning in women (13, 14) and diurnal rodents (15) and late afternoon in nocturnal rodents (reviewed in Ref. 16). In species that ovulate spontaneously, the timing of the LH surge is controlled by the master circadian pacemaker in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus (17–22). During most of the ovulatory cycle, ovarian estradiol acts through negative feedback to maintain low-amplitude, pulsatile release of GnRH and LH (22, 23). However, during a limited time window on the day of ovulation, estradiol integrates with circadian signaling upstream of the GnRH system to positively drive the LH surge and, subsequently, ovulation (24–26).
The SCN communicates with the GnRH system via both monosynaptic and multisynaptic projections. The observations that GnRH neurons are directly innervated by vasoactive intestinal peptide (VIP)-ergic SCN projections (27, 28) and that VIP administration stimulates the LH surge (29) initially suggested that GnRH neurons may represent the neural locus, at which circadian and estrogenic signaling are integrated to initiate ovulation. However, GnRH neurons do no express estrogen receptor (ER)α (30), the receptor subtype required for positive feedback (31, 32), negating this possibility. In contrast, cells expressing the stimulatory neuropeptide, kisspeptin, in the anteroventral periventricular nucleus (AVPV) express ERα (33, 34), are innervated by vasopressin (AVP)-ergic SCN cells (35–37), exhibit a circadian pattern in Fos mRNA and protein expression (35, 38) with maximal colocalization coordinated with the GnRH/LH surge (39), and are required for GnRH cell activation to initiate the LH surge (40). Together, these findings suggest that AVPV kisspeptin cells represent an important upstream locus for the integration of circadian and steroidal signaling in the initiation of ovulation.
Central administration of AVP, a key SCN neuropeptide, stimulates the LH surge in a time-gated fashion, initiating the LH surge in the late afternoon but not the morning in rats (41). Although kisspeptin cells in the AVPV respond to AVP administration with increased cellular activation during both the morning and late afternoon, GnRH cells exhibit markedly greater cell activation with late afternoon kisspeptin treatment (35), indicating that time-dependent responsiveness to SCN AVP-ergic stimulation is mediated via differential responsiveness of GnRH cells to upstream kisspeptin signaling. Immortalized GnRH neurons (ie, GT1–7 cells) express the core clock genes driving circadian rhythms at the cellular level (42, 43), exhibit time-dependent sensitivity to kisspeptin and VIP stimulation (42), and display daily rhythms in kisspeptin receptor expression (44), suggesting that GnRH cells possess independent clocks to putatively mediate daily changes in sensitivity to upstream signaling.
In contrast to the mechanisms positively driving ovulation, the neurochemical systems mediating the timed removal of estradiol-mediated negative feedback are less well understood. Our previous findings suggest that the RFamide-related peptide 3 (RFRP-3), the mammalian ortholog of avian gonadotropin-inhibitory hormone (45), participates in circadian-controlled estradiol negative feedback (46). RFRP-3 cells express ERα (47), are directly innervated by the SCN (46), and communicate directly with GnRH cells to inhibit their activity (47–50). Estradiol decreases Rfrp mRNA levels (51) and RFRP-3 cellular activity is reduced at the time of the LH surge, suggesting that, in addition to driving the LH surge positively, the SCN concomitantly coordinates the removal of steroid-mediated RFRP-3 inhibition of the gonadotropic axis to permit the surge (46). However, exactly how the SCN regulates RFRP-3 neurons remains unknown.
In the present study, we examined the specific means by which the SCN coordinates the removal of RFRP-3 inhibition to permit the LH surge. We first asked whether or not two key SCN neuropeptides responsible for positively driving the LH surge, AVP and VIP, might concurrently coordinate the suppression of RFRP-3 cellular activation during the preovulatory GnRH/LH surge. Next, we explored whether the RFRP-3 system exhibits the potential for independent circadian time keeping and displays time-dependent sensitivity to AVP and VIP signaling, similar to that observed for the GnRH system in response to kisspeptin and VIP (35, 42). We hypothesized that AVP and VIP might act to stimulate RFRP-3 activity in the morning but not in the afternoon at the time of the LH surge, an effect mediated through independent time keeping of RFRP-3 cells. Such a finding would indicate that the RFRP-3 system restrains gonadotropic axis activity through tonic activation of this neuronal network by AVP and VIP and that this inhibition is removed during the LH surge by RFRP-3 cell insensitivity to AVP/VIP stimulation. Alternatively, we speculated that AVP and VIP might suppress RFRP-3 cellular activity, indirectly, via stimulation of an intermediary inhibitory circuit linked to RFRP-3 cells. In such a case, AVP/VIP would act to positively drive the LH surge through actions directly on kisspeptin and GnRH cells, while simultaneously, indirectly suppressing RFRP-3 activity.
Materials and Methods
Animals
Female Syrian hamsters (Mesocricetus auratus) were purchased at 100-g body mass from Charles River and singly housed in translucent propylene cages (48 × 27 × 20 cm) with a circulating ventilation system. Animals were provided ad libitum access to food and water, wood chips for floor cover, and bedding material. All animals were > 65 days old at the time of the experiment. Animals were maintained in a colony room at 23 ± 1°C with a 14-hour light, 10-hour dark cycle, with lights on at 8 am and lights off at 10 pm. Lights on is designated as zeitgeber time (ZT) 0. For the receptor colocalization experiments, a cohort of adult female C57BL6 mice was also used. The mice were housed on a 12-hour light, 12-hour dark cycle with food and water provided ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committees of the University of California, Berkeley and University of California, San Diego.
Examination of AVP-ergic and VIP-ergic SCN projections
To examine whether or not RFRP-3 cells receive AVP-ergic and/or VIP-ergic input, 17 female Syrian hamsters were ovariectomized and implanted with 10-mm Silastic capsules (Dow Corning) containing 17-β-estradiol benzoate (Sigma-Aldrich) using a ketamine cocktail (21-mg ketamine, 2.4-mg xylazine, and 0.3-mg acepromazine per mL injected ip at a dose of 0.25 mL per 100-g body mass) with buprenorphrine for pre- and postoperative analgesia. This procedure results in proestrous concentrations of estradiol and daily LH surges controlled by the SCN (52, 53). After a 2-week recovery, animals were deeply anesthetized with sodium pentobarbital (200 mg/kg) and perfused transcardially with approximately 150 mL of 0.9% saline followed by 300–400 mL of 4% paraformaldehyde in 0.1M PBS (pH 7.3). Brains were postfixed in 4% paraformaldehyde for 3 hours followed by cryoprotection in 30% sucrose in 0.1M PBS before slicing on a cryostat. Every fourth, 40-μm section was immunofluorescently double-labeled for either AVP/RFRP-3 (n = 6) or VIP/RFRP-3 (n = 11). Separate sections were first labeled with antibodies against either AVP (raised in guinea pig, 1:100 000; Peninsula Laboratories, Inc) or VIP (raised in guinea pig, 1:100 000; Peninsula Laboratories, Inc), followed by biotinyated tyramide amplification and labeled with cyanine (Cy)-2-conjugated streptavidin (Jackson ImmunoResearch) as a the secondary antibody/fluorophore as previously described (35). The specificity of both antibodies has been verified in this species previously (35). Subsequently, sections were incubated with Pacific Immunology (PAC) 1365 anti-RFRP-3 antiserum (1:16 000, PAC1365) with Cy3 antirabbit (Jackson ImmunoResearch) as the secondary antibody/fluorophore. The specificity of the PAC1365 anti-RFRP-3 antibody has been confirmed previously in this species (46). Antibody details are listed in Table 1.
Table 1.
Table of Antibodies
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| AVP | Anti-Arg8-vasopressin | Peninsula Laboratories, Inc; T-5048 | Guinea pig; polyclonal | 1:100 000 | |
| VIP | Anti-VIP | Peninsula Laboratories, Inc; T-5030 | Guinea pig; polyclonal | 1:100 000 | |
| PER1 | Anti-PER1 | Dr Michael Lehman, University of Mississippi Medical Center | Goat; polyclonal | 1:30 000 | |
| c-Fos | c-Fos sc-52 | Santa Cruz Biotechnology, Inc; sc-52 | Rabbit; polyclonal | 1:2000 | |
| RFRP-3 | PAC1365 | Dr Lance Kriegsfeld, University of California, Berkeley | Rabbit; polyclonal | 1:16 000 | |
| Guinea pig IgG | Biotinylated goat ant-iguinea pig | Vector Labs; BA-7000 | Goat; polyclonal | 1:200 (of reconstituted antibody) | |
| Goat IgG | Biotinylated horse anti-goat IgG | Vector Labs; BA-9500 | Horse; polyclonal | 1:200 (of reconstituted antibody) | |
| Rabbit IgG | Biotylated goat ant-irabbit IgG | Vector Labs; BA-1000 | Goat; polyclonal | 1:200 (of reconstituted antibody) | |
| Rabbit IgG | Cy2 donkey anti-rabbit | Jackson ImmunoResearch; 711-225-152 | Donkey; polyclonal | 1:333 (of reconstituted antibody) | |
| Rabbit IgG | Cy3 donkey anti-rabbit | Jackson ImmunoResearch; 711-165-152 | Donkey; polyclonal | 1:333 (of reconstituted antibody) |
To confirm that any identified AVP and/or VIP fiber appositions upon RFRP-3 cells arise from cells in the SCN, we directed bilateral lesions to the SCN in an additional set of hamsters to determine whether AVP or VIP fiber appositions were eliminated after complete SCN destruction. Ovariectomized and estrogen-implanted female Syrian hamsters (n = 11) were anesthetized with a ketamine cocktail (described above), the head was shaved and positioned in a stereotaxic apparatus (David Kopf Instruments), and the head prepared for aseptic surgery. Lesions were aimed at the next coordinates: 7.8 mm below dura, 0.8 mm anterior to bregma, and 0.1 mm lateral to midline. Bilateral electrolytic lesions were made by applying 100 mV for 15 seconds using a lesion generator (RFG-4A; Radionics) and stainless steel electrodes insulated with Epoxylite (The Epoxylite Corp), excluding the tip (0.20 mm). Hamsters were perfused 3 days after surgery and brains collected. Alternate 40-μm sections were labeled for AVP/RFRP-3 and VIP/RFRP-3 as described above. Lesion placements were confirmed histologically by examining the SCN for the elimination of VIP-immunoreactivity (ir) and AVP-ir that, together, define the entire nucleus.
Examination of the impact of AVP and VIP on RFRP-3 cell activity
To determine the specific impact of central AVP and VIP administration on RFRP-3 cellular activity, 34 hamsters were ovariectomized and treated with 10-mm Silastic capsules, as described above, using a ketamine cocktail with buprenorphrine for pre- and postoperative analgesia (anesthesia and analgesia procedures described above). After 2 weeks of recovery, all hamsters received guide cannulas (6 mm; Plastics One) aimed at the lateral ventricle (1.3 mm mediolateral and 1.1 mm posterior to bregma, and 3 mm ventral from the surface of the dura mater). To maintain patency, dummy cannulas were attached to guide cannulae after surgery. After a 7- to 10-day recovery period, cannula placements were confirmed by injections of 20-ng angiotensin II in 5-μL sterile saline (0.9%) and considered correctly placed in the ventricle if drinking occurred immediately. Placement of guide cannulas were also verified histologically at the conclusion of the study. To determine whether the SCN communicates an excitatory or inhibitory signal to RFRP-3 cells via VIP or AVP, hamsters were injected intracerebroventricularly with 5 μL vehicle (0.9% sterile saline; n = 11), 5-μL VIP (9 ng/μL, n = 11; V6130-250UG; Sigma-Aldrich), or 5-μL AVP (4 ng/μL, n = 12; V0377-50IU; Sigma-Aldrich) at the rate of 1 μL/min. To establish whether or not RFRP-3 cells exhibit time-dependent sensitivity to these peptides, half of the hamsters in each group were injected in the morning (9 am; ZT1) and the other half in the late afternoon (7 pm; ZT11) at the time of the LH surge (46).
One hour after injections, hamsters were perfused transcardially as described above and brains collected for examination of RFRP-3/Fos coexpression. Every fourth 40 μm section was incubated in an anti-RFRP-3 antiserum (raised in rabbit, 1:60 000; PAC1365) for 48 hours at 4°C followed by amplification using a biotinylated tyramide procedure previously described (46) with Cy3-conjugated streptavidin (Jackson ImmunoResearch) as the fluorophore. The dilution of the PAC1365 antibody was 10-fold greater than that which is optimal for direct immunohistochemistry (ie, ∼1:6000). This dilution prevented the second, secondary antibody from nonspecifically binding to the first primary. After RFRP-3 labeling, brains were incubated in an anti-Fos antibody (raised in rabbit, 1:2000; Santa Cruz Biotechnology, Inc) for 48 hours at 4°C and labeled with a Cy2 antirabbit secondary antibody/fluorophore (Jackson ImmunoResearch) (Table 1).
VIP receptor expression on RFRP-3 cells
Because VIP, but not AVP, altered RFRP-3 cellular activity, we investigated whether or not RFRP-3 cells express VPAC1 or VPAC2 receptor mRNA. Hamsters (n = 3) were ovariectomized and treated with Silastic capsules containing 17-β-estradiol benzoate as described above and sacrificed by an overdose of pentobarbital sodium/phenytoin sodium (Euthasol, Virbac, Inc) in the late afternoon (8 pm; ZT12), when the LH surge would normally occur. Brains collected and frozen immediately on dry ice were cut on a cryostat into 5 series spanning the entire dorsomedial hypothalamus (DMH), where RFRP-3 cell bodies reside. To validate the findings in another species, we also used a cohort of adult female mice that were ovariectomized and treated with an sc estradiol Silastic capsule known to evoke LH surges 2 days later in the late afternoon (40). Brains collected just before lights off were cut similarly to the hamster brains. Two full series of sections from each hamster and mouse were used for double-label in situ hybridization to examine the presence or absence of either VPAC1 or VPAC2 in Rfrp cells. Double-label in situ hybridization was performed as previously described (40, 51, 54) using a validated Rfrp riboprobe and VPAC1 or Vpac2 riboprobes designed to base pairs 299–803 and 720–1296 of the murine VPAC1 and VPAC2 sequences, respectively (GenBank accession numbers NM_011703 and NM_009511). Pilot studies confirmed that these murine probes all bind efficiently in hamster brain tissue. For each animal, the total number of Rfrp neurons was counted along with the number of Rfrp neurons coexpressing either VPAC1 or VPAC2. This was used to calculate the overall percentage of Rfrp neurons coexpressing either VIP receptor for each animal within each species. Rfrp cells were considered double-labeled if the signal-to-background ratio was greater than 3.
Examination of period 1 (PER1) protein expression in RFRP-3 cells
To investigate the possibility that RFRP-3 cells can keep circadian time, we examined whether or not RFRP-3 cells express the clock-controlled gene product, PER1, and whether or not the percentage of RFRP-3 cells coexpressing PER1 varied in a circadian manner. Brains from 5 ovariectomized, estradiol-treated hamsters were collected at 10 am (ZT2), 3 pm (ZT7), 8 pm (ZT12), and 3 am (ZT19). Every fourth, 40-μm section was immunofluorescently double-labeled as described above, with goat anti-PER1 (1:30 000 concentration; generous gift of Dr Michael Lehman, University of Mississippi Medical Center; specificity verified previously in Syrian hamsters) (55) amplified using biotinylated tyramide with Cy2 as fluorophore and anti-RFRP-3 (1:16 000 concentration, PAC1365) directly labeled with Cy3 as the secondary antibody/fluorophore (Table 1).
Microscopy and image analysis
For initial examination of whether AVP or VIP terminal fibers appose RFRP-3 perikarya, automated Z-stack photomicrographs were captured for every RFRP-3 cell at 0.5-μm increments at ×400 at the conventional light microscopy level with a Zeiss Axioimager M1 microscope. Standard wavelengths for Cy2 (488 nm) and Cy3 (568 nm) were used. Each RFRP-3 cell was examined for VIP or AVP terminal buttons within the same RFRP-3 cell focal plane. A random subset (20%) of contacts identified at the light level was confirmed using 0.5 μm confocal microscopy scans at ×400 with a LSM710 confocal microscope (Carl Zeiss, Inc). Appositions were defined as terminal fibers directly contacting RFRP-3 cell soma in the same 0.5-μm plane.
To examine the percentage of RFRP-3 cells expressing Fos and PER1, sections were examined at the conventional light microcopy level using the standard wavelengths for Cy2 (488 nm) and Cy3 (568 nm) with a Zeiss Axioimager M1 microscope. Photographs of all RFRP-3 cells were taken at ×400. Each label was captured as a single image without moving the position of the stage or plane of focus between captures. Images were superimposed digitally. For identification of Fos or PER1 colabeling with RFRP-3, the 3 sections with the highest number of RFRP-3 cells were examined using Photoshop software (Adobe Systems, Inc) by investigators “blind” to the treatment conditions to which the animals were exposed. Cells were considered colabeled if Fos labeling was clearly confined to the cell nucleus. RFRP-3 cells without a visible nucleus were not counted.
Statistics
Data were analyzed using SPSS 17.0 software (IBM). For analysis of the impact of AVP and VIP on RFRP-3 cellular activity a two-way (peptide × time) ANOVA was used. To examine the percentage of RFRP-3 cells expressing PER1, a one-way, between-subjects (ANOVA) was used. All percentage data were submitted to an arcsine transformation before analysis. Group differences were explored using ANOVA contrasts with post hoc Tukey and Mann-Whitney U tests. Results were considered statistically significant if P < .05.
Results
The SCN projects to RFRP-3 cells via VIP-ergic and AVP-ergic efferents
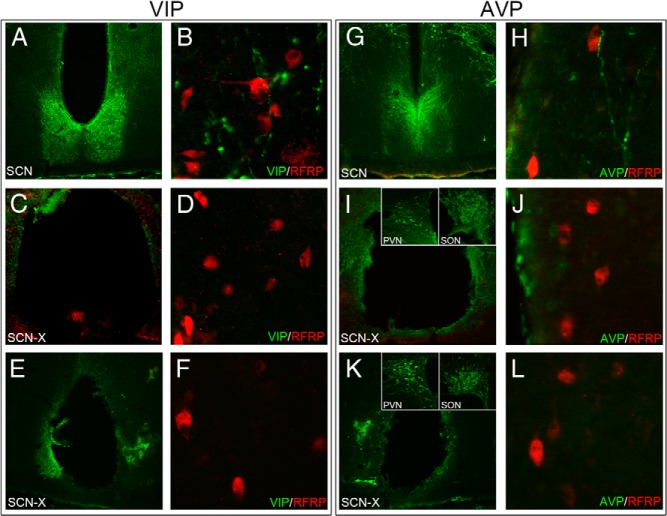
To examine whether or not AVP and/or VIP cells project to RFRP-3 cells as a potential means of removing estradiol negative feedback during the LH surge, separate immunofluorescently labeled sections were examined. Both VIP and AVP fibers contact RFRP-3-ir cell bodies within the DMH and periventromedial hypothalamus. Fiber contacts were relatively sparse, with 10.0 ± 1.0% of RFRP-3 cells receiving VIP contacts and 15.1 ± 1.0% of RFRP-3 cells receiving AVP contacts (Figure 1). All presumptive contacts identified at the conventional microscopy level were confirmed to be in the same 0.5-μm plane at the confocal microscopy level (Figure 1). To confirm that these appositions arise from the SCN, bilateral SCN ablations and subsequent immunofluorescent labeling for AVP/RFRP-3 and VIP/RFRP-3 was performed. Of those hamsters with lesions targeting the SCN, the SCN was completely bilaterally ablated in two individuals, as confirmed by SCN labeling for both VIP and AVP. After complete ablation of the SCN, there were no RFRP-3 cells that received close appositions by either AVP or VIP fibers, confirming that the origin of fibers identified in SCN-intact animals was the master circadian clock (Figure 2). VIP and AVP cell body labeling outside of the SCN did not differ between brains from intact and lesioned animals (Figure 2). Two additional animals with small SCN lesions eliminating VIP cells in the SCN showed no VIP fiber appositions upon RFRP-3 cells in the DMH. One of these partial lesions eliminated virtually all AVP cells and resulted in the complete abolition of AVP fiber appositions upon RFRP-3 cells in the DMH (Figure 2). Finally, 1 partial lesion that eliminated only a portion of AVP cells resulted in grossly reduced AVP fiber appositions onto RFRP-3 cells (3 contacts total, 1.1% of RFRP-3 neurons).
Figure 1.
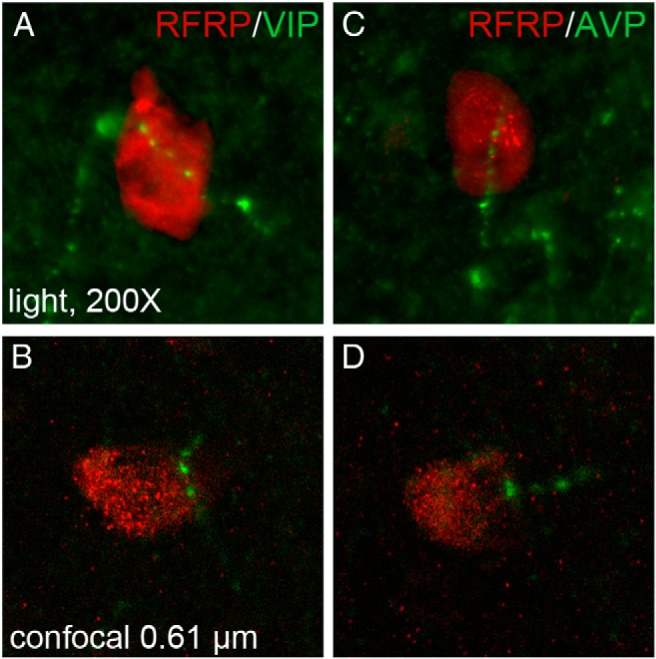
SCN fiber projections form close appositions with RFRP-ir cells in the DMH. A, Low-power conventional light photomicrograph (×200) depicting VIP fiber projections in close apposition to RFRP-3-ir cells in the DMH. B, Confocal microscopic image of VIP fiber within the same 0.5-μm plane as an RFRP-3-ir cell. C, Low-power conventional light photomicrograph of AVP fiber projections in close apposition to RFRP-3-ir cells in the DMH. D, Confocal microscopic image of AVP fiber within the same 0.5-μm plane as an RFRP-3-ir neuron.
Figure 2.
SCN lesions abolish AVP and VIP fiber projections to RFRP-3 cells. A, Low-power photomicrograph of VIP-ir labeling in an intact SCN. B, VIP fibers (green) and RFRP-3 cells (red) (×400) in the DMH. C, SCN lesion with elimination of VIP labeling. D, SCN lesions abolishes VIP fiber expression in the DMH. E, Small SCN lesion eliminating VIP in the SCN. F, Elimination of VIP labeling in DMH. G, Low-power photomicrograph of AVP-ir labeling in an intact SCN. H, AVP fibers (green) and RFRP-3 cells (red) (×400) in the DMH. I, SCN lesion with AVP labeling (green) and unperturbed AVP populations in the supraoptic nucleus (SON) and PVN (insets). J, SCN lesions abolishes VIP fiber expression in the DMH. K, Small SCN lesion eliminating virtually all AVP labeling (green) and unperturbed AVP populations in the SON and PVN (insets). L, Elimination of AVP labeling in DMH.
VIP, but not AVP, suppresses RFRP-3 activity in a time-dependent manner
Because RFRP-3 cells received AVP-ergic and VIP-ergic SCN projections, we examined whether or not central injection of these peptides alter RFRP-3 cellular activity. Additionally, we examined whether any impact of AVP or VIP on RFRP-3 cellular activity was time-dependent, as we have previously shown for GnRH system responsiveness to kisspeptin (35). For SAL (F1,19 = 6.68, P < .02) and VIP (F1,18 = 28.55, P < .001) injections, RFRP-3 cells exhibited reduced Fos expression in the afternoon, confirming our previous report that RFRP-3/Fos labeling is reduced around the time of the LH surge (P < .05) (Figure 3) (46). The percentage of RFRP-3 cells was significantly affected by injection of VIP (F1,18 = 7.18, P < .02). Specifically, whereas morning VIP injections did not alter the percentage of RFRP-3 cells expressing Fos (P > .05), afternoon injections of VIP markedly reduced the percentage of RFRP-3/Fos-labeled cells (P < .01) relative to SAL controls. In contrast to VIP, AVP injections did not alter the percentage of RFRP-3/Fos cells either in the morning or the evening (F1,19 = 0.06, P > .05) (Figure 3). Differences in percentages were not due to differences in the number of RFRP-3 cells visualized, as overall RFRP-3 cell numbers were not affected by time of injection (F2,28 = 0.006, P > .05) or injection type (F2,28 = 0.92, P > .05) (Table 2; P > .05 in all cases).
Figure 3.
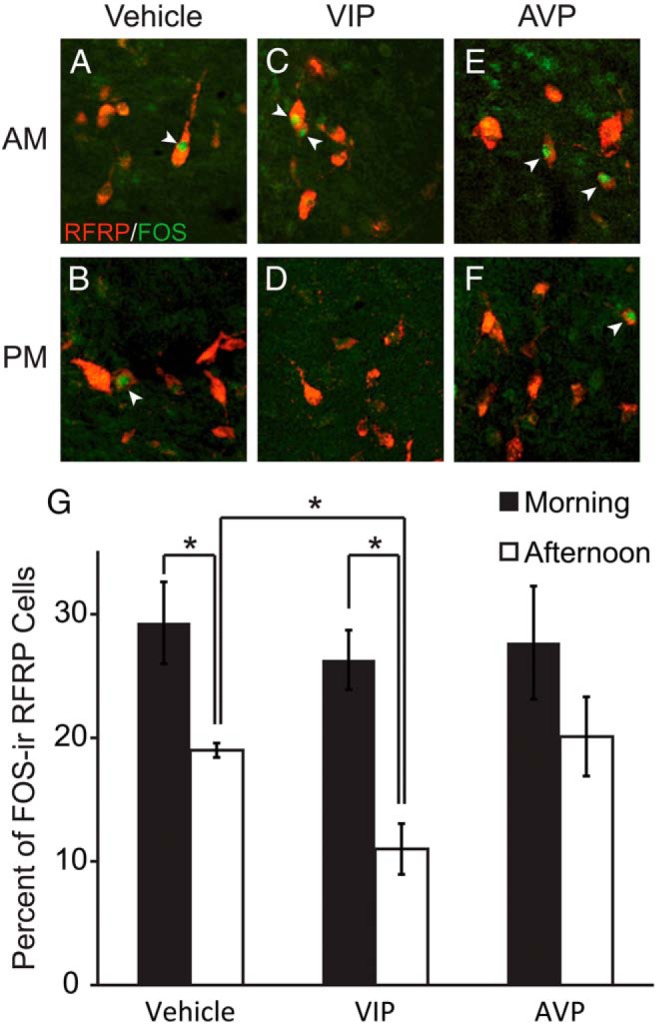
Representative photomicrographs depicting RFRP-3-ir (red) and Fos-ir (green) neurons 1 hour after intracerebroventricular injection of (A) saline (vehicle) at ZT2 (morning) and (B) ZT12 (late afternoon), (C) AVP (morning) and (D) AVP (late afternoon), and (E) VIP (morning) and (F) VIP (late afternoon). G, Mean (±SEM) percentage of RFRP-3 cells expressing Fos after saline (vehicle), AVP, or VIP injections in the morning or late afternoon. *, significantly different (P < .05).
Table 2.
Mean (±SEM) Number of RFRP-3 Cells From 3 Sections With the Highest RFRP-3 Cell Numbers
| Vehicle | VIP | AVP | |
|---|---|---|---|
| AM | 195.5 ± 23.4 | 158.0 ± 16.3 | 154.5 ± 21.8 |
| PM | 183.4 ± 41.7 | 159.4 ± 31.6 | 160.0 ± 24.6 |
Cell numbers did not significantly differ among groups (P > .05 in all cases).
Most RFRP-3 neurons do not coexpress VIP receptors
Because VIP treatment significantly altered neuronal activation of RFRP-3 neurons in female hamsters, we assessed whether this effect is mediated via VIP signaling directly to RFRP-3 neurons. We used double-label in situ hybridization to examine whether Rfrp neurons coexpress either of the VIP receptors, VPAC1 or VPAC2. Both receptors were detected in other regions of the brain, such as the SCN for VPAC2 (Figure 4, A and B), but were minimally expressed in the DMH. Indeed, only a very small minority of Rfrp neurons coexpressed VPAC2 (8.4 ± 1.9%) (Figure 4, C and E), and VPAC1 (3.4 ± 1.1%) (Figure 4G), suggesting that any effects of VIP on RFRP-3 neurons are primarily indirect. Moreover, the few Rfrp cells that did coexpress VPAC2 had very low levels of VIP receptor mRNA. This finding was confirmed in brains of female mice that were similarly sacrificed during the LH surge, with virtually no VPAC1 or VPAC2 coexpression identified in most Rfrp neurons (only 3.4 ± 0.9% and 3.9 ± 1.2%, respectively) (Figure 4, D, F, and H).
Figure 4.
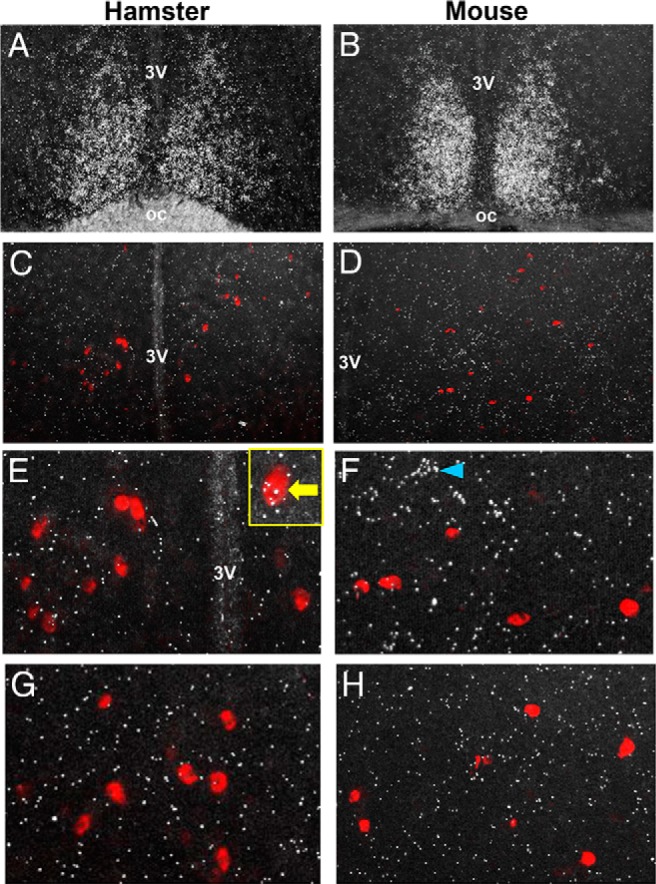
Representative photomicrographs of VPAC1 and VPAC2 mRNA expression in the brains of female hamsters and mice at the time of the LH surge. A and B, Abundant VPAC2 expression (silver grain clusters) in the SCN of both hamster and mouse, respectively. C and D, Rfrp-3 neurons (red fluorescence) in the DMH of hamster and mouse, respectively, with minimal observed VPAC2 expression in this region. E and F, Higher magnification of VPAC2 and Rfrp-3 expression in hamster and mouse, denoting virtually no coexpression of the 2 in either species. Yellow box inset in E denotes an example of 1 of the few Rfrp-3 cells weakly coexpressing VPAC2. Blue arrowhead in F denotes a VPAC2-expressing cell, which is not an Rfrp-3 cell. G and H, High magnification of VPAC1 and Rfrp-3 expression in hamster and mouse, denoting virtually no coexpression in either species.
RFRP-3 cells populations express the core clock gene, PER1
Because the response of RFRP-3 neurons to VIP was time-gated, we examined whether RFRP-3 neurons express PER1, the protein product of the essential clock gene, Period 1. We also explored whether or not the percentage of RFRP-3 cells expressing PER1 varied across the day, suggesting circadian timekeeping in RFRP-3 neurons. RFRP-3 cells readily expressed PER1, with a daily peak in coexpression at ZT12 (ZT0, lights on). The percentage of cells expressing PER1 was significantly greater at ZT12 than ZT7 (P < .05) and marginally significant relative to ZT2 and ZT19 time points (P = .052 and P = .057, respectively) (Figure 5).
Figure 5.
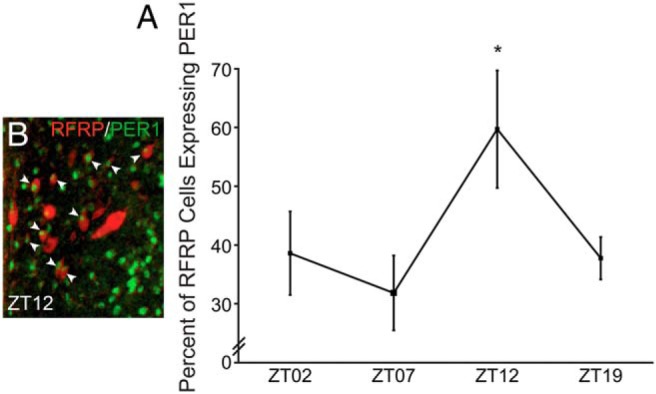
RFRP-3 cells rhythmically express PER1 protein. A, Representative low-power photomicrograph of RFRP-3-ir (red) and PER1 (green) immunofluorescence at ZT12. B, Mean (±SEM) percentage of RFRP-3-ir cells expressing PER1-ir at ZT2, ZT7, ZT12, and ZT19 in ovariectomized estradiol-treated female hamsters. *, significantly greater (P < .05) than ZT7, comparison with ZT2 and ZT19 nearing significance, P = .052 and P = .057, respectively.
Discussion
The present study revealed that a subset of RFRP-3 cells receive AVP-ergic and VIP-ergic projections originating from the SCN. Furthermore, the RFRP-3 system exhibits time-dependent responsiveness to SCN signaling, with afternoon, but not morning, injections of VIP reducing the percentage of RFRP-3 cells expressing Fos, whereas AVP injections at either time point did not differ from vehicle-injected controls. The present and previous findings that RFRP-3 cells express ERs (47) provide a mechanism for coordinated disinhibition of estrogen-mediated RFRP-3 suppression of reproductive axis activity (eg, negative feedback) at the time of the LH surge. That RFRP-3 cells express the core clock gene component, PER1, and expression of PER1 exhibits diurnal variation, suggests that these cells keep circadian time thereby coordinating daily changes in responsiveness to SCN signaling, as seen for the GnRH system (35, 42–44).
Contrary to our initial hypothesis that RFRP-3 cellular activity would be stimulated by AVP and/or VIP in the morning but not in the afternoon, the percentage of RFRP-3 cells expressing Fos was reduced by afternoon injections of VIP and unaffected by AVP administration at either time point. Because the receptors for VIP, VPAC1 and VPAC2, were not highly expressed in RFRP-3 neurons, and are coupled to stimulatory G proteins, it is unlikely that a suppressive action of VIP on RFRP-3 cells occurs directly. However, the present findings are consistent with VIP-ergic stimulation of an inhibitory intermediary system downstream of the SCN and upstream of RFRP-3 cells. Such a relation is in agreement with the present findings that the RFRP-3 population as a whole receives only a few VIP appositions. Such a putative circadian neural circuit is reminiscent of a similar mechanism of circadian control of corticotropin-releasing hormone (CRH), the central neuropeptide driving daily rhythms in glucocorticoid secretion, by AVP (20, 56–58). CRH neurons in the paraventricular nucleus of the hypothalamus (PVN) receive only sparse innervation by SCN AVP efferents (58–60). Furthermore, although AVP acts as an excitatory neuropeptide, AVP administration inhibits CRH secretion (56). Thus, an intermediate neuronal circuit was proposed (58, 61), and recent electrophysiological evidence suggests that this interneuronal system is likely gamma-aminobutyric acid-ergic (61).
The finding that VIP administration suppresses RFRP-3 cellular activity in a time-gated fashion suggests that either 1) the putative inhibitory intermediary system on which VIP acts directly displays time-dependent sensitivity to VIP signaling, 2) the RFRP-3 systems exhibits time-dependent sensitivity to this unspecified inhibitory signal, or 3) a combination of both mechanisms results in daily changes in the response of the RFRP-3 system to VIP. The observation that PER1 is expressed rhythmically in RFRP-3 cells suggests that the RFRP-3 system keeps daily time and likely participates in mediating daily changes in responsiveness to upstream neurochemical signaling. Whether this mechanism maintains circadian time in the absence of SCN input requires further investigation. Such a timekeeping system is analogous to that observed for GnRH cells (35, 42–44) and potentially in AVPV kisspeptin neurons (36, 62), suggesting that this means of temporal gating might be common to reproductively relevant systems subordinate to the SCN. A hierarchy of circadian control allows for greater precision in the timing of the LH surge than a single, master pacemaker, alone. Although such a gating mechanism is consistent with the present findings, additional studies in which circadian genes are manipulated specifically in RFRP-3 cells are necessary to confirm this possibility. Likewise, upon identification of an intermediate inhibitory system(s) on which SCN cells act to affect RFRP-3 cells, it will be possible to determine whether these systems participate in time-dependent transmission of SCN signaling.
The present results differ from those recently observed in female rats (63). Although a 3-hour VIP infusion rescues the LH surge in middle-aged rats and increases the cellular activation of GnRH cells (63, 64), Rfrp cell numbers, Rfrp mRNA levels per cell, and Rfrp cellular activation were unaffected by this VIP administration. Several procedural differences may account for these disparate findings. First, rats were injected with estradiol and then progesterone to induce a single LH surge, whereas hamsters in the present study were treated with constant proestrous concentrations of estradiol, without progesterone, to induce daily LH surges. Additionally, VIP was injected acutely in the present investigation but infused for several hours in rats. Moreover, because our present results indicate a time-dependent aspect of VIP effects on RFRP-3 neurons, it is possible that a significant decrease in RFRP-3 cellular activation would have similarly been observed in rats at other circadian times. Indeed, there was a trend for lower RFRP-3 cellular activation with VIP treatment in rats (63); because this was only assessed at a single time point, it is unknown whether more robust changes exist at other circadian times. Finally, it is possible that species differences exist in the mechanisms driving the LH surge across species. Future studies with both species investigated within the same experiments are necessary to select among these possibilities.
The SCN-derived AVP-ergic and VIP-ergic appositions observed for some RFRP-3 cells indicate an as-yet unspecified functional role for these SCN projections. One possibility is that the SCN projections identified communicate via other neurochemical/neurotransmitter signaling factors coreleased by SCN AVP and VIP cells. It is also possible that AVP and/or VIP stimulate cellular processes that do not utilize Fos and were therefore not detected with the present staining strategy. For nonreproductive subordinate systems, in the absence of the SCN, single-cell oscillators continue to function but the coordinated rhythm of the ensemble of cellular oscillators (eg, liver clocks, heart clocks) is lost (65). Thus, it is possible these SCN projections synchronize the activity/phase of independent RFRP-3 cellular oscillators to permit the maintenance of coordinated daily responsiveness of a small collection of RFRP-3 cells to upstream signaling. Alternatively, a small proportion of RFRP-3 cells may be synchronized by direct SCN projections and this timing information is then locally propagated throughout the network of RFRP-3 cells.
The present findings suggest that the SCN serves a dual role for ovulatory control, both coordinating timed gonadotropic axis stimulation with timed negative feedback disinhibition to initiate the preovulatory LH surge, and point to a novel, circadian-controlled neural circuit subserving these events. Based on our findings, we propose that the SCN signals the kisspeptin (35, 37) and GnRH systems (27, 66) via direct, neuronal projections to positively drive the LH surge. Concurrently, the SCN directly, and perhaps more likely, indirectly, signals the RFRP-3 system to coordinate the suppression of estradiol negative feedback with this positive drive. Both the GnRH and RFRP-3 systems maintain their own circadian time to further ensure that stimulation and disinhibition of the reproductive axis are precisely coordinated (Figure 6). Given that epidemiological and experimental findings indicate a pronounced negative impact of circadian disruption on female reproductive health, systematically uncovering the mechanisms underlying the circadian control of the female reproductive axis has potential clinical implications.
Figure 6.
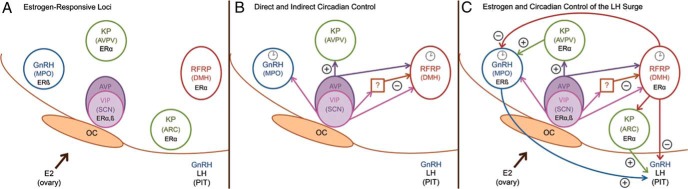
A proposed model depicting circadian control of the preovulatory LH surge and ovulation. A, Estrogen responsive circuitry. These cell populations integrate estrogenic, SCN-derived, and local endogenous circadian cues to appropriately time the preovulatory LH surge. B, Loci receiving direct and indirect input from the master clock in the SCN. In this model, the SCN projects directly to GnRH, kisspeptin, and RFRP-3 cells. Additionally, the SCN projects to a putative interneuron to drive the removal of RFRP-3 inhibition by SCN-derived VIP at the time of the surge. Clock icons indicate neural populations exhibiting circadian oscillations in clock genes/proteins and time-dependent sensitivity to SCN signaling, including the GnRH (35, 42, 43) and RFRP-3 systems (46). C, Estrogen- and clock-controlled hierarchy. RFRP-3 neurons project to GnRH cells and to the median eminence to regulate gonadotropin secretion. It is proposed that RFRP-3 neurons respond to estradiol by inhibiting the reproductive axis throughout most of the estrous cycle. At the time of the LH surge, the SCN inhibits the RFRP-3 system through indirect VIP-ergic signaling, removing negative feedback to allow concomitant, SCN-controlled surge generation. oc, optic chiasm; MPO, medial preoptic area; ARC, arcuate nucleus; PIT, pituitary; SON, supraoptic nucleus.
Acknowledgments
We thank Dr Irv Zucker for valuable comments on a previous version of this manuscript.
This work was supported by National Institutes of Health Grant R01 HD-050470 (to L.J.K.) and National Science Foundation Grants IOS-1257638 (to L.J.K.) and IOS-1025893 (to A.S.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVP
- vasopressin
- AVPV
- anteroventral periventricular nucleus
- CY
- cyanine
- DMH
- dorsomedial hypothalamus
- ER
- estrogen receptor
- ir
- immunoreactivity
- PAC
- Pacific Immunology
- PER1
- period 1
- PVN
- paraventricular nucleus of the hypothalamus
- RFRP-3
- RFamide-related peptide 3
- SCN
- suprachiasmatic nucleus
- VIP
- vasoactive intestinal peptide
- ZT
- zeitgeber time.
References
- 1. Ahlborg G, Jr, Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. Int J Epidemiol. 1996;25(4):783–790. [DOI] [PubMed] [Google Scholar]
- 2. Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. J Occup Environ Med. 1996;38(4):352–358. [DOI] [PubMed] [Google Scholar]
- 4. Nurminen T. Shift work and reproductive health. Scand J Work Environ Health. 1998;24(suppl 3):28–34. [PubMed] [Google Scholar]
- 5. Endo A, Watanabe T. Effects of non-24-hour days on reproductive efficacy and embryonic development in mice. Gamete Res. 1989;22(4):435–441. [DOI] [PubMed] [Google Scholar]
- 6. Summa KC, Vitaterna MH, Turek FW. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLoS One. 2012;7(5):e37668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci. 1977;198(1132):279–296. [DOI] [PubMed] [Google Scholar]
- 8. Gray GD, Söderstein P, Tallentire D, Davidson JM. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology. 1978;25(3):174–191. [DOI] [PubMed] [Google Scholar]
- 9. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34(6):395–404. [DOI] [PubMed] [Google Scholar]
- 10. Kawakami M, Arita J, Yoshioka E. Loss of estrogen-induced daily surges of prolactin and gonadotropins by suprachiasmatic nucleus lesions in ovariectomized rats. Endocrinology. 1980;106(4):1087–1092. [DOI] [PubMed] [Google Scholar]
- 11. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol. 1977;20(2):224–234. [DOI] [PubMed] [Google Scholar]
- 13. Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil Steril. 1998;70(1):56–59. [DOI] [PubMed] [Google Scholar]
- 14. Kerdelhué B, Brown S, Lenoir V, et al. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75(3):158–163. [DOI] [PubMed] [Google Scholar]
- 15. Mahoney MM, Sisk C, Ross HE, Smale L. Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthis niloticus, and in a nocturnal rodent, Rattus norvegicus. Biol Reprod. 2004;70(4):1049–1054. [DOI] [PubMed] [Google Scholar]
- 16. Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31(4):544–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147(3):1148–1153. [DOI] [PubMed] [Google Scholar]
- 19. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69(6):1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. [DOI] [PubMed] [Google Scholar]
- 21. Samson WK, McCann SM. Effects of suprachiasmatic nucleus lesions on hypothalamic LH-releasing hormone (LHRH) content and gonadotropin secretion in the ovariectomized (OVX) female rat. Brain Res Bull. 1979;4(6):783–788. [DOI] [PubMed] [Google Scholar]
- 22. Williams WP, 3rd, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne). 2012;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69(6):1771–1778. [DOI] [PubMed] [Google Scholar]
- 24. Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56(2):293–302. [DOI] [PubMed] [Google Scholar]
- 25. Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56(2):303–309. [DOI] [PubMed] [Google Scholar]
- 26. Kriegsfeld LJ. Circadian regulation of kisspeptin in female reproductive functioning. Adv Exp Med Biol. 2013;784:385–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol. 1993;5(2):137–144. [DOI] [PubMed] [Google Scholar]
- 28. Kriegsfeld LJ, Silver R, Gore AC, Crews D. Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurones increase following puberty in female rats. J Neuroendocrinol. 2002;14(9):685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samson WK, Burton KP, Reeves JP, McCann SM. Vasoactive intestinal peptide stimulates luteinizing hormone-releasing hormone release from median eminence synaptosomes. Regul Pept. 1981;2(4):253–264. [DOI] [PubMed] [Google Scholar]
- 30. Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50(2):283–298. [DOI] [PubMed] [Google Scholar]
- 31. Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor α in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78(4):204–209. [DOI] [PubMed] [Google Scholar]
- 32. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 34. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. [DOI] [PubMed] [Google Scholar]
- 35. Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152(2):595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology. 2012;153(6):2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vida B, Deli L, Hrabovszky E, et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 2010;22(9):1032–1039. [DOI] [PubMed] [Google Scholar]
- 38. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res. 2001;901(1–2):109–116. [DOI] [PubMed] [Google Scholar]
- 42. Zhao S, Kriegsfeld LJ. Daily changes in GT1–7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89(4):448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1–7 cell line. J Neurosci. 2003;23(35):11202–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tonsfeldt KJ, Goodall CP, Latham KL, Chappell PE. Oestrogen induces rhythmic expression of the kisspeptin-1 receptor GPR54 in hypothalamic gonadotrophin-releasing hormone-secreting GT1–7 cells. J Neuroendocrinol. 2011;23(9):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsutsui K, Saigoh E, Ukena K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–667. [DOI] [PubMed] [Google Scholar]
- 46. Gibson EM, Humber SA, Jain S, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kriegsfeld LJ, Mei DF, Bentley GE, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103(7):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–2804. [DOI] [PubMed] [Google Scholar]
- 49. Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(pt 7):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51(1):171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153(4):1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Norman RL, Blake CA, Sawyer CH. Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology. 1973;93(4):965–970. [DOI] [PubMed] [Google Scholar]
- 53. Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. [DOI] [PubMed] [Google Scholar]
- 54. Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J Neuroendocrinol. 2013;25(10):876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci. 2005;25(39):9017–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580(1–2):62–67. [DOI] [PubMed] [Google Scholar]
- 57. Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol. 1993;335(1):42–54. [DOI] [PubMed] [Google Scholar]
- 58. Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349(1):20–29. [DOI] [PubMed] [Google Scholar]
- 59. Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Prog Brain Res. 2000;126:117–132. [DOI] [PubMed] [Google Scholar]
- 60. Vrang N, Larsen PJ, Mikkelsen JD. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995;684(1):61–69. [DOI] [PubMed] [Google Scholar]
- 61. Hermes ML, Ruijter JM, Klop A, Buijs RM, Renaud LP. Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. J Neurophysiol. 2000;83(2):705–711. [DOI] [PubMed] [Google Scholar]
- 62. Smarr BL, Gile JJ, de la Iglesia HO. Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: possible role as an integrator for circadian and ovarian signals timing the luteinising hormone surge. J Neuroendocrinol. 2013;25(12):1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kauffman AS, Sun Y, Kim J, Khan AR, Shu J, Neal-Perry G. Vasoactive intestinal peptide modulation of the steroid-induced LH surge involves kisspeptin signaling in young but not in middle-aged female rats. Endocrinology. 2014;155(6):2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun Y, Shu J, Kyei K, Neal-Perry GS. Intracerebroventricular infusion of vasoactive intestinal Peptide rescues the luteinizing hormone surge in middle-aged female rats. Front Endocrinol (Lausanne). 2012;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14(24):2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384(4):569–579. [DOI] [PubMed] [Google Scholar]