Abstract
The adrenal glands consist of an outer cortex and an inner medulla, and their primary purposes include hormone synthesis and secretion. The adrenal cortex produces a complex array of steroid hormones, whereas the medulla is part of the sympathetic nervous system and produces the catecholamines epinephrine and norepinephrine. In the mouse, GATA binding protein (GATA) 4 and GATA6 transcription factors are coexpressed in several embryonic tissues, including the adrenal cortex. To explore the roles of GATA4 and GATA6 in mouse adrenal development, we conditionally deleted these genes in adrenocortical cells using the Sf1Cre strain of animals. We report here that mice with Sf1Cre-mediated double deletion of Gata4 and Gata6 genes lack identifiable adrenal glands, steroidogenic factor 1-positive cortical cells and steroidogenic gene expression in the adrenal location. The inactivation of the Gata6 gene alone (Sf1Cre;Gata6flox/flox) drastically reduced the adrenal size and corticosterone production in the adult animals. Adrenocortical aplasia is expected to result in the demise of the animal within 2 weeks after birth unless glucocorticoids are provided. In accordance, Sf1Cre;Gata4flox/floxGata6flox/flox females depend on steroid supplementation to survive after weaning. Surprisingly, Sf1Cre;Gata4flox/floxGata6flox/flox males appear to live normal lifespans as vital steroidogenic synthesis shifts to their testes. Our results reveal a requirement for GATA factors in adrenal development and provide a novel tool to characterize the transcriptional network controlling adrenocortical cell fates.
The mature adrenal (also suprarenal) glands are paired endocrine organs located anteriomedially to the respective kidneys (Latin r̄en̄es). Each adrenal is composed of 2 interdependent parts, the outer cortex and the internal medulla, enclosed in a fibrous capsule. The cortex and medulla have separate embryonic origins and separate functions and are involved in the synthesis and secretion of different hormones. The neuroendocrine medullar portion of the gland is not essential for viability. However, cortical hormones are required for life. Transgenic mouse models played a major role in establishing the requirements for the specific compartments of the adrenal gland. In rodents, the glucocorticoid (GC) corticosterone is required for normal lung development of the fetus (1), and the mineralocorticoid aldosterone regulates sodium retention and water balance after birth (1–3). Recent research also highlighted the role for GCs in stress and social adaption during adolescence (4, 5).
The pioneering work by the Parker and Morohashi laboratories (6–8) spearheaded the subsequent research effort that firmly established the commanding position of the transcription factor steroidogenic factor 1 (SF1) (SF1/adrenal 4-binding protein/nuclear receptor subfamily 5, group A, member 1) in adrenal development. SF1 defines steroidogenic cell identity in the fetal and adult adrenal cortices, acts to promote steroidogenic cell proliferation, and activates steroidogenic gene expression. In addition to SF1, several other transcription factors and paracrine and morphogenic pathways figure prominently in adrenocortical development (reviewed in Ref. 9). The transcription factor Wilm's tumor 1 (WT1) is required for the initial specification of the adrenocortical cell progenitors but has to be down-regulated to commence steroidogenic differentiation (10). GATA binding protein (GATA) 4 and GATA6 transcription factors have long been implicated in adrenal development (11) and adult adrenal function (12–14; reviewed in Ref. 15). Recently, a role for GATA4 (along with WT1, zinc finger protein GLI1, and transcription factor 21) in the long-living progenitor population within the adrenal gland has been proposed (10). To determine the function of the GATA proteins in the adrenal gland, we used a Cre recombination approach to ablate both Gata4 and Gata6 genes. We determined that adrenal loss of GATA function is incompatible with adrenocortical development. Specifically, a combined loss of GATA4 and GATA6 in the precursor cells results in the loss of SF1 expression, decreased adrenocortical proliferation, and adrenal agenesis in both sexes. The Sf1Cre;Gata4flox/floxGata6flox/flox females (conditional double mutants) harboring this double deletion died. However, Sf1Cre;Gata4flox/floxGata6flox/flox males survived and had normal lifespans, likely due to the shift of the vital steroidogenic synthesis to their testes. GATA6 loss alone is compatible with adrenal development and leads to adrenal hypoplasia. In summary, GATA6 serves as a principal driver of adrenocortical cell maintenance, whereas GATA4 protein acts in an ancillary role, carrying out basic regulatory functions to support the requisite number of steroidogenic cells to assure animal viability in the absence of GATA6.
Materials and Methods
Mouse strains
All animal experiments were approved by the University of Florida Institutional Animal Care and Use Committee. The Gata4flox/flox (16) and Gata6flox/flox (17) strains were obtained from The Jackson Laboratory, and the transgenic Sf1Cre mice were a kind gift from the late Dr Keith Parker (18). Sf1Cre;Gata4flox/+Gata6flox/flox males were backcrossed with Gata4flox/floxGata6flox/flox (double flox) females to generate Sf1Cre;Gata4flox/floxGata6flox/flox (conditional double mutant) animals. ROSAmT/mG males (19) (also from The Jackson Laboratory) were crossed with double flox females to obtain ROSAmT/mG;Gata4 flox/floxGata6flox/flox animals. ROSAmT/mG;Gata4flox/floxGata6flox/flox were backcrossed with Sf1Cre;Gata4flox/+Gata6flox/flox to generate the Cre-reporter animals (ROSAmT/mG;Sf1Cre;Gata4flox/flox Gata6flox/flox) with Gata deletions. Gata4flox/floxGata6flox/flox (Sf1Cre-negative) animals were used as experimental the controls. The primers used for genotyping were obtained from Integrated DNA Technologies and are shown in Supplemental Table 1.
Immunofluorescence (IF) staining
Torsos and adrenal glands were collected from the controls, Sf1Cre;Gata4flox/+Gata6flox/flox, Sf1Cre;Gata4flox/floxGata6flox/flox, ROSAmT/mG;Sf1Cre;Gata6flox/flox, and ROSAmT/mG;Sf1Cre;Gata4flox/+Gata6flox/flox mice at embryonic day (E)13.5, E15.5, and fixed overnight in 4% (wt/vol) paraformaldehyde. Samples were dehydrated and then rehydrated in graded methanol series followed by overnight saturation in 30% (wt/vol) sucrose. Optimal cutting temperature-embedded sections were processed as previously described (20, 21). The primary and secondary antibodies used are listed in the antibody table (Table 1).
Table 1.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| GATA 4 | GATA 4 (C-20) | Santa Cruz Biotechnology, Inc; SC-1237 | Goat; polyclonal | 1:300 |
| GATA 6 | GATA 6 | Cell Signaling; 5851 | Rabbit; monoclonal | 1:300 |
| 3βHSD | 3βHSD (P-18) | Santa Cruz Biotechnology, Inc; SC-30820 | Goat; polyclonal | 1:300 |
| Tyrosine hydroxylase | Antityrosine hydroxylase | Millipore; AB152 | Rabbit; polyclonal | 1:300 |
| Steroidogenic factor 1 (SF1) | Antimouse Nr5a1 (Ad4BP/SF-1) | Transgenic, Inc; KO610 | Rat; monoclonal | 1:300 |
| Neurofilament 68 | Anti-68-kDa NF | Abcam; ab72997 | Chicken; polyclonal | 1:300 |
| Bromodeoxyuridine (BrdU) | Anti-BrdU proliferation marker | Abcam; ab1893 | Sheep; polyclonal | 1:300 |
| CYP21A2 | Anti-CYP21A2 | Sigma-Aldrich; HPA048979 | Rabbit; polyclonal | 1:300 |
| Ki67 | Anti-Ki67 antibody | Abcam; 66155 | Rabbit; polyclonal | 1:300 |
| Goat IgG | Alexa Fluor 488 donkey antigoat IgG | Life Technology; A11055 | Donkey; polyclonal | 1:500 |
| Goat IgG | Alexa Fluor 555 donkey antigoat IgG | Life Technology; A21432 | Donkey; polyclonal | 1:500 |
| Rabbit IgG | Alexa Fluor 488 goat antirabbit IgG | Life Technology; A11070 | Goat; polyclonal | 1:500 |
| Rabbit IgG | Alexa Fluor 555 goat antirabbit IgG | Life Technology; A21429 | Goat; polyclonal | 1:500 |
| Chicken IgG | Alexa Fluor 488 goat antichicken | Life Technology; A11039 | Goat; polyclonal | 1:500 |
| Chicken IgG | Alexa Fluor 555 goat antichicken | Life Technology; A21437 | Goat; polyclonal | 1:500 |
| Rat IgG | Alexa Fluor 488 donkey antirat | Life technology; A21208 | Donkey; polyclonal | 1:500 |
| Rat IgG | Alexa Fluor 594 donkey antirat | Life Technology; A21209 | Donkey; polyclonal | 1:500 |
| Sheep IgG | Alexa Fluor 488 donkey antisheep | Life Technology; A11015 | Donkey; polyclonal | 1:500 |
RNA extraction and cDNA synthesis
Total RNA was isolated from controls and Sf1Cre;Gata4flox/floxGata6flox/flox testes at E15.5 and 18.5. Similarly, RNA was isolated from controls, Sf1Cre;Gata4flox/+Gata6flox/flox and Sf1Cre;Gata4flox/floxGata6flox/flox adrenal glands using the TRI Reagent (Sigma-Aldrich) following the manufacturer's instructions. The details of the procedure are described in Supplemental Materials and Methods.
Quantitative RT-PCR (qPCR)
Power SYBR Green PCR Master Mix (Applied Biosystems) was used to perform qPCRs in an ABI 7500 system (Applied Biosystems). The primers (Integrated DNA Technologies) used for qPCRs are listed in Supplemental Table 2. The details of the procedure are described in Supplemental Materials and Methods.
Immunohistochemistry
Ovaries at postnatal day (PND)4 and testes at E18.5 from controls and Sf1Cre;Gata4flox/floxGata6flox/flox animals were fixed as described above for the IF experiments. OCT-embedded samples were sectioned on the cryostat (5–7 μm) and mounted on slides. Sections were blocked with BLOXALL (Vector Laboratories, Inc) for 30 minutes at room temperature followed by one-hour incubation with a rabbit anti-Cytochrome P450, family 21, subfamily a, polypeptide 1 (CYP21A2) antibody (Sigma-Aldrich) in Dako diluting buffer (Dako North America, Inc) at room temperature. CYP21A2 antibody was detected with the immPRESS 3, 3′-diaminobenzidine peroxidase reagent (Vector Laboratories, Inc). Hematoxylin (Fisher Scientific) was used for counterstaining.
Proliferation assays
5-Bromo-2′-deoxyuridine (BrdU) and antigen Ki67 (Ki67) proliferation assays
Pregnant females were sacrificed after 2 hours of receiving an ip injection of BrdU (Sigma-Aldrich) at 0.1 mg/g of body weight. Embryos were harvested and processed as described above for the IF experiments. The staining procedure and statistical analysis are described in detail in Supplemental Materials and Methods. For Ki67, IF analysis was performed as described above, with adrenal sections from the controls and Sf1Cre;Gata4flox/+Gata6flox/flox fetuses (n = 3) costained with the antibody against the proliferation-associated Mki67 protein and the antibodies against SF1, GATA4, and neurofilament (NF)68 proteins and counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Terminal deoxynucleotidyl transferase [TdT]-mediated dUTP nick end labeling (TUNEL) assay
Cryosections from the control, Sf1Cre;Gata4flox/+Gata6flox/flox, and Sf1Cre;Gata4flox/floxGata6flox/flox adrenals at E15.5 were processed for TUNEL staining using an in situ cell death detection kit (TUNEL; Roche Diagnostics Corp), as previously described (21, 22)
Rescue of Sf1Cre;Gata4flox/floxGata6flox/flox females by GC supplementation
The controls and Sf1Cre;Gata4flox/floxGata6flox/flox females were identified by genotyping at PND2 and treated with a rescue cocktail containing 2 hormone analogs: fludrocortisone acetate (Sigma-Aldrich) and dexamethasone 21-phosphate (Santa Cruz Biotechnology, Inc) at 0.025 and 0.02 mg/kg, respectively. The details of the rescue protocol are provided in Supplemental Materials and Methods.
Glucose concentration
Glucose concentration was determined from whole blood of the female animals used in the GC supplementation procedure and from the control and Sf1Cre;Gata4flox/floxGata6flox/flox males (n = 3 from each genotype) at PND60 using a human commercial over the counter device (TRUEresult and TRUEtest system) following the manufacturer's instructions. The details of the procedure are described in Supplemental Materials and Methods.
Plasma and intratesticular corticosterone concentration
Corticosterone concentration was determined by using the competitive Cayman's corticosterone kit (Cayman Chemical Co) according to the vendor's specifications. The details of the procedure and the statistical analysis are described in Supplemental Materials and Methods.
Results
Mice with SF1Cre-mediated loss of GATA4 and GATA6 proteins lack adrenal glands.
In the course of experiments aimed at the characterization of gonadal development in animals with Sf1Cre-mediated deletion of Gata4 and Gata6 genes (21, 23), we crossed Sf1Cre;Gata4flox/+Gata6flox/flox males with Gata4flox/floxGata6flox/flox females. Upon genotyping the progeny at weaning, we noted that the sex ratio of the Sf1Cre;Gata4flox/floxGata6flox/flox animals was skewed from the expected 50:50 Mendelian distribution. Specifically, Sf1Cre;Gata4flox/flox;Gata6flox/flox male (XY) animals were present at the expected ratio (∼1/8). However, Sf1Cre;Gata4flox/floxGata6flox/flox female (XX) animals of the same genotype were absent. Upon examination of the genotypes at E15.5, XX animals were also observed at the expected ratio of approximately 1/8. Because we never found dead or resorbed fetuses at E19.5 in the females carrying double mutant litters we concluded that, Sf1Cre;Gata4flox/floxGata6flox/flox females died between the time of birth and the weaning (PND21).
The adrenal gland is one of the few organs where the Sf1Cre transgene is active (18), and both GATA4 and GATA6 transcription factors are expressed (11). Examination of animals at PND9 animals revealed that macroscopic adrenal glands were absent in both genetic sexes of the Sf1Cre;Gata4flox/floxGata6flox/flox genotype (Figure 1, A–D). Therefore, we concluded that adrenal organogenesis is incompatible with the loss of both GATA proteins. Although Sf1Cre;Gata4flox/floxGata6flox/flox females died, males of the same genotype survived after weaning and appeared to have a normal lifespan in the absence of the adrenal glands.
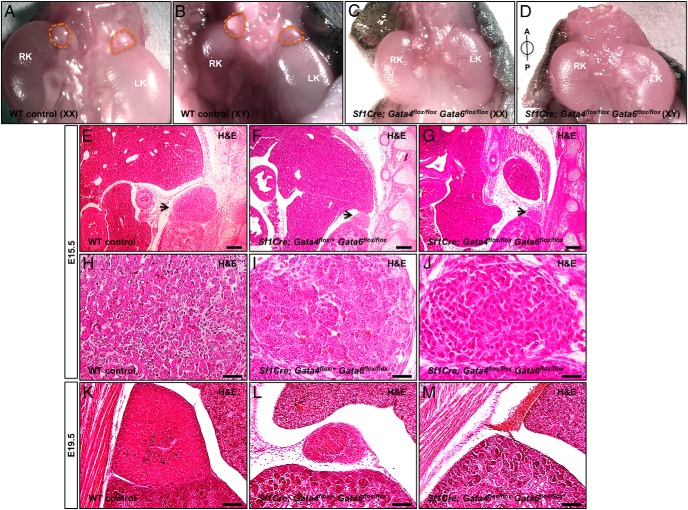
Figure 1.
Adrenal gland aplasia in Sf1Cre;Gata4flox/flox Gata6flox/flox mice. Representative pictures of torsos from the control (A and B) and Sf1Cre;Gata4flox/floxGata6flox/flox (C and D) of female (XX; A and C) and male (XY; B and D) mice at PND9. A and B, Adrenal glands are encircled in dashed orange lines. Note the absence of adrenal glands in Sf1Cre;Gata4flox/floxGata6flox/flox (C and D) animals. RK, right kidney; LK, left kidney; A, anterior; P, posterior. E–M, Histological appearance of the abdominal area of the control (E, H, and K), Sf1Cre;Gata4flox/+Gata6flox/flox (F, I, and L), and Sf1Cre;Gata4flox/floxGata6flox/flox (G, J, and M) fetuses at E15.5 (E–J) and 19.5 (K–M). Sections were stained with hematoxylin and eosin (H&E). Arrows in panels E–G point to the adrenal gland. Panels H–J are higher magnification of the adrenal gland showed in E–G, respectively. Scale bars, 200 μm (E–G), 100 μm (K–M), and 50 μm (H–J).
Embryonic analysis of adrenal development in E15.5 Sf1Cre;Gata4flox/floxGata6flox/flox mice
The adrenal development of the control and Sf1Cre;Gata4flox/floxGata6flox/flox animals was compared at E15.5. The controls had clearly identifiable adrenal glands in the characteristic position juxtaposed against the developing kidneys (Figure 1E). Histological examination of the adrenals revealed the expected heterogeneous cell types corresponding to the developing steroidogenic and adrenergic compartments. The cells with steroidogenic appearance (large polyhedral eosinophilic cells containing vacuoles) comprised most the population, with other cell types also present (Figure 1H). In contrast, Sf1Cre;Gata4flox/floxGata6flox/flox fetuses had underdeveloped rudimentary tissue located either anterior or medially to the kidneys at the transverse level where adrenal glands normally form (Figure 1G). Tight homogenous cell clusters that appeared nonsteroidogenic occupied the adrenal location (Figure 1J). E15.5 fetuses retaining 1 functional allele of Gata4 in the adrenal (Sf1Cre;Gata4flox/+Gata6flox/flox) were also analyzed (Figure 1, F and I). The adrenal primordia that developed in these fetuses were greatly diminished compared with the controls and were of a similar size as the adrenal primordia in the Sf1Cre;Gata4flox/floxGata6flox/flox (Figure 1, G and J). However, these adrenals harbored islands of cells with steroidogenic appearance, suggesting that, in the absence of Gata6, the remaining Gata4 allele is sufficient to support some degree of cortical differentiation (Figure 1I). Furthermore, the examination of the sections obtained from later stage fetuses (E19.5, Figure 1, K–M) identified normal organs in the controls (Figure 1K) and greatly diminished adrenal tissue in the Sf1Cre;Gata4flox/+Gata6flox/flox (Figure 1L); no identifiable structures were observed in this location in the Sf1Cre;Gata4flox/floxGata6flox/flox fetuses (Figure 1M). In contrast, animals retaining a single functional Gata6 allele (Sf1Cre;Gata4flox/floxGata6flox/+) had unremarkable adrenal development indistinguishable from that in the controls (data not shown).
Simultaneous deletion of Gata genes leads to a loss of adrenocortical steroidogenic markers
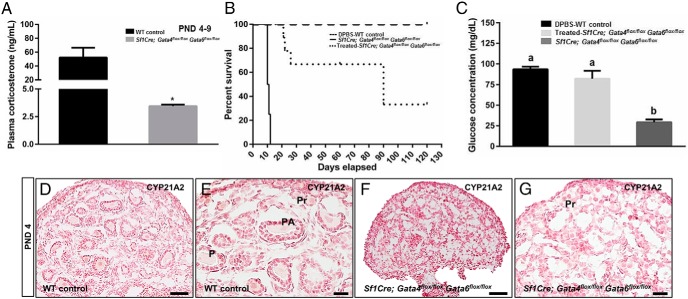
Expression of Gata genes in the developing adrenal glands has been previously examined by in situ hybridization (ISH) and immunohistochemistry at E17 (11). This work showed that Gata6 is strongly expressed in the steroidogenic cells in the developing adrenal gland, whereas Gata4 is mostly restricted to small clusters of subcapsular steroidogenic cells. As shown here, immunofluorescent analysis of GATA protein expression in the control E15.5 adrenal was in general agreement with the previously reported data (11) (Figure 2, A–I). In the capsule, most cells expressed GATA6, with GATA4 present only in a subset of these cells (Figure 2, A–H). Interestingly, GATA4 expression appeared to be notably enriched in the anterior capsular cells compared with the posterior (adjacent to kidney) layer (Figure 2D). The role of GATA4-positive cells as a source of the adrenogonadal progenitor-like population in the adrenal has been recently proposed (10). Adrenocortical cells in the control gland coexpress GATA6 with the steroidogenic master regulator SF1 (Figure 2, A–C), and these SF1- and 3β-hydroxysteroid dehydrogenase/Δ-5-4 isomerase (3βHSD)-positive cells (Figure 2, A–F and I) surround the medulla. 3βHSD is a requisite enzyme for corticosteroid synthesis.
Figure 2.
Loss of steroidogenic cells in Sf1Cre;Gata4flox/floxGata6flox/flox adrenals. Representative sections from controls (A–I) and Sf1Cre;Gata4flox/floxGata6flox/flox (J–O) adrenals at E15.5 were stained for GATA6 (red) and SF1 (green) (A–C, J, and M); GATA4 (red) and SF1 (green) (D–F, K, and N); GATA6 (red) and GATA4 (green) (G); and 3βHSD (red) (I, L, and O) and SF1 (green) (I). DAPI (blue) was used as nuclear staining. In the control adrenals, most SF1-positive cells express GATA6 but not GATA4 (compare B and E). Only anterior capsular cells consistently express GATA4 (arrow in E), whereas most the capsular cells express GATA6 (arrows in B and G). G, A small subset of subcapsular cells (arrowheads) and rare capsular cells (asterisk) coexpresses both GATA factors. In J–O, note that steroidogenic (SF1- or 3βHSD-positive) cells are absent in the Sf1Cre;Gata4flox/floxGata6flox/flox adrenals. B and C, and E and F, are higher magnifications of A and D, respectively, and M–O are higher magnifications of J–L, respectively. Scale bars, 100 μm (A and J–L), 50 μm (D and M–O), and 20 μm (B, C, and E–I).
The examination of the residual tissue present in the location corresponding to the adrenal gland in E15.5 Sf1Cre;Gata4flox/floxGata6flox/flox mice showed the presence of numerous scattered GATA6-positive cells, but the expression of SF1 and 3βHSD was not detected (Figure 2, J, L, M, and O). In the anterior part of the capsule, residual GATA4-positive cells in the Sf1Cre;Gata4flox/floxGata6flox/flox adrenal were primarily retained (Figure 2, K and N). Additionally, adrenocortical protein expression was compared in control and Sf1Cre;Gata4flox/floxGata6flox/flox fetuses at early stages of embryonic development (E13.5) (Supplemental Figure 1). Similarly, we observed no adrenocortical-specific steroidogenic expression in these Sf1Cre;Gata4flox/floxGata6flox/flox fetuses. We concluded that Sf1Cre-mediated loss of Gata4 and Gata6 in the adrenal cortex leads to an early demise of the steroidogenic gene expression program.
Medullar cells arrive in the adrenal space in the Sf1Cre;Gata4flox/floxGata6flox/flox mice
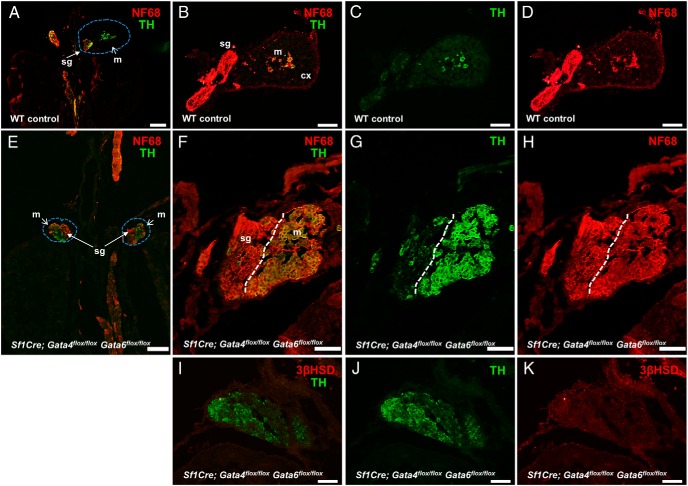
Cells harboring steroidogenic expression were not observed anterior to the kidneys in the Sf1Cre;Gata4flox/floxGata6flox/flox animals (Figure 2, J–O). However, residual tissue was present at this location. As previously reported, the neural crest-derived chromaffin cells migrated into their normal position even in the absence of adrenocortical cells (24, 25), suggesting that the collection of cells in the GATA4 and GATA6 loss-of-function Sf1Cre;Gata4flox/floxGata6flox/flox animals may represent chromaffin tissue.
The developing neurons express a set of genes that is similar to the expression observed in adrenal chromaffin cells. Normally, the intraadrenal location allows one to easily distinguish between the committed chromaffin cells and the adjoining neurons. However, in the complete absence of cortical mass, the developing medullar cells would no longer be partitioned within the defined space. In a similar situation, the neuronal cells were previously definitively distinguished from the closely associated sympathetic neurons by tyrosine hydroxylase (TH) immunoreactivity in combination with NF mRNA ISH (24). In this assay, the adrenal chromaffin progenitor cells were TH-positive but NF-negative (TH+/NF−). However, the sympathetic neuron progenitors were positive for both markers (TH+/NF+) as early as E13.5. We conducted a similar approach to characterize the residual cell clusters in the E15.5 Sf1Cre;Gata4flox/floxGata6flox/flox adrenals, except that the NF68 antibody was used to detect the NF. Unlike previously described NF RNA hybridization (24), chromaffin cell progenitors were also positive for the NF68 pan-neuronal marker, most likely due to a higher sensitivity of the IF assay compared with ISH (Figure 3, A–D). In the control adrenal, the 2 areas were clearly distinguishable as THhigh/NFlow (chromaffin cell location) and THlow/NFhigh (sympathetic neurons). However, in the Sf1Cre;Gata4flox/floxGata6flox/flox adrenal location, 2 types of cells could be identified with the THhigh group located juxtaposed and lateral to the THlow cells; both groups equally stained for NF68 (Figure 3, E–H). These results are in agreement with the previous work that established that the lack of an adrenal cortex upon Sf1 loss is compatible with the generation and differentiation of chromaffin cells (24). In summary, collective data strongly suggest that the lateral THhigh cells are chromaffin progenitors that, even in the absence of the adrenocortical cells in the Sf1Cre;Gata4flox/floxGata6flox/flox mice, arrive in the correct anatomical location, differentiate, and coalesce to form a compact cluster (Figure 3, E–H). None of the NF68-positive cells (whether THlow or THhigh) expressed steroidogenic markers (Figure 3, I–K).
Figure 3.
The adrenal area in Sf1Cre;Gata4flox/floxGata6flox/flox fetuses is occupied by medullar cells. Torso sections of control (A–D) and Sf1Cre;Gata6flox/flox (E–K) fetuses at E15.5 were stained for TH (green) and NF (NF68) (red) (A, B, E, and F), 3βHSD (red) and TH (green) (I). In A and E, adrenals are encircled by a blue dashed line, and B–D and F–H are higher magnifications of A and E, respectively. In the control, the medullar (THhigh/NFlow) and the nervous (THlow/NFhigh) zones are separated by the negative adrenocortical mass (A and B). Note that the Sf1Cre;Gata4flox/floxGata6flox/flox animals lack of adrenocortical cells, these 2 populations are directly juxtaposed (E and F). Also note in I and K the absence of steroidogenic cells (defined by 3βHSD staining) in the adrenal glands of Sf1Cre;Gata4flox/floxGata6flox/flox fetuses. cx, cortex; m, medulla; sg, sympathetic ganglion. Scale bars, 200 μm (A and E), 100 μm (B–D), and 50 μm (F–K).
The loss of Gata6 in steroidogenic cell progenitors is compatible with steroidogenesis
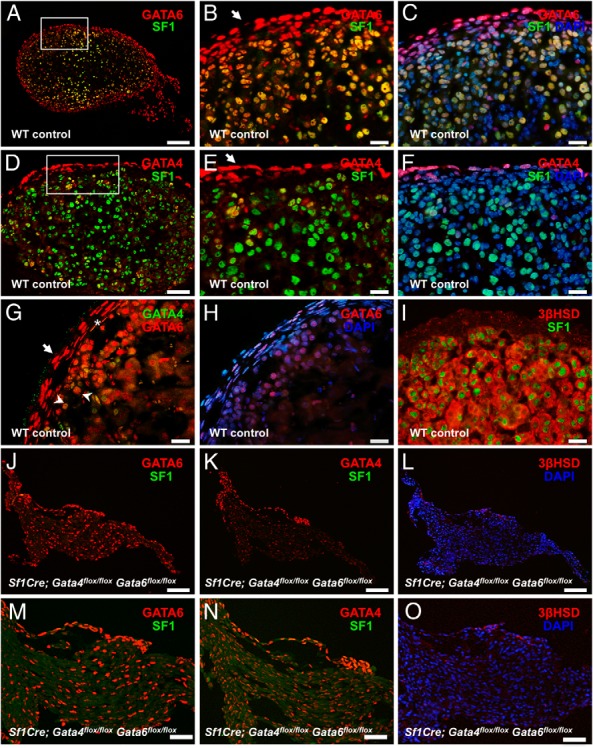
Previous work using Gata4−/− embryonic stem cells in chimera complementation experiments led to the conclusion that GATA4 is dispensable for early adrenocortical differentiation (11). Our studies have suggested that Gata4 and Gata6 are required for the initiation or the early maintenance of steroidogenic fate in the adrenal gland. To better understand the mechanism underlying GATA protein function, fetuses carrying single deletions of either transcription factor were examined. The adrenal glands were present in the E15.5 Sf1Cre;Gata4flox/flox fetuses and were indistinguishable from the control organs (data not shown). In contrast, the adrenal glands in the Sf1Cre;Gata6flox/flox mice were notably smaller compared with the controls (Supplemental Figure 2). Similar to the previously reported animals harboring adrenal-specific deletion of Gata6 (12), the Sf1Cre;Gata6flox/flox animals of both sexes were viable and fertile.
To examine the adrenal phenotype in the Sf1Cre;Gata6flox/flox animals, the glands in E15.5 ROSAmT/mG;Sf1Cre;Gata6flox/flox fetuses were analyzed. In these animals, Sf1Cre-mediated recombination of the mT/mG reporter (19) permanently induced enhanced green fluorescent protein (EGFP) expression. This manipulation enabled the tracking of cell fate, in which Cre recombinase was active (Figure 4, G–L). SF1 staining of the sectioned adrenal glands from the E15.5 ROSAmT/mG;Sf1Cre;Gata6flox/flox fetuses revealed that steroidogenic (SF1-positive) adrenocortical cells developed in the absence of GATA6 (Figure 4, G–L). No ectopic steroidogenic expression was observed outside of the EGFP-positive cells. Although numerous GATA6-positive cells were present in the suprarenal location, the sparse adrenocortical (EGFP/SF1-positive) cells did not express GATA6 (compare Figure 4, J and K). We concluded that GATA6 expression is required for the development of the adrenal cortex and the generation of the full complement of adrenocortical cells. The presence of SF1-positive; GATA6-negative cells (Figure 4J, arrows) implies that GATA6 is not absolutely required for establishing steroidogenic cell fate in the developing adrenal gland (see also Ref. 12).
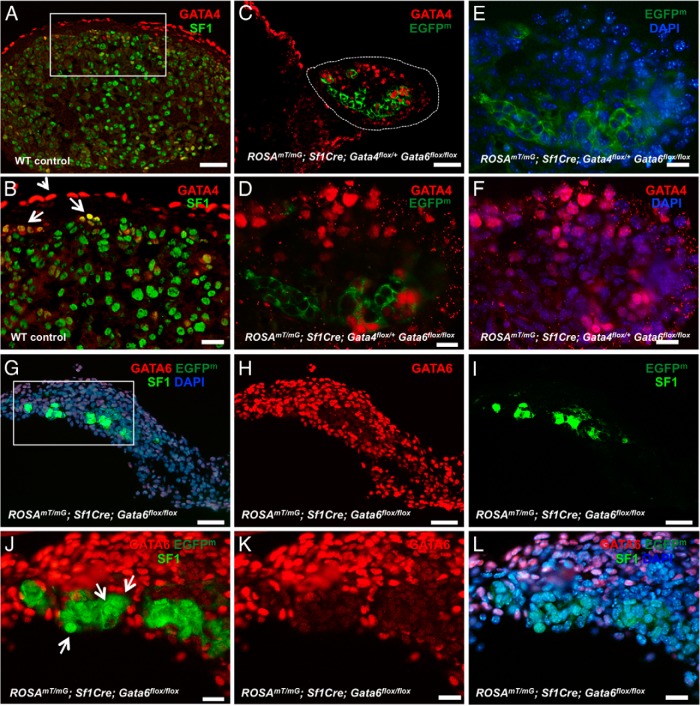
Figure 4.
Adrenocortical development is severely impaired in the absence of GATA6. A–F, Representative sections from control (A and B) and ROSAmT/mG; Sf1Cre;Gata4flox/+Gata6flox/flox (C–F) adrenals at E15.5 were stained for SF1 (bright green) (A and B) and GATA4 (red) (A–D and F). Cells that underwent Sf1Cre-mediated recombination are traced by membrane EGFP (EGFPm) (dark green). In B note that GATA4 expression is mostly limited to capsular cells (arrowhead), with rare cortical cells expressing the protein (arrows). In contrast, numerous cortical cells are GATA4-positive in the ROSAmT/mG; Sf1Cre;Gata4flox/+Gata6flox/flox adrenal (C, D, and F; encircled in C). In C–E only some EGFP-positive cells express GATA4 (see also Supplemental Figure 4). G–L, A limited number of steroidogenic cells is present in the ROSAmT/mG; Sf1Cre;Gata6flox/flox adrenals. Adrenal sections of ROSAmT/mG; Sf1Cre;Gata6flox/flox at E15.5 were stained with antibodies against GATA6 (red; G, H, and J–L) and SF1 (bright green) (G, I, J, and L). EGFPm expression (dark green) traces SF1Cre-mediated excision (G, I, J, and L). In J, arrows point to SF1-positive nuclei that are strictly confined to the EGFPm-positive/GATA6-negative cells. J–L, Higher magnifications of a rectangular area in G. Scale bars, 50 μm (A, C, and G–I) and 20 μm (B, D–F, and J–L).
Steroidogenic development in animals that retain a sole functional Gata4 allele
Adrenocortical development in animals with a Gata4 conditional deletion is normal, and in mice with a Gata6 deletion, adrenocortical development is severely impaired but not completely abrogated. In contrast, the simultaneous loss of Gata4 and Gata6 gene expression leads to a complete demise of adrenocortical cells. To reconcile these 2 observations, we hypothesized that in the absence of GATA6, GATA4 takes over the functions of its primary adrenal counterpart, GATA6, in organizing cortical development.
To explore this possibility, animals with a Gata6 deletion that carry only 1 functional allele of Gata4, Sf1Cre;Gata4flox/+Gata6flox/flox, were examined. Similar to the single Gata6 deletion, Sf1Cre;Gata4flox/+Gata6flox/flox animals are fertile and were used as breeders to generate conditional double knockout Sf1Cre;Gata4flox/floxGata6flox/flox animals in this project. At E15.5, Sf1Cre;Gata4flox/+Gata6flox/flox fetuses harbored severely underdeveloped organs (Figure 1F, arrow) that were similar in size to residual adrenal tissue in the Sf1Cre;Gata4flox/flox Gata6flox/flox animals (Figure 1G, arrow). However, in contrast to the Sf1Cre;Gata4flox/floxGata6flox/flox fetuses (Figure 2, J–O), adrenal glands in animals retaining GATA4 expression harbored a small number of steroidogenic cells that express SF1 and 3βHSD (Figure 5, C–J). These cell clusters developed side-by-side with the medullar (TH-positive) cells (Figure 5, C–H). Similar to ROSAmT/mG;Sf1Cre;Gata6flox/flox adrenals (Figure 4, G–L), SF1 and 3βHSD expression in the ROSAmT/mG;Sf1Cre;Gata4flox/+Gata6flox/flox mutants was confined to the EGFP-positive cells.
Figure 5.
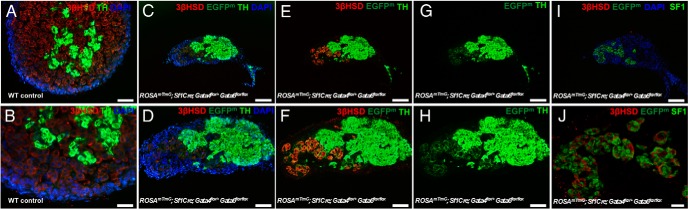
The sole functional allele of the Gata4 gene is sufficient to support adrenocortical development and steroidogenesis. Adrenal sections from control (A and B) and ROSAmT/mG; Sf1Cre;Gata4flox/+Gata6flox/flox (C–J) fetuses at E15.5 were stained for 3βHSD (red) (A–F) and TH (bright green) (A–H); SF1 (bright green) (I and J) and 3βHSD (red) (I and J). In the ROSAmT/mG; Sf1Cre;Gata4flox/+Gata6flox/flox adrenals, EGFP expression traces SF1Cre-mediated gene excision only by displaying membrane staining (EGFPm, dark green) (C–J). DAPI was used as nuclear staining (blue). Scale bars, 200 μm (C, E, G, and I), 100 μm (A, D, F, and H), 50 μm (D, F, and H), and 20 μm (B and J).
GATA4 expression in the control embryonic adrenal gland is mostly limited to the SF1-negative capsular cells (Figure 4, A and B, arrowhead; see also Figure 2, D–H). Rare doubly GATA4- and SF1-positive cells are clustered in the subcapsular region of the developing adrenal glands and likely represent the stem cell/progenitor population (10, 11, 19) (Figure 4B, arrows). In contrast, in the Sf1Cre;Gata4flox/+Gata6flox/flox adrenal, the cells that express GATA4 protein were more numerous and observed throughout the adrenal cortex (Figure 4, C–F). GATA4 expression only partially overlaps with the EGFP-positive staining that marks the cells with steroidogenic fate. In general, GATA4 presence in the steroidogenic cells is not required to maintain SF1 expression (Supplemental Figure 3, A–F); in fact, most the residual SF1-positive cells in the E15.5 Sf1Cre;Gata4flox/+Gata6flox/flox adrenals no longer express GATA4 (Supplemental Figure 3, G–L). The compensatory expression of the single Gata4 allele in Sf1Cre;Gata4flox/+Gata6flox/flox adrenals is better revealed at E13.5 (Supplemental Figure 4). Numerous doubly positive GATA4; SF1 cells are present in the Sf1Cre;Gata4flox/+Gata6flox/flox adrenals (Supplemental Figure 4, E–H) and are not observed in the controls (Supplemental Figure 4, A–D). Therefore, GATA6 is likely providing the necessary GATA function for steroidogenic differentiation under normal conditions. These findings further support the hypothesis that GATA-dependent cell commitment to steroidogenic fate is restricted early in the adrenal development.
Quantitative analysis of RNA expression upon Gata genes deletion
To quantify the RNA expression of cortical and medullar enzymes in the adrenal tissue, we performed qPCR. The decrease in the expression of steroidogenic genes upon double deletion was significant (P < .01 to P < .001) for Star, Cyp11a1, Cyp21a1, and Cyp11b1, confirming the agenesis of the adrenal cortex upon GATA loss (Figure 6). In animals retaining 1 functional Gata4 allele (Sf1Cre;Gata4flox/+Gata6flox/flox) a similar, but less comprehensive, reduction in steroidogenic expression was also observed, corresponding to a drastic decrease in the number of SF1-positive cells in this genotype (Figure 5). In both classes of adrenal mutant samples (Sf1Cre;Gata4flox/+Gata6flox/flox and Sf1Cre;Gata4flox/floxGata6flox/flox), the expression of medullar Nefl (Nf68) and Th genes was increased (P < .001 and P < .05, respectively) corresponding to the greater ratio of medullar cells in these samples (Figure 3). One gene that was differentially regulated between the Sf1Cre;Gata4flox/floxGata6flox/flox and Sf1Cre;Gata4flox/+Gata6flox/flox was Cyp17a1. The expression of this gene was decreased in the Sf1Cre;Gata4flox/floxGata6flox/flox but significantly up-regulated in Sf1Cre;Gata4flox/+Gata6flox/flox adrenals (P < .01). This result agrees with the previous report describing the deletion of Gata6 in the adult adrenal and likely reflects the acquisition of a Leydig-like phenotype in these GATA4-positive cells (12).
Figure 6.

Gene expression analysis of adrenal glands. RNA expression analysis via qPCR for the steroidogenic enzymes Star, Cyp11a1, Cyp11b1, Cyp21a2, and Cyp17a1 and the medullar-specific genes Th and Nefl. Adrenal glands were obtained from controls (at least n = 5), Sf1Cre;Gata4flox/+ Gata6flox/flox (at least n = 4), and Sf1Cre;Gata4flox/flox Gata6flox/flox (n = 3) at E15.5. Bars represent mean ± SEM of fold change of Sf1Cre;Gata4flox/+Gata6flox/flox (black bar) and Sf1Cre;Gata4flox/floxGata6flox/flox (gray bar) relative to the controls. Data were analyzed by ANOVA (one-way) followed by Tukey's multiple comparisons test, with significance at *, P < .05; **, P < .01; and ***, P < .001. Statistical difference between Sf1Cre;Gata4flox/+Gata6flox/flox and Sf1Cre;Gata4flox/floxGata6flox/flox is shown by the respective P value.
Postnatal development of Sf1Cre;Gata4flox/floxGata6flox/flox males is supported by the expansion of the adrenal-like cell population in the testis
The adrenal gland is absent in Sf1Cre;Gata4flox/floxGata6flox/flox animals regardless of sex (Figure 1, A–D). However, in contrast to Sf1Cre;Gata4flox/floxGata6flox/flox females, male animals of the same genotype (Sf1Cre;Gata4flox/floxGata6flox/flox) have a normal lifespan. The fetal rodent testis has been recently shown to harbor a small number of adrenal-like cells (26–28). We hypothesized that in the Sf1Cre;Gata4flox/floxGata6flox/flox male mice, this population is activated, thus supporting animal viability. Therefore, we examined the testes of the Sf1Cre;Gata4flox/floxGata6flox/flox males during embryonic development for the presence of adrenal-like cells. We observed a remarkable expansion of the cell population positive for the adrenal specific marker, a key adrenocortical enzyme CYP21A2 (Figure 7). At E18.5, the number of CYP21A2-positive cells in the control testis is quite limited, with rare isolated CYP21A2-positive cells residing in the interstitial space (Figure 7, A and B). In contrast, in the Sf1Cre;Gata4flox/floxGata6flox/flox testis, the number of CYP21A2 cells is greatly increased, and clusters of positive cells are widely distributed throughout the entire organ (Figure 7, C and D). Gene expression analysis of the adrenal steroidogenic pathway in embryonic Sf1Cre;Gata4flox/floxGata6flox/flox testes revealed a significant up-regulation of Cyp21a1 (P < .05) and Hsd3b6 (P < .001) at E15.5 (Figure 7E) and Mc2r (P < .05), Cyp21a1 (P < .01), and Hsd3b6 (P < .001) at E18.5 (Figure 7F).
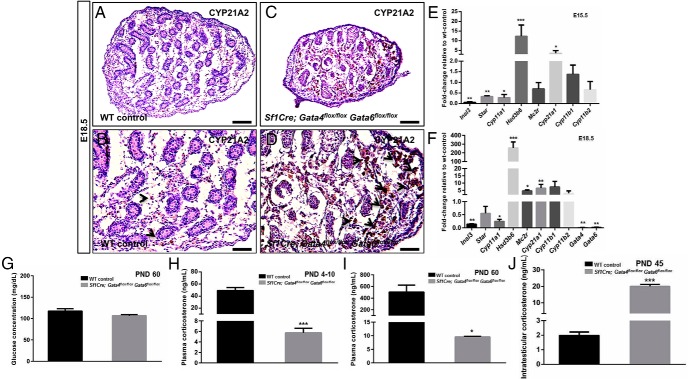
Figure 7.
Presence of adrenal-like cells in the embryonic Sf1Cre;Gata4flox/floxGata6flox/flox testes. Representative testicular sections from controls (A and B) and Sf1Cre;Gata4flox/floxGata6flox/flox (C and D) males at E18.5 were stained for the adrenal enzyme CYP21A2. B and D, Higher magnification of A and C, respectively. In B and D, arrowheads point to CYP21A2-positive cells. Scale bars, 100 μm (A and C) and 50 μm (B and D). E and F, Quantitative changes in the expression of Insl3, Star, Cyp11a1, Hsd3b6, Mc2r, Cyp21a1, Cyp11b1, Cyp11b2, Gata4, and Gata6 in Sf1Cre;Gata4flox/floxGata6flox/flox testes at E15.5 (E) and E18.5 (F). The results are plotted as the mean ± SEM of the fold change relative to the controls from n = 3 biological replicates of each genotype. G, Glucose concentration (mg/dL) from whole blood of the controls (n = 3; black bar) and Sf1Cre;Gata4flox/floxGata6flox/flox (n = 3; gray bar) males at PND60. H and I, Corticosterone concentration in plasma (ng/mL) from the controls (black bar) and Sf1Cre;Gata4flox/flox Gata6flox/flox (gray bar) male mice at PND4–PND10 (n = 4) (L) and 60 (n = 3) (M). J, Intratesticular corticosterone concentration (ng/mL) of the controls (n = 3; black bar) and Sf1Cre;Gata4flox/flox Gata6flox/flox (n = 3; gray bar) mice at PND45. Results are shown as the mean ± SEM, and datasets were analyzed by Student's t test, with significance considered at *, P < .05; **, P < .01; and ***, P < .001.
In addition, we determined the intratesticular corticosterone concentrations in Sf1Cre;Gata4flox/floxGata6flox/flox animals using ELISA (Figure 7J). In Sf1Cre;Gata4flox/floxGata6flox/flox testes, it was significantly elevated (∼20 ng/mL; P < .001) when compared with the control group (∼2 ng/mL). Additionally, plasma corticosterone was measured at PND4–PND10 and PND60 (Figure 7, H and I, respectively). The plasma corticosterone level in Sf1Cre;Gata4flox/floxGata6flox/flox animals was still significantly decreased compared with the controls. However, the circulating level of corticosterone corresponded with the level synthesized by the testis (Figure 7J). In the absence of a functional adrenal gland, the regulation of glucose metabolism by the adrenal hormones is compromised (reviewed in Ref. 9). We determined glucose concentration in whole blood from the controls and Sf1Cre;Gata4flox/floxGata6flox/flox males at PND90, with no difference between the 2 groups (Figure 7G). We concluded that the amount of corticosterone synthesized by the testis is likely sufficient to sustain the life of the Sf1Cre;Gata4flox/floxGata6flox/flox males.
GC supplementation rescues Sf1Cre;Gata4flox/floxGata6flox/flox females
Analysis of adrenal development and steroidogenic gene expression in fetuses harboring the Sf1Cre-mediated double deletion of both Gata genes strongly suggests that adrenocortical function in these animals is completely impaired. Absence of the adrenal gland at the time of birth is currently recognized to be lethal in rodents (7), and this premise is true for Sf1Cre;Gata4flox/floxGata6flox/flox females in which the deletion of both Gata4 and Gata6 leads to death before weaning. We determined corticosterone concentration in plasma from controls and Sf1Cre;Gata4flox/floxGata6flox/flox females between PND4 and PND9 by ELISA (Figure 8A). The mean concentration of corticosterone in Sf1Cre;Gata4flox/floxGata6flox/flox was significantly lower (∼3.4 ng/mL; P < .05) when compared with the control group (∼52 ng/mL). To examine whether the lethality of the Sf1Cre;Gata4flox/floxGata6flox/flox females is due to a lack of hormonal synthesis, we performed a rescue experiment with a steroid supplementation regimen initiated shortly after birth (based on Ref. 3). All of the untreated Sf1Cre;Gata4flox/floxGata6flox/flox females died between PND11 and PND15. However, all of the treated Sf1Cre;Gata4flox/floxGata6flox/flox females were alive at weaning and then required no further supplementation (Figure 8B). Four of these females survived the 3- to 4-month mark, and 2 remained alive at PND150. This result confirms that the lethality of the Sf1Cre;Gata4flox/floxGata6flox/flox females is due to adrenal hormone insufficiency.
Figure 8.
Rescue of Sf1Cre;Gata4flox/floxGata6flox/flox female mice with GC treatment. A, Plasma corticosterone concentration (ng/mL) of the controls (n = 3; black bar) and Sf1Cre;Gata4flox/floxGata6flox/flox (n = 3; gray bar) females between PND4 and PND9. Results are shown as the mean ± SEM, and the data were analyzed by Student's t test, with significance considered at *, P < .05. B, Percent survival of the controls treated with DPBS vehicle (n = 7; dashed line), Sf1Cre;Gata4flox/floxGata6flox/flox females that were injected daily with a cocktail of GCs (fludrocortisone acetate + dexamethasone 21-phosphate) (n = 7; dotted line), and nontreated Sf1Cre;Gata4flox/floxGata6flox/flox females (n = 6; solid line). C, Glucose concentration (mg/dL) in whole blood from the controls treated with DPBS vehicle (black bar), treated Sf1Cre;Gata4flox/floxGata6flox/flox females (light gray bar), and noninjected Sf1Cre;Gata4flox/flox Gata6flox/flox females (dark gray bar). The data are presented as the mean ± SEM, and datasets were analyzed with ANOVA (one-way) followed by Tukey's test for multiple comparisons. Bars with different superscripts differ significantly (***, P < .001). D–G, Representative ovarian sections from the controls (D and E) and Sf1Cre;Gata4flox/floxGata6flox/flox (F and G) at PND4. Sections were stained for the adrenal enzyme CYP21A2. E and G, Higher magnification of D and F, respectively. Note the lack of CYP21A2-positive cells in the ovaries of both genotypes. Pr, primordial; P, primary; PA, preantral. Scale bars, 50 μm (D and F) and 20 μm (E and G).
The glucose concentration was determined in whole blood from the Dulbecco's phosphate-buffered saline (DPBS)-vehicle control, rescued Sf1Cre;Gata4flox/floxGata6flox/flox and noninjected Sf1Cre;Gata4flox/floxGata6flox/flox females 2 weeks after birth. The control females had an average glucose concentration of 93 mg/dL, which is within the normal range. In contrast, the glucose concentration in the nontreated Sf1Cre;Gata4flox/floxGata6flox/flox females was significantly reduced to 29 mg/dL (P < .001), which was barely within the limit of detection. Similarly, glucose levels were measured in the hormone-injected females (Figure 8C). The mean concentration of glucose in the treated group between PND30 and PND90 (82 mg/dL) was improved compared with the nontreated Sf1Cre;Gata4flox/floxGata6flox/flox females (29 mg/dL; P > .001). Thus, glucose level maintenance is compromised in Sf1Cre;Gata4flox/floxGata6flox/flox females in the absence of hormonal supplementation. We also performed IHC experiments to determine the presence of CYP21A2-positive cells in the ovaries of the controls and Sf1Cre;Gata4flox/floxGata6flox/flox females at PND4 and detected no CYP21A2-positive cells in either group (Figure 8, D–G).
In summary, the rescue experiments performed in Sf1Cre;Gata4flox/floxGata6flox/flox females along with the analysis of CYP21A2 expression in Sf1Cre;Gata4flox/floxGata6flox/flox animals strongly suggest that the demise of the adrenal cortex in the Sf1Cre;Gata4flox/floxGata6flox/flox males is adequately compensated by the expansion of the adrenal-like cell population in their testis. These testicular cells are capable of corticosterone synthesis and could be sufficient to sustain life in the absence of the functional adrenal cortex.
Proliferation and apoptosis of the adrenal cells upon GATA protein loss
A dramatic decrease in adrenal size reflected either decreased proliferation or increased cell death upon Gata gene deletion. Adrenocortical cell proliferation in Sf1Cre;Gata4flox/+Gata6flox/flox fetuses was analyzed using the BrdU cell proliferation assay (Supplemental Figure 5). Statistical analysis showed that the ratio between total and proliferating cells was similar in the control and Sf1Cre;Gata4flox/+Gata6flox/flox mice adrenals (Supplemental Figure 5, A and B). However, the ratio of steroidogenic cells among the proliferating cells was significantly decreased (P < .001) (Supplemental Figure 5, C and D). Additionally, we observed a higher number of TUNEL-positive cells in the adrenal location in the Sf1Cre;Gata4flox/floxGata6flox/flox adrenal (Supplemental Figure 5, H–J). At E15.5, most capsular/subcapsular adrenal cells remained highly proliferative in the control and mutant adrenals and many of these were GATA4(+) (Supplemental Figure 6). However, examination of proliferation in Sf1Cre;Gata4flox/+Gata6flox/flox adrenals in cells located outside of the subcapsular area showed no correlation between the cell proliferation status (Ki67-positive) and GATA4 expression (Supplemental Figure 6). The bulk of highly proliferative cells in the E15.5 control and Sf1Cre;Gata4flox/+Gata6flox/flox adrenal core consists of medullar NF68(+)/TH(+) cell (Supplemental Figure 7). We concluded that, upon GATA6 loss, the sole functional GATA4 allele is insufficient to support the normal proliferation of SF1-positive adrenocortical cells. In the absence of the steroidogenic cells, cell death is likely a contributing factor in the ultimate adrenal demise during embryogenesis (eg, Ref. 25) and likely accounts for the absence of medullar components in the Sf1Cre;Gata4flox/floxGata6flox/flox adrenals at E19.5 (Figure 1M).
Discussion
GATA transcription factors (GATA1–GATA6) execute transcriptional control of critical developmental decisions in multiple tissues in vertebrates where they often conduct functions that partially overlap but are not completely redundant (29–32; reviewed in Refs. 15, 33, 34; see also Refs. 35, 36). Although GATA proteins are broadly expressed, SF1 is a master regulator of steroidogenic expression in the adrenal cortex and is required for normal adrenocortical differentiation (7). During adrenal development, the transcription factor WT1 likely serves to initially up-regulate Sf1 expression, and the ability of WT1 to directly activate Sf1 transcription in the testis was described in the past (37).
Hormonal synthesis by the adrenal cortex is required for animal viability, and mice with dysfunctional adrenal glands normally do not survive after 2 weeks after birth without hormonal supplementation (3, 25, 38–40). Unlike their female littermates that die of hormonal insufficiency and subsequent hypoglycemia, Sf1Cre;Gata4flox/floxGata6flox/flox males appear to have a normal lifespan. Both the embryonic and postnatal (23) Sf1Cre;Gata4flox/floxGata6flox/flox testes harbor an expanded adrenal-like cell population that expresses adrenocortical enzymes and is capable of synthesizing GCs. Cells with adrenal-like properties are normally found in the fetal testis. However, their number is very limited (26–28). Furthermore, it has been long suggested (41) that these cells result from the incomplete partition of the adrenal and gonadal progenitor populations during adrenogonadal progenitor separation. However, this hypothesis has not been experimentally proven. The population of cells present in the Sf1Cre;Gata4flox/floxGata6flox/flox testes is reminiscent of a testicular tumor caused by adrenogenital syndrome or testicular adrenal rest tumor cells in human congenital adrenal hyperplasia patients and is the primary reason for their infertility (42). Steroidogenic cells invading Wnt4 mutant ovaries have adrenal-like properties (43), which led to the hypothesis that the WNT pathway is accountable for repelling these cells from entering the ovary. This may explain the absence of adrenal-like cells in the female organ. In contrast, the fate mapping of adrenal cells indicates that the same pathway is unlikely to function in the testis (44).
Previous research has convincingly demonstrated that Nr5a1/Sf1 expression in steroidogenic organs is guided by at least 3 conserved regulatory elements. The proximal Sf1 promoter located within the first 674 bp upstream of the Sf1 transcription site drives Sf1 expression in indifferent (undifferentiated) gonad. The regulatory role of this element appears to be limited to Sertoli and granulosa cell expression (37). Pioneering studies in the Morohashi laboratory identified a fetal adrenal enhancer in the fourth intron of the Sf1 gene. This short (∼650 bp) fragment from the forth intron is sufficient to drive cortico-specific expression of the LacZ reporter in the fetal adrenal gland (45). Lastly, the third element active in fetal Leydig cells has also been described (46).
Our inspection identified a highly conserved GATA site in all of the 3 regulatory elements guiding Sf1 gene expression. The functional significance of GATA binding sites in Sf1 regulatory elements has not been determined yet. The ability of GATA proteins to activate Sf1 expression through different enhancer elements at different developmental times is likely accountable for the survival of adrenal-like population in the testis, but not in the adrenal itself. In this respect, although SF1-positive cells are no longer present in the Sf1Cre; Gata4flox/flox; Gata6flox/flox adrenals (Figure 2, J–O), testicular cells in these animals retain SF1 expression (23).
The most parsimonious scenario for the emergence of the adrenal-like cells in the testis is similar to the pathway presumed to be active in congenital adrenal hyperplasia patients. Namely, the demise of adrenal cortex and GC synthesis leads to a loss of GC-induced feedback inhibition of ACTH production. High ACTH concentration in the fetus stimulates the expansion of the ACTH-responsive progenitor cell population in the testis. At present, the mechanism underlying the execution of this transition is not well understood. Similarly, the relationship between these cells and the fetal or adult Leydig cell progenitors remains to be established. The misallocated adrenal progenitors residing in the testis may be destined to expand into adrenocortical-like cells under certain conditions. Alternatively, the adrenal-like and Leydig cells may share a common progenitor that is directed into an adrenal fate under high ACTH conditions. In addition, whether the loss of GATA factors in the testis is a contributing factor in actively promoting this transition remains unknown. Recapitulating chronic ACTH exposure is technically challenging in neonatal mice, and poor hormonal control is likely to be another factor that provides favorable conditions for the expansion of the adrenal-like testis population. In other words, the animals with functional adrenal glands will respond with increased GC synthesis in response to high ACTH and prevent the expansion of the adrenal tissue in the testis. An increase in the expression of an adrenal marker was observed in the testis of Cyp11a1 null mice (40). However, these animals (being deficient in a key steroidogenic enzyme) did not survive and did not synthesize active hormones.
We believe that Sf1Cre;Gata4flox/floxGata6flox/flox presents a unique experimental model and the opportunity to produce testicular adrenal rest tumor-like tissue in rodents and to understand its origin. This work provides conclusive evidence that the formation of the functional adrenal gland in mice is incompatible with the simultaneous deletion of GATA4 and GATA6 factors in adrenocortical cells. The functions of the 2 proteins in the adrenal do not completely overlap. Loss of GATA6 alone impairs but does not completely preclude adrenocortical formation and steroidogenesis. Hypoplastic adrenal glands in the Sf1Cre;Gata6flox/flox mice contain a diminished number of active steroidogenic cells that express the master regulator SF1 and sustain viability. In the Sf1Cre; Gata6flox/flox adrenal, steroidogenic cells that are GATA6-negative are intermixed with numerous GATA6-positive cells that do not express the steroidogenic markers SF1 or 3βHSD. In contrast, Gata4 deletion is dispensable for adrenal organogenesis. In summary, GATA6 protein is a principal driver of adrenocortical cell maintenance and fully compensates for the absence of GATA4 protein. GATA4 protein is not required for adrenocortical steroidogenic differentiation and hormone synthesis. However, this protein performs essential regulatory functions in the absence of GATA6 and supports the requisite number of adrenal steroidogenic cells to assure viability of Sf1Cre;Gata4flox/+Gata6flox/flox females. The conditional double mutant Sf1Cre;Gata4flox/floxGata6flox/flox males appear to live normal lifespans even in the absence of both GATA proteins as vital steroidogenic synthesis shifts to their testes. In addition to the adrenal phenotype described in this work, Sf1Cre;Gata4flox/floxGata6flox/flox males exhibit a lack of testis functionality, with a loss of normal steroidogenic testis function (23).
Acknowledgments
Present address for M.B.P.: Department of Surgery, School of Medicine, Indiana University, Indianapolis, IN 46202.
This work was supported by University of Florida and by National Institutes of Health Grant R01HD042751.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-bromo-2′-deoxyuridine
- Cyp21a1
- Cytochrome P450, family 21, subfamily a, polypeptide 1
- DAPI
- 4′,6-diamidino-2-phenylindole
- DPBS
- Dulbecco's phosphate-buffered saline
- E
- embryonic day
- EGFP
- enhanced green fluorescent protein
- GATA
- GATA binding protein
- GC
- glucocorticoid
- 3βHSD
- 3β-hydroxysteroid dehydrogenase/Δ-5-4 isomerase
- Ki67
- antigen Ki67
- IF
- immunofluorescence
- ISH
- in situ hybridization
- Ki67
- antigen Ki67
- NF
- neurofilament
- PND
- postnatal day
- qPCR
- quantitative RT-PCR
- SF1
- steroidogenic factor 1
- TH
- tyrosine hydroxylase
- TUNEL
- Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
- WT1
- Wilm's tumor 1.
References
- 1. Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373(6513):427–432. [DOI] [PubMed] [Google Scholar]
- 2. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18(3):361–377. [DOI] [PubMed] [Google Scholar]
- 3. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94(21):11540–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barik J, Marti F, Morel C, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339(6117):332–335. [DOI] [PubMed] [Google Scholar]
- 5. Niwa M, Jaaro-Peled H, Tankou S, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339(6117):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8(5):654–662. [DOI] [PubMed] [Google Scholar]
- 7. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. [DOI] [PubMed] [Google Scholar]
- 8. Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1(7):663–671. [DOI] [PubMed] [Google Scholar]
- 9. Yates R, Katugampola H, Cavlan D, et al. Adrenocortical development, maintenance, and disease. Curr Top Dev Biol. 2013;106:239–312. [DOI] [PubMed] [Google Scholar]
- 10. Bandiera R, Vidal VP, Motamedi FJ, et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiiveri S, Liu J, Westerholm-Ormio M, et al. Transcription factors GATA-4 and GATA-6 during mouse and human adrenocortical development. Endocr Res. 2002;28(4):647–650. [DOI] [PubMed] [Google Scholar]
- 12. Pihlajoki M, Gretzinger E, Cochran R, et al. Conditional mutagenesis of Gata6 in SF1-positive cells causes gonadal-like differentiation in the adrenal cortex of mice. Endocrinology. 2013;154(5):1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura Y, Xing Y, Sasano H, Rainey WE. The mediator complex subunit 1 enhances transcription of genes needed for adrenal androgen production. Endocrinology. 2009;150(9):4145–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kiiveri S, Siltanen S, Rahman N, et al. Reciprocal changes in the expression of transcription factors GATA-4 and GATA-6 accompany adrenocortical tumorigenesis in mice and humans. Mol Med. 1999;5(7):490–501. [PMC free article] [PubMed] [Google Scholar]
- 15. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22(4):781–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci USA. 2004;101(34):12573–12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sodhi CP, Li J, Duncan SA. Generation of mice harbouring a conditional loss-of-function allele of Gata6. BMC Dev Biol. 2006;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44(9):419–424. [DOI] [PubMed] [Google Scholar]
- 19. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 20. Efimenko E, Padua MB, Manuylov NL, Fox SC, Morse DA, Tevosian SG. The transcription factor GATA4 is required for follicular development and normal ovarian function. Dev Biol. 2013;381(1):144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Padua MB, Fox SC, Jiang T, Morse DA, Tevosian SG. Simultaneous gene deletion of Gata4 and Gata6 leads to early disruption of follicular development and germ cell loss in the murine ovary. Biol Reprod. 2014;91(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol. 2011;353:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Padua MB, Jiang T, Morse DA, Fox SC, Hatch HM, Tevosian SG. Combined loss of the GATA4 and GATA6 transcription factors in male mice disrupts testicular development and confers adrenal-like function in the testes. Endocrinology. 2015;156:1873–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gut P, Huber K, Lohr J, et al. Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development. 2005;132(20):4611–4619. [DOI] [PubMed] [Google Scholar]
- 25. Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602. [DOI] [PubMed] [Google Scholar]
- 26. Hu L, Monteiro A, Johnston H, King P, O'Shaughnessy PJ. Expression of Cyp21a1 and Cyp11b1 in the fetal mouse testis. Reproduction. 2007;134(4):585–591. [DOI] [PubMed] [Google Scholar]
- 27. Johnston H, King PJ, O'Shaughnessy PJ. Effects of ACTH and expression of the melanocortin-2 receptor in the neonatal mouse testis. Reproduction. 2007;133(6):1181–1187. [DOI] [PubMed] [Google Scholar]
- 28. Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299(1):250–256. [DOI] [PubMed] [Google Scholar]
- 29. Cantor AB, Orkin SH. Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins. Semin Cell Dev Biol. 2005;16(1):117–128. [DOI] [PubMed] [Google Scholar]
- 30. Chlon TM, Crispino JD. Combinatorial regulation of tissue specification by GATA and FOG factors. Development. 2012;139(21):3905–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275(50):38949–38952. [DOI] [PubMed] [Google Scholar]
- 32. Zaytouni T, Efimenko EE, Tevosian SG. GATA transcription factors in the developing reproductive system. Adv Genet. 2011;76:93–134. [DOI] [PubMed] [Google Scholar]
- 33. LaVoie HA. The role of GATA in mammalian reproduction. Exp Biol Med (Maywood). 2003;228(11):1282–1290. [DOI] [PubMed] [Google Scholar]
- 34. Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates). Curr Opin Genet Dev. 2002;12(4):416–422. [DOI] [PubMed] [Google Scholar]
- 35. Carrasco M, Delgado I, Soria B, Martín F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122(10):3504–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xuan S, Borok MJ, Decker KJ, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122(10):3516–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16(14):1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luo X, Ikeda Y, Schlosser DA, Parker KL. Steroidogenic factor 1 is the essential transcript of the mouse Ftz-F1 gene. Mol Endocrinol. 1995;9(9):1233–1239. [DOI] [PubMed] [Google Scholar]
- 39. Huang CC, Shih MC, Hsu NC, Chien Y, Chung BC. Fetal glucocorticoid synthesis is required for development of fetal adrenal medulla and hypothalamus feedback suppression. Endocrinology. 2012;153(10):4749–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu MC, Hsu NC, El Hadj NB, et al. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol. 2002;16(8):1943–1950. [DOI] [PubMed] [Google Scholar]
- 41. Wilkins L, Fleischmann W, Howard JE. Macrogenitosomia precox associated with hyperplasia of the androgenic tissue of the adrenal and death from corticoadrenal insufficiency. Endocrinology. 1940;26:385–395. [Google Scholar]
- 42. Claahsen-van der Grinten HL, Hermus AR, Otten BJ. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2009;2009:624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358–4365. [DOI] [PubMed] [Google Scholar]
- 44. Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28(23):7030–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shima Y, Miyabayashi K, Baba T, et al. Identification of an enhancer in the Ad4BP/SF-1 gene specific for fetal Leydig cells. Endocrinology. 2012;153(1):417–425. [DOI] [PubMed] [Google Scholar]