Abstract
Bone morphogenetic proteins (BMPs) comprise one of the largest subgroups in the TGF-β ligand superfamily. We have identified a functional BMP system equipped with the ligand (BMP4), receptors (BMP type II receptor, BMP type IA receptor, also called ALK3) and the signaling proteins, namely the mothers against decapentaplegic homologs 1, 4, and 5 in the human adrenal gland and the human adrenocortical cell line H295R. Microarray, quantitative RT-PCR, and immunohistochemistry confirmed that BMP4 expression was highest in the adrenal zona glomerulosa followed by the zona fasciculata and zona reticularis. Treatment of H295R cells with BMP4 caused phosphorylation of the mothers against decapentaplegic and a profound decrease in synthesis of the C19 steroids dehydroepiandrosterone (DHEA), DHEA sulfate, and androstenedione. Administration of BMP4 to cultures of H295R cells also caused a profound decrease in the mRNA and protein levels of 17α-hydroxylase/17,20-lyase (CYP17A1 and P450c17, respectively) but no significant effect on the mRNA levels of cholesterol side-chain cleavage cytochrome P450 (CYP11A1) or type 2 3β-hydroxysteroid dehydrogenase (HSD3B2). Furthermore, Noggin (a BMP inhibitor) was able to reverse the negative effects of BMP4 with respect to both CYP17A1 transcription and DHEA secretion in the H295R cell line. Collectively the present data suggest that BMP4 is an autocrine/paracrine negative regulator of C19 steroid synthesis in the human adrenal and works by suppressing P450c17.
The human adrenal cortex is anatomically and functionally classified into three zones, the zona glomerulosa (ZG), the fasciculata (ZF) and the reticularis (ZR), each producing a different class of steroids. ZG and ZF have been shown to synthesize mineralocorticoids and glucocorticoids, respectively (1, 2). The human ZR is considered the primary source for the C19 steroids like dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEA-S) (3–5). Although some steroidogenic enzymes and cofactor proteins are common to all zones of the cortex, the zone-specific production of steroids is a result of the differential expression of key steroidogenic enzymes (6–9). The pathway leading to the synthesis of DHEA requires only two steroidogenic enzymes, namely cholesterol side-chain cleavage cytochrome P450 and cytochrome P450 17α-hydroxylase/17,20-lyase (P450c17). Whereas cholesterol side-chain cleavage cytochrome P450 has been shown to be present in all three zones of the adrenal cortex, P450c17 is expressed in ZF and ZR (10). It is known that the 17α-hydroxylase activity of P450c17 is required for the production of the glucocorticoid cortisol in the ZF, and both the 17α-hydroxylase and 17,20-lyase activities of the P450c17 enzyme are mandatory for the synthesis of C19 steroids in the ZR (11–13). Other vital factors that contribute toward the C19 steroid production by the ZR are the ZR dominance of cytochrome b5, an allosteric regulator of P450c17, enhancing the 17,20-lyase activity of the enzyme, and the distinctly reduced expression/activity of type 2 3β-hydroxysteroid dehydrogenase (HSD3B2) in the ZR (8, 14, 15).
Bone morphogenetic proteins (BMPs) comprise one of the largest subgroups in the TGF-β ligand superfamily. The name BMP was initially given to three proteins purified from a bovine bone preparation that were independently capable of inducing the formation of cartilage and bone in vivo (16). Currently 15 BMPs have been described and recognized as multifunctional regulators involved in a myriad of processes in numerous tissues, including cell growth, apoptosis, differentiation, and migration (17). BMPs are known to act through a combination of type II and type I BMP receptors. Binding of BMP to its type II receptor in concert with a type I receptor leads to formation of a receptor complex. The constitutively active kinase domains of the type II receptor phosphorylate type I receptor, rendering it active. The active type I receptor subsequently phosphorylate a receptor-regulated mothers against decapentaplegic homolog (R-SMAD; eg, SMAD 1/5/8), allowing it to associate with the co-SMAD (SMAD 4) to form a heteromeric complex that translocates to the nucleus and stimulates the expression of target genes (18).
The expression levels of individual members of the BMP superfamily not only vary among different tissues, but each BMP member also differs in its functional effect, depending on the cell type. Some BMPs (BMP2, BMP4, BMP6, BMP7, BMP15) have also been shown to regulate steroidogenesis in the human ovarian tissue and cell lines (19–21). However, currently there is limited knowledge regarding the role of the BMP system in human adrenal steroidogenesis. BMP6 has been shown to augment angiotensin II (AngII)-induced aldosterone secretion in the human adrenocortical H295R cell line (22, 23). In contrast, BMP2 and BMP5 were able to suppress forskolin-stimulated steroidogenesis (aldosterone, cortisol and DHEA-S) in H295R cells (24).
In this study, we identified a functional BMP system equipped with the ligand (BMP4), receptors [BMP type II receptor, activin receptor-like kinase receptor 3 (ALK3), also called BMP type IA receptor] and the signaling proteins (SMAD 1, SMAD 4, and SMAD 5) in the human adrenal gland. Using the H295R human adrenocortical cell line, we demonstrate that BMP4 can act as an autocrine/paracrine negative regulator of adrenal C19 steroid synthesis.
Materials and Methods
Human tissue preparation
After receiving informed consent from the family, human adult adrenal glands were obtained from cadaveric kidney donors that were transplanted at the Georgia Regents University (Augusta, Georgia). Use of these tissues was approved by the Institutional Review Board of Georgia Regents University.
Isolation of ZG, ZF, and ZR tissues by laser-capture microdissection from human adult adrenals
Adrenal pieces of 3 mm were cut from donor adrenal glands and frozen in optimum cutting temperature compound (O.C.T. block; Sakura Finetek). Frozen adrenal glands in the optimum cutting temperature compound were cut into 7-μm sections and mounted onto Superfrost Plus microscope slides (Fisher Scientific). To recognize enriched populations of aldosterone-producing ZG, the sections were immunostained for CYP11B2 (aldosterone synthase; monoclonal mouse antibody generously provided by Dr Celso Gomez-Sanchez, University of Mississippi Medical Center, Jackson, Mississippi). For the immunohistochemistry, sections were fixed with 100% acetone and incubated with primary/secondary antibodies without antigen retrieval. The remaining tissue sections were stained with cresyl violet and used for laser-capture microdissection as previously reported (25, 26). ZG cells were captured from CYP11B2-positive cells based on CYP11B2-stained sections. ZF and ZR were captured for transcriptome comparison from lipid-rich cells in the middle layer (ZF) or compact cells outside of the medulla (ZR).
Transcriptome analysis of laser-captured ZG, ZF, and ZR
RNA from ZG, ZF, and ZR cells was isolated using a PicoPure RNA isolation kit (Life Technologies). Total RNA (1–10 ng) from ZG, ZF, and ZR samples was submitted to the University of Michigan DNA Sequencing and Microarray Cores for RNA amplification to reverse transcription, which were performed using the Ovation Pico WTA System V2 (NuGEN Technologies). The cDNA was purified using QIAquick PCR purification kit (QIAGEN) and biotin labeled using an Encore Biotin Module (NuGEN Technologies), followed by hybridization to GeneChip Human Genome U133 Plus 2.0 Array (Affymetrix). This array was designed to investigate 21 702 genes including 18 802 distinct, unambiguous genes on the human genome, with 54 675 probe sets comprised of 11 perfect match probes per probe set. Expression values for each probe set were calculated using a robust multiarray average method (27). We fit two-way ANOVA models with terms for three tissues (ZG, ZF, and ZR) and four people (respective patients) to log-transformed data for each probe set and used the resulting F tests to compare pairs of tissues. To identify the differences among the three zones, a list of ZG, ZF, and ZR markers was created by using transcripts satisfying the conditions including fold expression differences and statistical differences between the respective zones.
Residual RNA, which was prepared in the transcriptome analysis, was used for confirmation quantitative RT-PCR (qPCR) of BMP4. These RNAs were again amplified and reverse transcribed to cDNA as described above. For qPCR, 1 ng of prepared cDNA was mixed with Fast Universal PCR master mix (Applied Biosystems), following the manufacturer's recommendations, and stored at −80°C until further use.
Cell culture and treatments
The H295R human adrenocortical cell line was cultured in DME/F12 medium (Life Technologies) supplemented with 10% cosmic calf serum (GE Healthcare Life Sciences, HyClone Laboratories) and antibiotics including penicillin/streptomycin and gentamicin (Sigma-Aldrich). The cells were incubated under a humid atmosphere of 5% CO2, at 37°C, and the medium was changed every 2 days. H295R cells were plated at a density of 100 000 cells/well (48 well dish) in growth medium and grown to 60% confluence after which they were starved in low serum medium (1% cosmic calf serum and antibiotics) 18 hours prior to treatment with BMP4 (ProSpec).
Primary adrenal cells were isolated as previously described (28). Briefly, adrenal tissue was minced and dissociated into a single-cell suspension by repeated exposure of the tissue fragments to DMEM/F12 medium containing 1 mg/mL collagenase dispase and 0.25 mg/mL deoxyribonuclease 1 (Hoffmann-La Roche Ltd). Four 1-hour cycles of digestion at 37°C and mechanical dispersion were performed. Cells were collected between each digestion and combined prior to storage at −150°C. Primary adrenal cells were plated at a density of 20 000 cells/well (48 well dish) in growth medium and grown to 60% confluence after which they were starved in low serum medium 18 hours prior to treatment with BMP4.
To study the effect of BMP4 on dibutyryl cAMP (dbcAMP)-stimulated steroidogenesis, H295R cells were initially treated with 30 ng/mL BMP4 for 24 hours. The medium was then replaced with that containing 30 ng/mL BMP4, with and without 1 mM dbcAMP (Sigma-Aldrich). After 24 hours, media and cells were collected. For the inhibitor studies, cells were preincubated with the BMP inhibitor, Noggin (ProSpec), for 30 minutes before BMP4 addition.
For the liquid chromatography-tandem mass spectrometry (LC-MS/MS) studies, H295R cells were plated at a density of 100 000 cells/well (48 well dish) in growth medium and grown to 60% confluence after which they were starved in low serum medium (1% cosmic calf serum and antibiotics) 18 hours prior to treatment with 30 ng/mL BMP4 (ProSpec). After 24 hours, the medium was replaced with that containing fresh 30 ng/mL BMP4. Medium was then collected after 24 hours for steroid quantitation. At the end of each treatment, the medium was collected from each well and stored at −20°C for steroid immunoassays or LC-MS/MS, whereas cells were frozen at −80°C for protein assay, Western blot, or RNA isolation, with subsequent qPCR analysis, as described below.
To quantify the basal BMP4 secretion in the adrenal cell types, the H295R and primary adrenal cells were incubated in low serum medium for 48 hours after reaching 60% confluence. BMP4 content in the experimental media was quantified by an enzyme immunoassay (EIA).
qPCR analysis
For cell culture studies, total RNA (100 ng) was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies) following manufacturer's instructions and used for qPCR analysis. For qPCR, 5 ng cDNA was mixed with Taqman Fast Universal Master Mix (Life Technologies) following manufacturer's recommendations. qPCR was performed using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems). The primer and probe sets for BMP4, CYP11A1, HSD3B2, and CYP17A1 were designed using Primer Express 3.0 (Applied Biosystems) or purchased from Integrated DNA Technologies as published previously (29). Quantitative normalization of cDNA in each tissue-derived sample and cell sample was performed using expression of peptidylprolyl isomerase A (PPIA, cyclophilin A), purchased from Applied Biosystems, as an internal control.
Protein extraction and protein assay
Cells were lysed in 100 μL mammalian protein extraction reagent (Pierce Chemical Co), and the protein content was estimated by the bicinchoninic acid protein assay using the microbicinchoninic acid protocol (Thermo Scientific).
Steroid immunoassays for cell culture experiments
The aldosterone content of the cell culture medium was analyzed using Coat-a-Count RIA kits from Siemens, and radioactivity was quantified using a Wallac Wizard 1470 multicrystal γ-counter (PerkinElmer). Cortisol and DHEA were quantified by an EIA (ALPCO). Assays were conducted following the manufacturer's recommendations except that the standard curves were prepared in the experimental cell culture medium. The results were normalized to protein content and shown as fold change or percentage change over basal condition.
BMP4 immunoassay for cell culture experiments
The BMP4 content of the cell culture medium was analyzed using the RayBio EIA kit (Raybiotech). Assays were conducted following the manufacturer's recommendations except that the standard curves were prepared in the experimental cell culture medium. The results were normalized to total protein content.
Steroid measurement by LC-MS/MS
The C21 and C19 steroids were also measured by LC-MS/MS to determine the effects of BMP4 on H295R steroidogenesis, where specified. The LC-MS/MS method is described in detail in the Supplemental Methods section.
Western blot analysis
H295R cells were plated at a density of 200 000 cells/well (24 well dish) in growth medium and grown to 60% confluence after which they were starved in low serum medium 18 hours prior to treatment with BMP4 for the indicated times. Immunoblotting was performed using an XCell SureLock system (Life Technologies) following the manufacturer's recommendation. Briefly, samples were lysed with lysis buffer (2% sodium dodecyl sulfate, 62.5 μM Tris, 0.04% bromophenol blue, 0.5 M dithiothreitol) and heated at 95°C for 5 minutes. Proteins were then loaded in equal amounts on 10% Bis-Tris gel and allowed to separate for 1.5 hours before transferring to polyvinylidene difluoride membranes. The membranes were then blocked with 5% BSA for 1 hour and incubated with either pSMAD 1/5/8 antibody (polyclonal rabbit antihuman, 1:1000 in BSA; Cell Signaling) or P450c17 antibody (polyclonal rabbit antihuman, 1:1000; generously provided by Dr Michael R Waterman, Vanderbilt University School of Medicine, Nashville, Tennessee) (Table 1) and secondary antibody (goat antirabbit, 1:2000; Life Technologies). The Pierce ECL Western blotting substrate kit (Life Technologies) was used for signal development.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| BMP4 | Thermo Scientific, catalog number PA5-32279 | Polyclonal; rabbit | 1:100 (IHC) | ||
| P450c17 | Dr Michael Waterman (Vanderbilt University) | Polyclonal; rabbit | 1:1000 (WB) | ||
| CYP11B2 | Dr Celso Gomez-Sanchez (University of Mississippi Medical Center) | Monoclonal; mouse | 1:100 (IHC) | ||
| pSMAD 1/5/8 | Cell Signaling, catalog number 9511 | Polyclonal; rabbit | 1:1000 (WB) |
Abbreviations: IHC, immunohistochemistry; WB, Western blot.
Immunohistochemistry
Rabbit polyclonal antibody against human BMP4 (Table 1) was diluted 1:100 with the antibody diluent solution (DAKO). The slides with tissue sections were first heated at 225°F for 15 minutes. After deparaffinization, slides were incubated in target retrieval solution (diluted 1:100 with distilled water, pH 9) (DAKO) for antigen retrieval. Immunohistochemical analysis was performed using an Envision+ kit (DAKO) as per the manufacturer's instructions. Nonimmunized rabbit antiserum was used as a negative control for immunohistochemistry.
Data analysis and statistical methods
Results are presented as mean ± SEM where appropriate. For the microarray analysis, we fit two-way ANOVA models with terms for three tissues (ZG, ZF, and ZR) and four people (respective patients) to log-transformed data for each probe set and used the resulting F tests to compare pairs of tissues. Significance was accepted at the P < .05 level of probability. Statistical analysis for BMP4 mRNA comparison between ZG, ZF, and ZR by qPCR was determined using one-way ANOVA followed by a Holm-Sidak correction. Significance was accepted at the P < .05 level of probability.
Comparison between two treatment groups with H295R cells for enzyme expression and steroid analysis was performed using an unpaired t test, and significance was accepted at the P < .05 level of probability. For comparison between more than two treatment groups with H295R or primary cells, one-way ANOVA was used followed by a Holm-Sidak correction. Significance was accepted at the P < .05 level of probability.
Results
A functional BMP system in the human adrenal
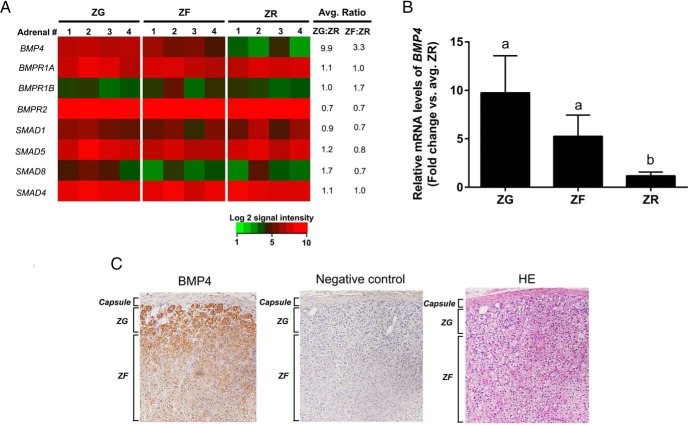
Laser-captured, microdissected ZG, ZF, and ZR from human adrenals were analyzed by a microarray for the presence of different BMPs as well as the entities involved in BMP signaling (Supplemental Figure 1 and Figure 1A, respectively) to define potential candidates for functional studies. We demonstrated that the ligand BMP4 was up-regulated in the ZG and ZF compared with ZR, whereas there was an abundant expression of the receptors ALK3 (BMP type IA receptor) and BMPR2, and the signaling proteins SMAD 1, -4, and -5 in all the three zones (Figure 1A). SMAD 8 expression was mainly restricted to the ZG. We validated the microarray results obtained for BMP4 using qPCR on the ZG, ZF and ZR (Figure 1B) and showed that the expression of BMP4 in the ZR is lower than the outer two zones of the adrenal cortex. In accordance with the expression analysis, BMP4 immunoreactivity was highest in the ZG (Figure 1C). Cultured primary adult adrenal cells and H295R cells were also found to secrete abundant BMP4 under basal conditions (Table 2).
Figure 1.
A, Heat map representation of microarray analysis for transcripts encoding genes involved in the BMP signaling pathway in adrenal ZG, ZF, and ZR. Normalized log2 signal intensity acquired from microarray analysis is represented by color (see color bar). Average ratio was calculated as average of normalized ZG or ZF signal intensities divided by that of normalized ZR signal intensities (n = 4) as indicated. A two-fit ANOVA (P < .05) confirmed that BMP4 was higher in the ZG as compared with both ZF and ZR. BMPR1A, BMP type IA receptor; BMPR1B, BMP type IB receptor; BMPR2, BMP type II receptor. B, Quantitative RT-PCR analysis for BMP4 in the adrenal ZG, ZF, and ZR obtained by laser capture microdissection. Validation of microarray analysis by real-time qPCR for BMP4 in ZG vs ZF vs ZR. BMP4 showed higher expression in ZG and ZF as compared with ZR. Three ZG, ZF, and ZR samples were used for qPCR analysis. Data are represented as mean ± SEM. Different letters above the bars indicate statistically significant differences (P < .05) between the zones (n = 4). Statistical significance was determined using a one-way ANOVA followed by a Holm-Sidak test. C, Immunohistochemical localization for BMP4 in the adrenal capsule, ZG, and ZF. Immunohistochemical analysis showed that BMP4 levels (left panel) were highest in ZG. Middle and right panels represent corresponding negative control (rabbit antiserum used instead of primary antibody) and hematoxylin-eosin (HE) stained sections, respectively.
Table 2.
Basal BMP4 Secretion in Primary Adult Adrenal Cell Cultures and the H295R Adrenocortical Cell Line Cultures
| Cell Type | BMP4, pg/mg Total Protein per 48 h |
|---|---|
| Primary adrenal cells | 893 ± 134 |
| H295R | 507 ± 82 |
An EIA was used to quantify the BMP4 in the medium from the primary adrenal cells and the H295R adrenocortical cell line after 48 hours of incubation. Results are normalized to total cellular protein content. Data are presented as mean ± SEM (n = 3 experiments).
BMP pathways are inducible in H295R cells
We examined and confirmed the expression of transcripts associated with BMP signaling in the H295R human adrenocortical cell line, primary adrenal cells, and normal adrenal tissue by microarray (Supplemental Figure 2). To ensure that the BMP-dependent pathways are intact in an adrenocortical tumor cell model, the H295R cells were incubated with 30 ng/mL of recombinant human BMP4, for increasing time intervals (5–30 min). A time-dependent BMP-induced phosphorylation of downstream SMAD 1/5/8 proteins was observed (Figure 2), indicating that the BMP pathways are active in H295R cells.
Figure 2.
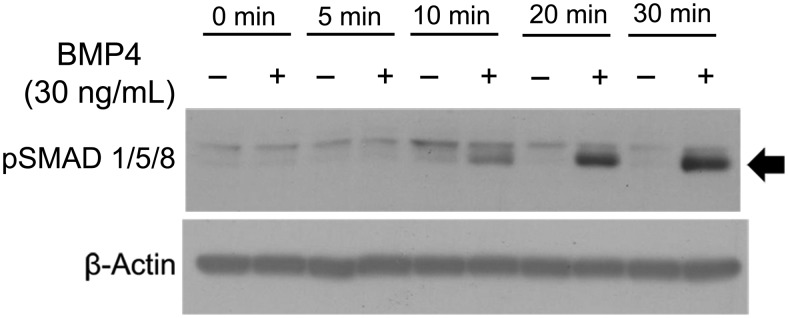
Effects of BMP4 on SMAD 1/5/8 phosphorylation in H295R adrenocortical cells. Cells were incubated with BMP4 (30 ng/mL) for the indicated time points. The cells were then lysed and subjected to SDS-PAGE and immunoblotting analysis using an anti-pSMAD 1/5/8 antibody. Treatment of adrenocortical tumor cells with BMP4 shows integrity of BMP-dependent pathways by phosphorylation of SMAD 1/5/8. Results shown are representative of those obtained from three independent experiments.
Concentration-dependent effects of BMP4 on steroidogenesis in H295R cells
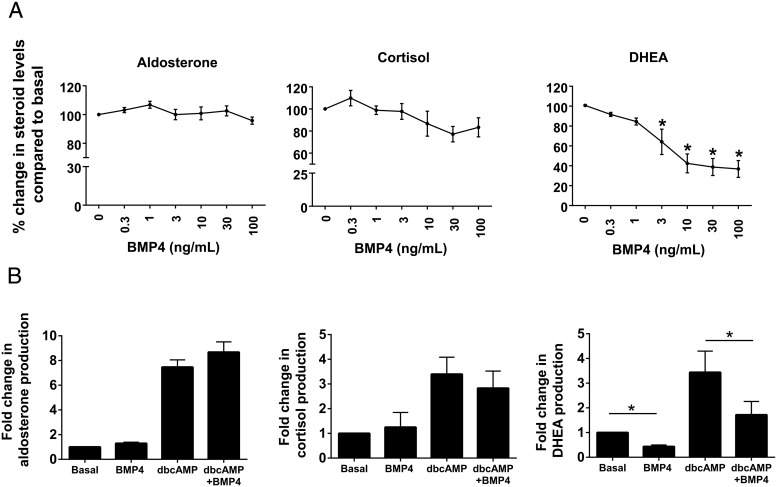
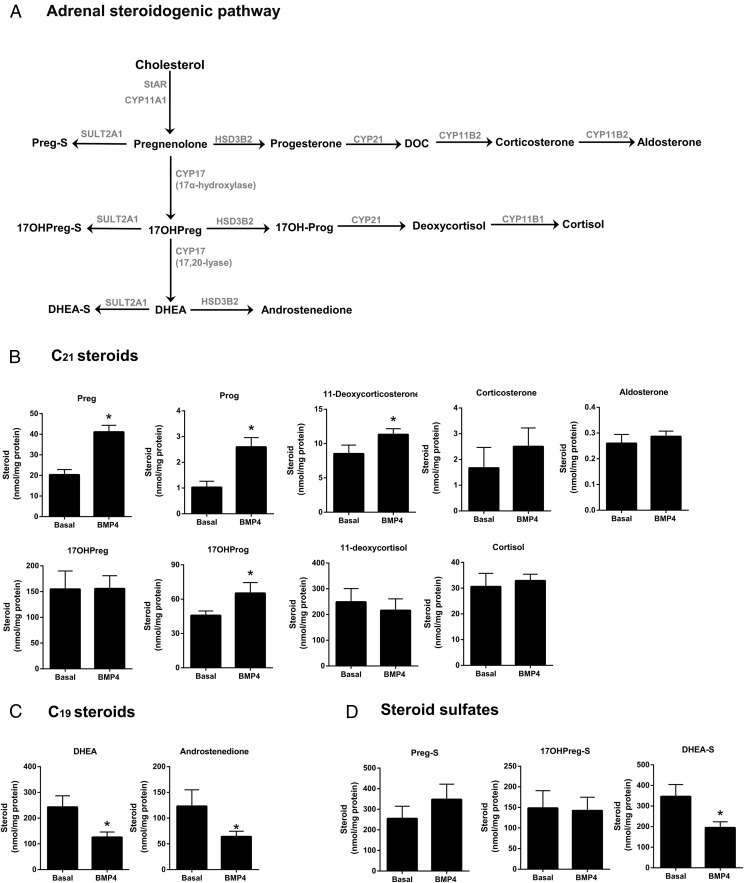
To examine the concentration-dependent effect of BMP4 on steroidogenesis, H295R cells were treated for 48 hours with control medium plus BMP4 in increasing doses (0.3, 1, 3, 10, 30, and 100 ng/mL). Accumulation of aldosterone, cortisol, and DHEA in the culture medium was then evaluated using immunoassays. No significant changes were found in the aldosterone and cortisol levels after treatment with increasing concentrations of BMP4 (Figure 3A). However, BMP4 was shown to significantly reduce the level of DHEA (Figure 3A) in a dose-dependent manner for all concentrations of 3 ng/mL or greater (P < .001).
Figure 3.
A, Dose-dependent effects of BMP4 on aldosterone, cortisol, and DHEA production in H295R adrenal cells. Cells were treated with the indicated concentrations of BMP4 for 48 hours. Steroid content of media was measured by immunoassays. Data are shown as the percentage fold change compared with no treatment. Results represent the mean ± SEM from four independent experiments. Statistical significances with the no-treatment control were determined using a one-way ANOVA followed by a Holm-Sidak test. *, P < .001. B, Effect of BMP4 on dbcAMP-induced steroidogenic hormone production in H295R cells. Cells were initially treated with the 30 ng/mL BMP4 for 24 hours. The medium was then replaced with that containing 30 ng/mL BMP4, with and without 1 mM dbcAMP. After 24 hours, media and cells were collected. Data are shown as the fold change compared with no treatment. Results represent the mean ± SEM from four independent experiments. Statistical significance with the no-treatment control was determined using a one-way ANOVA followed by a Holm-Sidak test. *, P < .001.
We further examined the effect of BMP4 on DHEA levels after treatment with dbcAMP. dbcAMP is an analog of cAMP that stimulates cAMP-dependent protein kinase-stimulated steroid production. Treatment of H295R cells with BMP4 inhibited both the basal and dbcAMP-increased DHEA production (Figure 3B), whereas the levels of aldosterone and cortisol remained unchanged under both basal and dbcAMP-stimulated conditions (Figure 3B) (P < .001).
Effects of BMP4 on the expression of key steroidogenic enzymes in H295R cells
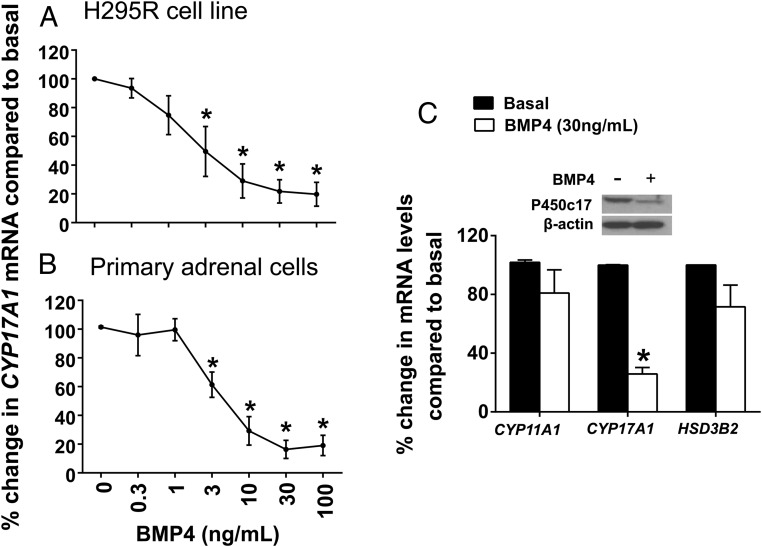
We sought to elucidate the effect of BMP4 on H295R cell expression of key determinants of steroidogenesis, namely CYP11A1, CYP17A1, and HSD3B2. In accordance with the decreasing levels of DHEA (Figure 3A), qPCR revealed the BMP4-dependent down-regulation of CYP17A1 in a dose-dependent manner in H295R cells (Figure 4A). We observed a similar dose-dependent decrease in the mRNA levels of CYP17A1 in primary adrenal cells (Figure 4B). Western analysis indicated that although BMP4 was able to decrease the levels of P450c17 in the H295R cells, it was not able to completely abolish its cellular expression after 48 hours of incubation (Figure 4C, top panel).
Figure 4.
Effects of BMP4 on the expression of key steroidogenic enzymes. A and B, Dose-dependent effects of BMP4 on CYP17A1 mRNA levels in human H295R adrenal cells and primary human adrenal cells, respectively. Cells were treated with the indicated concentrations of BMP4 for 48 hours. CYP17A1 mRNA levels were quantified by real-time qPCR analysis. Data are shown as the percentage fold change compared with no treatment. Results represent the mean ± SEM from at least three independent experiments. Statistical significance was determined using a one-way ANOVA followed by a Holm-Sidak test. *, P < .001. C, Effects of BMP4 on mRNA levels of key steroidogenic enzymes CYP11A1, CYP17A1, and HSD3B2. The top panel represents effect of BMP4 on P450c17 protein levels in H295R adrenal cells. Cells were treated with or without BMP4 (30 ng/mL) for 48 hours. The cells were then lysed and subjected to SDS-PAGE and immunoblot analysis using an anti-P450c17 antibody. Results shown are representative of those obtained from three independent experiments.
To further detail the BMP4-dependent effects on steroidogenesis, we also evaluated the regulation of other enzymes located at key branch points in the steroidogenic pathway: CYP11A1 and HSD3B2. Contrary to CYP17A1, we found that there was no significant change in the expression of these enzymes (Figure 4C).
Noggin prevents the negative regulatory effects of BMP4 on adrenal CYP17A1 mRNA levels and DHEA synthesis
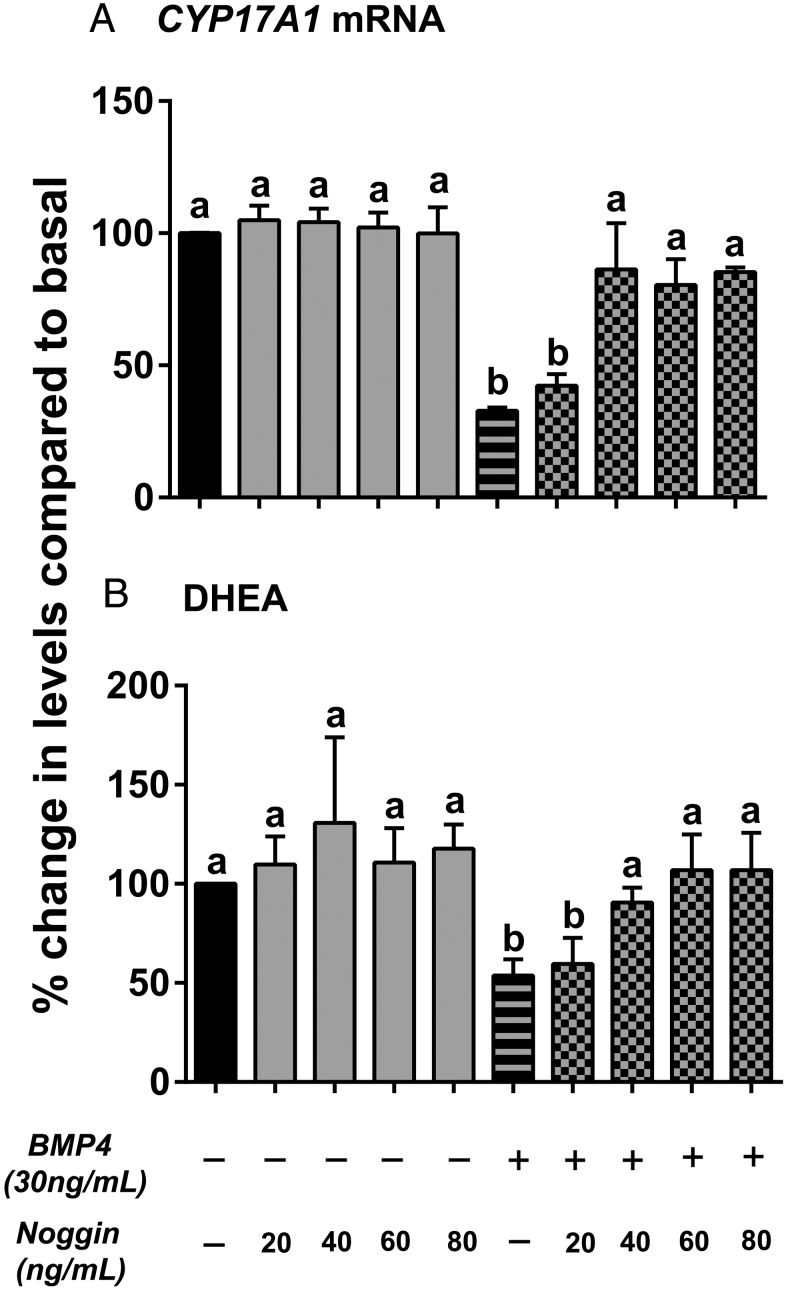
To further confirm the role of BMP4 in decreasing C19 steroid levels in H295R cells, a BMP antagonist, Noggin (which acts by limiting BMP signaling by ligand sequestration), was used in combination with BMP4 followed by the analysis of CYP17A1 mRNA and DHEA steroid levels. We observed that Noggin alone (20–80 ng/mL) did not affect the CYP17A1 mRNA and DHEA levels (Figure 5). However, 40–80 ng/mL of Noggin was able to reverse the negative effects of BMP4 (30 ng/mL) with respect to both CYP17A1 transcription and DHEA secretion (Figure 5) (a vs b; P < .01).
Figure 5.
Action of Noggin on BMP4-induced effects on CYP17A1 (A) mRNA levels and DHEA production (B) in H295R cells. Cells were treated with the indicated doses of noggin followed by 30 ng/mL of BMP4 for 48 hours. qPCR was used to quantify cellular CYP17A1 mRNA, and media content of DHEA was measured by immunoassay. Data are shown as the percentage fold change compared with no treatment (basal). Results represent the mean ± SEM from three independent experiments. Statistical significance was determined using a one-way ANOVA followed by Holm-Sidak test. a vs b, P < .01.
BMP4 effects on the H295R steroid metabolome
We also defined the global effects of BMP4 on steroidogenesis in the H295R adrenocortical cell line using LC-MS/MS analysis of secreted steroids (Figure 6). We measured nine C21 and two C19 unconjugated steroids along with three steroid sulfates. Our LC-MS/MS studies confirmed that the incubation of H295R cells with BMP4 (30 ng/mL) for 48 hours decreased the levels of both C19 steroids: DHEA and androstenedione (Figure 6C) (P < .05). DHEA-S was also decreased after BMP4 treatment (Figure 6D) (P < .05). On the other hand, we detected a discernible increase in the concentrations of pregnenolone, progesterone, 17α-hydroxyprogesterone, and 11-deoxycorticosterone (Figure 6B) (P < .05). Levels of other unconjugated and sulfated C21 steroids, including aldosterone and cortisol, did not change with BMP4 treatment (Figure 6, B and D).
Figure 6.
Effects of BMP4 on global steroidogenesis in H295R cells. The adrenal steroidogenic pathway including C21 and C19 steroids and steroid sulfates is described in panel A, Cells were incubated with BMP4 (30 ng/mL) for 48 hours with medium/BMP4 changed after 24 hours. Medium steroid content from the final 24 hours was determined using LC-MS/MS (panels B–D) and normalized with cellular protein content. Results represent the mean ± SEM from three independent experiments. Statistical significance with the untreated control was determined using an unpaired t test. *, P < .05.
Discussion
The purpose of this study was to demonstrate the existence of an endogenous BMP system in the human adrenal gland and to investigate its autocrine/paracrine role in the regulation of adrenal C19 steroid biosynthesis via modulation of key enzymes involved in steroidogenesis.
The fetal and adult human adrenals have been shown to produce a variety of factors that affect growth and proliferation as well as steroidogenic capacity (30–37). Members of the TGF-β family, including TGFβ1, activin, and BMPs, have been shown to play a significant role in the regulation of key steroidogenic enzymes and thereby human adrenal steroidogenesis (22–24, 30, 31, 35, 36, 38–41). The present study has demonstrated the existence of a complete BMP system composed of ligand (BMP4), type I receptor (ALK3), type II receptor (BMPRII), and signaling proteins (SMAD 1, SMAD 4, and SMAD 5) in the human adrenal cortex.
In addition, we observed the existence of the same entities in the H295R cell line by microarray. Our results are partially in agreement with studies by Suzuki et al (22) that suggest that the H295R cell line expresses entities like ALK2, ALK3, ALK4, and BMP type II receptor. However, they also demonstrate that the H295R cells express the ligand BMP6, rather than BMP4. The expression of other BMP ligands like BMP2 and BMP5 has also been established in the adrenal cortex (24). We have also showed that the H295R cells and the primary adult adrenal cells secrete significant quantities of the BMP4 protein.
In the present study, the treatment of H295R cells with BMP4 caused a concentration-dependent inhibition of the two adrenal C19 steroids, DHEA and androstenedione. The effect of BMPs on adrenal C19 steroids has been evaluated by only one other study (24). Johnsen et al (24) showed that BMP2 and BMP5 were able to abate the production of aldosterone, cortisol, and DHEA-S in a dose-dependent manner in the H295R cell line. In contrast, our studies show that aldosterone and cortisol levels remained unchanged after BMP4 treatment. BMP2 and BMP5 have been shown to suppress the overall forskolin-induced steroidogenic capacity of H295R cells (24). In other studies BMP6 has been shown to augment AngII-induced aldosterone secretion through SMAD 1/5/8 (22, 23). However, potassium-induced aldosterone secretion remained unchanged. An interesting follow-up study by Otani et al (38) demonstrated that the endogenous BMP6 in the H295R cells was involved in aldosterone breakthrough caused by chronic blockage of AngII type 1 receptors. It would be interesting to determine whether BMP4 is able to cause similar effects in H295R cells under AngII induction.
Despite the substantial reduction in the mRNA levels of CYP17A1 with BMP4 treatment, LC-MS/MS profiles showed that BMP4 prominently decreased synthesis of only the C19 steroids, DHEA, DHEA-S, and androstenedione, but not cortisol. Interestingly, BMP4 did not have any effect on the mRNA levels of CYB5A, an allosteric regulator of the 17,20-lyase activity of P450c17 (data not shown). Our results are predictable for changes in P450c17, an enzyme that catalyzes two sequential reactions. Reduced P450c17 activity with sustained CYP11A1 and HSD3B2 expression allowed precursors pregnenolone and progesterone to rise. Steroids that require only the 17α-hydroxylase activity of P450c17 for their production either remained unchanged (cortisol) or showed a slight increase (17α-hydroxyprogesterone) upon BMP4 treatment. The dominant C21 steroid product of H295R cells, 11-deoxycortisol, fell slightly but not significantly. Thus, precursor to product ratios rose for both the 17α-hydroxylase and 17,20-lyase reactions. Our results mirror the consequences of treatment with the P450c17 inhibitor abiraterone acetate in men with prostate cancer (42).
P450c17 executes the switch between the production of mineralocorticoids, glucocorticoids, and C19 steroids. The production of mineralocorticoids in the ZG relies on low levels of P450c17 expression, whereas C19 steroid production in the ZR is controlled through the differential regulation of its 17α-hydroxylase and 17,20-lyase activities. One interpretation correlating our immunohistochemical analysis and the decreased CYP17A1 mRNA could be that BMP4 acts as a paracrine/autocrine factor involved in suppression of P450c17 in the ZG and outer ZF. It can thereby contribute to functional adrenocortical zonation.
The intracellular mechanism of BMP4 action on steroidogenic enzymes is still unknown. The BMP2/5 study by Johnsen et al (24) showed that along with decreasing the expression of the steroidogenic enzymes (P450c17 and HSD3B2) and adrenocorticotropic hormone receptors [melanocortin 2 receptor (MC2R)] BMP2/5 increased H295R cell ID1 transcript levels under both basal and forskolin-stimulated conditions. The inhibitor of DNA binding 1 (ID1; a member of the inhibitor of DNA binding protein family) can inhibit proteins of the basic helix-loop-helix (bHLH) family. The same study (24) also examined the MC2R promoter and showed that it possesses two consensus sites for E-box elements that could be targeted by the transcription factors of the bHLH family. Hence, they speculated that the inhibition of MC2R and the genes encoding the steroidogenic enzymes could be a direct consequence of the BMP-dependent up-regulation of the ID1 levels because ID1 could in turn inhibit the stimulating transcription factors of the bHLH family (24). Interestingly, the bHLH factor, brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1, is required for circadian glucocorticoid secretion through E-box elements on the steroidogenic acute regulatory protein promoter (43, 44). Hence, it would be interesting to determine whether the BMP4 down-regulation of P450c17 occurs by a similar mechanism.
Studies by Glister et al (45) using ovarian cells indicate an alternate mechanism by which BMP4 could regulate CYP17A1 mRNA levels in the human adrenal. This microarray study involving bovine theca cells cultivated in the presence and absence of BMP6, suggested a link between insulin-like factor 3 (INSL3) signaling and thecal cell steroidogenesis because the treated cells showed a reduction in INSL3 as well as CYP17A1 and the mRNA levels of other steroidogenic enzymes along with a concomitant decrease in androstenedione secretion (45). The authors not only proved that INSL3-RXFP2 (Relaxin/insulin-like family peptide receptor) signaling plays a pivotal role in thecal androgen secretion but also confirmed that autocrine/paracrine INSL3-RXFP2 signaling increases theca cell androgen synthesis under both basal and LH-stimulated conditions (45). However, microarray analysis of the three zones of the human adrenal showed that the endogenous levels of both INSL3 and RXFP2 are very low (data not shown), suggesting that the INSL3-RXFP2 signaling pathway does not operate in the adrenal. Thus, it appears that CYP17A1 is likely regulated differently between theca and adrenal cells.
Collectively we have demonstrated the presence of a BMP system in the human adrenal gland. BMP4 was able to decrease adrenal C19 steroid production without significantly affecting aldosterone and cortisol synthesis at basal conditions. Such an endogenous BMP system might play a crucial role in the regulation of adrenal steroidogenesis in an autocrine/paracrine manner and could thereby modulate functional adrenocortical zonation.
Acknowledgments
We thank Dr Mary Bassett for her editorial assistance.
This work was supported by Grants DK43140 and DK069950 from the National Institutes of Health (to W.E.R.). Mass spectrometry used core services supported by Grant DK089503 from the National Institutes of Health (to the University of Michigan under the Michigan Nutrition Obesity Center).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ALK3
- activin receptor-like kinase receptor 3
- AngII
- angiotensin II
- bHLH
- basic helix-loop-helix
- BMP
- bone morphogenetic protein
- CYP11A1
- cytochrome P450 cholesterol side-chain cleavage
- CYP17A1
- 17α-hydroxylase/17,20-lyase mRNA
- dbcAMP
- dibutyryl cAMP
- DHEA
- dehydroepiandrosterone
- DHEA-S
- DHEA sulfate
- EIA
- enzyme immunoassay
- HSD3B2
- type 2 3β-hydroxysteroid dehydrogenase
- ID1
- inhibitor of DNA binding protein 1
- INSL3
- insulin-like factor 3
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MC2R
- melanocortin 2 receptor
- P450c17
- 17α-hydroxylase/17,20-lyase protein
- qPCR
- quantitative RT-PCR
- SMAD
- mothers against decapentaplegic homolog
- ZF
- zona fasciculata
- ZG
- zona glomerulosa
- ZR
- zona reticularis.
References
- 1. Vinson GP. Adrenocortical zonation and ACTH. Microsc Res Tech. 2003;61:227–239. [DOI] [PubMed] [Google Scholar]
- 2. Vinson GP, Ho MM. Origins of zonation: the adrenocortical model of tissue development and differentiation. Clin Exp Pharmacol Physiol Suppl. 1998;25:S91–S96. [DOI] [PubMed] [Google Scholar]
- 3. Hornsby PJ. Biosynthesis of DHEA(S) by the human adrenal cortex and its age-related decline. Ann NY Acad Sci. 1995;774:29–46. [DOI] [PubMed] [Google Scholar]
- 4. Parker LN, Odell WD. Control of adrenal androgen secretion. Endocr Rev. 1980;1:392–410. [DOI] [PubMed] [Google Scholar]
- 5. Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ. Differentially expressed genes in zona reticularis cells of the human adrenal cortex. Mol Cell Endocrinol. 2001;173:127–134. [DOI] [PubMed] [Google Scholar]
- 6. Aiba M, Fujibayashi M. Alteration of subcapsular adrenocortical zonation in humans with aging: the progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. J Histochem Cytochem. 2011;59:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Domalik LJ, Chaplin DD, Kirkman MS, et al. Different isozymes of mouse 11β-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol. 1991;5:1853–1861. [DOI] [PubMed] [Google Scholar]
- 8. Gell JS, Carr BR, Sasano H, et al. Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83:3695–3701. [DOI] [PubMed] [Google Scholar]
- 9. Nishimoto K, Nakagawa K, Li D, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Sasano H, Takeyama J, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf). 2000;53:739–747. [DOI] [PubMed] [Google Scholar]
- 11. Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606. [DOI] [PubMed] [Google Scholar]
- 12. Miller WL. Early steps in androgen biosynthesis: from cholesterol to DHEA. Baillieres Clin Endocrinol Metab. 1998;12:67–81. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord. 2009;10:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81:3558–3565. [DOI] [PubMed] [Google Scholar]
- 15. Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the δ5 and δ4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. [DOI] [PubMed] [Google Scholar]
- 16. Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. [DOI] [PubMed] [Google Scholar]
- 17. Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. [DOI] [PubMed] [Google Scholar]
- 18. Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. [DOI] [PubMed] [Google Scholar]
- 19. Khalaf M, Morera J, Bourret A, et al. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur J Endocrinol. 2013;168:437–444. [DOI] [PubMed] [Google Scholar]
- 20. Dooley CA, Attia GR, Rainey WE, Moore DR, Carr BR. Bone morphogenetic protein inhibits ovarian androgen production. J Clin Endocrinol Metab. 2000;85:3331–3337. [DOI] [PubMed] [Google Scholar]
- 21. Chang HM, Cheng JC, Klausen C, Leung PC. BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol. 2013;27:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki J, Otsuka F, Inagaki K, Takeda M, Ogura T, Makino H. Novel action of activin and bone morphogenetic protein in regulating aldosterone production by human adrenocortical cells. Endocrinology. 2004;145:639–649. [DOI] [PubMed] [Google Scholar]
- 23. Inagaki K, Otsuka F, Suzuki J, et al. Involvement of bone morphogenetic protein-6 in differential regulation of aldosterone production by angiotensin II and potassium in human adrenocortical cells. Endocrinology. 2006;147:2681–2689. [DOI] [PubMed] [Google Scholar]
- 24. Johnsen IK, Kappler R, Auernhammer CJ, Beuschlein F. Bone morphogenetic proteins 2 and 5 are down-regulated in adrenocortical carcinoma and modulate adrenal cell proliferation and steroidogenesis. Cancer Res. 2009;69:5784–5792. [DOI] [PubMed] [Google Scholar]
- 25. Nishimoto K, Rigsby CS, Wang T, et al. Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology. 2012;153:1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishimoto K, Harris RB, Rainey WE, Seki T. Sodium deficiency regulates rat adrenal zona glomerulosa gene expression. Endocrinology. 2014;155:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. [DOI] [PubMed] [Google Scholar]
- 28. Bassett MH, Suzuki T, Sasano H, et al. The orphan nuclear receptor NGFIB regulates transcription of 3β-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004;279:37622–37630. [DOI] [PubMed] [Google Scholar]
- 29. Bassett MH, Mayhew B, Rehman K, et al. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–5455. [DOI] [PubMed] [Google Scholar]
- 30. Feige JJ, Vilgrain I, Brand C, Bailly S, Souchelnitskiy S. Fine tuning of adrenocortical functions by locally produced growth factors. J Endocrinol. 1998;158:7–19. [DOI] [PubMed] [Google Scholar]
- 31. Penhoat A, Ouali R, Viard I, Langlois D, Saez JM. Regulation of primary response and specific genes in adrenal cells by peptide hormones and growth factors. Steroids. 1996;61:176–183. [DOI] [PubMed] [Google Scholar]
- 32. Spencer SJ, Rabinovici J, Mesiano S, Goldsmith PC, Jaffe RB. Activin and inhibin in the human adrenal gland. Regulation and differential effects in fetal and adult cells. J Clin Invest. 1992;90:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45:1005–1019. [DOI] [PubMed] [Google Scholar]
- 34. Mesiano S, Mellon SH, Jaffe RB. Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J Clin Endocrinol Metab. 1993;76:968–976. [DOI] [PubMed] [Google Scholar]
- 35. Lebrethon MC, Jaillard C, Naville D, Begeot M, Saez JM. Effects of transforming growth factor-β1 on human adrenocortical fasciculata-reticularis cell differentiated functions. J Clin Endocrinol Metab. 1994;79:1033–1039. [DOI] [PubMed] [Google Scholar]
- 36. Stankovic AK, Dion LD, Parker CR., Jr Effects of transforming growth factor-β on human fetal adrenal steroid production. Mol Cell Endocrinol. 1994;99:145–151. [DOI] [PubMed] [Google Scholar]
- 37. Mesiano S, Jaffe RB. Role of growth factors in the developmental regulation of the human fetal adrenal cortex. Steroids. 1997;62:62–72. [DOI] [PubMed] [Google Scholar]
- 38. Otani H, Otsuka F, Inagaki K, et al. Aldosterone breakthrough caused by chronic blockage of angiotensin II type 1 receptors in human adrenocortical cells: possible involvement of bone morphogenetic protein-6 actions. Endocrinology. 2008;149:2816–2825. [DOI] [PubMed] [Google Scholar]
- 39. Vanttinen T, Liu J, Kuulasmaa T, Kivinen P, Voutilainen R. Expression of activin/inhibin signaling components in the human adrenal gland and the effects of activins and inhibins on adrenocortical steroidogenesis and apoptosis. J Endocrinol. 2003;178:479–489. [DOI] [PubMed] [Google Scholar]
- 40. Hofland J, Steenbergen J, Hofland LJ, et al. Protein kinase C-induced activin A switches adrenocortical steroidogenesis to aldosterone by suppressing CYP17A1 expression. Am J Physiol Endocrinol Metab. 2013;305:E736–E744. [DOI] [PubMed] [Google Scholar]
- 41. Derebecka-Holysz N, Lehmann TP, Holysz M, Trzeciak WH. TGF-β inhibits CYP17 transcription in H295R cells acting via activin receptor-like kinase 5. Endocr Res. 2009;34:68–79. [DOI] [PubMed] [Google Scholar]
- 42. Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. [DOI] [PubMed] [Google Scholar]
- 43. Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology. 2009;150:1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Son GH, Chung S, Choe HK, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA. 2008;105:20970–20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glister C, Satchell L, Bathgate RA, et al. Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci USA. 2013;110:E1426–E1435. [DOI] [PMC free article] [PubMed] [Google Scholar]