Abstract
KNDy neurons facilitate tail skin vasodilation and modulate the effects of estradiol on thermoregulation. We hypothesize that KNDy neurons influence cutaneous vasodilation via projections to neurons in the median preoptic nucleus (MnPO) that express the neurokinin 3 receptor (NK3R). In support of this hypothesis, focal microinjections of senktide, an NK3R agonist, into the MnPO lowers core temperature (TCORE) in the female rat. To further study the role of MnPO NK3R neurons in thermoregulation, these neurons were specifically ablated using a conjugate of a selective NK3R agonist and saporin (NK3-SAP). NK3-SAP or blank-SAP (control) was injected into the MnPO/medial septum. Tail skin temperature (TSKIN) and TCORE were measured in ovariectomized rats exposed to 3 ambient temperatures (TAMBIENT) before and after estradiol-17β (E2) treatment. Before killing, we injected senktide (sc), monitored TCORE for 70 minutes, and harvested brains for Fos immunohistochemistry. Ablation of MnPO NK3R neurons lowered TSKIN at neutral and subneutral TAMBIENT regardless of E2 treatment. However, ablation did not prevent the effects of E2 on TCORE and TSKIN. In control rats, senktide injections induced hypothermia with numerous Fos-immunoreactive cells in the MnPO. In contrast, in NK3-SAP rats, senktide did not alter TCORE and minimal Fos-immunoreactive neurons were identified in the MnPO. These data show that NK3R neurons in the MnPO are required for the hypothermic effects of senktide but not for the E2 modulation of thermoregulation. The lower TSKIN in NK3-SAP–injected rats suggests that MnPO NK3R neurons, like KNDy neurons, facilitate cutaneous vasodilation, an important heat-dissipation effector.
Hot flushes occur in most menopausal women and in a variety of individuals after withdrawal of estrogens (1, 2). A hot flush is characterized by sudden, inappropriate activation of heat-dissipation effectors, including skin vasodilation, sweating, and cold-seeking behavior (2, 3). Understanding how estrogen withdrawal alters the activation of heat-dissipation effectors will ultimately shed light on the etiology of flushes (4).
In the human infundibular (arcuate) nucleus, a subpopulation of neurons expressing estrogen receptor-α, kisspeptin, and neurokinin B (NKB) mRNA exhibits hypertrophy and increased kisspeptin and NKB gene expression after menopause (5–7). The neuronal hypertrophy and increased kisspeptin and NKB gene expression in postmenopausal women are due to estrogen withdrawal because identical changes occur in response to ovariectomy of young monkeys and are reversed by replacement with estrogens (7–9). A homologous population of estrogen-responsive neurons exists in the arcuate nucleus of rodents, goats, and ewes, which are termed KNDy neurons based on coexpression of kisspeptin, NKB, and dynorphin (10–14). Extensive colocalization of kisspeptin and NKB is also observed in the infundibular nucleus of postmenopausal women, with dynorphin identified in a small number of kisspeptin-immunoreactive (ir) fibers (15). However, there is hypertrophy of neurons expressing dynorphin mRNA in the infundibular nucleus of postmenopausal women, with decreased dynorphin gene expression (16). These data suggest that the KNDy neuron phenotype may also exist in the human.
Because kisspeptin and NKB gene expression in the human hypothalamus changes so dramatically in response to estrogen withdrawal, we have hypothesized that KNDy neurons participate in the generation of hot flushes (4, 6). Further evidence is the close link between LH pulses and flushes in humans (17) and the putative role of KNDy neurons in pulsatile LH secretion in animal models (14, 18, 19). We have also shown that ablation of KNDy neurons in the rat reduces tail skin vasodilation (20). Moreover, KNDy neuron ablation prevents the reduction in tail skin temperature (TSKIN) by estradiol during the light phase and blocks the effects of estradiol on core temperature (TCORE) during heat stress (20). These data provide the first evidence that KNDy neurons play a role in the estradiol-17β (E2) modulation of body temperature and facilitate cutaneous vasodilation, a cardinal feature of the menopausal flush.
KNDy neurons project to thermoregulatory centers in the rostral hypothalamus, including the median preoptic nucleus (MnPO) and medial preoptic area (21, 22). Recent studies have established the MnPO to be an important component of a thermosensory heat-defense pathway (23). The MnPO is the first hypothalamic area to receive information from peripheral warm-sensitive thermoreceptors via the lateral parabrachial nucleus (23). In turn, the MnPO projects to the medial preoptic area (24), the dorsomedial nucleus (25), and premotor sympathetic neurons in the rostral medullary raphe that control tail skin vasomotion (26). Fos expression in the MnPO is increased in response to warm environmental temperatures and modulated by estradiol (27). Interestingly, MnPO neurons express NK3R mRNA (28) and protein (29), suggesting that KNDy neurons could modulate the heat-defense pathway through NKB signaling in the MnPO. In support of this hypothesis, focal microinfusion of the NK3R agonist, senktide, activates MnPO neurons and markedly reduces body temperature consistent with the activation of heat-dissipation effectors (29).
To further study the role of MnPO NK3R-expressing neurons in body temperature regulation, we studied the effects of ablating these neurons using focal microinjections of NK3-SAP. NK3-SAP is a conjugate of saporin, a ribosome-inactivating toxin, with [MePhe7]-NKB, a potent and selective NK3R agonist (30, 31). The use of this conjugate for the selective ablation of NK3R neurons has been described previously (20, 32). TSKIN and TCORE were measured in ovariectomized (OVX) rats exposed to various ambient temperatures (TAMBIENT) before and after treatment with E2. We also evaluated whether NK3R neurons in the MnPO are essential for the drop in body temperature that occurs after injections of senktide. If MnPO NK3R neurons participate in the heat-defense pathway, we predicted that ablation of these neurons would decrease tail skin vasodilation and prevent the hypothermic effects of senktide.
Materials and Methods
Young adult female Sprague-Dawley rats (approximately 12 weeks old, 200–250 g; Harlan Laboratories) were individually housed in a quiet, temperature-controlled room (21.1–22.5°C) in the University of Arizona Animal Care Facility with a 12:12 hour light/dark cycle (lights on at 7:00 am). Rats had ad libitum access to water and a low-phytoestrogen diet (Harlan Teklad rodent diet 2014; Harlan Laboratories). All protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to National Institutes of Health guidelines.
Rats (n = 16) received general anesthesia by the injection of a cocktail (1.0 mL/kg im) containing ketamine (33.3 mg/mL), xylazine (10.7 mg/mL), and acepromazine (1.3 mg/mL). The rats were OVX and a PhysioTel telemetry transmitter (TA-F40; Data Sciences International) was inserted into the peritoneal cavity. Stereotaxic surgery was used to make 2 injections of NK3-SAP (10 ng/100 nL PBS; n = 8) into the MnPO/septum. Control rats (n = 8) received injections of blank-SAP (10 ng/100 nL PBS), a scrambled peptide conjugated to saporin. Based on the diffusion of NK3-SAP (32), the injections were designed to ablate NK3R neurons in the MnPO but avoid direct needle tract damage to this nucleus. The coordinates for the 2 midline injections were 0.84 mm anterior to bregma, 6.4 mm ventral to the skull surface and 0.12 mm anterior to bregma, and 7.0 mm ventral to the skull surface. Injections were performed using a NanoFil 10-μL syringe with a 33-gauge beveled tip needle (World Precision Instruments) inserted into an Ultra Micro Pump with a Micro 4 controller (UMP3; World Precision Instruments). Injections were made over 5 minutes (20 nL/min), and the needle was left in place for an additional 5 minutes before removal. The incision was closed, and animals were given postoperative analgesia (buprenorphine, 0.03–0.05 mg/kg sc).
TCORE was measured via telemetry using the implanted intraperitoneal transmitter. Each rat was placed on an RPC-1 Physiotel receiver board connected by a Data Exchange Matrix to a computer equipped with Dataquest A.R.T. Software (Data Sciences International). TSKIN was recorded with a datalogger (SubCue) housed in nylon casing fitted to the animal's tail as described previously (33). This device was placed 4.0 cm distal to the base of the animal's tail under isoflurane anesthesia (<5 minutes duration). TAMBIENT was recorded with a thermocouple inserted into a TC4000 or QuadTemp data logger (Madgetech, Inc). The temperature recording devices were calibrated according to the manufacturer's specifications and validated against a National Institute of Standards and Technology certified TC4000 thermocouple (Madgetech, Inc).
Experiment 1: Body temperature regulation in NK3-SAP or blank-SAP rats exposed to various TAMBIENT, with and without estradiol replacement
The TAMBIENT exposures began 14 days after the initial surgery. Freely moving rats were placed in plastic grid cages (6 inches × 6 inches × 4 inches) with unrestricted airflow and ad libitum access to food and water. These cages were placed on the Physiotel receiver boards in an environmental chamber (Forma model 3940; Thermo Scientific). The environmental chamber was equilibrated to 1 of 3 different TAMBIENT (26, 11, or 33°C, in that order) with the humidity set at 50%. Rats were habituated to the experimental procedures on 3 occasions before recording. Rats were exposed to 1 TAMBIENT each morning. TCORE, TSKIN, and TAMBIENT were measured every 10 minutes over a 3-hour period.
Approximately 22 days after the initial surgery, rats were implanted with 2 subcutaneous Silastic capsules (each 20 mm effective length, 1.57 mm inner diameter, and 3.18 mm outer diameter; Dow Corning) containing E2 dissolved in sesame oil (360 μg/mL). This capsule regimen has been shown to produce low physiological levels of estrogen (33). Eight days after E2 treatment, rats were again exposed to the 3 different TAMBIENT as described above.
Experiment 2: Effects of NK3-SAP or blank-SAP injections in the MnPO/septum on serum LH and body weight
Our previous studies showed that ablation of NK3R-expressing KNDy neurons in the arcuate nucleus resulted in profound alterations in the E2 modulation of LH secretion and body weight. To determine whether similar changes occurred after ablation of NK3R neurons in the MnPO, rats were weighed, and blood samples were taken at the time of ovariectomy, 3 weeks later, and 11 days after E2 replacement. The first and second blood samples were collected via saphenous vein puncture using a 5-mm lancet (Braintree Scientific) and a Microvette capillary tube (Sarstedt). The final sample was taken from a cardiac puncture at the time of perfusion. Blood was allowed to clot at room temperature for 90 minutes and was centrifuged. Serum was stored at −20°C and shipped on dry ice to the Ligand Assay and Analysis Core Laboratory at the University of Virginia Center for Research in Reproduction (Charlottesville, VA). Serum LH (10-μL sample, in singlet) was assayed using a multiplex panel assay (Milliplex MAP for Luminex xMAP Technology, RPT-86K-02). The LH assay had a sensitivity of 0.24 ng/mL and intra-assay variability of 6.9%.
Experiment 3: Effects of senktide on TCORE and Fos in rats injected with NK3-SAP or blank-SAP in the MnPO/septum
Rats were injected with senktide, a selective NK3R agonist, at a concentration (0.5 mg/kg sc) previously shown to elicit central effects (34). Immediately after injection, the rats were placed into clear shoebox style cages on the Physiotel receiver boards with clean bedding and ad libitum access to food and water (TAMBIENT of 22.0–23.0°C). TCORE was measured every minute for 70 minutes using the implanted telemetry probes. Both NK3-SAP and blank-SAP rats exhibited wet-dog shakes, confirming the central effect of senktide injections (34). Ninety minutes after senktide, rats were injected with an overdose of sodium pentobarbital (100 mg/kg ip). The rats were perfused through the ascending aorta with 200 mL of heparinized 0.1 M PBS followed by 400 mL of 4% paraformaldehyde in phosphate buffer, pH 7.4. The brains were removed, postfixed for 1 hour in the same fixative, and then cryoprotected in ascending concentrations of sucrose in PBS (10% up to 30%). Brain blocks were frozen and sectioned (40-μm thickness) using a sliding microtome, and every 10th section was stained with cresyl violet. The rest of the free-floating sections were stored in cryoprotectant solution (35) at −20°C until immunohistochemical analysis.
Immunohistochemical studies
The antiserum used to visualize Fos protein (cfos Ab-5, lot D00007099; EMD Biosciences, Inc) was generated in rabbit against amino acids 4 to 17 of the human c-Fos protein (Table 1). The specificity of this antiserum was verified by preadsorption experiments (36, 37) and negative staining in Fos knockout mice (36). The NK3R antiserum was generously donated by Philippe Ciofi (IS-7/7, bleed 040595) and was raised in rabbit against amino acids 443 to 452 of rat NK3R (38). Omission of the primary antiserum prevented staining, as did preadsorption using a synthetic peptide corresponding to amino acids 443 to 452 of rat NK3R protein (Bio-Synthesis). The staining pattern observed with this antiserum matched previous reports of the distribution of NK3R mRNA (28) and protein (38, 39) in the rat. The NKB antiserum (NB300–201 lot A2; Novus Biologicals) was raised in rabbit against residues 50 to 79 of mouse pro-NKB. The distribution of pro-NKB immunoreactivity was similar to that in previous reports (32, 40–42). Specific labeling was prevented by preadsorption with the synthetic peptide used for immunization (Novus Biologicals).
Antibody Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Fos | Amino acids 4–17 of the human c-Fos | Ab-5 | EMD Biosciences, Inc | Rabbit polyclonal | 1:15 000 |
| Neurokinin 3 receptor | Amino acids 443–452 of Rat NK3R | IS-7/7 | Philippe Ciofi | Rabbit polyclonal | 1:1000 |
| Neurokinin B | Amino acids 50–79 of mouse proNKB | NB300-201 | Novus Biologicals | Rabbit polyclonal | 1:15 000 |
Fos and NK3R immunohistochemical analysis with tyramide signal amplification was performed on sections matched to plates 30, 32, 34, and 36 of a rat brain atlas (43). Multiple rinses with PBS were performed before the procedure and between each step. For antigen retrieval, sections were incubated in 15 mM sodium citrate at 80°C for 30 minutes and left to come to room temperature. After incubation in 0.3% H2O2 to remove endogenous peroxidases, sections were blocked in 3% normal goat serum plus 0.4% Triton X-100 in PBS (blocking solution). Sections were incubated with either Fos (1:15 000) or NK3R (1:1000) antiserum in blocking solution at 4°C for 48 hours and then were incubated overnight with a biotinylated goat anti-rabbit secondary antiserum (Invitrogen) in blocking solution. The following morning, sections were incubated in an avidin-biotin complex solution (Vector Laboratories) and exposed to a biotinyl tyramide solution (1:200 in 0.005% H2O2; PerkinElmer) for 20 minutes. Finally, sections were incubated in Alexa 488–conjugated (Fos) or Alexa 568–conjugated (NK3R) streptavidin (Invitrogen) for 3 hours at 37°C. After an overnight PBS wash (4°C), sections were exposed to a 3 μM 4′,6-diamidino-2-phenylindole (DAPI) solution, mounted on subbed slides, dried, and coverslipped using ProLong Gold Antifade (Invitrogen).
Fluorescence labeling (Fos or NK3R) was visualized with a Nikon E1000 microscope equipped with a VFM epifluorescent attachment, a LUDL motorized stage (Ludl Electronic Products), a Uniblitz model VMM-D1 shutter driver (Vincent Associates), and a Photometrics CoolSNAP FX camera (Roper Scientific). Two sections were analyzed for each rat, matched to plates 32 and 34 (43). Digital images were captured in a systematic stepwise fashion using a ×20 Nikon Plan Fluor objective (numerical aperture 0.50) and the appropriate filter sets for Alexa Fluor 488, Alexa Fluor 568, or DAPI stains. Images were assembled into montages using MetaMorph software (Molecular Devices). The regions of interest were identified and outlined using the DAPI counterstain as described previously (27). Fos-ir cell nuclei were manually counted on these images.
NKB immunohistochemical analysis was performed on 2 sections for each rat that were matched to the midlevel (plate 56) of the arcuate nucleus (43). Sections were rinsed in PBS, incubated in 0.3% H2O2 for the removal of endogenous peroxidases, rinsed, and blocked in 3% blocking solution. Sections were incubated with the rabbit NKB antiserum (1:15 000) in blocking solution at 4°C for 48 hours. Sections were then rinsed and incubated in blocking solution containing a biotinylated goat antirabbit antiserum (1:600), followed by rinsing and incubation in an avidin-biotin complex solution. After rinsing with PBS and 0.175 M sodium acetate, filtered nickel-intensified 3,3′-diaminobenzidine solution was used for visualization (1.25 g of nickel ammonium sulfate, 10 mg of 3,3′-diaminobenzidine in 50 mL of sodium acetate solution, and 41.5 μL of 30% H2O2). Sections were rinsed, mounted on subbed slides, dehydrated, and coverslipped.
Morphologic analysis of NKB-ir neurons was conducted using an image-combining computer microscope system equipped with a LUDL motorized stage, a Lucivid miniature CRT, and Neurolucida software (MBF Science). Cells were manually marked for counting using a 20× Plan apochromat objective (numerical aperture 0.50). The number of cells per unilateral arcuate section was averaged for each rat, and these values were used to compare groups. Groups were compared using a Student t test (α = .05).
Temperature data analysis
To allow 1 hour of acclimation in the environmental chamber, only the second and third hours of temperature recordings were used for data analysis. The heat loss index (HLI) was calculated by the formula, HLI = (TSKIN − TAMBIENT)/(TCORE − TAMBIENT) (44). The HLI is strongly correlated with blood flow and ranges between 0 (corresponding to maximal skin vasoconstriction) and 1 (maximal skin vasodilation). The mean TCORE, TSKIN, and HLI of each rat was used to calculate group averages. Group averages were compared using a two-way ANOVA (SAP treatment × E2 status) with a Tukey post hoc analysis (α = .05).
To determine the effects of subcutaneous senktide injections, a baseline TCORE value was first calculated by averaging all TCORE values from the 30 minutes before injection. To calculate treatment response from baseline, TCORE values for each animal were subtracted from the baseline TCORE of that animal. Data were analyzed using two-way ANOVA with repeated measures (treatment over time) and Tukey's post hoc analysis (α = .05).
Results
NK3-SAP injections ablated NK3R-ir neurons in the MnPO and septum
Light microscopy of Nissl-stained sections revealed no qualitative differences between control (blank-SAP) and NK3-SAP rats (Figure 1, A and B). Importantly, no parenchymal damage was observed, other than the cannula tract in the medial septum. Using immunohistochemical analysis, the experimental groups could be clearly distinguished by the pattern of NK3R cell loss (Figure 1, C and D). In control rats, large NK3R neurons were identified in the medial septum, septohypothalamic nucleus, and nucleus basalis (39). Small NK3R neurons were identified in the bed nucleus of the stria terminalis, and lightly labeled neurons were scattered in the medial preoptic area. Control rats also had dense NK3R fiber labeling in the organum vasculosum of the lamina terminalis and light punctate staining in the MnPO, consistent with previous reports (29). In rats injected with NK3-SAP, there was loss of NK3R labeling in the medial septum, septohypothalamic nucleus, and MnPO. For simplicity, this region of NK3R cell loss will be referred to as the MnPO/septum. Variable loss of NK3R neurons was observed in the bed nucleus of the stria terminalis of NK3-SAP rats. Limited diffusion of toxin was demonstrated by the preservation of NK3R immunoreactivity in the nucleus basalis and organum vasculosum of the lamina terminalis. The loss of NK3R-ir elements, with no perceptible changes in Nissl morphology, is consistent with previous studies demonstrating the selectivity of NK3-SAP for ablation of NK3R neurons in the rat brain (32).
Figure 1.
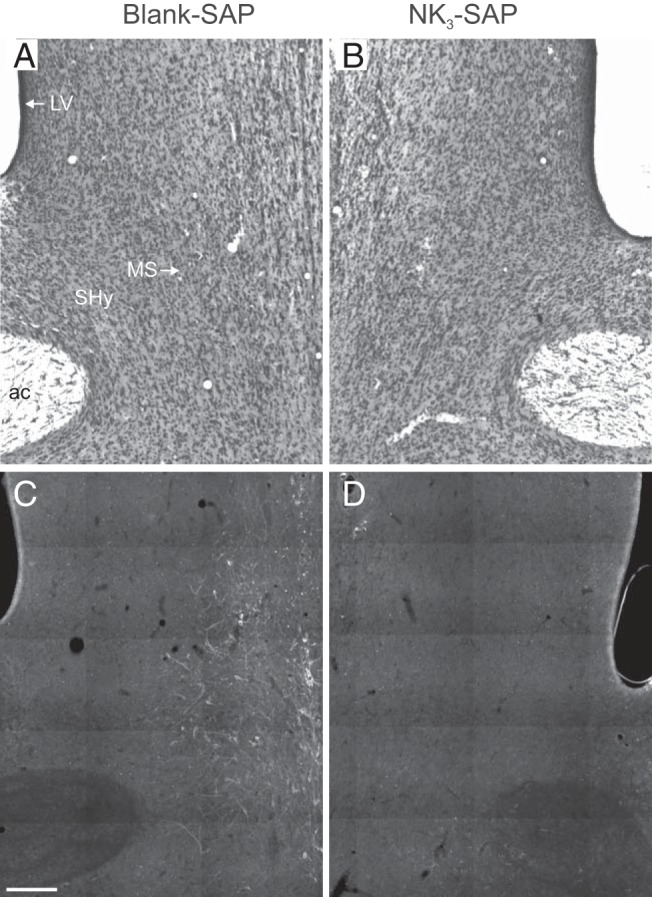
Representative coronal photomicrographs of the septum from rats injected with blank-SAP (left) or NK3-SAP (right). Cresyl violet–stained sections (A and B) show no qualitative change in the Nissl architecture between blank-SAP and NK3-SAP rats. In contrast, immunohistochemical studies (C and D) reveal a marked reduction in NK3R-ir cells in rats with NK3-SAP injections. Sections are shown as mirror images with landmarks labeled in A: ac, anterior commissure; LV, lateral ventricle; MS, medial septum, SHy, septohypothalamic nucleus. Scale bar corresponds to 250 μm.
NK3-SAP injections did not produce retrograde degeneration of KNDy neurons
Because loss of NKB-ir cells and fibers in the arcuate nucleus is a sensitive marker for KNDy neuron ablation (32), we conducted NKB immunohistochemical analysis to evaluate KNDy neurons in the injected animals. Qualitatively, there was no difference in the labeling of NKB-ir cell bodies and fibers in the arcuate nucleus between rats receiving NK3-SAP or blank-SAP injections in the MnPO/septum (Figure 2, A and B). Cell counting confirmed that NK3-SAP did not produce retrograde degeneration of arcuate NKB-ir neurons (28.6 ± 2.0 neurons/unilateral arcuate section [control] vs 32.0 ± 1.7 neurons/unilateral arcuate section [NK3-SAP], n = 6–7 rats/group; mean ± SEM).
Figure 2.
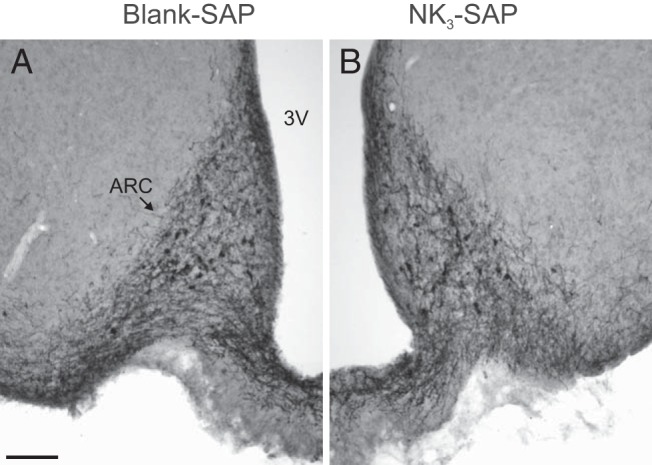
Representative photomicrographs of NKB-ir cells and fibers in the arcuate nucleus of rats injected with blank-SAP (A) or NK3-SAP (B). There was no retrograde degeneration of arcuate NKB neurons in rats with NK3-SAP injections into the MnPO/septum. Sections are shown as mirror images with landmarks labeled in A: 3V, third ventricle; ARC, arcuate nucleus. Scale bar corresponds to 100 μm.
Ablation of NK3R neurons in the MnPO/septum mildly decreased peak levels of serum LH in OVX rats but had no effect on body weight
Three weeks after ovariectomy, control animals displayed a 15-fold increase in serum LH, and this value decreased to intact levels after E2 treatment (Figure 3A). A similar pattern of serum LH was observed in the NK3-SAP rats, but the peak levels after ovariectomy were slightly (but significantly) reduced, compared with those of controls (19.7 ± 1.3 ng/mL [NK3-SAP] vs 23.7 ± 1.2 ng/mL [control], n = 5–7 rats/group). Control animals gained 18% to 22% of their original body weight after ovariectomy, and weight loss occurred after E2 treatment (Figure 3B). Identical changes in body weights occurred in rats injected with NK3-SAP. Body weights were not significantly different between control and NK3-SAP animals at any time point (Figure 3B).
Figure 3.
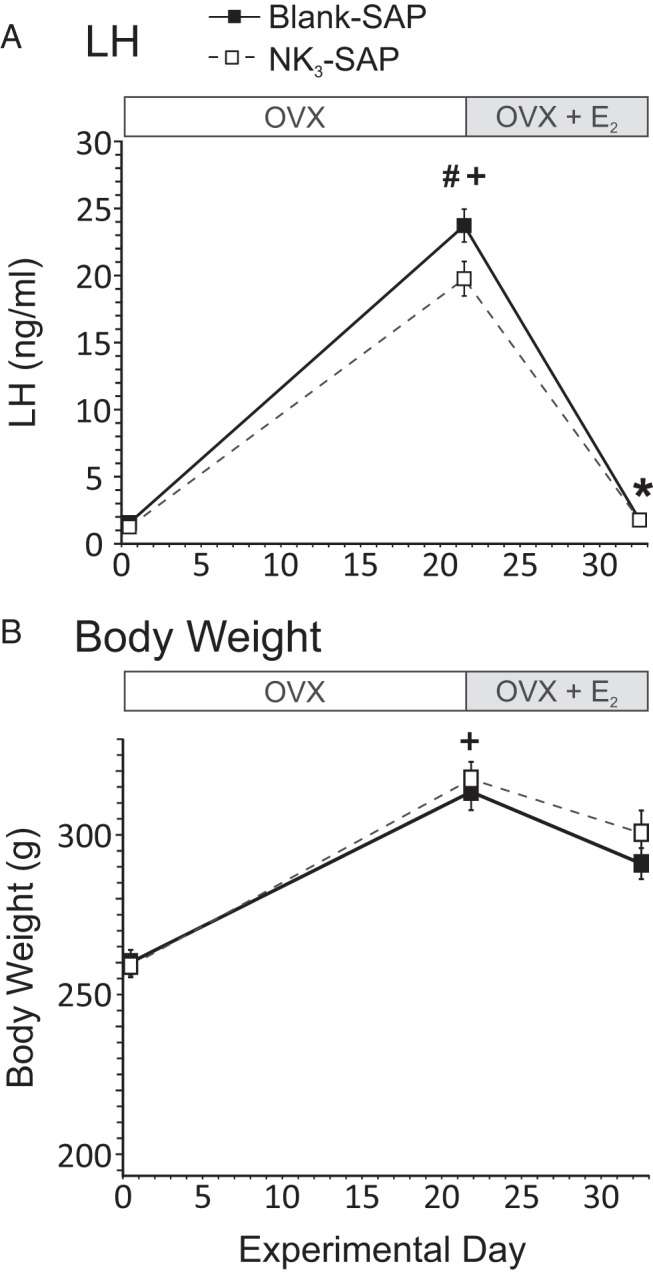
Effects of ablating MnPO/septal NK3R neurons on serum LH (A) and body weight (B). Rats were OVX and injected with blank-SAP (control) or NK3-SAP in the arcuate nucleus on day 0, implanted with subcutaneous E2 capsules on day 22, and killed 11 days later. Serum LH and body weight were markedly increased after 22 days of ovariectomy and reduced by E2 treatment in control rats. Similar changes were seen in NK3-SAP rats; however, the peak levels of serum LH after OVX were slightly reduced compared with those of controls. Body weights were similar between control and NK3-SAP rats. #, P ≤ .05, significantly different, NK3-SAP vs blank-SAP; +, P ≤ .01, significantly different from intact values on day 0; *, P ≤ .01, significantly different after E2 (vs OVX).
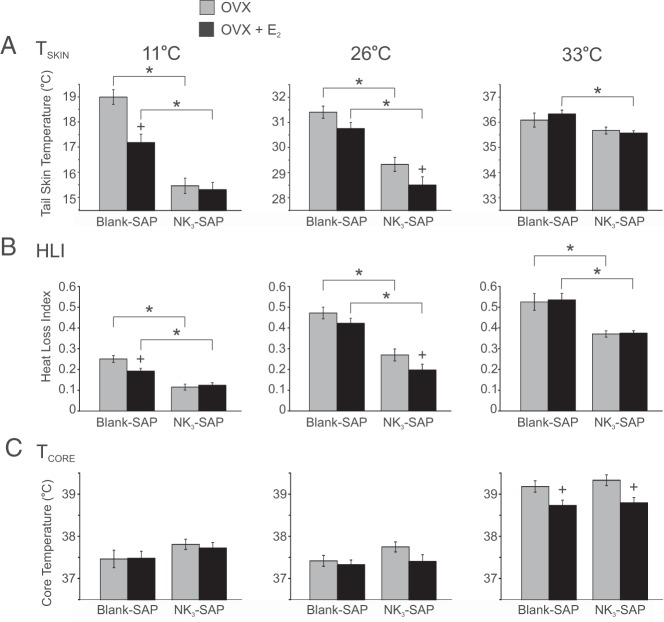
Ablation of NK3R neurons in the MnPO/septum decreased tail skin vasodilation but did not prevent the E2 modulation of TSKIN or TCORE
At TAMBIENT of 11°C and 26°C, the TSKIN of NK3-SAP rats was lower than that of controls, in both OVX and OVX + E2 conditions (Figure 4A). At 33°C, the TSKIN of NK3-SAP rats was significantly lower than that of control rats, but only in OVX + E2 rats. At all 3 TAMBIENT and in both OVX and OVX + E2 conditions, the HLI was significantly lower in NK3-SAP rats than in controls (Figure 4B). These data indicate a consistent decrease in tail skin vasodilation in NK3-SAP rats, regardless of E2 status or TAMBIENT. E2 treatment significantly reduced TSKIN and HLI in NK3-SAP rats (at the TAMBIENT of 26°C) and control rats (at the TAMBIENT of at 11°C). Thus, despite the consistently lower levels of tail skin vasodilation in NK3-SAP rats, the TSKIN and HLI were still responsive to E2 at a neutral TAMBIENT.
Figure 4.
Effects of blank-SAP (control) or NK3-SAP injections on TSKIN (A), HLI (B), and TCORE (C) in rats exposed to various TAMBIENT. A, At TAMBIENT of 11°C and 26°C, TSKIN was lower in NK3-SAP than in control rats. E2 reduced TSKIN in control rats at the TAMBIENT of 11°C and in NK3-SAP rats at the TAMBIENT of 26°C. B, At all 3 TAMBIENT, the lower levels of HLI in NK3-SAP rats indicates decreased skin vasodilation. C, At the high TAMBIENT of 33°C, the TCORE was reduced by E2 in both control and NK3-SAP rats. No differences were detected in TCORE between control and NK3-SAP rats. Values represent means ± SEM; n = 6 to 8 rats/group. *, Significantly different, blank-SAP vs NK3-SAP; +, significantly different after E2 treatment (within blank-SAP or NK3-SAP).
At the high TAMBIENT of 33°C, the average TCORE of OVX control rats rose to above 39.0°C, and this value was reduced by E2 treatment (Figure 4C). These changes agree with previous descriptions that E2 improves TCORE regulation of OVX rats at supraneutral TAMBIENT (20, 45). Similar to controls, E2 lowered TCORE in NK3-SAP rats at the TAMBIENT of 33°C, and there was no significant difference in TCORE between NK3-SAP and control rats at any TAMBIENT.
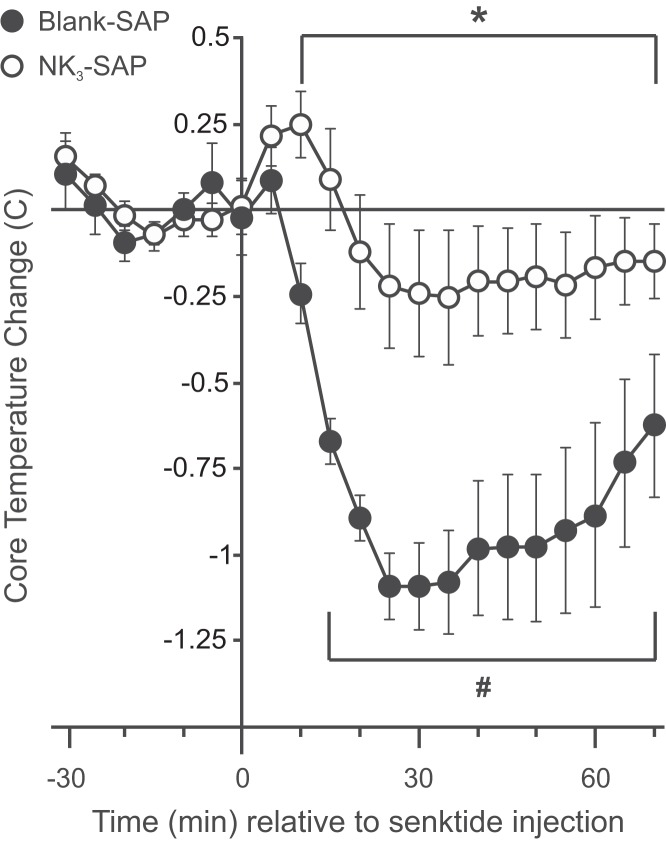
Ablation of NK3R neurons in the MnPO/septum blocked the hypothermic effect of senktide and reduced the number of Fos-ir cells in the MnPO
Subcutaneous injection of the NK3R agonist, senktide, caused a rapid decrease in TCORE in blank-SAP rats (Figure 5). The changes in TCORE after the subcutaneous senktide injections were nearly identical to those observed previously after direct microinfusion of senktide into the MnPO of female rats (29). In contrast, senktide did not significantly lower TCORE in NK3-SAP rats. The average TCORE of blank-SAP rats was lower than that of NK3-SAP rats from 10 to 70 minutes after senktide injections. Wet-dog shakes were observed in both blank-SAP and NK3-SAP rats, indicative of the central effects of senktide (34).
Figure 5.
Effects of subcutaneous injections of the NK3R agonist, senktide, in blank-SAP and NK3-SAP rats. Subcutaneous senktide injections significantly reduced TCORE in blank-SAP control rats similar to senktide microinfusion directly into the MnPO (29). In contrast, subcutaneous senktide injections did not significantly reduce TCORE in rats with ablation of NK3R-expressing MnPO neurons. Data represent changes from baseline in average TCORE (± SEM; n = 6–8 rats/group). #, Significantly different from baseline in control rats; *, significantly different, control rats vs NK3-SAP rats.
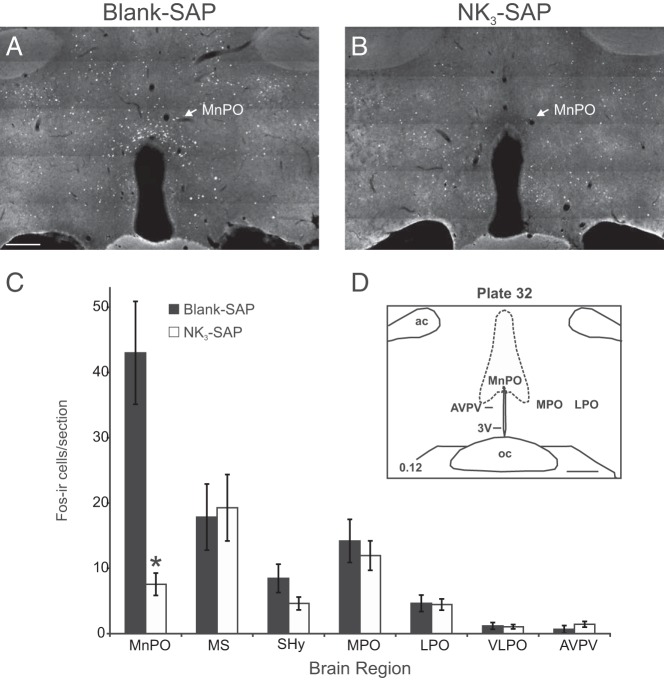
Numerous Fos-ir cells highlighted the MnPO of blank-SAP rats after subcutaneous senktide injections, with smaller numbers of scattered Fos-ir cells in adjacent nuclei (Figure 6A). These findings were similar to those of our previous studies, in which direct microinfusion of senktide into the MnPO induced marked Fos activation in the MnPO and little induction of Fos in adjacent nuclei (29). In contrast, in NK3-SAP rats, there were few Fos-ir cells detected in the MnPO after subcutaneous senktide injections (Figure 6B).
Figure 6.
Representative photomicrographs of Fos immunofluorescence in blank-SAP (A) and NK3-SAP (B) rats injected subcutaneously with senktide 90 minutes before killing. After senktide injections, numerous Fos-ir cells were identified in the MnPO of control rats (A) but not in that of NK3-SAP rats (B). C, Average numbers of Fos-ir cells counted in blank-SAP and NK3-SAP rats injected with senktide. In the MnPO, the number of Fos-ir neurons was markedly decreased in NK3-SAP rats, compared with those in controls. No significant differences were detected in other areas. D, Schematic diagram of landmarks at this level of the hypothalamus (43). Scale bar corresponds to 250 μm for A and B. Scale bar corresponds to 500 μm for D. Values represent means ± SEM; n = 8 rats/group. *, P < .05, significantly different, blank-SAP vs NK3-SAP. ac, anterior commissure; AVPV, anteroventral periventricular nucleus; LPO, lateral preoptic area; MnPO, median preoptic nucleus; MPO, medial preoptic area; MS, medial septum; oc, optic chiasm; SHy, septohypothalamic nucleus; VLPO, ventrolateral preoptic area; 3V, third ventricle.
Cell counts showed significantly more Fos-ir nuclei in the MnPO of blank-SAP rats after senktide injections than in that of NK3-SAP rats (Figure 6C). This effect was significant at 2 different levels of the MnPO, corresponding to plates 32 and 34 (43). In contrast, there was no difference in the numbers of Fos-ir nuclei between NK3-SAP and control rats in the medial septum, septohypothalamic nucleus, medial preoptic area, lateral preoptic area, ventrolateral preoptic area, or anteroventral periventricular nucleus (Figure 6C).
Discussion
Ablation of estrogen-responsive arcuate KNDy neurons reduces tail skin vasodilation, prevents the effects of E2 on TSKIN during the light phase, and blocks the E2 reduction in TCORE during heat stress (20). These data provide evidence that KNDy neurons influence the activation of heat-dissipation effectors and therefore could play a role in the mechanism of flushes (4). We have hypothesized that KNDy neurons facilitate cutaneous vasodilation via projections to NK3R-expressing neurons in the preoptic hypothalamus (4, 20). To further investigate the function of NK3R neurons in the MnPO, we ablated these neurons using stereotaxic injections of NK3-SAP. KNDy neurons were preserved in these animals, consistent with previous studies showing that peptide-conjugated SAP does not produce retrograde neuronal degeneration (46). Remarkably, the HLI (an index of tail vasomotion) of NK3-SAP rats was lower than that of controls at all TAMBIENT, indicating decreased levels of cutaneous vasodilation. Although ablation of these neurons lowered tail skin vasodilation, there was no global impairment in the ability of the tail vessels to dilate, because the HLI was appropriately higher in the warm ambient temperature of 33°C (relative to 26°C). Moreover, compensatory mechanisms were able to maintain TCORE, despite the relative vasoconstriction. These findings are remarkably similar to the effects of KNDy neuron ablation (20) and show that NK3R neurons in the MnPO/septum, like KNDy neurons, facilitate tail skin vasodilation.
In our previous studies, ablation of KNDy neurons prevented the rise in serum LH after ovariectomy and reduced tonic LH secretion in E2-treated rats (20). In contrast, when NK3R neurons were ablated in the MnPO/septum, there was only a mild reduction (approximately 16%) in the peak levels of serum LH, which could be secondary to degeneration of a small subpopulation of GnRH neurons in the rostral hypothalamus that express NK3R (39). Another major difference is that KNDy neuron ablation blocked the effects of E2 on body weight and girth (20), but no changes occurred in these parameters when MnPO/septal neurons were lesioned. Clearly, NK3R-expressing KNDy neurons and NK3R-expressing MnPO/septal neurons play very different roles in LH secretion and body weight.
Our studies show that NK3R-expressing neurons in the MnPO/septum are required for the NK3R agonist, senktide, to produce hypothermia. We have previously shown that direct microinfusion of senktide into the MnPO selectively activates Fos in MnPO neurons and results in hypothermia (29). In the present study, subcutaneous injections of senktide in blank-SAP rats induced a rapid drop in core temperature that was virtually identical to that seen after direct infusion into the MnPO (29). In contrast, the hypothermic effect of subcutaneous senktide was completely blocked in NK3-SAP rats, with TCORE exhibiting changes similar to those for the direct MnPO infusion of vehicle in previous studies (29). Moreover, the extensive Fos labeling in the MnPO in blank-SAP rats after senktide injections was not observed in NK3-SAP rats. These data provide further evidence that NK3-SAP successfully ablated NK3R neurons in the MnPO and supports the hypothesis that activation of NK3R-expressing MnPO neurons mediates the hypothermic effect of senktide.
In a warm environment, E2 protects against heat stress by reducing body temperature (20, 45, 47). Similar effects were seen in the present study: E2 reduced the TCORE of both control and NK3-SAP rats at the high TAMBIENT of 33°C. Moreover, E2 reduced TSKIN and HLI in both control and NK3-SAP rats, although at different TAMBIENT (11°C in controls and 26°C in NK3-SAP rats). These data are consistent with the variable effects of E2 on TSKIN during the light phase (33). Thus, we were unable to convincingly demonstrate that NK3R neuron ablation in the MnPO/septum blocked the effects of estradiol on TCORE or TSKIN, in contrast to the effects of KNDy neuron ablation (20). Whereas these data indicate that NK3R neurons in the MnPO/septum are not essential for the modulation of thermoregulation by E2, a role is not excluded because there may be compensatory mechanisms or redundant circuits. Because KNDy neurons are preserved in these animals, activation of other preoptic neurons expressing the receptors for kisspeptin or dynorphin could continue to mediate E2 effects (48, 49). Alternatively, the medial preoptic area, another important thermoregulatory structure, has NK3R-expressing neurons and there is preliminary evidence that warm-sensitive medial preoptic area neurons express the gene encoding NK3R (50). Further studies will be needed to determine whether ablation of NK3R neurons in both the MnPO and medial preoptic area blocks the E2 modulation of body temperature.
The MnPO regulates multiple autonomic functions, including thermoregulation, water balance, cardiovascular homeostasis, circadian rhythms, and sleep (51–56). Importantly, the MnPO participates in the thermosensory heat-defense pathway that relays information from cutaneous thermoreceptors to brainstem areas controlling cutaneous vasodilation (23). Our studies support a specific role of a subpopulation of NK3R-expressing neurons in the MnPO in this heat-defense pathway. KNDy neurons project to the MnPO in the rat (10, 21), but it is not known whether a homologous projection pathway exists in the human. Many features of KNDy neurons are conserved among mammalian species, however, including the expression of estrogen receptor-α (6, 10, 11, 19, 57), the modulation of NKB and kisspeptin mRNA by estrogens (13, 18, 58–61), and the projections of KNDy neurons to GnRH terminals in the median eminence (21, 39, 62–64). Moreover, the morphological appearance of the MnPO is similar in a wide range of species including rat, dog, monkey, and human (52, 65). Although studies are needed to determine whether KNDy neurons project to the MnPO in the human, our studies provide basic information on how increased KNDy neuron activity in postmenopausal women (6, 7, 66) could modulate preoptic thermoregulatory pathways and thus be involved in the generation of hot flushes (4). Further support for a role of NK3R signaling in flushes comes from the recent demonstration that infusion of NKB triggers hot flushes in women (67).
In summary, our studies show that ablation of NK3R neurons in the MnPO/septum consistently decreased tail skin vasodilation, much like that seen after ablation of KNDy neurons in the arcuate nucleus. Moreover, ablation of NK3R neurons in the MnPO/septum blocked both the hypothermic effects of senktide and the activation of MnPO neurons by senktide. Combined with previous studies (23, 27, 29), these data suggest a role of MnPO NK3R neurons in the activation of heat-dissipation effectors and support the hypothesis that KNDy neurons could influence thermoregulatory vasodilation via projections to NK3R-expressing neurons in the MnPO. However, unlike the effects of KNDy neuron ablation, ablation of MnPO NK3R neurons did not influence E2 modulation of TSKIN or TCORE. Thus, the essential pathways for the modulation of thermoregulation by E2 remain to be determined.
Acknowledgments
We gratefully acknowledge Dr Phillipe Ciofi for the donation of the NK3R antiserum.
This work was supported by the National Institutes of Health National Institute on Aging (Grants R01 AG032315 and R01 AG047887). The hormone assays were performed at the Ligand Assay and Analysis Core at the University of Virginia Center for Research in Reproduction (supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54-HD28934).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DAPI
- 4′,6-diamidino-2-phenylindole
- E2
- estradiol-17β
- HLI
- heat loss index
- ir
- immunoreactive
- KNDy
- neurons coexpressing kisspeptin, NKB, and dynorphin
- MnPO
- median preoptic nucleus
- NKB
- neurokinin B
- NK3R
- neurokinin 3 receptor
- OVX
- ovariectomized
- TAMBIENT
- ambient temperature
- TCORE
- core temperature
- TSKIN
- skin temperature.
References
- 1. Santoro N. Symptoms of menopause: hot flushes. Clin Obstet Gynecol. 2008;51:539–548. [DOI] [PubMed] [Google Scholar]
- 2. Kronenberg F. Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J Nutr. 2010;140:1380S–1385S. [DOI] [PubMed] [Google Scholar]
- 3. Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13:453–464. [DOI] [PubMed] [Google Scholar]
- 4. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., 3rd Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71:79–85. [DOI] [PubMed] [Google Scholar]
- 6. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. [DOI] [PubMed] [Google Scholar]
- 7. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. [DOI] [PubMed] [Google Scholar]
- 8. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118. [DOI] [PubMed] [Google Scholar]
- 9. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–153. [DOI] [PubMed] [Google Scholar]
- 10. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. [DOI] [PubMed] [Google Scholar]
- 11. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. [DOI] [PubMed] [Google Scholar]
- 12. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 13. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skrapits K, Borsay BA, Herczeg L, Ciofi P, Liposits Z, Hrabovszky E. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front Neurosci. 2015;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casper RF, Yen SS, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science. 1979;205:823–825. [DOI] [PubMed] [Google Scholar]
- 18. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romanovsky AA, Almeida MC, Garami A, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dacks PA, Krajewski SJ, Rance NE. Ambient temperature and 17β-estradiol modify fos immunoreactivity in the median preoptic nucleus, a putative regulator of skin vasomotion. Endocrinology. 2011;152:2750–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372:395–414. [DOI] [PubMed] [Google Scholar]
- 29. Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152:4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drapeau G, d'Orléans-Juste P, Dion S, Rhaleb NE, Regoli D. Specific agonists for neurokinin B receptors. Eur J Pharmacol. 1987;136:401–403. [DOI] [PubMed] [Google Scholar]
- 31. Corboz MR, Rivelli MA, Eckel SP. Bronchoconstrictor effect of the tachykinin NK3-receptor agonists [MePhe7]-neurokinin B and senktide in the isolated guinea pig lung. Exp Lung Res. 2010;36:509–521. [DOI] [PubMed] [Google Scholar]
- 32. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams H, Dacks PA, Rance NE. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. 2010;151:5389–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stoessl AJ, Dourish CT, Iversen SD. The NK-3 tachykinin receptor agonist senktide elicits 5-HT-mediated behaviour following central or peripheral administration in mice and rats. Br J Pharmacol. 1988;94:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. [DOI] [PubMed] [Google Scholar]
- 36. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patronas P, Horowitz M, Simon E, Gerstberger R. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res. 1998;798:127–139. [DOI] [PubMed] [Google Scholar]
- 38. Griffond B, Ciofi P, Bayer L, Jacquemard C, Fellmann D. Immunocytochemical detection of the neurokinin B receptor (NK3) on melanin-concentrating hormone (MCH) neurons in rat brain. J Chem Neuroanat. 1997;12:183–189. [DOI] [PubMed] [Google Scholar]
- 39. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. [DOI] [PubMed] [Google Scholar]
- 40. Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience. 1992;51:317–345. [DOI] [PubMed] [Google Scholar]
- 41. Marksteiner J, Saria A, Krause JE. Comparative distribution of neurokinin B-, substance P- and enkephalin-like immunoreactivities and neurokinin B messenger RNA in the basal forebrain of the rat: evidence for neurochemical compartmentation. Neuroscience. 1992;51:107–120. [DOI] [PubMed] [Google Scholar]
- 42. Ciofi P, Krause JE, Prins GS, Mazzuca M. Presence of nuclear androgen receptor-like immunoreactivity in neurokinin B-containing neurons of the hypothalamic arcuate nucleus of the adult male rat. Neurosci Lett. 1994;182:193–196. [DOI] [PubMed] [Google Scholar]
- 43. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th ed Burlington, MA: Elsevier Inc; 2007. [Google Scholar]
- 44. Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. [DOI] [PubMed] [Google Scholar]
- 45. Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–1191. [DOI] [PubMed] [Google Scholar]
- 47. Baker MA, Dawson DD, Peters CE, Walker AM. Effects of estrogen on thermoregulatory evaporation in rats exposed to heat. Am J Physiol. 1994;267:R673–R677. [DOI] [PubMed] [Google Scholar]
- 48. Csabafi K, Jászberényi M, Bagosi Z, Lipták N, Telegdy G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav Brain Res. 2013;241:56–61. [DOI] [PubMed] [Google Scholar]
- 49. Xin L, Geller EB, Adler MW. Body temperature and analgesic effects of selective mu and kappa opioid receptor agonists microdialyzed into rat brain. J Pharmacol Exp Ther. 1997;281:499–507. [PubMed] [Google Scholar]
- 50. Eberwine J, Bartfai T. Single cell transcriptomics of hypothalamic warm sensitive neurons that control core body temperature and fever response: signaling asymmetry and an extension of chemical neuroanatomy. Pharmacol Ther. 2011;129:241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Travis KA, Johnson AK. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. Am J Physiol. 1993;264:R1200–R1205. [DOI] [PubMed] [Google Scholar]
- 52. McKinley MJ, McAllen RM, Davern P, et al. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:1–122. [DOI] [PubMed] [Google Scholar]
- 53. Lazarus M, Yoshida K, Coppari R, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. [DOI] [PubMed] [Google Scholar]
- 54. Whyte DG, Johnson AK. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (AV3V) during acute heat stress in the rat. Clin Exp Pharmacol Physiol. 2005;32:457–461. [DOI] [PubMed] [Google Scholar]
- 55. Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. [DOI] [PubMed] [Google Scholar]
- 56. Ployngam T, Collister JP. An intact median preoptic nucleus is necessary for chronic angiotensin II-induced hypertension. Brain Res. 2007;1162:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. [DOI] [PubMed] [Google Scholar]
- 59. Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol. 2003;15:749–753. [DOI] [PubMed] [Google Scholar]
- 60. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145:736–742. [DOI] [PubMed] [Google Scholar]
- 61. Alçin E, Sahu A, Ramaswamy S, et al. Ovarian regulation of kisspeptin neurones in the arcuate nucleus of the rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2013;25:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. [DOI] [PubMed] [Google Scholar]
- 64. Borsay BÁ, Skrapits K, Herczeg L, et al. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin b and substance p immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology. 2014;100:141–152. [DOI] [PubMed] [Google Scholar]
- 65. Nauta WJH, Haymaker W. Hypothalamic nuclei and fiber connections. In: Haymaker W, Anderson E, Nauta WJH. eds. The Hypothalamus. Springfield, IL: Charles C Thomas; 1969:136–209. [Google Scholar]
- 66. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]