Highlights
-
•
A combination of laurate and oleate synergistically enhances lipotoxicity.
-
•
Cell death pathways influenced by a combination of oleate and laurate vary among cell types.
-
•
Laurate increases oleate uptake.
Abbreviations: LC-SFAs, long-chain saturated fatty acids; MC-SFAs, medium-chain saturated fatty acids; ROS, reactive oxygen species; SFAs, saturated fatty acids; TG, triacylglycerol
Keywords: Fatty acid, Lipotoxicity, Laurate, Oleate
Abstract
Free fatty acids have been reported to induce cell death (lipotoxicity), but the effects depend on the carbon chain length and number of double bonds. Medium-chain saturated fatty acids (MC-SFAs), such as laurate, have less lipotoxicity than long-chain saturated fatty acids (LC-SFAs), such as palmitate. Monounsaturated fatty acids, such as oleate, have also been reported not only to exert cytotoxic effects, but also to reduce the lipotoxicity of LC-SFA. However the interaction between MC-SFA and oleate with respect to cell death is unclear. In this report, we found that lipotoxicity was enhanced by a combination of laurate and oleate relative to either fatty acid alone. The possible mechanisms involved were examined by measuring the production of reactive oxygen species, mitochondrial depolarization, caspase-3 activity, and lipid droplet formation. Although the stress signals and cell death pathways were distinct among different cell types, we found a common phenomenon of enhanced lipid droplet formation in all cells tested. Using fluorescent- or radioisotope-labeled fatty acids, we found that oleate, but not laurate, increased the uptake of fluorescent-labeled fatty acids, and the combinatory effect was more efficient than with oleate alone. We also found that laurate increased oleate uptake, but the effect of oleate on laurate uptake varied among cell types. These results suggest that laurate enhances the influx rate of oleate, the increased intracellular concentration of which not only enhances lipid storage, but also induces cell death by lipotoxic stress responses, which vary according to cell type.
1. Introduction
Saturated fatty acids (SFAs) represent endogenously synthesized and dietary compounds that substantially contribute to tissue building and serve as an energy source as well as facilitating the development of diseases [1–3]. In mammalian cells, SFAs vary in their carbon chain lengths from 2 to 24 carbons, and the different chain lengths impart various physiological activities to the fatty acids in many cell types [1–3]. Current evidence suggests that an accumulation of long-chain (LC)-SFAs (more than 14 carbons) leads to lipid-mediated cell dysfunction [1–3]. Many investigators have also demonstrated that LC-SFAs, especially palmitate and stearate, induce apoptosis within a variety of cell types [4–14]. LC-SFAs must be activated to long-chain acyl-CoA for oxidation or use in cellular processes such as membrane formation and lipid storage. Many studies have reported that LC-SFAs induce cell death by triggering production of reactive oxygen species (ROS) [15], causing dysfunction of membrane fluidity by changing the fatty acid content [4,16], and inducing stress signals [5–8,20].
On the other hand, long chain monounsaturated fatty acids (MUFAs), such as oleate and palmitoleate, have lower cytotoxicity than LC-SFAs, but a combination results in an even lower cytotoxic effect, suggesting that MUFAs can inhibit LC-SFA cytotoxicity [4,9,17–20,37]. Following treatment of cells with palmitate and oleate, the intracellular membrane composition of oleate increases to maintain high fluidity and suppress stress signals induced by the treatment with palmitate alone [21,22]. Thus, it appears that MUFAs can promote detoxification of LC-SFAs by altering their partitioning within cells.
In contrast to LC-SFAs, medium chain-saturated fatty acids (MC-SFAs) such as caprylate (8 carbons), caproate (10 carbons), and laurate (12 carbons), are thought to be less cytotoxic than LC-SFAs [23]. MC-SFAs have physical and metabolic properties that are distinct from those of LC-SFAs, which make them a readily available cellular energy source. Few papers have studied cell death induction by MC-SFAs [24], and any interaction between MC-SFA and oleate in the context of cell death has not yet been evaluated precisely. In this report, we found that co-stimulation of laurate with oleate promoted more cytotoxic activity than each alone in several cell types. We also showed that the combination of fatty acids enhanced the formation of lipid droplets and increased fatty acid uptake, especially laurate, which greatly enhanced oleate uptake. Our results highlight the ability of laurate to increase fatty acid uptake and decrease the threshold of oleate cytotoxicity.
2. Materials and methods
2.1. Cell culture
Cells were obtained from the RIKEN Cell Bank (Tsukuba, Japan) and the Health Science Research Resources Bank (HSRRB) (Osaka, Japan) and maintained at 37 °C in a humidified 5% (v/v) CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 10 U/ml penicillin, and 10 g/ml streptomycin, as described previously [25,26].
2.2. Fatty acid treatment
Fatty acids were obtained from Wako (Osaka, Japan), Sigma (St. Louis, MO), and Tokyo Chemical Industry Co., Ltd. (TCI, Tokyo, Japan). Stock fatty acid solutions (100 mM) in dimethyl sulfoxide (DMSO) were diluted in medium containing 0.5% (w/v) fatty acid-free bovine serum albumin. Cells were seeded at 2 × 105/well into 96-well plates. After 24 h of culture, fatty acids (0.5–1 mM) were added to the media. All experiments were carried out at 37 °C under a humidified 5% (v/v) CO2 atmosphere.
2.3. MTT assay
The MTT assay was used to determine cell viability; the assay is based on bioreduction of the tetrazolium compound MTT [3-(4,5-dimethylthiazolyl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] and the electronic coupling reagent phenazine ethosulfate (PES) to yield a colored formazan product, the absorbance of which is proportional to the number of viable cells. Briefly, after fatty acid treatment for 24–48 h, MTT solution was added to media, the plates were incubated at 37 °C for 2–4 h, and the absorbance of each supernatant was measured at 535 nm, according to the manufacturer’s instructions.
2.4. Flow cytometry
Cells were treated with fatty acids for 1–6 h and stained with NucView™ 488 (Biotium, Hayward, CA) to determine the extent of caspase-3 activation; with tetramethylrhodamine methylester (TMRM, Invitrogen) to assess the integrity of the mitochondrial membrane; or with 2′,7′dichlorofluorescin diacetate (DCFDA, Invitrogen) to detect reactive oxygen species (ROS), according to the respective manufacturers’ instructions. Fluorescent cells were detected using a JSAN platform (Bay Bioscience, Kobe, Japan) and cell populations were analyzed with the aid of Flowjo software (TreeStar, Ashland, OR).
2.5. Measurement of fatty acid uptake
Cells were incubated with a fluorescent fatty acid analog, C1-BODIPY 500/510 dodecanoic acid (Molecular Probes, Eugene, OR), or radioisotope-labeled fatty acids, thus [1-14C]-oleate, laurate, or palmitate (PerkinElmer, Waltham, MA), with or without addition of other fatty acids, for 1–4 h [27]. Then the cells were washed three times with PBS, lysed in NaOH, and the suspensions were neutralized with acetic acid. Then the cell lysates were transferred to either 96-well black microwell-plates or scintillation vials containing Sintisol (Nacarai Tesque, Kyoto, Japan). Fluorescence intensities and radioactivity levels were measured using a Fluoroscan Ascent platform (Thermo-Scientific, Rochester, MA) or a liquid scintillation counter, respectively.
3. Results and discussion
Previous reports have shown that palmitate, an LC-SFA, induced cell death in several cell types but that its cytotoxicity was ameliorated when combined with oleate [2,3]. Although the MC-SFAs (caprylate, caproate, and laurate) were less cytotoxic than palmitate, any combinatory effect with oleate on cytotoxicity remains unknown. To test the combinatory effect of oleate with MC-SFAs on cell viability, we stimulated HT1080 cells, a human fibrosarcoma cell line, using MC-SFAs with or without oleate. When the cells were stimulated with each fatty acid alone, palmitate was more cytotoxic than laurate and oleate, but these fatty acids still exhibited cytotoxic effects at higher concentrations (Fig. 1). As shown in previous studies, co-stimulation by palmitate (0.5 mM) with oleate (1 mM) caused much less lipotoxicity (more than 80% viability) than that by palmitate alone; however, a mixture of MC-SFA and oleate effectively induced cell death, especially in the case of laurate (less than 10% viability; Fig. 2a and b).
Fig. 1.
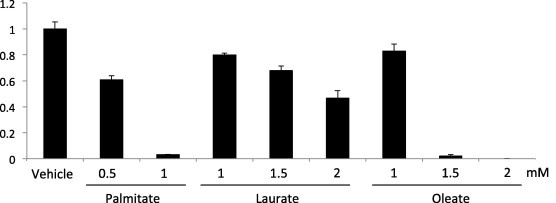
Lipotoxicity induced by palmitate, laurate, or oleate alone. HT1080 cells were treated with palmitate, laurate, or oleate for 24 h. Cell viability was assessed using the MTT assay, as described in Section 2. The data are the means ± SDs (n = 3).
Fig. 2.
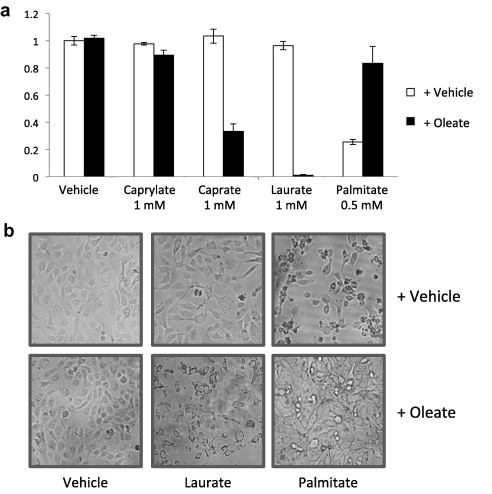
Effect of oleate on lipotoxicity induced by caprylate, caprate, laurate, or palmitate. (a) HT1080 cells were treated with caprylate, caprate, laurate, or palmitate in the presence or absence of oleate for 24 h. Cell viability was assessed using the MTT assay, as described in Section 2. The data are the means ± SDs (n = 3). (b) Representative phase-contrast micrographs taken at the conclusion of the experiments.
To investigate whether the combination of laurate and oleate exerts the same effects in other cell lines, we tested the combination on 10 additional human and mouse cancer cell lines (A549, Caco2, Ehrlich, HeLa, HepG2, Jurkat, Neuro2a) and cell lines derived from normal tissues (JB6, MRC-5, NIH-3T3). The combination induced a variable but appreciable degree of cell death in all cell lines tested (Fig. 3). These results suggest that the effect of palmitate is different from that of laurate when mixed with oleate.
Fig. 3.
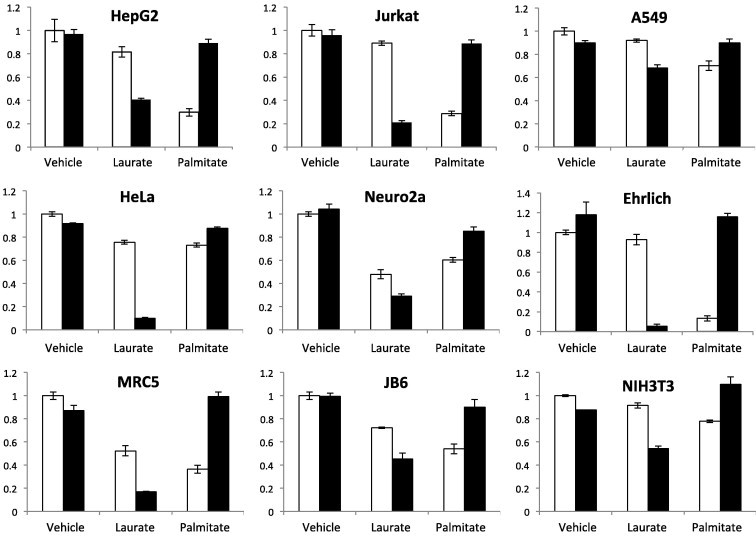
Viabilities of human and mouse cell lines upon co-exposure to laurate or palmitate, and oleate. The viabilities of human (A549, Caco2, Ehrlich, HeLa, HepG2, and Jurkat) and mouse (Neuro2a) cancer cell lines, and normal human (MRC-5) and mouse (JB6, NIH3T3) cell lines, were assessed as described in the legend to Fig. 1. The data are the means ± SDs (n = 3).
Long-chain saturated fatty acids have been shown in several cell types to produce reactive oxygen species (ROS), decrease mitochondrial membrane potential, and induce caspase-3 activation, which are hallmark features of LC-SFA-induced cell death [2,3,15]. To assess the effects of a combination of laurate and oleate on ROS production, HT1080, HepG2, and Jurkat cells were treated with laurate in combination with oleate for 1 h. As shown in Fig. 4, ROS levels were increased to a greater extent by exposure of Jurkat cells to the combination (6-fold); however, the effects were less in HT1080 and HepG2 cells (less than 3-fold) than Jurkat cells. The caspase-3 activation induced by laurate with oleate occurred at 6 h in Jurkat cells (more than 60%), but not in HT1080 or HepG2 cells (less than 20%, Fig. 5). The loss of mitochondrial membrane potential is one of the steps preceding caspase activation during apoptosis. As shown in Fig. 6, the combination of laurate and oleate induced a significant increase in the percentage of Jurkat cells with low mitochondrial membrane potential (more than 60%) at 6 h; however, the treatment did not induce depolarization of the membrane potential in HT1080 and HepG2 cells (less than 10%), suggesting that apoptotic processes such as ROS production, caspase-3 activation, and decreased mitochondrial membrane potential induced by the combination of laurate and oleate occur in a cell-type-dependent manner.
Fig. 4.
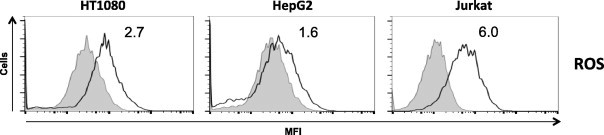
ROS production upon co-exposure to laurate and oleate. Cells were treated with fatty acids for 1 h and next treated with DCFDA. The histograms show the mean fluorescence intensities of untreated cells (shaded boxes) and co-stimulated cells (white boxes). Each data point is the average MFI (in arbitrary units, AUs) of ROS-producing cells compared to untreated cells (AU = 1). The data are representative of at least three separate experiments.
Fig. 5.
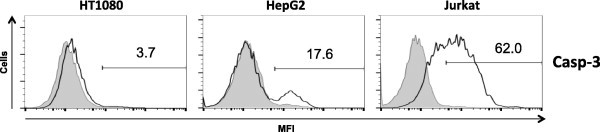
Caspase-3 activation upon co-addition of laurate and oleate. Cells were stimulated by fatty acids for 6 h and next treated with NucView™ 488. The histograms show the mean fluorescence intensities of untreated cells (shaded boxes) and co-stimulated cells (white boxes). Each data point is the population (%) of cells with the increased mean fluorescence intensity (MFI) characteristic of caspase-3 activated cells. The data are representative of at least three separate experiments.
Fig. 6.
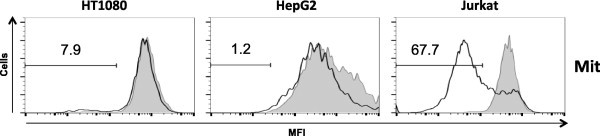
Mitochondrial membrane dysfunction upon co-addition of laurate and oleate. Cells were stimulated by fatty acids for 6 h and then treated with TMRM. The histograms show the mean fluorescence intensities of untreated cells (shaded boxes) and co-stimulated cells (white boxes). Each data point is the population (%) of cells with the decreased mean fluorescence intensity (MFI) characteristic of mitochondria that have lost their membrane potential (Mit). The data are representative of at least three separate experiments.
It has been shown that triacylglycerol (TG) synthesis and the formation of lipid droplets are stimulated by oleate as well as laurate in tissue culture cells [28–31]. Therefore, we investigated the effect of the combination of fatty acids on lipid droplet formation using a fluorescent indicator. Lipid droplet formation was increased by treatment with either laurate or oleate alone; however co-treatment with laurate and oleate dramatically enhanced lipid formation in all cell types (Fig. 7).
Fig. 7.
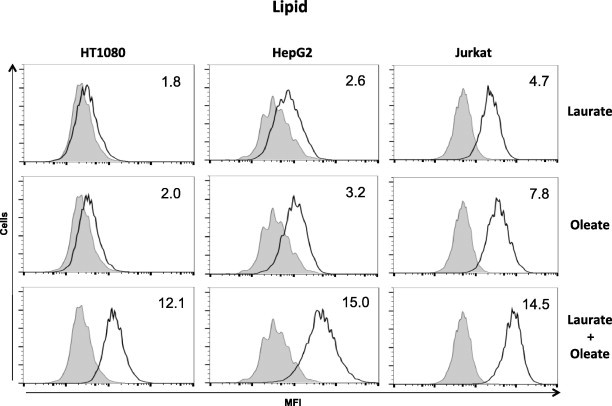
Lipid accumulation following co-addition of laurate and oleate. Cells were stimulated with fatty acids for 6 h and then stained with Nile Red to detect lipid droplets. The histograms show the mean fluorescence intensities (MFIs) of untreated cells (shaded boxes) and stimulated cells (white boxes) with laurate (upper), oleate (middle), or the combination (lower). Each number is the average MFI (in arbitrary units, AUs) compared to that of untreated cells (AU = 1), reflecting lipid droplet accumulation (lipid). The data are representative of at least three separate experiments.
Next, we examined the effect of fatty acid uptake by each fatty acid alone or by a mixture of laurate and oleate. Although the uptake of fluorescent BODIPY-labeled fatty acid was enhanced by treatment with oleate alone, but not laurate or palmitate, the fluorescence intensity by oleate was considerably greater upon co-stimulation with laurate (Fig. 8). We also investigated how the uptake of each fatty acid was affected by other fatty acids. Co-incubation of 14C-labeled oleate with palmitate did not affect the influx of 14C-labeled oleate; however, laurate dramatically enhanced its incorporation (Fig. 9a). On the other hand, the effect of oleate on the influx of 14C-labeled laurate influx was cell type-dependent (Fig. 9b). We also confirmed that laurate did not enhance the influx of 3H-labeled 2-deoxyglucose (Fig. 10).
Fig. 8.
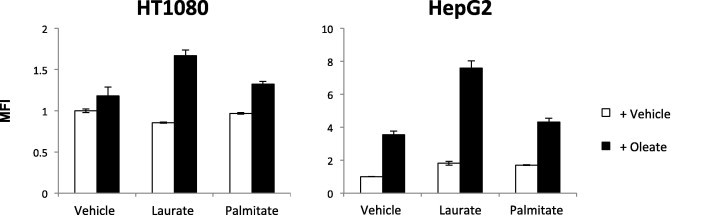
Fatty acid uptake upon co-addition of laurate and oleate. Cells were treated with the indicated fatty acids and BODIPY for 4 h. BODIPY fluorescence was detected using a Fluoroscan. BODIPY uptakes are presented as MFIs (in arbitrary units, AUs) compared to that of untreated cells (AU = 1). The data are the means ± SDs (n = 3).
Fig. 9.
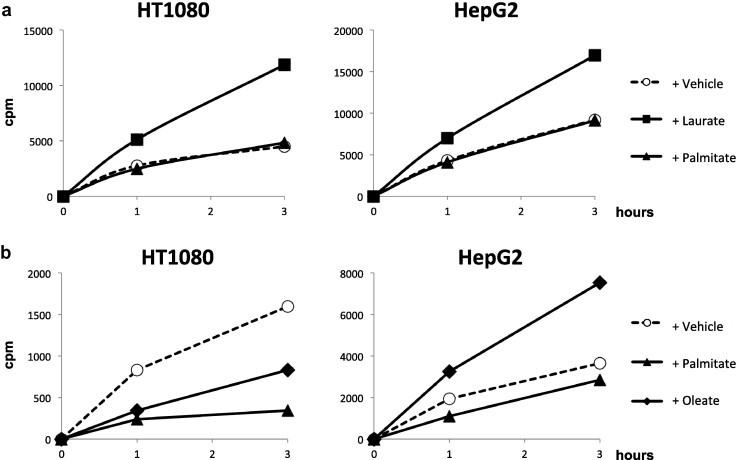
Oleate influx is enhanced by laurate. Cells were treated with the indicated fatty acids and 14C-labeled oleate (a) or laurate (b) for 1–3 h. Radioactivity levels (cpm) were measured using a scintillation counter as described in Section 2.
Fig. 10.
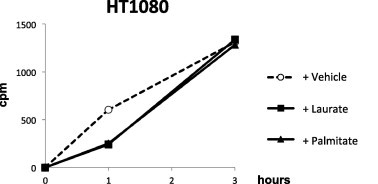
2-Deoxyglucose uptake by laurate. Cells were treated with the indicated fatty acids and 3H-labeled 2-deoxyglucose for 1–3 h. Radioactivity levels (cpm) were measured using a scintillation counter as described in Section 2.
We have herein shown that lipotoxicity exerted by a combination of laurate and oleate was dramatically greater than the lipotoxicity of either fatty acid alone; in contrast to what was noted using a mixture of palmitate and oleate. Because non-metabolized palmitate is unable to induce cell death, LC-SFA must be converted into acyl-CoA and then incorporated into membrane lipids or lipid storage [4,10,20]. The partitioned LC-SFA is believed to induce ROS, and endoplasmic reticulum stress, caused by membrane dysfunction attributable to decreased membrane fluidity [4,16]. Oleate has been thought to suppress the lipotoxicity of LC-SFA by competing with the undesired localization of LC-SFA to maintain the membrane fluidity, suggesting that the balance between saturated and unsaturated fatty acids in the membrane is critical in terms of cell death (from stress) or survival (when stress is absent) [21,22].
Although oleate can detoxify LC-SFA, that fatty acid was toxic to several cell lines, but less so to LC-SFA. In addition, the mechanisms of oleate-induced cell death differ among cell types [23,34–36]. We also found that oleate had cytotoxic effects at higher concentrations than laurate, and the lipotoxicity of oleate was greater than that of laurate (Fig. 1). Formation of lipid droplets, in addition to lipotoxicity, was enhanced by a combination of laurate and oleate. Laurate has been shown to enhance rectal absorption of propranolol [32], to improve intestinal absorption of thyrotropin [33], and to stimulate triacylglycerol synthesis [28,29]. We have clearly shown that laurate also enhances fatty acid uptake. Although laurate also acts as a detergent, we found that laurate increased the uptake of fatty acids, but not glucose, suggesting that laurate somehow affects the machinery of fatty acid uptake. Several studies have shown that fatty acid-induced cell death is not attributable to overloading of cells with lipid droplets [12,19,30,31,37], suggesting that lipid droplet formation by the laurate/oleate combination probably does not induce cell death. From these results, we hypothesize that when laurate enhances the cellular uptake of oleate, the excess free oleate which has not been stored as lipid droplets induces stress, causing the cells to eventually die. Stress symptoms, including ROS production, mitochondrial dysfunction, and caspase activation differed among various cell types treated with laurate and oleate, suggesting that the stress signals and cell death pathways are activated by excess oleate, and depend upon the cell type. Although susceptibility to stress caused by excess oleate requires lipid droplet formation, the precise role played by such droplets in cell death requires further investigation.
In this report, the combination of laurate and oleate increased not only the cytotoxic effects, but also lipid droplet formation and fatty acid uptake, compared to treatment with each fatty acid alone. However the co-stimulation-induced stress signals and cell death pathways differed among cell types. The findings of the present study suggest that laurate increases fatty acid uptake, allowing the cells to obtain excess amounts of oleate to lower the threshold for the cytotoxic effects of oleate. Given these findings, laurate may be useful for promoting intestinal absorption of fatty acids in cases of malnutrition. Future studies are needed to elucidate the exact mechanism by which laurate affects fatty acid uptake.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (Nos. 23780132 and 25504005 to Y.K., and No. 22650152 to Y.S.) from the Japan Society for the Promotion of Science (JSPS), the Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) (Grant No. AS232Z01558G to Y.K.) from the Japan Science and Technology Agency (JST), and Japanese Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP Project ID 14533567 to Y.S.).
Y.K. and Y.S. conceived and supervised the study; Y.K. and K.I. designed the experiments; Y.K., K.I., N.K., and N.M. performed the experiments and analyzed data; Y.K. wrote the manuscript; and Y.S. revised the manuscript.
References
- 1.Flock M.R., Kris-Etherton P.M. Diverse physiological effects of long-chain saturated fatty acids: implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(2):133–140. doi: 10.1097/MCO.0b013e328359e6ac. [DOI] [PubMed] [Google Scholar]
- 2.Savary S., Trompier D., Andréoletti P., Le Borgne F., Demarquoy J., Lizard G. Fatty acids-induced lipotoxicity and inflammation. Curr. Drug Metab. 2012;13(10):1358–1370. doi: 10.2174/138920012803762729. [DOI] [PubMed] [Google Scholar]
- 3.Masi L.N., Rodrigues A.C., Curi R. Fatty acids regulation of inflammatory and metabolic genes. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(4):418–424. doi: 10.1097/MCO.0b013e32836236df. [DOI] [PubMed] [Google Scholar]
- 4.Hardy S., El-Assaad W., Przybytkowski E., Joly E., Prentki M., Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J. Biol. Chem. 2003;278(34):31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- 5.Wei Y., Wang D., Topczewski F., Pagliassotti M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006;291(2):E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 6.Cazanave S.C., Elmi N.A., Akazawa Y., Bronk S.F., Mott J.L., Gores G.J. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(1):G236–G243. doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H., Wu M., Zhang L., Thompson R., Nath A., Chan C. Signaling dynamics of palmitate-induced ER stress responses mediated by ATF4 in HepG2 cells. BMC Syst. Biol. 2013;7:9. doi: 10.1186/1752-0509-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu X., Li K., Laybutt D.R., He M.L., Zhao H.L., Chan J.C., Xu G. Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci. 2010;87(23–26):724–732. doi: 10.1016/j.lfs.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 9.El-Assaad W., Buteau J., Peyot M.L., Nolan C., Roduit R., Hardy S., Joly E., Dbaibo G., Rosenberg L., Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144(9):4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 10.Cunha D.A., Hekerman P., Ladrière L., Bazarra-Castro A., Ortis F., Wakeham M.C., Moore F., Rasschaert J., Cardozo A.K., Bellomo E., Overbergh L., Mathieu C., Lupi R., Hai T., Herchuelz A., Marchetti P., Rutter G.A., Eizirik D.L., Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J. Cell Sci. 2008;121(Pt 14):2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharroubi I., Ladrière L., Cardozo A.K., Dogusan Z., Cnop M., Eizirik D.L. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 12.Malhi H., Bronk S.F., Werneburg N.W., Gores G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 13.Mishra R., Simonson M.S. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc. Diabetol. 2005;4(1):2. doi: 10.1186/1475-2840-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai W., Liu Z. P38 mitogen-activated protein kinase mediates palmitate-induced apoptosis but not inhibitor of nuclear factor-kappaB degradation in human coronary artery endothelial cells. Endocrinology. 2007;148(4):1622–1628. doi: 10.1210/en.2006-1068. [DOI] [PubMed] [Google Scholar]
- 15.Listenberger L.L., Ory D.S., Schaffer J.E. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 2001;276(18):14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 16.Ostrander D.B., Sparagna G.C., Amoscato A.A., McMillin J.B., Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J. Biol. Chem. 2001;276(41):38061–38067. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 17.Staiger K., Staiger H., Weigert C., Haas C., Häring H.U., Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55(11):3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 18.Miller T.A., LeBrasseur N.K., Cote G.M., Trucillo M.P., Pimentel D.R., Ido Y., Ruderman N.B., Sawyer D.B. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem. Biophys. Res. Commun. 2005;336(1):309–315. doi: 10.1016/j.bbrc.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 19.Ricchi M., Odoardi M.R., Carulli L., Anzivino C., Ballestri S., Pinetti A., Fantoni L.I., Marra F., Bertolotti M., Banni S., Lonardo A., Carulli N., Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 20.Akazawa Y., Cazanave S., Mott J.L., Elmi N., Bronk S.F., Kohno S., Charlton M.R., Gores G.J. Palmitoleate attenuates palmitate-induced Bim and PUMA upregulation and hepatocyte lipoapoptosis. J. Hepatol. 2010;52(4):586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzer R.G., Park E.J., Li N., Tran H., Chen M., Choi C., Solinas G., Karin M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147(1):173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leamy A.K., Egnatchik R.A., Shiota M., Ivanova P.T., Myers D.S., Brown H.A., Young J.D. Enhanced synthesis of saturated phospholipids is associated with ER stress and lipotoxicity in palmitate treated hepatic cells. J. Lipid Res. 2014;55(7):1478–1488. doi: 10.1194/jlr.M050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima T.M., Kanunfre C.C., Pompéia C., Verlengia R., Curi R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol. In Vitro. 2002;16(6):741–747. doi: 10.1016/s0887-2333(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 24.Yang J.Y., Rayalam S., Della-Fera M.A., Ambati S., Baile C.A. Octanoate and decanoate induce apoptosis in 3T3-L1 adipocytes. J. Med. Food. 2009;12(5):959–966. doi: 10.1089/jmf.2008.0262. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi C., Sheng Z., Horan T.P., Kitayama H., Maki M., Hitomi K., Kitaura Y., Takai S., Sasahara R.M., Horimoto A., Ikawa Y., Ratzkin B.J., Arakawa T., Noda M. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc. Natl. Acad. Sci. USA. 1998;95(22):13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitaura Y., Matsumoto S., Satoh H., Hitomi K., Maki M. Peflin and ALG-2, members of the penta-EF-hand protein family, form a heterodimer that dissociates in a Ca2+-dependent manner. J. Biol. Chem. 2001;276(17):14053–14058. doi: 10.1074/jbc.M008649200. [DOI] [PubMed] [Google Scholar]
- 27.Stahl A., Evans J.G., Pattel S., Hirsch D., Lodish H.F. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev. Cell. 2002;2(4):477–488. doi: 10.1016/s1534-5807(02)00143-0. [DOI] [PubMed] [Google Scholar]
- 28.Hornung B., Amtmann E., Sauer G. Medium chain length fatty acids stimulate triacylglycerol synthesis in tissue culture cells. Biochem. Pharmacol. 1992;43(2):175–181. doi: 10.1016/0006-2952(92)90275-n. [DOI] [PubMed] [Google Scholar]
- 29.Guo W., Huang N., Cai J., Xie W., Hamilton J.A. Fatty acid transport and metabolism in HepG2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(3):G528–G534. doi: 10.1152/ajpgi.00386.2005. [DOI] [PubMed] [Google Scholar]
- 30.Yonezawa T., Yonekura S., Kobayashi Y., Hagino A., Katoh K., Obara Y. Effects of long-chain fatty acids on cytosolic triacylglycerol accumulation and lipid droplet formation in primary cultured bovine mammary epithelial cells. J. Dairy Sci. 2004;87(8):2527–2534. doi: 10.3168/jds.S0022-0302(04)73377-9. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto Y., Onoduka J., Homma K.J., Yamaguchi S., Mori M., Higashi Y., Makita M., Kinoshita T., Noda J., Itabe H., Takanoa T. Long-chain fatty acids induce lipid droplet formation in a cultured human hepatocyte in a manner dependent of Acyl-CoA synthetase. Biol. Pharm. Bull. 2006;29(11):2174–2180. doi: 10.1248/bpb.29.2174. [DOI] [PubMed] [Google Scholar]
- 32.Ogiso T., Iwaki M., Kashitani Y., Yamashita K. Enhancement effect of lauric acid on the rectal absorption of propranolol from suppository in rats. Chem. Pharm. Bull. (Tokyo) 1991;39(10):2657–2661. doi: 10.1248/cpb.39.2657. [DOI] [PubMed] [Google Scholar]
- 33.Yamada K., Murakami M., Yamamoto A., Takada K., Muranishi S. Improvement of intestinal absorption of thyrotropin-releasing hormone by chemical modification with lauric acid. J. Pharm. Pharmacol. 1992;44(9):717–721. doi: 10.1111/j.2042-7158.1992.tb05506.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y., Schwarz S., Ahlemeyer B., Grzeschik S., Klumpp S., Krieglstein J. Oleic acid causes apoptosis and dephosphorylates Bad. J. Neurochem. Int. 2005;46(2):127–135. doi: 10.1016/j.neuint.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Maris M., Waelkens E., Cnop M., D’Hertog W., Cunha D.A., Korf H., Koike T., Overbergh L., Mathieu C. Oleate-induced beta cell dysfunction and apoptosis: a proteomic approach to glucolipotoxicity by an unsaturated fatty acid. J. Proteome Res. 2011;10(8):3372–3385. doi: 10.1021/pr101290n. [DOI] [PubMed] [Google Scholar]
- 36.Reinbold M., Hufnagel B., Kewitz T., Klumpp S., Krieglstein J. Unsaturated fatty acids liberated from VLDL cause apoptosis in endothelial cells. J. Mol. Nutr. Food Res. 2008;52(5):581–588. doi: 10.1002/mnfr.200700321. [DOI] [PubMed] [Google Scholar]
- 37.Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Jr., Ory D.S., Schaffer J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 2003;100(6):3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]


