Abstract
Lycium barbarum polysaccharide (LBP) has traditionally been used in Chinese medicine as a chief ingredient of L. barbarum (wolf berry/goji berry) for the treatment of various diseases with the symptoms of frequent drinking and urination. This study was conducted as a randomized, controlled clinical trial. A total of 67 patients with type 2 diabetes (30 in control group and 37 in LBP group) were enrolled in this prospective, randomized, double-blind study (administration at 300mg/day body weight). In order to observe the hypoglycemic and lipid-lowering activity of LBP in patients with type 2 diabetes after dinner, various tests were conducted between control and LBP intervention groups in 3 months. Although, the study had small sample size and short follow-up, significant findings were observed. The results of our study indicated a remarkable protective effect of LBP in patients with type 2 diabetes. Serum glucose was found to be significantly decreased and insulinogenic index increased during OMTT after 3 months administration of LBP. LBP also increased HDL levels in patients with type 2 diabetes. It showed more obvious hypoglycemic efficacy for those people who did not take any hypoglycemic medicine compared to patients taking hypoglycemic medicines. This study showed LBP to be a good potential treatment aided-agent for type 2 diabetes.
Keywords: Lycium barbarum polysaccharide, type 2 diabetes, glucose, lipids, cytokines.
INTRODUCTION
Lycium barbarum (L. barbarum, wolf berry/Goji berry) has been used for the treatment of various diseases with the symptom of frequent drinking and urination as the traditional Chinese medicine for many years. L. barbarum poly-saccharide (LBP) is recognized as the active ingredient to the efficacy of L. barbarum. Derived from natural plants, LBP has no toxicity to the liver and kidney as hypoglycemic substance [1,4]. Numerous studies demonstrated hypo-glycemic effects of LBP in cell or animal experiment [1-5], but scarce studies reported about the antidiabetic efficacy of LBP in patients with type 2 diabetes [6, 7]. Diabetes has been a global concern due to unsuitable living environment, diet and lifestyle [8]. It is reported that 347 million people worldwide had diabetes by the end of 2008 [9]. WHO predicts that diabetes will be the 7th leading cause of death by 2030 [10]. We have reported the effect of LBP on fasting blood glucose and cytokines in type 2 diabetics previously [11]. In the present study, we investigated the effect of LBP on postprandial glucose and lipid metabolism in type 2diabetics after meal. The results showed that even if fasting lipids are normal, postprandial hyperlipemia is still an independent risk factor for early development of athero-sclerosis in patients with diabetes [12]. Postprandial lipid metabolism is the key period for development of athero-sclerosis in patients with diabetes. Oral metabolic tolerance test (OMTT) is a useful method to assess the status of postprandial metabolism in patients [13]. The metabolic ability can be shown by postprandial metabolic curves after high-fat load meal for patients with diabetes.
Therefore, the present study aims to get further insight on the postprandial hypoglycemic activity of LBP in patients with type 2 diabetes. Using OMTT, we tested the hypoglycemic and lipid-lowering activities of LBP in patients with type 2 diabetes after high-fat load meal. Postprandial metabolism is completely discussed.
MATERIALS AND METHODS
Preparation of LBP
L. barbarum fruit was purchased from Ningxia province, China. LBP used in the study was produced by a pharmaceutical company in Shanghai, China and its characteristics were detected in our laboratory. The powder of L. barbarum was mixed with water (60 oC). It was decocted by a traditional method for Chinese medicinal herbs after degreasing. After centrifuging and filtering by regenerated cellulose membranes of 300 kDa, 100 kDa, 80 kDa, 50 kDa and 30 kDa (0.2 MPa, 60 oC), the molecular weight of 30 kDa to 50 kDa fraction remained. The resulting fraction was retained and vacuum-dried at 40 oC, giving brown powder. It was identified to be homogeneous by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which showed a single band after staining with Ag [14] and periodic acid-Schiff [15]. LBP is composed of neutral sugars (78.23 %), acidic sugars (14.83 %) and proteins (6.92 %). Neutral sugars were determined by phenol–H2SO4, [16] acidic sugars by carbazole [17] and proteins by coomassie brilliant blue G-250 method [18]. Arabinose, galactosamine, lactose and glucose linked together with glycosidic linkages to compose the polysaccharides. Hygienic quality assessment of LBP was carried out by Center for Disease Control and Prevention of Nanjing.
Patient Selection
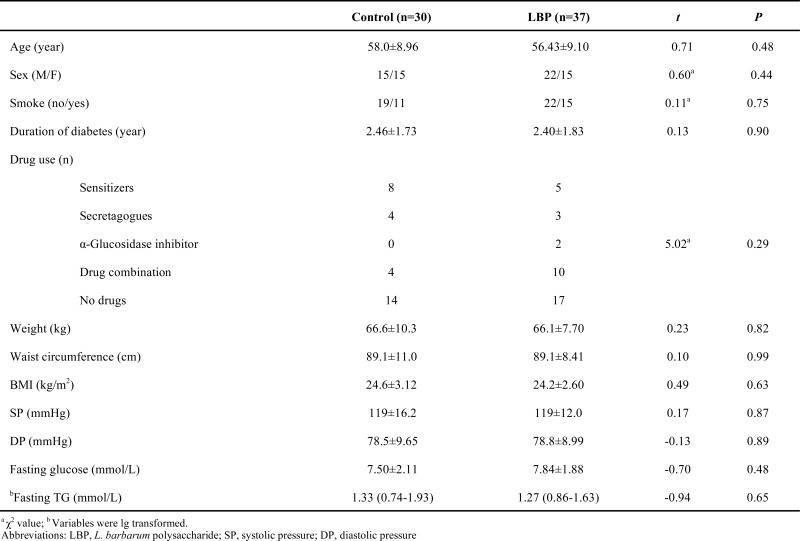
Subjects were recruited by voluntary sampling method. They were patients who were diagnosed with type 2 diabetes less than 5 years. Patients were classified as having diabetes if they met the following criteria: Fasting serum glucose greater than 7.0 mmol/L and/or 11.1 mmol/L after meal. The exclusion criteria were as follows: Type 1 diabetes, insulin treatment, known impairment of liver or kidney function, history of cancer, recent history of alcohol or drug abuse, disorders of the hematologic, digestive or central nervous system, pregnancy or nursing, or complications of diabetes (diabetic encephalopathy, oculopathy, nephropathy, cardiopathy, diabetic foot and skin disorder). All eligible subjects under went 1-week washout period before the beginning of the study. During this week, all of the participants were asked to monitor their diet. At the end of the week, blood routine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), uric acid (UA) were tested at fasting status in Nanjing Zhongda Hospital. Those people with large scale of transaminase, Cr and UA or having acute infection were excluded.
Trial Design
Eligible subjects took LBP or placebo capsule twice daily for 3 consecutive months (grouped by sequential balanced method as Borm et al. described [19]). Age, sex and duration of diabetes were divided into: young (<40 years) vs. middle (40-60 years) vs. old (>60 years), male vs. female, over 3 years vs. 2-3 years and under 2 years for duration of diabetes. Each subject belonged to exactly one class for each factor. If for age, there was a difference of more than one between the numbers of subjects in the class who were on each treatment, the treatment that reduces the difference was allocated. If the difference was one or less, next factor should be considered. If for gender, there was a difference of more than one between the numbers of subjects in the class who were on each treatment, the treatment that reduces the difference was allocated. If the difference was one or less, next factor should be considered. The last step in the algorithm was to allocate a treatment at random. Drug use, hypertension and serum triglyceride were analyzed after the random process. Randomization was implemented by statistician. Sample size was determined by fasting blood glucose (N=33). N= 2(Zα+Zβ)2σ2 / d2 (Zα=1.64, Zβ=1.28,σ=1.81, d =1.3).
Each LBP capsule contained 150 mg of LBP and 150 mg of microcrystalline cellulose. Placebo contained 300 mg of microcrystalline cellulose. Placebo was similar with LBP capsule in surface and flavor. The research personnel and the patients were unaware about the capsule that was given to the patients. During the invention period, they were asked not to change their previous therapy. Patients were followed-up every two weeks. People were excluded at any time when they had reactions (ALT, AST, Cr or UA exceed mark). Three days dietary survey method was used to record people’s dietary intake before and after intervention. Exercise and its duration were recorded within a week during intervention on a questionnaire. Average daily energy expenditure and balance were calculated according to rate of basal metabolism and daily activity energy consumption.
Assessment of Outcomes
An OMTT was performed with a high-fat load liquid meal on the first day. Overnight fasting (10h) before OMTT and any oral hypoglycemic agents were forbidden during the day. Meal was composed of 31.7 g of protein (12.6 % of energy), 76.7 g of carbohydrate (30.5 % of energy; 93 % saccharose and 7 % lactose), 60.2 g of fat (56.9 % of energy; 65 % saturated and 35 % unsaturated fatty acids) and 513.8 mg cholesterols in 1000 kcal of energy. Patients were asked to take the meal (20 kcal/kg) in 5 minutes. A catheter was placed into patient’s brachial vein to allow frequent blood sampling. Blood samples were collected at 0 h, 0.5 h, 1 h, 2 h, 4 h, 6 h and 8 h after the high-fat load liquid meal. Subjects were kept fasting all the day and strenuous activity was forbidden. Insulin, triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and apoprotein B (ApoB) were quantified at postprandial status. Serum glucose and lipids were determined using separate enzymatic assays by automatic biochemical analyzer (LX20, BECKMAN). Serum insulin, leptin, TNF-α and IL-6 were measured by radioimmunoassay (HTA CO., LTD., Peking, China). ADPN was measured by sandwich ELISA (Mithras LB 940, Berthold, Germany). A repeated OMTT was performed after 3-months intervention.
The local ethical committee of Southeast university affiliated Zhongda hospital approved the protocol and the protocol had been registered on Chinese clinical trial registry (2012ZD11KY17.0). A total of 74 subjects participated in the study and gave written informed consent.
Anthropometric Measurements
Anthropometric measurements were performed during OMTT. The anthropometric data included body weight, height, waist circumference, hip circumference, pulse, systolic pressure, diastolic pressure and so on. Following that, the clinical information was ascertained, including past medical history and treatment of diabetes. Blood samples were collected by the authorized hospital. Standardized physical examination was recommended by World Health Organization [20].
Statistical Analyses
Data are presented as mean ± SD. Some data indicated abnormal distributions as presented by media (25th-75th percentile). Normality was evaluated by Shapiro-Wilk test. Baseline characteristics were assessed by independent samples t-test or χ2 test. The areas under the curve (AUC) of serum glucose, insulin, TG, TC, HDL and ApoB were computed by the trapezoid method. Changes (∆) of AUC existing before and after intervention and homeostasis model assessment index of insulin resistance (HOMA-IR) were calculated with the following formula.
Changes (∆)=AUC after 3 months –AUC of beginning
HOMA-IR=fasting glucose×fasting insulin/22.5
The differences of changes were assessed by non-parametric test on 2-independent samples test as well as by dietary intake. The interventional efficacy of LBP was tested by paired t-test (for normally distributed data) or Wilcoxon signed rank tests (for skewed data). P<0.05 was considered significant. All statistical analyses were performed with SPSS 13.0 software.
RESULTS
There were 74 eligible subjects in this study. Among them, 5 subjects withdrew and 2 subjects were unable to contact in control group during the study. Finally, total 67 subjects completed the study, 30 in control group and 37 in LBP group. No adverse events were observed during the study.
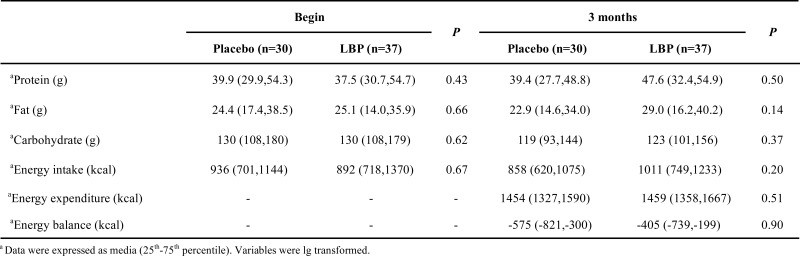
Baseline characteristics of two groups are showed in Table 1. No differences were observed between the two groups (χ2=5.02, P>0.05). Patients with type 2 diabetes who did not have any oral hypoglycemic agents amounted to 46.67 % and 45.95 %, respectively in placebo and LBP groups. As indicated in Table 2, average daily intake of protein, fat and carbohydrate does not produce any differences before and after the intervention (P>0.05). Average daily energy intake, expenditure and balance in placebo and LBP group have no differences during intervention (P>0.05).
Table 1.
Baseline characteristics of 67 patients with type 2 diabetes mellitus for Control and LBP groups.
Effects of LBP on Serum Glucose, Insulin and Lipids
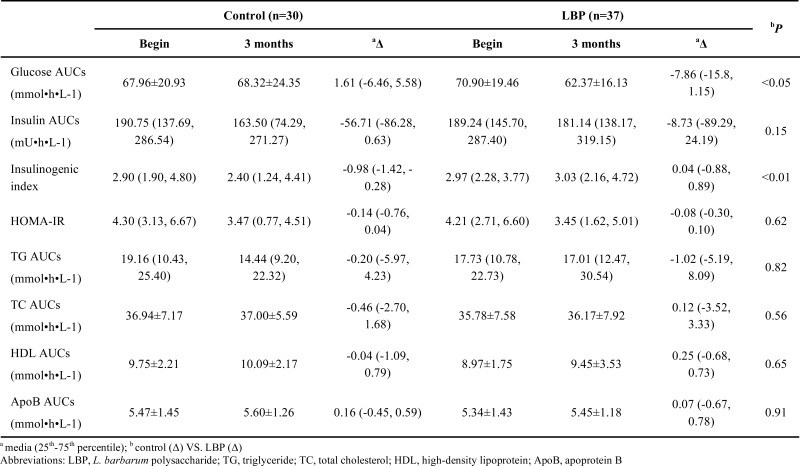
Serum glucose, insulin and lipids were measured during OMTT. Glucose AUC decreased noticeably compared with placebo (-7.86 % vs. 1.61 %, P<0.05) after intervention. The insulinogenic index (Insulin-AUCs / glucose-AUCs) increased from -0.98 % to 0.04 % (P<0.05) although the insulin response to the meal was not changed (Table 3). Serum lipids AUCs (TG, TC, HDL, ApoB) showed no significant differences between LBP treatment and placebo (P>0.05).
Table 3.
Comparison of areas under the curve (AUCs) of 67 patients with type 2 diabetes for glucose, insulin and lipids after oral metabolic tolerance test.
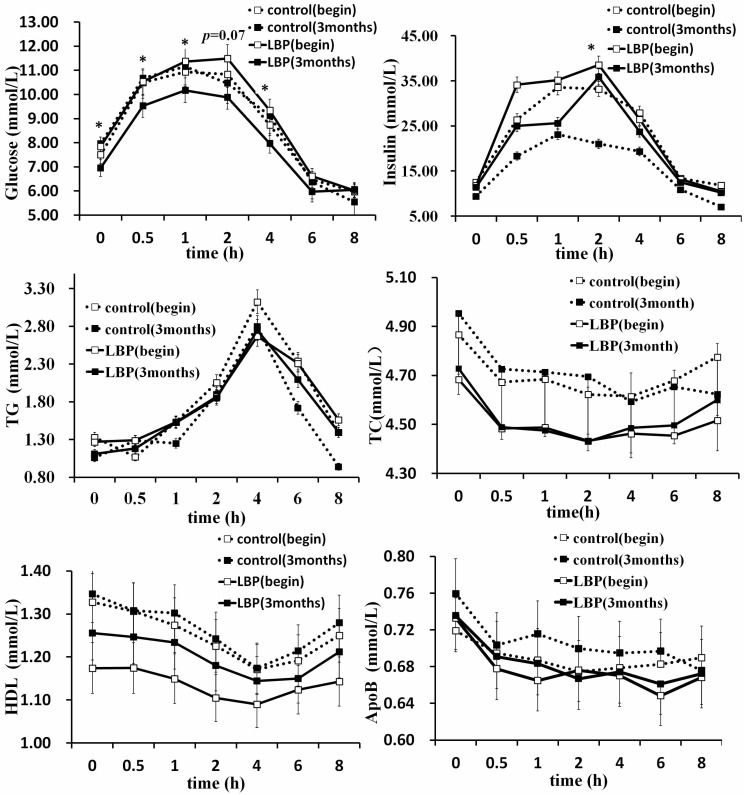
Changes of glucose at each time are presented in Fig. 1A. Differences of glucose levels were significant at 0 h, 0.5 h, 1 h and 4 h during OMTT between placebo and LBP (P<0.05). Difference of glucose level was closed to 0.05 at 2 h in the two groups (P=0.07). Difference of insulin level was significant at 2 h between placebo and LBP groups (P<0.05) (Fig. 1B). Changes of TG, TC and ApoB at each time showed no significant difference (Fig. 1C, D, F). However, HDL levels showed a significant difference at 0 h, 0.5 h and 1 h (Fig. 1E).
Fig. (1).
Oral metabolic tolerance test of 67 patients with type 2 diabetes mellitus for control and LBP groups before and after LBP administration. (Data were presented as mean or media. * P<0.05 vs. controls.) White squares represented baseline measurements and black squares represented a period of 3 months later. Control group is shown with a dotted line and LBP with a real line. Abbreviations: LBP, L. barbarum polysaccharide; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; ApoB, apoprotein B.
Table 4 shows the HDL profile after 3 months intervention with either placebo or LBP. Increases of HDL after LBP treatment were higher than placebo at 0 h, 0.5 h and 1 h (P<0.05). No significant changes were observed at 2 h, 4 h, 6 h and 8 h (P>0.05).
Table 4.
Effects of LBP on serum HDL of 67 patients with type 2 diabetes after oral metabolic tolerance test at different times.

Effects of LBP on Adipokines
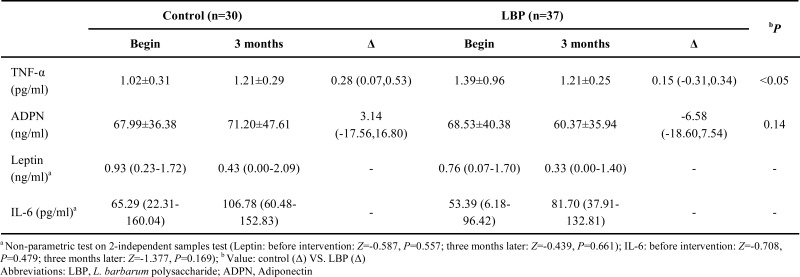
Effects of LBP on adipokines at 0 h are showed in Table 5. TNF-α was reduced in LBP group, compared to placebo (0.28 % vs. 0.15 %). There were no significant differences in ADPN (P>0.05), leptin (before intervention: Z=-0.587, P=0.557; three months later: Z=-0.439, P=0.661) and IL-6 (before intervention: Z=-0.708, P=0.479; three months later: Z=-1.377, P=0.169) between placebo and LBP groups after LBP administration.
Table 5.
Effects of LBP on adipokines of 67 patients with type 2 diabetes after intervention.
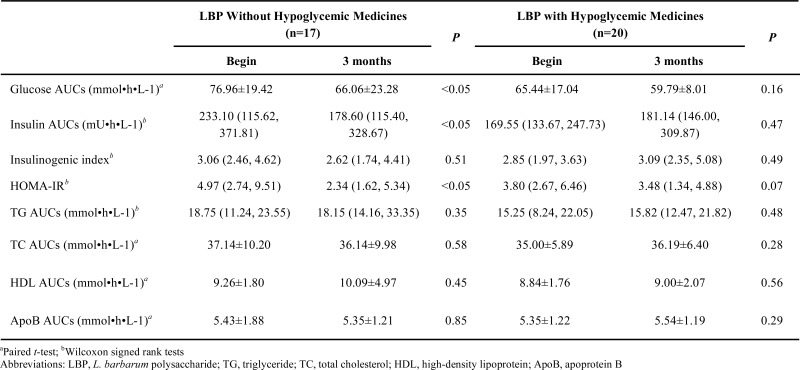
The Interventional Efficacy of LBP for Patients Taking Hypoglycemic Medicines Or Not
The interference efficacy of LBP for patients taking hypoglycemic medicines or not is presented in Table 6. Compared with the beginning of intervention, LBP reduced AUCs of glucose, insulin and HOMA-IR in patients not taking hypoglycemic medicines. Glucose AUCs and HOMA-IR were decreased in patients taking hypoglycemic medicines, but there were no significant differences after 3 months.
Table 6.
The interference efficacy of LBP for patients taking hypoglycemic medicines or not.
DISCUSSION
This study reports that LBP administration resulted in a significant reduction in postprandial levels of serum glucose at a dose of 300 mg/day for 3 months. LBP also increased HDL levels at 0 h, 0.5 h and 1 h in patients with type 2 diabetes.
The present study suggests that LBP favorably affected glucose levels. These findings were similar to observations from animal studies, in which treatment of LBP on diabetes mellitus animals was associated with a significant reduction in fasting blood glucose level [1, 5, 21, 22]. Our present study and previous study [11] confirmed that LBP has antidiabetic efficacy in patients with type 2 diabetes. LBP could reduce fasting and postprandial blood glucose. Serum insulinogenic index was significantly increased although no significant change was observed in postprandial insulin AUC after LBP treatment. The results suggest that LBP did not affect postprandial insulin responses. It was associated with a significant improvement in HOMA-IR along with a significant reduction in glucose response to a meal. The combined effect of LBP to reduce postprandial glucose levels while sustaining postprandial insulin suggests a relative stimulation of insulin secretion and an increased insulin sensitivity. These indicated improvement in β-cell responsiveness. [23].
Improved HDL levels were observed in LBP group. In diabetes mellitus, hyperglycemia is accompanied with dyslipidemia that is characterized with increases in TC, LDL cholesterol and TG and decrease in HDL cholesterol [24]. High levels of TC and LDL cholesterol are thought to be the major risk factors for cardiovascular disease, which is a major death cause for patients with diabetes [25], the increases HDL cholesterol is associated with lower cardiovascular disease risk [26]. HDL plays an important role in the transport of cholesterol from the peripheral blood to the liver by the reverse cholesterol transport pathway [27]. Thus, increased HDL is associated with a decrease in cardiovascular disease risk. In the present study, fasting and postprandial serum HDL were elevated by LBP at 0 h, 0.5 h and 1 h. The findings highlight the potential favorable effects of LBP on HDL levels, which may result in reduced mortality from cardiovascular disease among patients with diabetes.
Many animal studies indicated that LBP remarkably decreased serum TG, TC, and LDL-C levels, whereas the HDL-C level increased significantly after short-term administration of LBP [1, 28]. However, no significant changes were observed in TG, TC and ApoB level in type 2 diabetes patients during OMTT.
Adipokines are cytokines or hormones secreted by white adipocytes. It is reported that some adipokines such as TNF-α, leptin, IL-6 and ADPN are believed to be involved in the pathogenesis of insulin resistance and subsequent development of type 2 diabetes [29]. Few studies have reported about the effect of LBP on these adipokines in diabetics. In our previous study, we found that LBP decreased the level of TNF-α in patients with type 2 diabetes [11]. In the present study, TNF-α level was also reduced by continuous administration of LBP in patients with type 2 diabetes. TNF-α has been closely associated with inhibition of the insulin signaling pathway and insulin resistance [30]. It diminishes insulin-induced IRS-1 and IRS-2 tyrosine phosphorylation in hepatocytes and adipocytes, resulting in impaired insulin action [31]. Furthermore, it decreases insulin stimulation of cellular glucose uptake. Reduction of TNF-α could result in increased cellular glucose uptake and inhibition of transient insulin resistance [32]. Combining the two studies we consider that may be one of the methods for LBP will be effective to alleviate insulin resistance in patients with type 2 diabetes. In the present study, effect of LBP on leptin, IL-6 and ADPN were also analyzed. However, no significant differences were found.
In addition, LBP efficacy was also analyzed for patients taking hypoglycemic medicines or not. LBP reduced AUCs of glucose, insulin and HOMA-IR in patients without taking hypoglycemic medicines (P<0.05). However, there was not obvious efficacy in patients taking hypoglycemic medicines.
There are limitations to our study. The interventional time and dose are small, and this may have led to weaker associations for LBP with some of the variables, such as TG, TC, ApoB and so on. Nevertheless, our findings provided a foundation for understanding efficacy of LBP for patients with type 2 diabetes. In order to fully understand the biological role of LBP in patients with type 2 diabetes, we need a prospective study with a significantly larger interventional time and dose.
CONCLUSION
In conclusion the randomized and controlled clinical trial indicated remarkable protective effects of LBP in patients with type 2 diabetes. Serum glucose decreased significantly and insulinogenic index increased after 3 months administration with LBP during OMTT. LBP also increased HDL levels of patients with type 2 diabetes. The hypoglycemic efficacy was more significant for patients not taking hypoglycemic medicines than for patients taking hypoglycemic medicines.
The present study shows that LBP is a potentially good treatment aided-agent for type 2 diabetes.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
Table 2.
Comparison of dietary intake of 67 patients with type 2 diabetes before and after the intervention.
ACKNOWLEDGEMENTS
We would like to acknowledge Zhang Peng, Schrezenmeir. J and other innominate medical personnel for supporting the study. The national natural science fund (30872119) and research fund for Ph.D. Program Foundation of Ministry of Education of China (20100092110039) supported this research.
REFERENCES
- 1.Jin ML, Huang QS, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Liu W, Yu JP, Zou S, Wang JJ, Yao WB, Gao XD. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013;98(1 ): 8–16. doi: 10.1016/j.carbpol.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 3.Amagase H, Farnsworth NR. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji) Food Res. Int. 2011;44(7): 1702–17. [Google Scholar]
- 4.Zhao R, Li QW, Li J, Zhang T. Protective effect of Lycium barbarum polysaccharide 4 on kidneys in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2009;87(9 ): 711–9. doi: 10.1139/y09-068. [DOI] [PubMed] [Google Scholar]
- 5.Luo Q, Cai YZ, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76(2): 137–49. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Wang L, Deng X, Jiang L, Zhang C, Zhang C. Study of the fragility and abnormality rate of red blood cells in patients with type-2 diabetes and the effects of Lycium barbarum polysaccharides. Hebei. J. Tradit. Chin. Med. 2000;22(8): 585–6. [Google Scholar]
- 7.Wang L, Zhang C, Li W. Regulating effect of Lycium barbarum Polysaccharide on T lymphocyte subpopulation and cytokines in patients with type-2 diabetes. Hebei. J. Tradit. Chin. Med. 2001;23(12): 888–890. [Google Scholar]
- 8.Weaver RR, Lemonde M, Payman N, Goodman WM. Health capabilities and diabetes self-management The impact of economic, social, and cultural resources. Soc. Sci. Med. 2014;102:58–68. doi: 10.1016/j.socscimed.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2 million participants. Lancet. 2011;378(9785): 31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: 2011. [Google Scholar]
- 11.Cai HZ, Liu FK, Lu HX, Guo F, Hu LJ, Han C, Sun GJ. Short-Term Intervention of Lycium barbarum Polysaccharide on Type 2 Diabetes. Food science (in Chinese) 2013;33(13 ):259–62. [Google Scholar]
- 12.Anderson RA, Evans ML, Ellis GR, Graham J, Morris K, Jackson SK, Lewis MJ, Rees A, Frenneaux MP. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atheroscleosis. 2001;154(2): 475–83. doi: 10.1016/s0021-9150(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes. Metab. 2011;13(4 ): 366–73. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 14.Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 1981;118(1): 197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 15.Mcmanus JF A. Histological demonstration of mucin after periodic acid. Nature. 1946;158:202. doi: 10.1038/158202a0. [DOI] [PubMed] [Google Scholar]
- 16.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28(3): 350–6. [Google Scholar]
- 17.Chaplin MF. Oxford: Carbohydrate Analysis; 1986. Monosaccharides. [Google Scholar]
- 18.Noble JE, Bailey MJ A. 2nd ed. Vol. 463. USA: Academic Press; 2009. In Quantitation of Protein. Methods Enzymol; pp. 73–95. [DOI] [PubMed] [Google Scholar]
- 19.Borm GF, Hoogendoorn EH, den Heijer M, Zielhuis GA. Sequential balancing a simple method for treatment allocation in clinical trials. Contemp. Clin. Tials. 2005;26(6 ): 637–45. doi: 10.1016/j.cct.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Guide to physical examination. who.int/chp/steps/manual/en/index3.html .
- 21.Ma M, Liu GH, Yu ZH, Chen G, Zhang X. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009;113(4): 872–7. [Google Scholar]
- 22.Li XM. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007;40(5): 461–5. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.D'Alessio DA, Denney AM, Hermiller LM, Prigeon RL, Martin JM, Tharp WG, Saylan ML, He Y, Dunning BE, Foley JE, Pratley RE. Treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin improves fasting islet-cell function in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009;94(1): 81–8. doi: 10.1210/jc.2008-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Fu JF, Koonen DP, Kuivenhoven JA, Snieder H, Hoflker MH. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atheroscleosis. 2014;233(1 ): 130–8. doi: 10.1016/j.atherosclerosis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Preis SR, Hwang SJ, Coady S, Pencina MJ, D'Agostino RB, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study 1950 to 2005. Circultion. 2009;119:1728–35. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiner Ž. Managing the residual cardiovascular disease risk associated with HDL-cholesterol and triglycerides in statin-treated patients A clinical update Nutrition. Nutr. Metab. Cardiovas. 2013;23(9):799–807. doi: 10.1016/j.numecd.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Catalamo G, Guerin M. Biological Functions and Clinical Implications; USA: Academic Press; 2010. HDL and Reverse Cholesterol Transport: Physiological Modulation; The HDL Handbook. [Google Scholar]
- 28.Cheng D, Kong H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Moleules. 2011;16(3):2542–50. doi: 10.3390/molecules16032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch S P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans a cross-sectional study. Cytkine. 2012;57:136–42. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh JM, McGowan CA, Byrne JA, Rath A, McAuliffe FM. The association between TNF-α and insulin resistance in euglycemic women. Cytkine. 2013;64(1 ): 208–12. doi: 10.1016/j.cyto.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.White MF. Handbook of Cell Signaling. USA: Academic Press:; 2003. IRS-Protein Scaffolds and Insulin/IGF Action. [Google Scholar]
- 32.del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am. J. Physiol. 1999;276:E849–55. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]