Abstract
We report a patient with epilepsy who experienced interictal and postictal psychoses. Her psychiatric symptoms consisted of grandiose and fantastic delusions during both psychotic states. During remission, electroencephalography showed bitemporal epileptiform discharges that were predominant in the right temporal region. Epileptiform discharges present during the psychotic states were predominant in the left temporal region. Single-photon emission computed tomography showed hyperperfusion in the left basal ganglia during the interictal psychotic state and hyperperfusion in the right temporal lobe and left basal ganglia during the postictal psychotic state. We suggest that the occurrence of postictal and interictal psychotic states in this patient were associated with a common change in electrographic activity and blood flow.
Keywords: Postictal psychosis, Interictal psychosis, Epilepsy, Temporal lobe, Electrical activity, Blood flow
1. Introduction
Psychoses in patients with epilepsy are classified as interictal psychosis (IIP) or postictal psychosis (PIP). Postictal psychosis develops after complex partial seizures, often secondary generalized tonic–clonic seizures, and often after a cluster of these seizures [1].
Previous studies have described the characteristics of IIP and PIP and suggested a difference in the pathology of these two types of psychosis. Postictal psychosis is associated with elevated mood and religious and grandiose delusions, and patients usually have a lucid interval, i.e., a nonpsychotic period, between the last seizure and the beginning of the psychotic state [1,2]. Psychiatric symptoms of PIP typically remit within 1 week [1]. By contrast, IIP occurs with no direct relation to seizures. The psychiatric symptoms of IIP are mainly auditory hallucinations and delusions of reference, which are similar to the symptoms of schizophrenia. The onset of IIP occurs earlier in the course of the disease than the onset of PIP [3]. In most patients, the psychiatric symptoms of IIP continue for a few months, but, in some patients, they progress chronically.
If psychosis occurs in accordance with an improvement of seizures, it is called alternative psychosis. When electroencephalography (EEG) improves during psychosis, this is called forced normalization [4]. Spikes and slow waves in EEG generally increase during PIP episodes [1]. Most single-photon emission computed tomography (SPECT) studies report hyperperfusion of the frontal and temporal lobes during PIP [5–9], and hyperperfusion of the left temporal lobe during IIP has also been reported [10].
Some patients have both PIP and IIP. Adachi et al. referred to this as bimodal psychoses and described that bimodal psychoses had characteristics similar to those of either PIP or IIP [3]. However, the changes in electrographic activity and blood flow that occur in each psychotic episode are unclear. Here, we investigated EEG and SPECT in a patient with both PIP and IIP episodes.
2. Case report
The patient was a right-handed woman who was first treated at our hospital at the age of 34 years. The patient had encephalitis at the age of 9 years and had epilepsy and mental retardation since this time. The patient has no family history of epileptic or psychiatric disorders. Informed consent was obtained from this patient and her family, and we have omitted information that may reveal the identity of the patient.
At the age of 25 years, the patient experienced her first psychotic episode, during which she said “someone is roasted” and “someone told me that you were a dragon”. It is unclear whether epileptic seizures occurred in the days preceding this episode. Psychotic episodes then recurred and always occurred within a few days of an epileptic seizure or cluster of seizures.
In the lucid interval that occurred in the days following this cluster of seizures, the patient reported insomnia and developed a psychotic state that was characterized by visual and auditory hallucinations, delusions of reference, and grandiose delusions, typified by speaking phrases such as “my eyes were stolen” and “someone orders me”. In this period, the patient behaved as if she was a character in an animation. These symptoms persisted for a few days and then disappeared.
The patient consulted our hospital at the age of 34 years for her epileptic seizures and psychiatric symptoms. At this time, she had no psychiatric symptoms. Her habitual seizures consisted of complex partial and secondary generalized tonic–clonic seizures, which were preceded by cephalic sensation. Her seizures tended to occur just before menses and episodically in clusters.
Neurological examination revealed no definite deficit. Interictal scalp EEG showed sporadic sharp waves at F8 and T4 (Fig. 1A) and also at F7 and T3, though rarely. Magnetic resonance imaging showed no definite structural abnormality. The patient was taking valproic acid 800 mg/day (serum concentration = 111.3 μg/ml), clobazam 20 mg/day, nitrazepam 15 mg/day, brotizolam 0.25 mg/day, and risperidone 4 mg/day. Despite being treated with these anticonvulsants, secondary generalized tonic–clonic seizures occurred monthly.
Fig. 1.
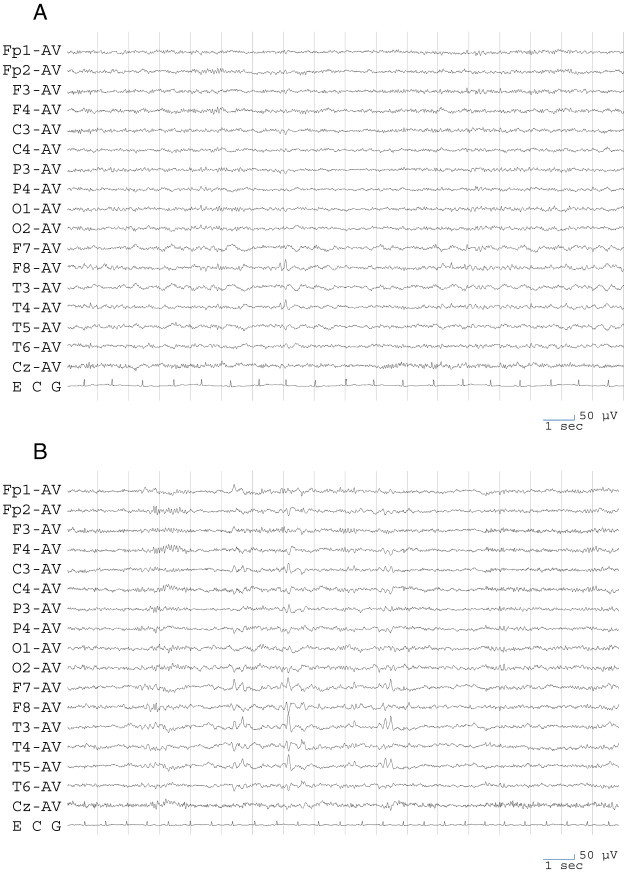
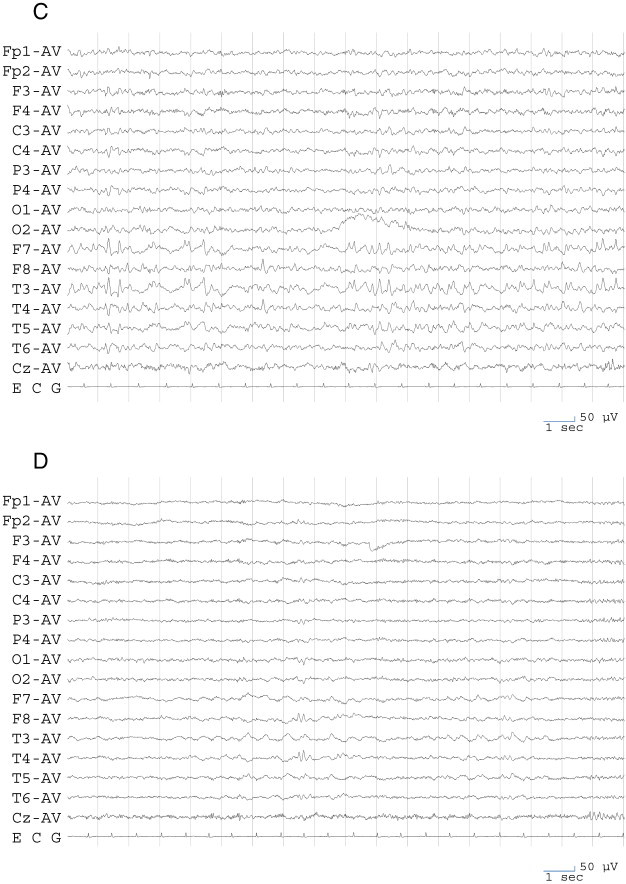
Electroencephalogram findings. A. An electroencephalogram recorded during remission of psychosis at the first visit to our hospital. Sporadic sharp waves are evident at F8 and T4 and also at F7 and T3, though rarely. B. An electroencephalogram recorded during IIP. Frequent independent bitemporal sharp waves are evident and are particularly remarkable on the left side. C. An electroencephalogram recorded during PIP. Frequent independent bitemporal sharp waves are evident and are particularly remarkable on the left side. D. An electroencephalogram recorded during remission of psychosis after starting lamotrigine. Epileptiform discharge remarkably decreased, and only right temporal sharp waves are evident.
Valproic acid and risperidone were stopped, and carbamazepine was started and gradually increased to a dose of 1000 mg/day (serum concentration = 10.54 μg/ml). After 3 months at this dose, the seizures disappeared.
2.1. Details of IIP
From about the time of the disappearance of seizures, the patient had insomnia and exhibited dullness, fluctuations of emotion, and a silly smile. She responded slowly, had depressive feelings, was incoherent, and exhibited bizarre behaviors. She reported that she was possessed and was spoken to by someone, that she was spoken to by an alien, and that she herself was an alien or a magician. She was episodically excited and aggressive.
Interictal scalp EEG showed frequent independent bitemporal sharp waves, which were remarkable in the left side (Fig. 1B). Risperidone was reintroduced, but the psychiatric symptoms persisted for a further 5 months. These symptoms resolved after recurrence of an epileptic seizure.
2.2. Details of PIP
At 36 years of age, the patient deteriorated into a psychotic state after experiencing several seizures over a series of days. There was no definite lucid interval; however, symptomatology and clinical course of this episode closely resembled those of PIP. The patient showed dullness, was depressed, and reported that she saw a fairy and heard voices. Electroencephalography showed frequent independent bitemporal sharp waves, which were remarkable in the left side (Fig. 1C).
The psychotic state remitted within 3 weeks. After the episode, lamotrigine was added to her medication and gradually increased in dose to 400 mg/day. This dose provided insufficient control of seizures but slightly reduced the intensity of the seizures relative to before the medication. The patient experienced no visual or auditory hallucinations while on this medication. In this remitted state, EEG showed that epileptiform discharges remarkably decreased and were present just in the right temporal region and not in the left side (Fig. 1D).
2.3. SPECT findings
Single-photon emission computed tomography scans were performed using iodo-123-n-isopropyl-p-iodoamphetamine (123I-IMP). Single-photon emission computed tomography during remission showed hypoperfusion of the left temporal lobe. Single-photon emission computed tomography during IIP revealed hyperperfusion of the left basal ganglia, and SPECT during PIP revealed hyperperfusion of the left basal ganglia and right temporal lobe (Fig. 2). The clinical course of this patient is shown in Fig. 3.
Fig. 2.
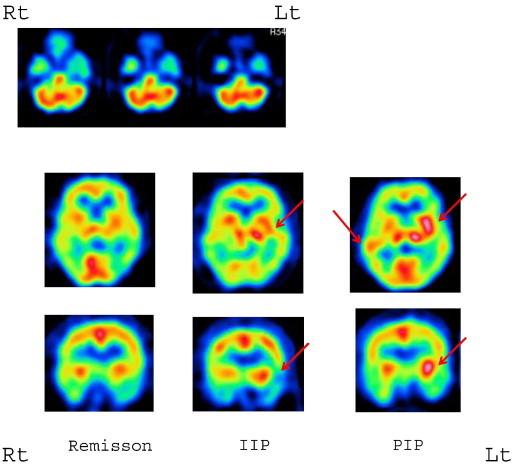
Iodo-123-n-isopropyl-p-iodoamphetamine SPECT findings. Hypoperfusion of the left temporal lobe was evident during remission of psychosis (top). Hyperperfusion of the left basal ganglia was evident during IIP (center; red arrows). Hyperperfusion of the left basal ganglia and right temporal lobe was evident during PIP (right; red arrows).
Fig. 3.
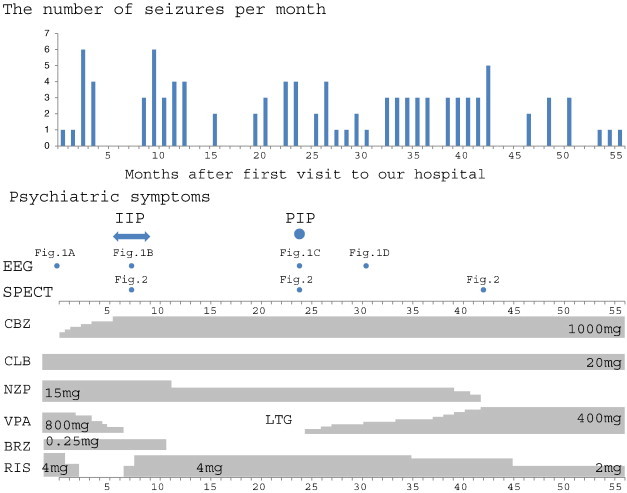
The clinical course of the patient. The number of seizures per month is shown in the top panel. The occurrence of psychotic episodes and the performance of examinations are shown in the middle panel. Medication is shown in the bottom panel. CBZ, carbamazepine; CLB, clobazam; NZP, nitrazepam; VPA, valproic acid; LTG, lamotrigine; BRZ, brotizolam; RIS, risperidone.
3. Discussion
3.1. Psychiatric symptoms
Postictal psychosis occurs soon after an epileptic seizure or a cluster of epileptic seizures. Logsdail and Toone defined PIP as a psychotic state that develops within a week of an epileptic seizure [1]. Typical PIP has a lucid interval, which is a period of normal mental state, in the few days preceding the development of psychosis. In typical PIP, seizures do not occur, while psychiatric symptoms are present [11]. Characteristic psychiatric symptoms of PIP are severe aggression and emotional response, grandiose delusion and religious delusion [2]. The case described in the current report first developed a psychotic state at the age of 25 years and experienced repetition of typical PIP. She experienced PIP again at the age of 36 years; however, this PIP had no lucid interval, and seizures occurred during PIP; therefore, this was not typical PIP. Oshima et al. categorized PIP as nuclear, which is preceded by a lucid interval and mood elevation, and atypical, which has no lucid interval, has seizures during psychotic episodes, and involves frequent episodes [11]. They speculated that nuclear PIP reflects secondary alterations in response to seizure activity and that atypical PIP represents direct seizure activity [11]. The PIP episode experienced by our case at the age of 36 years would be considered atypical according to this classification and may represent direct seizure activity. In fact, this psychotic episode did not occur after a seizure cluster or generalized tonic–clonic seizure but occurred during a period in which seizures occurred frequently. Strictly speaking, this episode does not fit the criteria for PIP and may be better categorized as periictal psychosis.
Our case developed psychosis after inhibition of seizures, and psychotic symptoms resolved after the recurrence of seizures at the age of 34 years. The features of this psychotic state fit the characteristics of an alternative psychosis.
Adachi et al. stated that patients with episodes of both PIP and IIP have bimodal psychosis [3,12]. Bimodal psychosis accounts for 3–8% of psychoses with epilepsy [3,13]. According to Adachi et al., the factors related to this type of psychosis are family history of psychosis, partial epilepsy, complex partial seizures, and low intelligence. These factors are common to PIP and IIP. The onset of epilepsy and psychosis is similar to that of IIP and earlier than that of PIP [3].
Typical symptoms in IIP are auditory hallucinations and delusions of reference, which are similar to the symptoms of schizophrenia. By contrast, auditory hallucinations, grandiose and fantastic delusions, and fluctuations of emotion and aggression were found during IIP in the current case and were similar to the symptoms of PIP in this case. Previous studies have also described that the psychiatric symptoms of PIP and IIP were similar in patients with both PIP and IIP [12,14].
3.2. EEG and SPECT findings
In this case, EEG showed independent bilateral sharp waves in the temporal region that were predominant on the right side. Single-photon emission computed tomography showed decreased perfusion in the left temporal lobe during remission of psychosis. Epileptogenic focus was unclear, but the SPECT finding suggests the left temporal lobe. By contrast, EEG during psychosis showed frequent bilateral sharp waves in the temporal regions that were particularly remarkable on the left side. Single-photon emission computed tomography showed increased perfusion in the left basal ganglia during both PIP and IIP episodes and increased perfusion in the right temporal lobe during PIP.
The relation between bilateral EEG abnormalities and development of psychosis has been indicated in previous studies [1,12,15,16]. Generally, increased epileptiform discharges and slow waves on EEG are present during PIP [1], although some studies have reported that EEG is unchanged or normalized during PIP [5,6,17,18]. Scalp EEG is commonly unchanged or normalized during IIP [4]. Our case showed increased bilateral interictal discharges, especially on the left side, not only during the postictal psychotic state but also during the alternative psychotic state. Interictal discharges were greater during PIP than during IIP.
Some intracranial EEG studies have reported that the development of PIP is due to ictal discharges [19,20]. However, one study reported that interictal discharges in depth electrode recordings increased during PIP [21], and another reported no change in interictal discharges during PIP [22]. These differences in EEG findings may be associated with heterogeneity in PIP, as described by Oshima et al. [11]. Furthermore, Wieser reported that ictal discharges occurred in depth electrode recordings during IIP even though scalp EEG was normal [23]. Wolf speculated that during forced normalization, a powerful inhibitory mechanism is activated, and epileptic discharge is still active subcortically [24].
The EEG findings in our case suggest that it is possible for the development of psychosis to be associated with direct epileptic activity. In addition, the increase in left temporal discharges may be important in the development of both PIP and IIP. By contrast, the more severe EEG abnormalities observed in PIP compared with IIP may suggest that the level of functional disturbance is more severe in PIP. Furthermore, the EEG abnormalities observed during IIP in our case were different from the usual change in scalp EEG observed in IIP, indicating the presence of heterogeneity in IIP.
Though it was possible that antipsychotics affected epileptic activity, epileptiform discharges increased during IIP, when risperidone, which usually lowers the threshold of seizures, was stopped. Furthermore, epileptiform discharges in EEG decreased during the remission of PIP, in spite of a fixed dose of risperidone. Therefore, we consider it unlikely that antipsychotics had a significant effect on epileptic activity. On the other hand, epileptiform discharges increased with the development of IIP when valproic acid was decreased and carbamazepine was increased. This change in antiepileptic drugs might have affected epileptic activity, leading to the development of psychosis. However, afterward, epileptiform discharges decreased with remission of IIP in spite of there being no remarkable change in antiepileptic drugs. Though seizures decreased after carbamazepine was added to the medication regimen, this medication was not sufficient to control the fluctuations in epileptic activity. On the other hand, after adding and increasing the dose of lamotrigine, the intensity of seizures was reduced in accordance with the improvement in EEG. Therefore, it is possible that lamotrigine inhibited epileptic activity.
Most SPECT studies have reported increased perfusion in the frontal and/or temporal lobe during PIP [5–9]. Oshima et al. reported that perfusion was especially increased in the right temporal lobe during PIP [8], and Jibiki et al. reported that perfusion was increased in the left temporal lobe during IIP [10]. Our case showed a remarkable increase in perfusion in the left basal ganglia during both PIP and IIP. Similar findings have been reported during PIP [7]. Leutmerzer et al. and Nishida et al. described how an increase in blood flow may reflect subcortical electrical activity [5,6]. Our results also suggest a subcortical functional change and indicate that increased subcortical blood flow may play an important role in the development of psychosis. In addition, our case showed hyperperfusion of the left basal ganglia during both PIP and IIP, and it is likely that the change in EEG and SPECT findings of the postictal and alternative psychotic state in our case have a common cause. Hyperperfusion of the basal ganglia in schizophrenia and other psychoses has previously been reported [25]. On the other hand, our case showed increased perfusion in the right temporal lobe during PIP but not during IIP, and this finding may be specific to PIP [8].
3.3. The duration of psychoses
In the current case, the psychiatric symptoms and EEG and SPECT findings were similar in PIP and IIP. However, there was a difference in the duration of the psychotic state of PIP and IIP. Though PIP remitted after about 3 weeks, IIP continued for about 5 months. This is a common difference between PIP and IIP. From the EEG findings in this case, we consider that both psychotic states were induced by an increase in epileptic activities. Regarding the difference in the duration of psychoses, we speculate that a powerful inhibition of seizure activity occurred during the postictal period and caused PIP to subside promptly, whereas a change in anticonvulsants caused a peculiar condition of continuous increased epileptic activity that did not cause clinical seizures but sustained IIP. According to Adachi et al., patients with bimodal psychosis tend to develop a psychotic state easily in response to events such as seizures and changes in medication because they are vulnerable to psychosis [12]. As described by Leutmerzer et al., a subtle change in epileptic activity may induce a change in the activity of a neuronal network, leading to occurrence of psychosis [6]. In our case, a subtle heightening of epileptic activity might have induced a psychotic state.
Our case showed similar changes in EEG and SPECT findings in IIP and PIP. We suggest that activity in the left temporal lobe and basal ganglia played an important role in the development of psychosis in our case.
In patients who have PIP and IIP, there are two patterns of psychosis development. Tarulli et al. described that, in most patients, IIP has a chronic course after repetitions of PIP [14]. Most cases seemed to have inhibition of seizures during psychoses. On the other hand, Adachi et al. reported patients who had PIP after remission of chronic interictal psychosis. They described that PIP and IIP seemed to occur independently in their cases and that psychotic states might tend to occur easily in these patients [12]. Differences of the clinical course in the development of psychoses may reflect heterogeneity in patients with both PIP and IIP. In these reports, as in our case, symptoms of PIP and IIP were similar; therefore, the two psychotic states may have occurred because of similar mechanisms. However, most cases of IIP continue chronically, differentiating them from PIP, and there may be different mechanisms between the two psychotic states. Furthermore, as described above, though our case showed similar changes in EEG and SPECT during the two types of psychoses, other possible investigations could involve patients with both psychoses which may show different changes in respective psychotic states. Furthermore, though our case showed increased epileptic activity in EEG during psychoses, other studies may show different findings. Whereas we consider the psychoses in our case to be related to direct epileptic activity, other studies may be considered where psychoses occur in relation to secondary alterations such as changes in neurotransmitters in response to events such as the occurrence of seizures and changes in medication. The hyperperfusion of the basal ganglia during psychosis in our case was different from that in most previous SPECT studies [5,6,8–10]. Furthermore, in this study, we described the relation between psychosis and dysfunction in the left side of the brain, but previous reports of the relation between laterality and psychoses are not consistent. In addition, we should consider the association with the epileptogenic zone. Nishida et al. described hyperperfusion in the side ipsilateral to the estimated epileptogenic zone during PIP [5]. Laterality, localization, and the relation to the epileptogenic zone are possibly associated with the development of psychosis, and conclusions on these factors cannot be made using only our single case.
In this study, EEG was performed five times when the patient was not in a psychotic state, and, on all occasions, epileptiform discharges remarkably decreased compared with during the psychotic state, and sporadic right temporal discharges were present. Therefore, we consider that the psychotic state was associated with changes in EEG. However, SPECT was performed only one time when the patient was not in a psychotic state, and we must, therefore, consider the possibility of a coincidental change.
Given that these conclusions are based on only one case, a collection of cases is needed for validation. A further limitation of this study is the visual analysis of SPECT. In addition, if SPECT could be performed more frequently, more detailed changes would be captured. Furthermore, although this case had a history of typical PIP, typical PIP was not investigated in this study. We expect that the accumulation of physiological data related to psychosis will uncover the pathophysiology of psychosis.
Conflict of interest
There is no conflict of interest that we should disclose.
References
- 1.Logsdail S.J., Toone B.K. Post-ictal psychoses: a clinical and phenomenological description. Br J Psychiatry. 1988;152:246–252. doi: 10.1192/bjp.152.2.246. [DOI] [PubMed] [Google Scholar]
- 2.Kanemoto K., Kawasaki J., Kawai I. Postictal psychosis: a comparison with acute interictal and chronic psychoses. Epilepsia. 1996;37(6):551–556. doi: 10.1111/j.1528-1157.1996.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 3.Adachi N., Matsuura M., Hara T., Oana Y., Okubo Y., Kato M. Psychoses and epilepsy: are interictal and postictal psychoses distinct clinical entities? Epilepsia. 2002;43(12):1574–1582. doi: 10.1046/j.1528-1157.2002.22402.x. [DOI] [PubMed] [Google Scholar]
- 4.Landolt H. Serial EEG investigations during psychotic episodes in epileptic patients and during schizophrenic attacks. In: de Haas AM Lorenz., editor. Lectures on epilepsy. Elsevier; Amsterdam: 1958. pp. P91–P133. [Google Scholar]
- 5.Nishida T., Kudo T., Inoue Y., Nakamura F., Yoshimura M., Matsuda K. Postictal mania versus postictal psychosis: differences in clinical features, epileptogenic zone, and brain functional changes during postictal period. Epilepsia. 2006;47(12):2104–2114. doi: 10.1111/j.1528-1167.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 6.Leutmerzer F., Prodreka I., Aserbaum S., Pitrzyk U., Lucht H., Back C. Postictal psychosis in temporal lobe epilepsy. Epilepsia. 2003;44(4):582–590. doi: 10.1046/j.1528-1157.2003.32802.x. [DOI] [PubMed] [Google Scholar]
- 7.Fong G.C., Fong K.Y., Mak W., Tsang K.L., Chan K.H., Cheung R.T. Postictal psychosis related regional cerebral hyperperfusion. J Neurol Neurosurg Psychiatry. 2000;68:100–101. doi: 10.1136/jnnp.68.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshima T., Motooka H., Kanemoto K. SPECT findings during postictal psychoses: predominance of relative increase of perfusion in right temporal lobe. Epilepsia. 2011;52:1192–1194. doi: 10.1111/j.1528-1167.2010.02962.x. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner C., Podreka I., Benda N., Olbrich A., Serles W., Novak K., Luger S., Lindinger G. Postictal psychosis: a SPECT study. Epilepsia. 1995;36(Suppl. 3):S218. [Google Scholar]
- 10.Jibiki I., Maeda T., Kubota T., Yamaguchi N. 123I-IMP SPECT brain imaging in epileptic psychosis: a study of two cases of temporal lobe epilepsy with schizophrenia-like syndrome. Neuropsychobiology. 1993;28(4):207–211. doi: 10.1159/000119025. [DOI] [PubMed] [Google Scholar]
- 11.Oshima T., Tadokoro Y., Kanemoto K. A prospective study of postictal psychoses with emphasis on the periictal type. Epilepsia. 2006;47(12):2131–2134. doi: 10.1111/j.1528-1167.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 12.Adachi N., Kato M., Sekimoto M., Ichikawa I., Akanuma N., Uesugi H. Recurrent postictal psychosis after remission of interictal psychosis: further evidence of bimodal psychosis. Epilepsia. 2003;44(9):1218–1222. doi: 10.1046/j.1528-1157.2003.66702.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanemoto K. Postictal psychoses, revisited. In: Trimble M., Scmitz B., editors. The neuropsychiatry of epilepsy. Cambridge University Press; Cambridge: 2002. pp. 117–131. [Google Scholar]
- 14.Tarulli A., Devinsky O., Alper K. Progression of postictal to interictal psychosis. Epilepsia. 2001;42(11):1468–1471. doi: 10.1046/j.1528-1157.2001.10701.x. [DOI] [PubMed] [Google Scholar]
- 15.Savard G., Andermann F., Oliver A., Reilmond G.M. Postictal psychosis after partial complex seizures: a multiple case study. Epilepsia. 1991;32(2):225–231. doi: 10.1111/j.1528-1157.1991.tb05249.x. [DOI] [PubMed] [Google Scholar]
- 16.Umbriricht D., Degreef G., Barr W.B., Lieberman J.A., Pollack S., Schaul N. Postictal and chronic psychoses in patients with temporal lobe epilepsy. Am J Psychiatry. 1995;152:224–231. doi: 10.1176/ajp.152.2.224. [DOI] [PubMed] [Google Scholar]
- 17.Kanner A.M., Stagno S., Kotagal P., Morris H.H. Postictal psychiatric events during prolonged video-electroencephalographic monitoring studies. Arch Neurol. 1996;53(3):258–263. doi: 10.1001/archneur.1996.00550030070024. [DOI] [PubMed] [Google Scholar]
- 18.Akanuma N., Kanemoto K., Adachi N., Kawasaki J., Ito M., Onuma T. Prolonged postictal psychosis with forced normalization (Landolt) in temporal lobe epilepsy. Epilepsy Behav. 2005;6:456–459. doi: 10.1016/j.yebeh.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Takeda Y., Inoue Y., Tottori T., Mihara T. Acute psychosis during intracranial EEG monitoring: close relationship between psychotic symptoms and discharges in amygdale. Epilepsia. 2001;42(6):719–724. doi: 10.1046/j.1528-1157.2001.08700.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanemoto K. Periictal Capgras syndrome after clustered ictal fear: depth-electroencephalogram study. Epilepsia. 1997;38(7):847–850. doi: 10.1111/j.1528-1157.1997.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 21.So N.K., Savard G., Andermann F., Olivier A., Quesney L.F. Acute postictal psychosis: a stereo EEG study. Epilepsia. 1990;31(2):188–193. doi: 10.1111/j.1528-1167.1990.tb06305.x. [DOI] [PubMed] [Google Scholar]
- 22.Mathern G.W., Pretorius J.K., Babb T.L., Quinn B. Unilateral hippocampal mossy fiber sprouting and bilateral asymmetric neuron loss with episodic postictal psychosis. J Neurosurg. 1995;82:228–233. doi: 10.3171/jns.1995.82.2.0228. [DOI] [PubMed] [Google Scholar]
- 23.Wieser H.G., Hailemariam S., Regard M., Landis T. Unilateral limbic epileptic status activity: stereo EEG, behavioral, and cognitive data. Epilepsia. 1985;26(1):19–29. doi: 10.1111/j.1528-1157.1985.tb05184.x. [DOI] [PubMed] [Google Scholar]
- 24.Wolf P. Vol. 55. Raven Press; New York: 1991. Acute behavioral symptomatology at disappearance of epileptiform EEG abnormality: paradoxical or “forced” normalization; pp. 127–142. (Advances in Neurology). [PubMed] [Google Scholar]
- 25.O'Connell R.A., Van Heertum R.L., Billick S.B., Holt A.R., Gonzalez A., Notardonato H. Single photon emission computed tomography (SPECT) with [123I] IMP in the differential diagnosis of psychiatric disorders. J Neuropsychiatry Clin Neurosci. 1989;1(2):145–153. doi: 10.1176/jnp.1.2.145. [DOI] [PubMed] [Google Scholar]


