Abstract
The adipose tissue is not only an inert storage depot for lipids, but also it secretes a variety of bioactive molecules, known as adipokines, which affect whole-body homeostasis. Adiponectin is the most abundant of these adipocytokines and is known to have a regulatory effect on the metabolism of glucose and lipid. The main objectives of this study were to evaluate the serum levels of adiponectin and to establish a correlation between adiponectin serum levels and the degree of insulin resistance in type 2 diabetic patients. Eighty participants were enrolled in this study; 61 type 2 diabetic patients and 19 apparently healthy subjects. Serum level of adiponectin was measured by enzyme-linked immunosorbent assay (ELISA) for each participant. Data collection sheet was filled with all required information for each participant. Adiponectin level in the diabetic patients (5.05 ± 2.61 μg/ml) was lower than in non-diabetic healthy controls (5.71 ± 2.35 μg/ml). When the results were compared according to gender, diabetic females showed significantly higher adiponectin levels (5.76 ± 2.64 μg/ml) than diabetic males (4.366 ± 2.43 μg/ml, P = 0.035). In addition, female diabetic patients with abdominal obesity (waist circumference (WC) ⩾ 88 cm) had lower adiponectin levels (5.58 ± 2.58 μg/ml) than diabetic females without abdominal obesity (6.96 ± 3.12 μg/ml). The correlation analysis indicated that adiponectin had a significant positive correlation with age (r = −0.450, P < 0.001). In conclusion, female diabetic patients had a statistically significant higher adiponectin level than male diabetic patients which could indicate a gender effect. Adiponectin levels were inversely related to insulin resistance; as patients with abdominal obesity had lower serum levels of adiponectin.
Keywords: Adiponectin, Diabetes mellitus, Abdominal obesity, Insulin resistance, Gender, Jordan
1. Introduction
The designation of the adipose tissue as a pivotal active endocrine organ has indeed drawn much scientific interest in the last few years. The adipose tissue is now viewed as the largest endocrine organ in the body (Oh et al., 2007). It secretes numerous bioactive proteins, collectively known adipocytes, into the circulation (Chandran et al., 2003). The most abundant of these adipocytokines is the adiponectin which is secreted by white adipose tissue and accounts for 0.01% of total plasma proteins (Gil-Campos et al., 2004; Heiker et al., 2010).
Adiponectin shares homology with collagen and complement 1q family (Simpson and Whitehead, 2010). It consists of 244 amino acids, which form four different domains (Sheng and Yang, 2008). Adiponectin modulates a number of metabolic processes via the activation of 5′-adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator activated receptor-α (PPAR-α) (Fagerberg et al., 2011). In addition, it plays an important role in the suppression of the metabolic derangements that cause insulin resistance and type 2 diabetes mellitus (type 2 DM) (Sheng and Yang, 2008).
Interestingly, although adiponectin is secreted by mature adipocytes, its plasma level shows a negative correlation with body fat mass. It has been found that adiponectin plasma level in obese individuals was lower than in non-obese ones (Arita et al., 2012). Adiponectin has been also shown to be negatively correlated with obesity-related diseases such as type 2 DM (Han et al., 2009). Furthermore, low adiponectin levels predicts the incidence of type 2 DM (Han et al., 2009). The latter findings have triggered a wealth of studies aiming at investigating the impact of circulating plasma adiponectin levels on enhancing insulin sensitivity in the diabetic patients (Fagerberg et al., 2011; Nayak et al., 2010; Weyer et al., 2001).
Diabetes types 2 as well as the impaired fasting glucose (IFG) are common among Jordanian population. The estimated age standardized prevalence rate of (IFG) and diabetes were 7.8% and 17.1%, respectively, with no significant gender differences according to a recent study (Ajlouni et al., 2008). To complicate things further, there are alarming rates of obesity and its associated co-morbidities among Jordanians, especially among women (Khader et al., 2008). This study aims to evaluate the serum levels of adiponectin in type 2 diabetic patients and to establish a correlation between adiponectin serum levels and insulin resistance in those patients. In contrast, previous studies had investigated the association of adiponectin serum levels and obesity and DM type 2. Jordan is an ideal place to conduct the current study due to the high prevalence of DM type 2 and prediabetes, as mentioned earlier.
2. Methods
2.1. Setting and participants
This is a cross-sectional study that has been conducted in the outpatient diabetes clinic of National Center for Diabetes, Endocrinology, and Genetics for 7 months. Eighty Jordanian subjects were included; 61 patients above 18 years diagnosed with type 2 diabetes for at least 6 months and 19 apparently healthy, medically free, and treatment naive individuals were recruited to serve as non-diabetic control. Type 1 diabetic patients, patients with acute or chronic kidney disease, patients with congestive heart failure, patients receiving thiazolidinedione (TZD) or exogenous insulin, and pregnant female patients all were excluded from the study.
Sample size calculation was conducted using an online tool (The Survey System, Creative Research Systems, www.surveysystem.com). Using a confidence level of 95%, a minimum sample size of 79 subjects was calculated to be sufficient to conduct the study. An Institutional Review Board (IRB) approval was granted and an informed consent form, written in lay Arabic language, was handed to and signed by each subject.
2.2. Anthropometric measurements
All the anthropometric parameters including weight, height, and waist circumference (WC) were measured in a situation where subjects were in the standing position and wearing light clothing without shoes.
WC was measured in centimeters using constant tension tape at the end of a normal expiration at the point between the rib cage and the pelvis. Abdominal obesity was defined as WC ⩾ 102 cm in males and ⩾88 cm in female.
Body weight was measured in kilograms to the nearest 0.5 kg using a calibrated balance. Height was measured in centimeters to the nearest 0.5 cm. Body mass index (BMI) for each subject was calculated as body weight (in kilograms) divided by the square of body height (in meters). The BMI was classified into normal (8–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (30–34.9 kg/m2) and morbidly obese (>35 kg/m2).
2.3. Sample collection and handling
Venous blood samples (6–8 ml) were collected in sterile plain tubes from all subjects after fasting for at least 10 h. Serum samples were separated by centrifugation of the coagulated blood samples at 4000 rpm for 5 min at 4 °C and then aliquoted and stored at −80 °C until the day of adiponectin level measurement using (ELIZA).
2.4. Measurement of serum adiponectin levels
Serum adiponectin concentration was measured using a commercially available human ELIZA kit (Adipo Bioscience, USA, Adipo Bioscience Incorporation, 2012).
The ELISA assay utilizes a monoclonal purified immunoglobulin G (IgG) against a specific determinant on human adiponectin molecule for capture on the micro plate wells and a mouse anti-adiponectin antibody conjugated to horseradish peroxidase (HRP) for detection. The serum samples were thawed at room temperature and then they were diluted by 5000 folds using dilution buffer supplied by the kit. 100 μL of diluted serum were added to each plate well. The assay was carried out according to the manufacturer procedures (Adipo Bioscience, USA). The developed color was measured spectrophotometrically using the micro plate reader (MQX200, Biotek, USA) at 450 nm. Adiponectin concentrations, in μg/ml, were calculated from the standard curve prepared using recombinant human adiponectin standards.
2.5. Data management and statistical analysis
All data were processed using the statistical package for social science (SPSS version 16, Chicago, USA software). Differences were considered statistically significant if P < 0.05. Continuous variables were expressed as mean ± SD while categorical variables were presented as percentage. For testing whether there was a normal distribution or not, Kolmogorov–Smirnov test was used. For comparison of two normally distributed means t-test was used. If distribution of variables was not normal, the statistical analysis was conducted using Kruskall–Wallis followed by Mann–Whitney. Continuous variables were compared using student’s t-test.
Chi-square was used to compare categorical variables. Pearson correlation coefficients were calculated to evaluate the relationship between serum adiponectin levels and study variables.
3. Results
The clinical and demographic characteristics of the study population are presented in Table 1. In the study population, diabetic patients had lower adiponectin serum level compared to non-diabetics control group. However, statistically this difference was not significant (Fig. 1A).
Table 1.
Clinical and demographic characteristics of the study population.
| Parameter | Diabetic patients, N (%) | Control group, N (%) | P-value |
|---|---|---|---|
| 61 (76.25) | 19 (23.75) | ||
| Agea (year) | 56.15 ± 12.02 | 43.05 ± 9.19 | 0.001 |
| Genderb | |||
| Male | 31 (50.8) | 12 (63.2) | 0.346 |
| Female | 30 (49.2) | 7 (36.8) | |
| Smoking status | |||
| Smoker | 19 (31.1) | 6 (31.6) | 0.972 |
| Non-smoker | 42 (68.9) | 13 (68.4) | |
| BMIa (kg/m2) | 28.36 ± 3.47 | 26.47 ± 3.52 | 0.043 |
| WCa (cm) | 97.79 ± 9.22 | 96.00 ± 7.79 | 0.447 |
| Adiponectina (μg/ml) | 5.05 ± 2.61 | 5.71 ± 2.35 | 0.331 |
Abbreviations: BMI – body mass index; WC – waist circumference; N – number of subjects.
Data were presented as means ± SD and t-test used for comparison.
Data were presented as percentage and compared via chi-square test.
Figure 1.
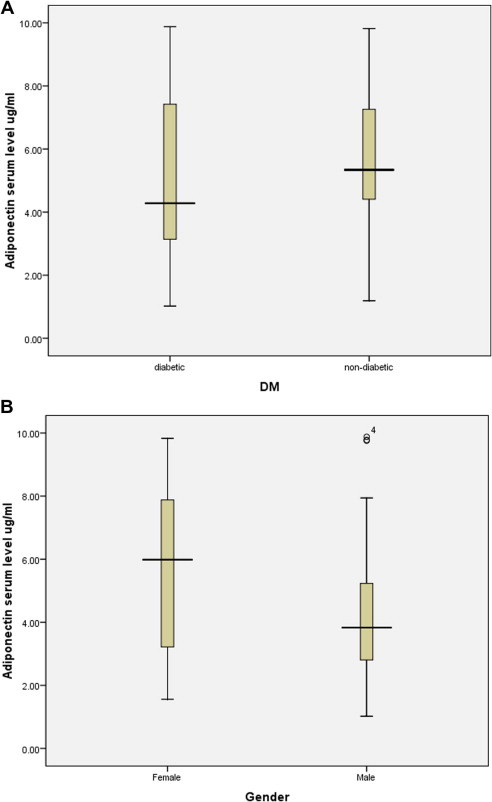
Box and whisker plot plots of adiponectin serum levels in different subject groups. (A) Adiponectin serum levels in diabetic and non-diabetic. (B) Adiponectin serum levels in diabetic females and males.
3.1. Association of adiponection with obesity (abdominal and general obesity), impact of gender
Interestingly, there was a statistically significant gender difference in adiponectin serum level among the study population. Diabetic females showed significantly high adiponectin serum level in contrast to diabetic males (5.76 ± 2.64 μg/ml versus 4.366 ± 2.43 μg/ml, respectively, P = 0.035) (Fig. 1B). In order to investigate this finding further, diabetic patients were classified according to the gender and the prevalence of the abdominal obesity into two groups: patients with abdominal obesity, which is a marker of insulin resistance, and patients without (Tables 2 and 3).
Table 2.
Adiponectin levels in Female diabetic patients according to the abdominal obesity prevalence.
| Diabetic females with abdominal obesitya | Diabetic females without abdominal obesitya | P-value | |
|---|---|---|---|
| N (%) | 26 (86.7) | 4 (13.3) | |
| Adiponectinb (μg/ml) | 5.58 ± 2.58 | 6.96 ± 3.12 | 0.882 |
Abdominal obesity was defined as WC ⩾ 102 cm in females.
Data were presented as means ± SD and t-test used for comparison.
Table 3.
Adiponectin levels in Male diabetic patients according to the abdominal obesity prevalence.
| Diabetic males with abdominal obesitya | Diabetic males without abdominal obesitya | P-value | |
|---|---|---|---|
| N (%) | 12 (38.7) | 19 (61.3) | |
| Adiponectinb (μg/ml) | 4.28 ± 2.22 | 4.42 ± 2.61 | 0.882 |
Abdominal obesity was defined as WC ⩾ 102 cm in males.
Data were presented as means ± SD and t-test used for comparison.
The results showed that abdominal obesity is associated with low circulating adiponectin levels in both genders. Moreover, when compared across gender, adiponectin level was higher in diabetic females with abdominal obesity than in males (Tables 2 and 3). Adiponectin serum levels correlated negatively with both WC and BMI in diabetic patients (Pearson correlation coefficient (r) = −0.125 and −0.222, respectively). However, these correlations were not statistically significant (p 0.337 and 0.086, respectively). As a measure for general obesity, diabetic patients were classified according to their BMI into two categories (obese and non-obese). Both normal weight and overweight patients were grouped under the “non-obese” patients group. Obese patients had a lower adiponectin serum level compared to non-obese patients, but statistically the difference was not significant (4.57 ± 2.23 μg/ml versus 5.38 ± 2.83 μg/ml, P = 0.416). On the other hand, serum adiponectin levels correlated positively with age (Pearson correlation (r) = 0.450, P < 0.001).
3.2. The variation in adiponectin serum levels among diabetic patients
The majority of patients (70.5%) had adiponectin serum levels within (1.02–6.9 μg/ml) while (29.5%) had adiponectin levels ⩾7 μg/ml (Fig. 2 and Table 4). In order to justify this finding, diabetic patients were divided into two groups according to the variance in adiponectin levels. A cut off value of 7 μg/ml was arbitrarily chosen (Semple et al., 2008) to designate a non-reduced level of adiponectin. Table 4 shows comparisons between these two groups. There was a statistically significant difference in age and gender between the two groups (P < 0.05). The majority of diabetic patients who had adiponectin serum level ⩾7 μg/ml were females (72%). Interestingly, patients in this group had lower mean of BMI than patients in the group of patients who had adiponectin serum level ⩽7 μg/ml (Table 4).
Figure 2.
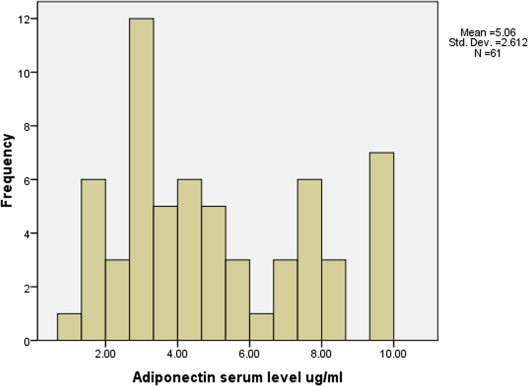
The distribution of adiponectin serum levels in diabetic patients.
Table 4.
Diabetic patients according to the variance in adiponectin levels.
| Variable | Diabetic patients with adiponectin level < 7 μg/ml | Diabetic patients with adiponectin level ⩾ 7 μg/ml | P-value |
|---|---|---|---|
| N (%) | 43(70.5) | 18 (29.5) | |
| Age (year)a | 52.93 ± 11.51 | 63.83 ± 9.68 | 0.001 |
| Genderb | |||
| Female | 17(39.5) | 13 (72.2) | 0.020 |
| Male | 26(60.5) | 5 (27.8) | |
| BMIa (kg/m2) | 28.91 ± 3.47 | 27.0 ± 3.18 | 0.054 |
| WCa (cm) | 98.98 ± 8.92 | 95.06 ± 9.59 | 0.135 |
Abbreviations: BMI – body mass index; WC – waist circumference; N – number of subjects.
Data were presented as means ± SD and t-test used for comparison .
Data were presented as percentage and compared via chi-sequre test.
4. Discussion
This study, as a few other series studies, has investigated adiponectin levels among type 2 diabetic patients. We have found that gender is a potentially important cofounder when assessing the levels of adiponectin and its correlation with waist circumference. The latter is an important determinant for insulin resistance.
Females had a significantly higher adiponectin serum levels than males. A possible explanation for this gender based difference in adiponectin serum levels might be due to the following reasons; first, is the effect of sex hormones on the production of adiponectin rate (Kadowaki et al., 2006). Experimental studies have proved that androgens have an inhibitory effect on adiponectin secretion (Bottner et al., 2004; Nishizawa et al., 2002). Second, is the different body fat distribution between males and females. It has been reported that the number of fat cells and their size are possible determinants of adiponectin production rates since it is mainly secreted from adipocytes (Cnop et al., 2003).
It is known that insulin resistance increases with age, which would predict the lower adiponectin levels in the elderly (Cnop et al., 2003). Interestingly, our study has shown that the adiponectin levels were correlated positively with age. The increase in adiponectin levels with age could be explained by the fact that the decline in sex steroidal hormones with age might rise the adiponectin levels in the elderly (Bottner et al., 2004; Nishizawa et al., 2002). Moreover, the decline in renal function with aging might reduce the adiponectin clearance by kidney (Isobe et al., 2005).
Generally, obesity is associated with insulin resistance (Cnop et al., 2002). Abdominal obesity, where the fat is centrally distributed, is particularly an important determinant of insulin resistance (Cnop et al., 2003; Gierach et al., 2014). It has been reported that abdominal obesity, measured by waist circumferences, is strongly associated with lower levels of adiponectin and decrease in insulin sensitivity among diabetic patients (Cnop et al., 2003; Mohammadzadeh and Ghaffari, 2014).
This study has shown low levels of adiponectin in both obese diabetic patients and patients with abdominal obesity and negative correlation between adiponectin levels and both BMI and WC. Given the fact that adiponectin is a major modulator of insulin action for its role in enhancing insulin sensitivity (Cnop et al., 2003; Schulze et al., 2004), it is therefore of critical importance to note that factors decreasing adiponectin levels such as obesity, and specially the abdominal obesity, could correlate with insulin resistance.
In addition, diabetic patients included in this study had a variable adiponectin serum levels. The majority of them had levels between (1.02–6.9) μg/ml while the remaining had levels ⩾7 μg/ml. This variability in adiponectin serum levels might indicate that there are some factors affect the adiponectin levels among type 2 diabetic patients. It is important to note that the majority of diabetic patients who have adiponectin levels ⩾7 μg/ml were elderly, females, and had lower mean of BMI compared to the second group (Table.4). Taken together, these characteristics of diabetic patients who have adiponectin levels ⩾7 μg/ml are possible factors for increasing adiponectin level in this subgroup of diabetic patients.
Semple et al., 2008 have found that adiponectin levels more than 7 μg/ml are associated with severe insulin resistance and has a 97% positive predictive value for insulin receptoropathy (Semple et al., 2008). While adiponectin levels less than 5 μg/ml have a 97% negative predictive value for insulin receptoropathy (Semple et al., 2008). It was suggested that patients with insulin receptor dysfunction, including diabetics, may have unexpectedly high levels of adiponectin (Semple et al., 2008).
Interestingly, a series of published studies have reported a paradoxical high level of adiponectin in diabetic patients with severe insulin resistance (Semple et al., 2008, 2007, 2006). This paradoxical hyperadiponectinemia is due to pathology in the insulin receptor “insulin receptoropathy” which occurs in select patients (Semple et al., 2008). In those patients, insulin resistance was caused either by mutation in the receptor or by auto antibodies against the receptor (Semple et al., 2008). In fact, experimental studies have showed that in knockout mice with adipose-specific deletion of the insulin receptor, there was as much as 60% increase in the serum adiponectin levels (Bluher et al., 2002). The latter finding clearly delineates a potential role for adiponectin in modulating the interaction between insulin and its receptor.
This finding indicates that the net effect on insulin resistance will be determined by the contribution of a constellation of factors that dictate the molecular interaction between insulin and its receptor in different effector tissues and organs.
5. Conclusion
Adiponectin could link intra-abdominal fat with insulin resistance. Gender and age are independent predictors of serum adiponectin levels and thus they need to be taken into account when assessing the level of adiponectin. This potential correlation between adiponectin levels and insulin resistance is a first step in establishing new treatment strategies and monitoring parameters in our fight against diabetes. The idea of using adiponectin serum level as a marker for both predicting and monitoring the proper therapeutic regimens in type 2 diabetic patients is highly plausible. Further studies are still needed to unravel the full therapeutic potential and clinical applications of adiponectin in the management of diabetes.
Acknowledgments
This project was sponsored by the Deanship of Scientific Research at the University of Jordan. The authors wish to thank the Deanship of Scientific Research for their generous funds.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adipo Bioscience Incorporation, 2012. Product Sheet Human Adiponectin (Total) ELISA Kit. www.Adipobioscience.com.
- Ajlouni K., Khader Y.S., Batieha A., Ajlouni H., El-Khateeb M. An increase in prevalence of diabetes mellitus in Jordan over 10 years. J. Diabetes Complications. 2008;22:317–324. doi: 10.1016/j.jdiacomp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 2012;425:560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Bluher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B., Kahn C.R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Bottner A., Kratzsch J., Muller G., Kapellen T.M., Bluher S., Keller E., Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J. Clin. Endocrinol. Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- Chandran M., Phillips S.A., Ciaraldi T., Henry R.R. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- Cnop M., Landchild M.J., Vidal J., Havel P.J., Knowles N.G., Carr D.R., Kahn S.E. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- Cnop M., Havel P.J., Utzschneider K.M., Carr D.B., Sinha M.K., Boyko E.J., Kahn S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Fagerberg B., Kellis D., Bergstrom G., Behre C.J. Adiponectin in relation to insulin sensitivity and insulin secretion in the development of type 2 diabetes: a prospective study in 64-year-old women. J. Int. Med. 2011;269:636–643. doi: 10.1111/j.1365-2796.2010.02336.x. [DOI] [PubMed] [Google Scholar]
- Gierach M., Gierach J., Ewertowska M., Arndt A., Junik R. Correlation between body mass index and waist circumference in patients with metabolic syndrome. ISRN Endocrinol. 2014;2014:514589. doi: 10.1155/2014/514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Campos M., Canete R.R., Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin. Nutr. 2004;23:963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Han S.H., Sakuma I., Shin E.K., Koh K.K. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Prog. Cardiovasc. Dis. 2009;52:126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Heiker J.T., Kosel D., Beck-Sickinger A.G. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol. Chem. 2010;391:1005–1018. doi: 10.1515/BC.2010.104. [DOI] [PubMed] [Google Scholar]
- Isobe T., Saitoh S., Takagi S., Takeuchi H., Chiba Y., Katoh N., Shimamoto K. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur. J. Endocrinol. 2005;153:91–98. doi: 10.1530/eje.1.01930. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader Y., Batieha A., Ajlouni H., El-Khateeb M., Ajlouni K. Obesity in Jordan: prevalence, associated factors, comorbidities, and change in prevalence over ten years. Metab. Syndr. Relat. Disord. 2008;6:113–120. doi: 10.1089/met.2007.0030. [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh G., Ghaffari M.A. Additional effect of diabetes mellitus type 2 on the risk of coronary artery disease: role of serum adiponectin. Iran Red. Crescent Med. J. 2014;16:e8742. doi: 10.5812/ircmj.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak B.S., Ramsingh D., Gooding S., Legall G., Bissram S., Mohammed A., Figaro K. Plasma adiponectin levels are related to obesity, inflammation, blood lipids and insulin in type 2 diabetic and non-diabetic Trinidadians. Prim. Care Diabetes. 2010;4:187–192. doi: 10.1016/j.pcd.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Nishizawa H., Shimomura I., Kishida K., Maeda N., Kuriyama H., Nagaretani H., Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- Oh D.K., Ciaraldi T., Henry R.R. Adiponectin in health and disease. Diabetes Obes. Metab. 2007;9:282–289. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- Schulze M.B., Rimm E.B., Shai I., Rifai N., Hu F.B. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- Semple R.K., Soos M.A., Luan J., Mitchell C.S., Wilson J.C., Gurnell M., O’Rahilly S. Elevated plasma adiponectin in humans with genetically defective insulin receptors. J. Clin. Endocrinol. Metab. 2006;91:3219–3223. doi: 10.1210/jc.2006-0166. [DOI] [PubMed] [Google Scholar]
- Semple R.K., Halberg N.H., Burling K., Soos M.A., Schraw T., Luan J., O’Rahilly S. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes. 2007;56:1712–1717. doi: 10.2337/db06-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple R.K., Cochran E.K., Soos M.A., Burling K.A., Savage D.B., Gorden P., O’Rahilly S. Plasma adiponectin as a marker of insulin receptor dysfunction: clinical utility in severe insulin resistance. Diabetes Care. 2008;31:977–979. doi: 10.2337/dc07-2194. [DOI] [PubMed] [Google Scholar]
- Sheng T., Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J. Genet. Genomics. 2008;35:321–326. doi: 10.1016/S1673-8527(08)60047-8. [DOI] [PubMed] [Google Scholar]
- Simpson F., Whitehead J.P. Adiponectin–it’s all about the modifications. Int. J. Biochem. Cell Biol. 2010;42:785–788. doi: 10.1016/j.biocel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R.E., Tataranni P.A. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]


