Abstract
Chikungunya is a mosquito-borne infection of humans, and its diffusion has increased worldwide. In 2007 an outbreak occurred in Italy. In this study, the antibody response of 133 patients followed up starting from the acute phase of infection was investigated. Antibody titres were periodically scored up to 1 year since the infection: 82.7% of the IgM antibody disappeared within 12 months, and the IgG response lasted longer than 12 months. Nevertheless, the IgG mean titre was lower in 95.5% of patients at the end of follow-up, thus suggesting a decrease within a relatively short period.
Keywords: Antibody response, chikungunya virus, infected patients, Italy, serological follow-up
Introduction
Chikungunya virus (CHIKV), an arthropod-borne virus (Togaviridae family) transmitted by Aedes spp. mosquitoes [1], represents an important reemerging public health problem in both tropical and temperate countries [2,3]. The first epidemic in a temperate area was reported in the Emilia-Romagna region of northeastern Italy in summer 2007 [4,5]. An active surveillance system for the identification of all suspected cases (patients with fever and joint paint) was implemented during the event, with suspected cases confirmed with molecular and serologic tests by the Clinical Microbiology Unit, Regional Reference Centre for Microbiological Emergencies, St Orsola-Malpighi University Hospital, Bologna.
During the course of CHIKV infection in humans, an antibody response develops in the early stages, but its persistence over time is not fully established [6], as differences in the length of permanence after onset of symptoms and in relation to the method used to measure CHIKV antibodies have been reported [6–8].
As far as the epidemiology of CHIKV occurring in areas that are not tropical is concerned, to our knowledge, ours is the first study to report on the lasting period of specific immunoresponse in a population of patients followed since the beginning of the acute onset of the disease in a temperate zone with autochthonous transmission.
We report the results of a serologic follow-up aimed at estimating the modification of the CHIKV-specific antibody titres with a follow-up time lasting 12 months since the acute phase.
This study was submitted to the Area Vasta Romagna ethical committee for approval (which was provided on 12 December 2007), and all participants signed an informed consent form.
Methods
The study population was composed of 133 patients for whom the history of CHIKV infection was recorded, as previously described [9]. Inclusion criteria included clinical evidence of CHIKV infection [9] and detection of an anti-CHIKV-specific IgM titre of ≥1/100 with a negative IgG response or the detection of CHIKV RNA in serum in the acute stage. All patients who met these criteria were enrolled and followed up as described below.
Of the 133 subjects included, 70 (52.6%) were female. Serum samples (three for each patient) were collected between September–December 2007 and September–December 2008, as previously described [9], within subsequent intervals of 2 months, 4–5 months and 12–13 months after the clinical onset of the infection. The anti-CHIKV-specific IgM and IgG antibodies were detected and titrated by a commercially available ELISA following the manufacturer's instructions (Diesse, Siena, Italy).
Results and discussion
At the first follow-up at 2 months, 94 (70.7%) patients were positive for both IgM and IgG and 39 (29.3%) were positive for IgM only. In these samples, the IgM-positive specimens had a titre ranging from 1/100 to 1/12 800 (Fig. 1), and the IgG positive titres ranged between negative and 1/12 800 (Fig. 2).
Fig. 1.
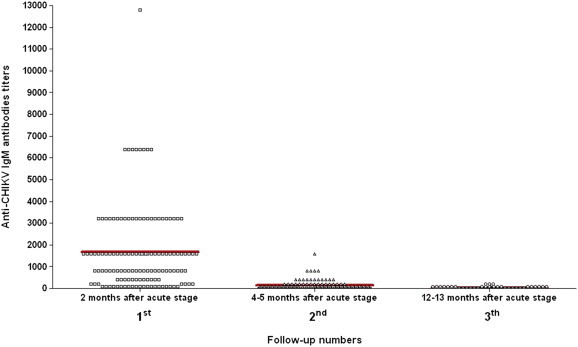
Variation of the IgM antibody titres in the three follow-up samplings obtained from 133 patients after their acute-phase chikungunya virus (CHIKV) infection. For each follow-up group of specimens, the mean value of the antibody titres was calculated (thick red line).
Fig. 2.
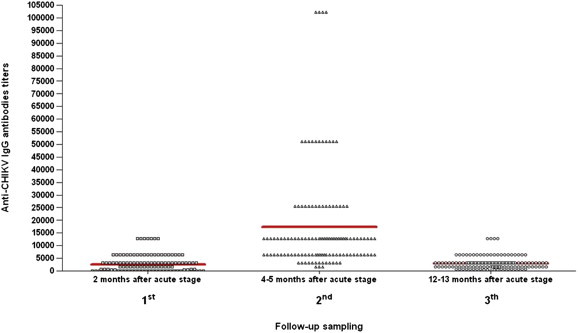
Variation of the IgG antibody titres in the three follow-up samplings obtained from 133 patients after their acute-phase chikungunya virus (CHIKV) infection. For each follow-up group of specimens, the mean value of the antibody titres was calculated (thick red line).
In the second sampling of this study (4–5 months from the acute stage), 93 (70%) patients were positive for both IgM and IgG, no sample was positive for IgM only and 40 (30%) were positive only for IgG. The IgM titres varied between negative and 1/1600 (Fig. 1) and the IgG titres ranged from 1/1600 to 1/102 400 (Fig. 2).
Finally, in the third sampling (12–13 months after the acute stage), 23 (17.3%) samples still showed an IgM and IgG immunoresponse, no sample was positive for IgM only (as expected, given the data collected during the second sampling) and 110 (82.7%) were positive for IgG only. The IgM-positive samples showed titres between negative and 1/200 (Fig. 1), while IgG titres varied between 1/100 and 1/12 800 (Fig. 2).
The results obtained at the end of the follow-up period clearly suggested that the IgG antibody titres decreased starting from the second to the last series of specimens in 127 (95.5%) samples. In addition, 110 (82.7%) patients showed a completely negative IgM response. Only in six (4.5%) patients did the IgG titre not show a modification: five patients demonstrated a stable titre, and in only one was the IgG titre increased. Samples that were still IgM positive at the end of follow-up (17.3%) showed quite a low titre (1/100–1/200) (Fig. 1). No sample became negative for IgG within the follow-up time frame, although the mean titre was considerably lower (about 4 times lower) than in the second sampling (Fig. 2). We stress that all specimens obtained during follow-up were tested in triplicate at the end of this period in two separate runs.
In addition, we believe that 1 year after the acute phase, the anti-CHIKV IgG response is mainly represented by neutralizing antibodies. Data in the literature show that neutralizing antibodies circulate up to 12 months after the acute stage [6,7] and that even in the presence of titres lower than those identified in the present study at the third sampling, this immunoresponse is still capable of neutralizing CHIKV in vitro[10].
In conclusion, this study showed that up to 1 year after the acute stage of CHIKV infection, a large percentage of the studied population had persistent anti-CHIKV-specific antibodies, even if the amount of immunoresponse decreased over time, as expected. To our knowledge, this is the first study to evaluate the CHIKV-specific immunoresponse among a population living in a temperate area, choosing as starting point for study inclusion either a laboratory confirmation of viremia or the detection of a specific IgM response. In addition, this evaluation was performed mainly among a white population, thus demonstrating that the immunoresponse against this virus is not substantially different in diverse geographic zones. Further studies are warranted to evaluate the variation of the CHIKV-specific antibody response over a longer period in order to assess the persistence of the immune-mediated protection.
Conflict of interest
None declared.
Acknowledgement
This study was supported by “Fondi Lab P3” from Regione Emilia-Romagna.
References
- 1.Cavrini F., Gaibani P., Pierro A.M., Rossini G., Landini M.P., Sambri V. Chikungunya: an emerging and spreading arthropod-borne viral disease. J Infect Dev Ctries. 2009;3(10):744–752. doi: 10.3855/jidc.169. [DOI] [PubMed] [Google Scholar]
- 2.Rougeron V., Sam I.C., Caron M., Nkoghe D., Leroy E., Roques P. Chikungunya, a paradigm of neglected tropical disease that emerged to be a new health global risk. J Clin Virol. 2015;64:144–152. doi: 10.1016/j.jcv.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Van Bortel W., Dorleans F., Rosine J., Blateau A., Rousset D., Matheus S. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill. 2014;19(13) doi: 10.2807/1560-7917.es2014.19.13.20759. [DOI] [PubMed] [Google Scholar]
- 4.Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A.C., Panning M. Infection with Chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 5.Sambri V., Cavrini F., Rossini G., Pierro A., Landini M.P. The 2007 epidemic outbreak of Chikungunya virus infection in the Romagna region of Italy: a new perspective for the possible diffusion of tropical diseases in temperate areas? New Microbiol. 2008;31:303–304. [PubMed] [Google Scholar]
- 6.Edelman R., Tacket C.O., Wasserman S.S., Bodison S.A., Perry J.G., Mangiafico J.A. Phase II safety and immunogenicity study of live Chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62(6):681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 7.Couderc T., Khandoudi N., Grandadam M., Visse C., Gangneux N., Bagot S. Prophylaxis and therapy for Chikungunya virus infections. J Infect Dis. 2009;200(4):516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kam Y.W., Lee W.W., Simarmata D., Harjanto S., Teng T.S., Tolou H. Longitudinal analysis of the human antibody response to Chikungunya virus infection: implications for sero-diagnosis assay and vaccine development. J Virol. 2012;86(23):13005–13015. doi: 10.1128/JVI.01780-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moro M.L., Grilli E., Corvetta A., Silvi G., Angelini R., Mascella F. Long-term Chikungunya infection clinical manifestation after an outbreak in Italy: a prognostic Cohort study. J Infect. 2012;65(2):165–172. doi: 10.1016/j.jinf.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama I., Uno K., Yumisashi T., Takasaki T., Lim C.K., Kurane I. A case of Chikungunya fever imported from India to Japan: follow-up of specific IgM and IgG antibodies over a 6-month period. Jpn J Infect Dis. 2010;63(1):65–66. [PubMed] [Google Scholar]


