Abstract
The incidence and significant morbidity of vertebral osteomyelitis are increasing despite the progress of diagnosis competences. Among the 50 cases of vertebral osteomyelitis managed in our centers over the past 5 years, 84% of the cases were in men. The mean age was 55 years. Sixty-two percent of patients had comorbidities and risk factors: diabetes mellitus (24%), malignancy (16%), intravenous drug use (10%) and alcoholism (4%). A source of infection was identified in 66% of cases, including postvertebral surgery infection (18%) and hematogenous infection (48%). The mean time to diagnosis was 36 days. Back pain were occurred in 90% of cases, fever (70%), neurologic deficits (40%), epidural abscesses (32%), completed vertebral bone destruction (26%) and psoas abscess (12%). A single organism was isolated in 92% of cases. Gram-positive bacteria were identified in 76% of cases, while Gram-negative bacilli (GNB) were found in 18% of cases. The presence of GNB was significantly associated with malignancy (p 0.041). The mean duration of antibiotic therapy was 123 days. Surgical treatment was performed in 41 cases: spinal stabilization (26%), drainage of abscesses (32%) and relief of compression (40%). Residual pain was found in 24% of cases, and neurologic sequelae in 22%. Cervical or thoracic localization was a risk factor for neurologic compromise (p 0.042). The epidemiology of vertebral osteomyelitis has changed; an increase in malignancy that was significantly associated with vertebral osteomyelitis due to GNB has been observed. Our study shows that the rate of neurologic complications remains high despite improved diagnostic capabilities and optimal treatment.
Keywords: Epidemiology of bacteria, human, malignancy, spondylodiscitis, vertebral osteomyelitis
Introduction
The incidence of vertebral osteomyelitis has increased in recent years, likely due to longer life expectancies, the increasing prevalence of chronic disease, improved diagnosis and more frequent use of indwelling intravascular catheters and immunosuppressive therapies [1]. Vertebral osteomyelitis is associated with significant morbidity, including prolonged need for antimicrobial therapy and decreased functional status [2].
Vertebral osteomyelitis is mainly caused by hematogenous seeding. Direct inoculation through iatrogenic procedures and contiguous spread from adjacent infection are rare [3]. In a systematic review, the source of infection was documented in 32% of the cases. However, details were presented for only 35% of the cases [4]. The urinary tract was the most frequently presumed source of infection in 17% of the cases [4].
The mortality rate of vertebral osteomyelitis is decreased, however neurologic compromise remains high. Approximately one-third of survivors have experienced residual disabilities that seriously affected their quality of life [5]. Despite improved recognition of the disease, management strategies vary widely across centers, particularly with regard to the choice and duration of antimicrobial treatment and the indication for surgical intervention [1]. Previous studies have reported that the residual back pain was more frequent in patient treated only by medical treatment (64%) than those treated by surgery (26%) [6].
We retrospectively reviewed the clinical, microbial features and outcome of vertebral osteomyelitis managed in an interregional referral center for bone and joint infections in the south of France.
Materials and methods
This study design was approved by the review board of our institute. We retrospectively reviewed the 50 cases of vertebral osteomyelitis with positive culture managed between January 2007 and December 2012 from among the 3778 patients with bone and joint infections (inpatients and outpatients >18 years old) managed in an interregional referral center for bone and joint infections in the south of France. This center is composed of two neurosurgery units, four orthopedic surgery units, two plastic surgery units and two infectious diseases units which located in University hospitals of Marseille.
The diagnosis of vertebral osteomyelitis was based on patients' medical history, including clinical evidence of infection based on biologic and/or radiologic data, with two or more positive blood cultures or two or more positive cultures from deep samples from surgical or percutaneous biopsy to exclude contaminating bacteria as previously described [7]. Three blood cultures, three deep samples from percutaneous biopsy and five deep samples from surgical biopsy on average per patient were taken.
After incubation, the bacteria species was identified through conventional phenotypic identification or matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Brüker Daltonik) as previously described [7]. A complete 16S rRNA gene sequencing and real-time PCR assays targeting Staphylococcus aureus performed on bacteria colony for the unknown bacteria identified through MALDI-TOF MS and on deep samples which were negative at culture, as previously described [7,8]. All the cases of vertebral osteomyelitis without bacterial documented by culture and biology molecular identification were excluded. We evaluated each patient's medical history, assessing factors such as demographic characteristics, comorbidities, risk factors associated with vertebral osteomyelitis and clinical characteristics. Routes of entry and dissemination for vertebral osteomyelitis were reviewed. We also recorded the location of infection and the presence of any orthopedic prostheses or devices. We reviewed the antimicrobial and/or surgical treatment approaches used in each case.
Data analyses were performed using IBM® SPSS® Statistics software v.20.0. Proportions were compared using two-tailed chi-squared tests. A value of p < 0.05 was considered statistically significant.
Results
Clinical characteristics
Among the 50 cases of vertebral osteomyelitis which represented 2.6% of 3778 patients with bone and joint infection managed in our centers overs 6-year study period, 42 (84%) were men, resulting in a male: female ratio of 5.25:1. The mean age of patients was 55 (±15 years, range 29–92). Thirty-one cases (62%) had comorbidities and risk factors such as diabetes mellitus in 12 cases (24%), solid cancer or hematologic malignancy in eight cases (16%), intravenous drug use (IDU) in five cases (10%) and alcoholism in two cases (4%) (Table 1). Eight cases of vertebral osteomyelitis co-occurred with malignancy, including three cases of head and neck cancer, two cases of hematologic malignancy, two cases of digestive cancer and one case of lung cancer.
Table 1.
Population characteristics
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 8 (16) |
| Male | 42 (84) |
| Comorbidity and risk factor | |
| Diabetes mellitus | 12 (24) |
| Solid cancer, hematologic malignancy | 8 (16) |
| Immunosuppressive therapy | 4 (8) |
| Intravenous drug users | 5 (10) |
| Alcoholism | 2 (4) |
| Source of infection | |
| Urinary tract infection | 15 (30) |
| Postvertebral surgery with infection of internal fixation or another artificial device | 9 (18) |
| Vascular catheter-related infection | 5 (10) |
| Respiratory tract infection | 4 (8) |
| Clinical presentation | |
| Pain | 45 (90) |
| Fever | 35 (70) |
| Neurologic deficits | 20 (40) |
| Epidural abscess | 16 (32) |
| Completed vertebral bone destruction | 13 (26) |
| Psoas abscess | 6 (12) |
| Endocarditis | 3 (6) |
| Septic cerebral embolism | 1 (2) |
| Pulmonary abscess | 1 (2) |
| Localization | |
| Cervical | 5 (10) |
| Thoracic | 13 (26) |
| Lumbar | 29 (58) |
| Multiple localization | 3 (6) |
Source of infection was identified in 33 cases (66%), including 15 cases (30%) of urinary tract infection, nine cases (18%) of after vertebral surgery with infection of internal fixation or another artificial device, five cases (10%) of vascular catheter-related infection and four cases (8%) of respiratory tract infection (Table 1).
The mean time from symptom onset to diagnosis of the 50 cases of vertebral osteomyelitis in our study was 36 days (±20 days, range 10–77 days). Forty-two cases (78%) were diagnosed within the first month of symptom onset. Back pain, which occurred in 45 cases (90%), was the most frequent symptom, followed by fever in 35 cases (70%), neurologic deficits in 20 cases (40%), epidural abscesses in 16 cases (32%), complete vertebral bone destruction in 13 cases (26%) and psoas abscess in six cases (12%). Concomitant infections, including endocarditis, pulmonary abscess and brain abscess, were observed in three cases, one case and one case, respectively. Among the 20 cases with neurologic deficits, spinal cord compression was observed in 11 cases (22%), caudal equine compression in five cases (10%) and isolated nerve root compressions in four cases (8%). We also recorded a case of femoral neuropathy and a case of paraplegia.
Vertebral osteomyelitis presented as a disease of a single region of the spine in 94% of cases. The lumbar region was affected in 29 cases (58%), followed by the thoracic region in 13 cases (26%) and cervical regions in five cases (10%). We observed three cases (6%) that affected multiple vertebra.
Among 50 cases of vertebral osteomyelitis, 45 cases (90%) had two or more positive vertebral biopsy findings, and 33 cases (66%) had two or more positive blood cultures. Twenty-eight cases (56%) had both two or more positive blood cultures and two or more positive vertebral biposy findings with the same microorganism. Five cases (10%) of vertebral osteomyelitis were diagnosed based on positive blood cultures alone. Among 45 positive vertebral biopsy findings, surgical biopsy was performed in 25 cases (56%), percutaneous biopsy in six cases (13%) and both in 14 cases (31%; Fig. 1).
Fig. 1.
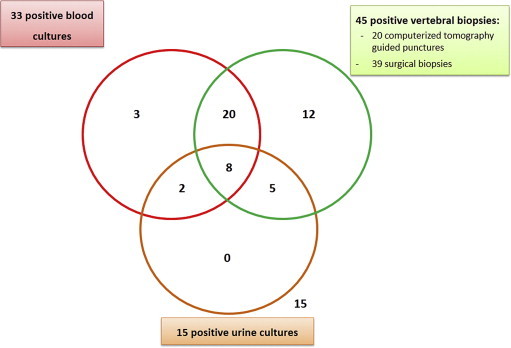
Diagnostic tools of vertebral osteomyelitis.
Microbiologic characteristics
A single organism was isolated from 37 cases (92%), while polymicrobial infection was reported in three cases (8%). Aerobic Gram-positive bacteria were found in 38 cases (76%), while aerobic Gram-negative bacilli (GNB) represented 18% of cases. Fifteen bacteria species have been identified and include Staphylococcus aureus (18 cases), S. epidermidis (10), S. hominis (1), Streptococcus agalactiae (5), S. mitis (1), S. sanguinis (1), Enterococcus faecalis (2), Klebsiella pneumoniae (5), K. oxytoca (1), Escherichia coli (2), Serratia marcescens (1), Pseudomonas aeruginosa (1), Peptoniphilus harei (1), Brevibacterium casei (1), Propionibacterium acnes (1) and Mycobacterium tuberculosis (3) (Table 2). All of 54 isolates, 11 (20%) were identified by conventional phenotypic identification and 43 (86%) were identified by MALDI-TOF MS. Two species (Brevibacterium casei and Peptoniphilus harei) were identified by MALDI-TOF MS and confirmed by molecular identification.
Table 2.
List of bacterial species identified in the 50 cases of vertebral osteomyelitis and the resistance to antibiotics
| Bacteria species | No. of isolates | Resistant to: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methicillin | Rifampicin | Clindamycin | Fusidic acid | Vancomycin | Quinolones | Cotrimoxazole | Aminoglycoside | Amoxicillin | Ceftriaxone | Imipenem | ||
| Staphylococcus aureus | 18 | 3 | 1 | 2 | 2 | 0 | 3 | 0 | 1 | — | — | — |
| Coagulase-negative staphylococci | 11 | 5 | 0 | 3 | 5 | 0 | 5 | 3 | 3 | — | — | — |
| Enterobacteriaceae | 9 | — | — | — | — | — | 4 | 2 | 1 | 6 | 2 | 0 |
| Klebsiella pneumoniae | 4 | — | — | — | — | — | 2 | 1 | 1 | 4 | 2 | 0 |
| Escherichia coli | 3 | — | — | — | — | — | 1 | 1 | 0 | 1 | 0 | 0 |
| Klebsiella oxytoca | 1 | — | — | — | — | — | 1 | 0 | 0 | 1 | 0 | 0 |
| Serratia marcescens | 1 | — | — | — | — | — | 0 | 0 | 0 | 0 | 0 | — |
| Pseudomonas aeruginosa | 1 | — | — | — | — | — | 0 | — | 0 | — | — | 0 |
| Streptococcus spp. | 7 | — | 1 | — | — | — | — | — | 1 | 1 | 0 | 0 |
| Streptococcus agalactiae | 5 | — | 1 | — | — | — | — | — | 1 | 0 | 0 | 0 |
| Streptococcus mitis | 1 | — | 0 | — | — | — | — | — | 0 | 1 | 0 | 0 |
| Streptococcus sanguinis | 1 | — | 0 | — | — | — | — | — | 0 | 0 | 0 | 0 |
| Enterococcus faecalis | 2 | — | 0 | — | — | — | — | — | 0 | 0 | — | 0 |
| Brevibacterium casei | 1 | — | 0 | — | — | 0 | 0 | — | 0 | 0 | 0 | 0 |
| Peptoniphilus harei | 1 | — | — | 0 | — | 0 | — | — | 0 | 0 | 0 | — |
| Propionibacterium acnes | 1 | — | 0 | 0 | — | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Mycobacterium tuberculosis | 3 | — | — | — | — | 0 | — | — | — | — | — | — |
Coagulase-negative staphylococci (CNS) was identified in 22% of our cases which was frequently occurred in postoperative implant-associated vertebral osteomyelitis than hematogenous native vertebral osteomyelitis (5/11 vs. 4/40, p 0.017). Six S. epidermidis isolates were identified in both blood culture and vertebral biopsy samples; one S. epidermidis isolate was isolated from blood culture; and one S. hominis was isolated from vertebral biopsy samples. All of the nine cases (18%) of vertebral osteomyelitis caused by aerobic GNB were occurred in the patients who had native vertebral osteomyelitis. Six of nine cases of aerobic GNB vertebral osteomyelitis were localized in lumbar compared to 25 of 41 cases of non-GNB vertebral osteomyelitis (p 1.000).
Of 18 S. aureus isolates, sensibility reduced to methicillin was found in three cases (17%), quinolones (three cases; 17%), rifampicin (one case, 6%), clindamycin (two cases, 11%), aminoglycoside (one case, 6%) and fusidic acid (two cases, 11%). No resistant to cotrimoxazole was found in S. aureus isolates (Table 2).
Among 11 cases of CNS isolates, methicillin resistant was found in five cases (45%), quinolones resistant (five cases; 45%), cotrimoxazole resistant (three cases, 27%), clindamycin resistant (three cases, 27%), aminoglycoside resistant (three cases, 27%) and fusidic acid resistant (five cases, 45%). No resistant to rifampicin was found in CNS isolates. No relevant of antimicrobial resistant found among streptococci isolates (Table 2).
Among nine isolates of Enterobacteriaceae, sensibility reduced to ceftriaxone were found in two cases (22%), aminoglycoside (one case, 11%), quinolones (four cases, 44%) and cotrimoxazole (two cases, 22%) (Table 2).
Medical and surgical treatment
The antibiotics that were most frequently used in our series were rifampicin, quinolones, amoxicillin and ceftriaxone, which were used in 25 cases (50%), 24 cases (48%), 12 cases (24%) and five cases (10%), respectively. Combination of antibiotic treatment was recorded in 41 cases (82%) and typically involved two antibiotics (37 cases, 74%). The combination of rifampicin and quinolones was used in 14 cases (28%). The mean overall duration of antibiotic treatment was 123 days (±44 days, range 90–183). Nine cases (18%) were administered only medical treatment without require surgery treatment. Forty-one cases (82%) were treated by surgical treatment included spinal stabilization in 13 cases (26%), drainage of abscesses in 16 cases (32%) and relief of compression in 20 cases (40%). Out of the 50 cases of vertebral osteomyelitis, the three cases of vertebral osteomyelitis caused by M. tuberculosis were treated by 9 months of antituberculosis drugs.
Clinical outcome
In 45 of 50 cases (90%), patients received complete follow-up until the end of treatment. The mean duration of follow-up for these cases was 36 months (±20 months; range 9–77). Four patients (8.5%) died; death was due to malignancy in three cases and to septic shock in one case. Four cases were lost during the follow-up treatment period. Thirty-six cases (78%) were discharged without any neurologic sequelae included eight cases in the group of medical treatment only and 28 cases in the surgery treatment group. Residual pain was observed in 11 cases (24%). Neurologic sequelae were observed in 10 cases (22%), including impaired mobility in nine cases (20%) and motor weakness in one case (2%). One case of neurologic sequelae was in the group of medical treatment only; and nine cases were in the surgery treatment group. The presence of neurologic sequelae was not significantly different between medical treatment groups and surgical treatment groups (p 0.659).
A recurrence of the symptoms of infection after apparent resolution developed after discharge in two patients (4%); thus, two relapses occurred. The intervals between the initial illness and relapse were 1 and 12 months. Relapse was confirmed in the two affected patients by positive cultures of samples obtained from bone, abscesses and blood. S. epidermidis strains isolated from both patients during the first episode were also recovered during relapse. In one patient, K. pneumoniae was isolated concomitantly from the infected intervertebral disk space. This patient exhibited severe destructive changes and deformities of the vertebrae that might have suppressed healing and facilitated the persistence of microorganisms, despite antibiotic treatment.
The mean time between the onset of symptoms and diagnosis was significantly longer for cases with neurologic sequelae (n = 10) than for cases that went into remission without neurologic sequelae (n = 35), i.e. 46 days (±23 days, range 14–76 days, 95% confidence interval 30–62) vs. 32 days (±19 days, range 10–77 days, 95% confidence interval 25–38), respectively (p 0.049).
The subgroup of 20 cases (40%) of neurologic deficits comprised five cases (25%) of S. aureus, four cases (25%) of S. epidermidis, two cases (10%) of streptococci and eight cases (40%) of Gram-negative bacillus infections. Vertebral osteomyelitis associated with GNB was also significantly associated with malignancy (p 0.041), including two cases of hematologic malignancy, one case of head and neck cancer, one case of digestive cancer and one case of lung cancer.
The frequency of motor dysfunction was highest among patients with infections of the lumbar spine (40% of cases), followed by infections of the thoracic (35%) and cervical spine (15%). In addition, spinal cord compression was significantly more frequent in vertebral osteomyelitis of the cervical and thoracic spine than in that of the lumbar spine (p 0.049).
Discussion
Herein, we presented a series of 50 cases of microbiologically proven vertebral osteomyelitis gathered over a 6-year period for which a long-term outcome was determined. The median time to diagnosis in the present study was 30 days, which is shorter than the time to diagnosis observed in a previous study (6 weeks) [3]. This phenomenon likely results from the fact that magnetic resonance imaging (MRI) is now more easily accessible. Almost all affected persons were asymptomatic for several days before diagnosis [9–12]. The clinical picture of vertebral osteomyelitis was often nonspecific, and for this reason, imaging has become an essential component of the diagnostic process.
Surgical biopsy was relevant to the microbiologic diagnosis of vertebral osteomyelitis in from 58% to 79% of cases [4]. A recent study reported that second percutaneous biopsies allow microbiologic diagnosis in 80% of cases [13]. In our study, microbiologic diagnosis of vertebral osteomyelitis was accomplished by surgical biopsy in 39 cases (78%), and positive cultures of computerized tomography-guided punctures preceded surgical treatment in 14 of 20 cases (70%). Eighty-two percent of our patients needed surgical treatment, such as spinal stabilization (26%), drainage of abscesses (32%) and relief of compression (40%); a microbiologic diagnosis is made in 90% of patients using surgical biopsy tissue because of this high rate of surgical intervention.
Sixty-six cases of vertebral osteomyelitis have positive blood culture in our study, which was comparable to the rate observed in previous studies [4,14], but lower than that of the biopsy samples. However, in our series, blood cultures taken before biopsy were used to diagnose five cases of vertebral osteomyelitis that had negative vertebral biopsy findings. Another benefit of positive blood cultures was that they encouraged us to look for potential complications, such as infective endocarditis, pulmonary abscess and septic cerebral embolism related to vertebral osteomyelitis in our series.
S. aureus remains the predominant causative microorganism (36%) in our series; this finding was consistent with the results of previous studies (35 to 75%) [15–18]. Thus, coagulase-negative staphylococci have been recorded in 22% of our patients, which is a higher rate than that observed previous studies (3 to 15%) [15–17,19], likely reflecting the high proportion of hospital-acquired infections included in our series: 38% of our patients presented with hospital-acquired infections (postvertebral surgery infections and vascular catheter-related infections). A similarly high proportion of hospital-acquired infections vertebral osteomyelitis has been previously reported [20].
K. pneumoniae was the predominant etiologic agent of infections with GNB in our series, in contrast with previous studies [4,21]. We have also observed an increase in Streptococci (14%) and anaerobic bacteria that may be indicative of epidemiologic change.
Forty-eight percent of vertebral osteomyelitis resulted from hematogenous dissemination from a remote site of infection, as was observed with vascular catheter-related infections (10%), respiratory tract infections (8%) and urinary tract infections (20%). Microbes can spread from the urinary system and seed the vertebrae, leading to the development of osteomyelitis via Batson's plexus [22].
Previous studies have suggested that the factors affecting the occurrence of vertebral osteomyelitis include prior infection, diabetes mellitus, rheumatic disease, immunodeficiency, IDU, alcoholism, and vertebral compression due to malignant metastasis [3]. The underlying diseases associated with vertebral osteomyelitis in this study were similar to those previously reported, with diabetes mellitus found in 24% of patients, followed by cancer (16%), IDU (10%) and corticosteroid treatment (7%) [15,19,21,23–25].
Among the well-known comorbidity and risk factors for vertebral osteomyelitis, we observed a significant association between malignancy and infection with GNB. This observation has not been reported in previous studies [1]. No association was found between bacterial species and clinical outcome.
Intravenous antibiotics were the most prescribed antibiotics in some studies [21,24,26]. The high bioavailability and adequate diffusion of rifampicin, quinolone, clindamycin, and fusidic acid have allowed oral administration early in the course of treatment [21,27]. Oral treatments with rifampicin, quinolones and amoxicillin were used in 25 (50%), 24 (48%) and 12 (24%) cases in our series, respectively. Twenty-eight percent of the cases in our series were treated with a combination of rifampicin and quinolones.
Conclusions
The epidemiology of vertebral osteomyelitis may be changing, with an increase in malignancy that is significantly associated with GNB in vertebral osteomyelitis. In our study, the rate of neurologic complications remains high despite improved diagnosis and optimal treatment. Vertebral osteomyelitis requires a multidisciplinary approach to improve the prognosis.
Conflict of interest
None declared.
Acknowledgements
The authors thank C. Leautier and C. Peruffo for technical support in data extraction.
References
- 1.Akiyama T., Chikuda H., Yasunaga H. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2012-002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasbarrini A., Boriani L., Salvadori C. Biopsy for suspected spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl. 2):26–34. [PubMed] [Google Scholar]
- 3.McHenry M.C., Easley K.A., Locker G.A. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 4.Mylona E., Samarkos M., Kakalou E., Fanourgiakis P., Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Cottle L., Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Hadjipavlou A.G., Mader J.T., Necessary J.T., Muffoletto A.J. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Seng P., Abat C., Rolain J.M. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2013;51:2182–2194. doi: 10.1128/JCM.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy P.Y., Fournier P.E., Fenollar F., Raoult D. Systematic PCR detection in culture-negative osteoarticular infections. Am J Med. 2013;126 doi: 10.1016/j.amjmed.2013.04.027. 1143.e25–e33. [DOI] [PubMed] [Google Scholar]
- 9.Friedman J.A., Maher C.O., Quast L.M., McClelland R.L., Ebersold M.J. Spontaneous disc space infections in adults. Surg Neurol. 2002;57:81–86. doi: 10.1016/s0090-3019(01)00681-4. [DOI] [PubMed] [Google Scholar]
- 10.Thueler A., Weber M., Ziehmann M., Gubler J. Common backache of unusual etiology. Prax Bern. 1994;2002(91):1219–1221. doi: 10.1024/0369-8394.91.29.1219. [DOI] [PubMed] [Google Scholar]
- 11.Fica A., Bozan F., Aristegui M., Bustos P. Spondylodiscitis. Analysis of 25 cases. Rev Med Chil. 2003;131:473–482. [PubMed] [Google Scholar]
- 12.Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 13.Gras G., Buzele R., Parienti J.J. Microbiological diagnosis of vertebral osteomyelitis: relevance of second percutaneous biopsy following initial negative biopsy and limited yield of post-biopsy blood cultures. Eur J Clin Microbiol Infect Dis. 2014;33:371–375. doi: 10.1007/s10096-013-1965-y. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Mejias M.E., de Dios Colmenero J., Sanchez-Lora F.J. Postoperative spondylodiskitis: etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29:339–345. doi: 10.1086/520212. [DOI] [PubMed] [Google Scholar]
- 15.Osenbach R.K., Hitchon P.W., Menezes A.H. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33:266–275. doi: 10.1016/0090-3019(90)90047-s. [DOI] [PubMed] [Google Scholar]
- 16.Sapico F.L., Montgomerie J.Z. Vertebral osteomyelitis. Infect Clin North Am. 1990;4:539–550. [PubMed] [Google Scholar]
- 17.Sapico F.L., Liquete J.A., Sarma R.J. Bone and joint infections in patients with infective endocarditis: review of a 4-year experience. Clin Infect Dis. 1996;22:783–787. doi: 10.1093/clinids/22.5.783. [DOI] [PubMed] [Google Scholar]
- 18.Chelsom J., Solberg C.O. Vertebral osteomyelitis at a Norwegian university hospital, 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30:147–151. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 19.Jensen A.G., Espersen F., Skinhoj P., Frimodt-Moller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158:509–517. doi: 10.1001/archinte.158.5.509. [DOI] [PubMed] [Google Scholar]
- 20.Roblot F., Besnier J.M., Juhel L. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum. 2007;36:269–277. doi: 10.1016/j.semarthrit.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Legrand E., Flipo R.M., Guggenbuhl P. Management of nontuberculous infectious discitis: treatments used in 110 patients admitted to 12 teaching hospitals in France. Jt Bone Spine. 2001;68:504–509. doi: 10.1016/s1297-319x(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee C.H., Su L.H., Lin W.C., Tang Y.F., Liu J.W. Refractory vertebral osteomyelitis due to CTX-M-14-producing Escherichia coli at ertapenem treatment in a patient with a coexisting urinary tract infection caused by the same pathogen. Int J Infect Dis. 2010;14(Suppl. 3):e183–e186. doi: 10.1016/j.ijid.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Bontoux D., Codello L., Debiais F. Infectious spondylodiscitis. Analysis of a series of 105 cases. Rev Rhum Mal Osteoartic. 1992;59:401–407. [PubMed] [Google Scholar]
- 24.Nolla J.M., Ariza J., Gomez-Vaquero C. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum. 2002;31:271–278. doi: 10.1053/sarh.2002.29492. [DOI] [PubMed] [Google Scholar]
- 25.Kowalski T.J., Berbari E.F., Huddleston P.M., Steckelberg J.M., Osmon D.R. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clin Infect Dis. 2006;43:172–179. doi: 10.1086/505118. [DOI] [PubMed] [Google Scholar]
- 26.Torda A.J., Gottlieb T., Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis. 1995;20:320–328. doi: 10.1093/clinids/20.2.320. [DOI] [PubMed] [Google Scholar]
- 27.Darley E.S., MacGowan A.P. Antibiotic treatment of Gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53:928–935. doi: 10.1093/jac/dkh191. [DOI] [PubMed] [Google Scholar]


