Abstract
Delivery of orally compromised therapeutic drug molecules to the systemic circulation via buccal route has gained a significant interest in recent past. Bioadhesive polymers play a major role in designing such buccal dosage forms, as they help in adhesion of designed delivery system to mucosal membrane and also prolong release of drug from delivery system. In the present study, HPMC (release retarding polymer) and mannitol (diluent and pore former) were used to prepare bioadhesive and controlled release buccal discs of buspirone hydrochloride (BS) by direct compression method. Compatibility of BS with various excipients used during the study was assessed using DSC and FTIR techniques. Effect of mannitol and HPMC on drug release and bioadhesive strength was studied using a 32 factorial design. The drug release rate from delivery system decreased with increasing levels of HPMC in formulations. However, bioadhesive strength of formulations increased with increasing proportion of HPMC in buccal discs. Increased levels of mannitol resulted in faster rate of drug release and rapid in vitro uptake of water due to the formation of channels in the matrix. Pharmacokinetic studies of designed bioadhesive buccal discs in rabbits demonstrated a 10-fold increase in bioavailability in comparison with oral bioavailability of buspirone reported.
Keywords: Buspirone hydrochloride, Buccal, HPMC, Mannitol, Bioadhesion
1. Introduction
Buccal route along with other transmucosal routes (such as nasal, pulmonary, rectal) has long been advocated as possible route of drug administration for systemic delivery of drugs with poor and/or erratic bioavailability. Buccal route has many advantages over other transmucosal route such as ease of application and removal (Harris and Robinson, 1992). Moreover, buccal route helps in avoidance of first pass metabolism, erratic oral absorption of drug due to food interactions and at the same time limits the exposure of drugs to harsh gastric environment. The relative immobility of buccal mucosa as compared to that of other transmucosal routes makes this site ideal for sustained delivery of drugs. Thus, adhesive dosage forms such as tablets (Choi and Kim, 2000), discs (Jaipal et al., 2013, Yehia et al., 2008), gels (Morishita et al., 2001), sprays (Pozzilli et al., 2005), solutions (Muchohi et al., 2008), patches or films (Giovino et al., 2012, Kianfar et al., 2013) have been reported for buccal delivery of drugs. Solid unit dosage forms (such as discs and tablets) are most preferred for buccal delivery as they supply the dose accurately when compared to other dosage forms listed above (Abruzzo et al., 2012). Moreover, buccal discs are flat, thin solid unit non-flexible compacts; and are similar to buccal tablets. Buccal discs are designed to minimize the discomfort caused due to bulky tablet buccal dosage forms (Jaipal et al., 2014). Solid dosage forms are easier to prepare and the process can easily be scaled up for large scale manufacturing.
Polymers with bioadhesive and drug release retarding properties have widely been reported in literature for effective delivery of orally compromised drugs via buccal route. These polymers help the delivery system adhere to buccal mucosa and at the same time retard the release of drug (Abruzzo et al., 2012, Andrews et al., 2009, Boyapally et al., 2010, Kianfar et al., 2013, Martin et al., 2003, Rossi et al., 2005, Salamat-Miller et al., 2005).
Hydroxy propyl methyl cellulose (HPMC) is a semi synthetic and inert bioadhesive polymer. HPMC is GRAS (generally recognized as safe) listed ingredient and included in FDA’s Inactive Ingredient database used in manufacturing of variety of dosage forms available commercially. HPMC is available in a wide range of viscosity grades (3–100,000 mPa) (Nafee et al., 2004). Biocompatible and biodegradable nature of HPMC along with bioadhesive and release rate retarding properties of HPMC make it a suitable excipient for designing buccal discs (Mumtaz and Ch’ng, 1995, Narendra et al., 2005, Taylan et al., 1996).
Mannitol is a white crystalline polyol synthesized industrially by catalytic hydrogenation of fructose or glucose syrup. Mannitol is water soluble, non toxic and non hygroscopic ingredient extensively used in food and pharmaceutical preparations (Debord et al., 1987). Mannitol is GRAS (generally recognized as safe) listed ingredient and is used in many dosage forms available commercially. It has been reported that mannitol enhances dissolution of drug from the dosage form as it forms pores within the dosage form matrix (Holgado et al., 1995).
Buspirone hydrochloride (BS), is an anxiolytic agent and a serotonin receptor partial agonist belonging to the azaspirodecanediones class of compounds (Blier and Ward, 2003, Sramek et al., 2002). BS does not possess anticonvulsant or muscle relaxant properties and also does not cause sedation or physical dependence like other anxiolytics (Riblet et al., 1983, Taylor et al., 1985). After oral administration, BS is rapidly and erratically absorbed in humans and undergoes extensive first pass metabolism resulting is very low oral bioavailability of approximately 4% (Mahmood and Sahajwalla, 1999). Following oral administration, plasma concentrations of unchanged BS are very low and with a very high inter and intra subject variability (Lilja et al., 1998, Ratey et al., 1989, Realmuto et al., 1989). Peak plasma concentration of BS varies in the presence of food (Lilja et al., 1998, Mahmood and Sahajwalla, 1999). These pharmacokinetic properties make BS a suitable candidate for delivery via buccal route.
The objective of the present investigation was to develop and evaluate buccal bioadhesive discs of BS for improvement of bioavailability. Buccal disc were formulated using HPMC as bioadhesive and rate controlling polymer and mannitol as a pore former. Effect of polymer amount and amount of mannitol on drug release behavior, drug release rate and bioadhesive properties of designed buccal discs was assessed. This effect of HPMC and mannitol was studied statistically using 32 full factorial design. Bioadhesive buccal discs of BS using xanthan gum (Jaipal et al., 2013) and HPMC with effervescent agents (Jaipal et al., 2014) were previously reported by our group. In the present research effect of HPMC and mannitol on drug release and bioadhesion behavior is reported. To the extent of our knowledge, the effect of HPMC and mannitol on drug release and bioadhesive behavior have not been reported for the drug BS using factorial design method. Moreover, in vivo pharmacokinetic parameters for the designed buccal discs are also reported in comparison with i.v bolus.
2. Materials and methods
Buspirone hydrochloride (BS) was provided as a gift sample by Astron Research Limited, India. Hydroxy propyl methyl cellulose (HPMC 15K) was provided as gift sample by Signet Chemical Corporation, India. Mannitol was purchased from Sigma–Aldrich Corporation. Cellulose microcrystalline (Avicel PH101) was purchased from FMC BioPolymer, India. HPLC grade acetonitrile was purchased from Merck, India and deionized water used for in vitro and analytical studies were obtained using a Millipore water purification system (Milli-Q). Nylon membrane filters of pore diameter 0.22 μm were purchased from Millipore India. Other reagents and chemicals used were of analytical grade. Solid phase extraction cartridges (HyperSep Retain PEP 30 mg, 1 mL capacity) were purchased from Thermo Scientific India Limited, India.
2.1. Instrumentation and chromatographic conditions
In-vitro sample analysis was performed using validated liquid chromatographic method. The liquid chromatographic system employed was Shimadzu HPLC (Shimadzu, Japan) with solvent delivery system of two pumps (Model LC 10AT VP), auto injector (Model SIL HT A) and UV–VIS detector (Model SPD 10A VP). Data collection and integration was accomplished using LC Solutions software. Column oven (Model CTO-10AS) was used to maintain column temperature at 25 °C. Chromatographic separation was performed using a C8 column (LiChroCART®; 250 mm × 4.6 mm ID, 5 μm) maintained at 25 °C using mobile phase flow rate of 1 mL/min. Drug was monitored at 238 nm with an injection volume of 50 μL. Analysis was performed in triplicate for all the samples. The mobile phase constituted of acetonitrile and 0.025 M potassium phosphate buffer pH 3.0 (25:75 v/v). The buffer pH was adjusted with orthophosphoric acid (0.1 M). The buffer solution was passed through 0.22 μm filters prior to use.
Liquid chromatographic method used for estimation of BS in rabbit plasma employed same chromatographic conditions as discussed above. Sumatriptan was used as an internal standard (IS) while estimating the drug content in in vivo samples. BS and IS in rabbit plasma were detected at 9.19 and 13.00 min of retention time respectively with tailing factor less than 1.36 ± 0.03.
2.2. Drug excipient compatibility studies
Compatibility of BS with various excipients was assessed using Differential Scanning Calorimetry (DSC, Shimadzu, Japan) and Fourier Transform Infra Red (FTIR) Spectroscopy (Shimadzu-Prestige 21, Japan). DSC thermograms and FTIR spectra of pure drug, pure excipients and 1:1 mixture of drug and excipient were compared to assess compatibility. For DSC study, sample (2–4 mg) was weighed accurately in aluminum pans and thermogram was acquired over a temperature range of 30–300 °C with a heating rate of 10 °C per minute in a nitrogen gas environment. FTIR spectroscopic studies were carried out by appropriately diluting the sample with dried potassium bromide and acquiring infra red (IR) spectrum in the range of 400–4000 cm−1 with 2 cm−1 resolution.
2.3. Preparation of buccal discs
Buccal discs containing 10 mg of BS were prepared by direct compression method. All the ingredients were passed through sieve (#18) and mixed geometrically; finally magnesium stearate (2%) was added as lubricant (Table 1). The resulting mix was compressed using a 10 station compression machine (Rimek Mini Press, India) equipped with 5 mm punches. Composition of all the designed buccal discs is presented in Table 1. Weight of the designed discs was kept constant for all the batches by adjusting final weight with cellulose microcrystalline, a directly compressible diluent having negligible effect on drug release and bioadhesive behavior over wide range of concentrations (Badhan et al., 2009).
Table 1.
Composition, drug content, thickness, friability and surface pH of the designed buccal discs.
| B. noa | HPMC 15 K (mg) | Mannitol (mg) | MCC (mg) | Drug content (%) | Thickness (mm) | Friability (%) | Surface pH |
|---|---|---|---|---|---|---|---|
| F1 | 15.00 | 22.50 | 26.00 | 100.40 | 2.511 ± 0.010 | 0.27 | 6.86 ± 0.15 |
| F2 | 15.00 | 30.00 | 18.50 | 98.06 | 2.496 ± 0.006 | 0.53 | 6.91 ± 0.20 |
| F3 | 15.00 | 37.50 | 11.00 | 100.14 | 2.501 ± 0.017 | 0.13 | 7.01 ± 0.15 |
| F4 | 18.75 | 22.50 | 22.25 | 98.80 | 2.395 ± 0.021 | 0.60 | 6.86 ± 0.10 |
| F5 | 18.75 | 30.00 | 14.75 | 100.06 | 2.467 ± 0.015 | 0.33 | 7.03 ± 0.11 |
| F6 | 18.75 | 37.50 | 7.25 | 99.67 | 2.505 ± 0.010 | 0.73 | 6.59 ± 0.24 |
| F7 | 22.50 | 22.50 | 18.50 | 101.23 | 2.506 ± 0.011 | 0.27 | 7.16 ± 0.23 |
| F8 | 22.50 | 30.00 | 11.00 | 98.80 | 2.516 ± 0.006 | 0.27 | 6.94 ± 0.16 |
| F9 | 22.50 | 37.50 | 3.50 | 101.81 | 2.488 ± 0.020 | 0.40 | 7.02 ± 0.23 |
All the batches contain 10 mg BS and 1.50 mg of magnesium stearate.
A 32 full factorial design was employed to study the effect of varying amount of HPMC and mannitol on drug release and bioadhesive behavior of buccal discs of BS. Amount of HPMC was varied at three levels viz. 15.00 mg, 18.75 mg and 22.50 mg. Similarly amount of mannitol was varied at three levels viz. 22.50 mg, 30.00 mg and 37.50 mg (Table 2). The amount of HPMC 15 K (Factor: X1) and mannitol (Factor: X2) was selected as independent variable and the dependent response variables measured were percent drug release at 5 h (Q5 in percent), time required to release 50% of drug (T50 in h) and bioadhesive strength (FN in Newton). The effect of formulation variables on the response variables was studied and statistically analyzed by applying one-way ANOVA at 0.05 level using Design-Expert® version 8.0.7.1 (Stat-Ease Inc.). The data obtained were fitted into quadratic model and were supported by 3D surface response plots to illustrate the effect of HPMC and Mannitol on dependent variables. Experiments were conducted in random sequence with levels of factors coded. In all the designed batches, discs weight (75 mg) was kept constant by adding appropriate amount of microcrystalline cellulose.
Table 2.
Factors selected for 32 full factorial design along with levels and amounts of respective factors.
| Factors | Levels (mg) |
||
|---|---|---|---|
| −1 | 0 | +1 | |
| X1: HPMC K15 | 15.00 | 18.75 | 22.50 |
| X2: mannitol | 22.50 | 30.00 | 37.50 |
To describe the response surface curvature, the design was evaluated by quadratic model given by polynomial equation (Eq. (1)).
| (1) |
where ‘Y’ is the dependent variable, B0 is arithmetic mean response of 9 batches and B1 is the estimated coefficient of factor X1. X1 and X2 are the main effects corresponding to the average effect of one factor at a time from low to high value (Narendra et al., 2005). The interaction terms (X1X2) show how response changes when 2 factors are changed simultaneously. The polynomial equations can be used to draw conclusions after considering the magnitude of coefficient and the mathematical sign it carries (i.e., positive or negative).
2.4. Physical characterization of buccal discs
2.4.1. Weight variation
From each batch randomly selected buccal discs (20) were evaluated for weight variation using an electronic digital weighing balance (Mettler Toledo TA 250D). Mean weight of the buccal discs and percentage deviation was calculated.
2.4.2. Thickness
Thickness of buccal discs (10) was measured in millimeter using Vernier Caliper (Mitutoyo Digimatic Caliper, Japan). The mean thickness of buccal discs and percent standard deviation was calculated.
2.4.3. Friability
Friability analysis of the designed discs was assessed using friability test apparatus (Campbell Electronics, India) for 20 discs using standard procedure (25 rpm for 4 min). The percentage loss on friability (F) was calculated.
2.4.4. Surface pH
Acidic or alkaline pH of the dosage form could cause uneasiness in the oral cavity and may lead to irritation. Surface pH for all the batches was determined in triplicate using a digital pH meter (Eutech Instruments, Singapore). The buccal discs were hydrated in 100 mL distilled water for 20 min. The electrode upon contact with the hydrated buccal discs surface was allowed to equilibrate for 1 min and the pH was recorded immediately. All the readings were recorded in triplicates.
2.4.5. Drug content
Randomly selected buccal discs (10) were crushed using mortar and pestle. Powder equivalent to 2 mg of BS was weighed accurately and BS was extracted using 10 mL of solvent system (acetonitrile and Phosphate buffer pH 3.0; 1:3 v/v). The resultant mix was centrifuged at 3000 rpm (Remi-R8C laboratory centrifuge) and 1 mL of supernatant was collected and suitably diluted for the determination of drug content by liquid chromatographic method mentioned in the previous section.
2.4.6. In-vitro water uptake studies
Water uptake studies of the designed buccal discs were performed on 2% aqueous agar plates maintained at 37 °C and 40% RH in a humidity chamber. The percent water uptake was calculated at different time points. All the readings were recorded in triplicate for each time point. Percent water uptake was calculated for all the batches using Eq. (1).
| (1) |
2.4.7. In-vitro bioadhesion studies
Bioadhesion studies of designed formulations were carried out using texture analyzer (Stable Micro Systems TA-XT Plus). Freshly excised porcine buccal mucosa was obtained from the local slaughterhouse. The tissue was placed in simulated salivary fluid and stored at −20 °C till further usage. The thawed mucosal membrane was fixed at the base of instrument using a Teflon hollow disc and screws in temperature controlled bath containing simulated salivary fluid with pH 6.75 as described elsewhere (Gohel et al., 2009). Designed buccal disc was attached to the base of the texture analyzer movable probe (SMSP/10) using a double sided adhesive tape. The probe was lowered at a speed of 0.5 mm/s. Upon contact of buccal disc with mucosal membrane a contact force of 0.01 N was applied for 300 s. The probe was then dragged in opposite direction and force required to detach the buccal disc from the mucosal surface was recorded.
2.4.8. In-vitro drug release studies
Previously reported in-house modified USP type-1 dissolution apparatus (Charde et al., 2008) was used to study the drug release rate from designed buccal bioadhesive discs. Briefly, the method uses 50 mL capacity screw cap tubes arranged concentric to the shaft of dissolution apparatus using in-house designed aluminum holder. Single unit of buccal disc was loaded in the basket attached to the instrument shaft and allowed to immerse in the prearranged tubes containing 50 mL pH 6.8 phosphate buffer maintained at 37 °C. The instrument was operated at 25 rpm and samples were collected at various time intervals of 0.5, 1, 2, 3, 4 and 5 h. The samples collected were analyzed for drug content using liquid chromatographic method described previously. All the drug release studies were performed in triplicates.
2.4.9. Drug release kinetics
Drug release rate from hydrophilic matrix systems depends on swelling behavior of the polymer, shape of the matrices, and diffusion and erosion properties of the polymer and dissolution characteristics of the drug. Dose and solubility of the drug, type and quantity of the fillers and the polymer characteristics influence the mechanism of the drug release (Baveja et al., 1988, Korsmeyer et al., 1983a, Korsmeyer et al., 1983b). Data obtained from the drug release studies were fitted into zero order, first order, Higuchi and Korsmeyer–Peppas models to predict the drug release kinetics and mechanism from the designed HPMC buccal discs (Gurny et al., 1982, Korsmeyer et al., 1983b, Peppas and Sahlin, 1996).
2.4.10. In-vivo pharmacokinetic study
The efficiency of HPMC buccal discs to deliver BS into systemic blood circulation was evaluated in rabbits. Male New Zealand white rabbits were provided by Central Animal Facility of the Institute with mean weight of 1.65 ± 0.15 kg. The study was conducted with an approval (Protocol approval number: IAEC/RES/16/04) and as per guidelines prescribed by Institutional Animal Ethics Committee, and under the supervision of registered veterinarian. Animals were issued 10 days prior to experimentation for acclimatization and were kept on standard pellet diet and water ab libitum. Animals were fasted 4–6 h prior to experimentation.
Rabbits were divided into two groups of three rabbits each. To the first group 1 mL of BS solution in sterile water (10 mg/mL) was administered intravenously. The second group was administered designed HPMC buccal bioadhesive controlled release discs of BS (Batch No.: F3) in the buccal position of oral cavity after light anesthetization of animals by an i.m injection of 1:5 mixtures of xylazine (1.5 mg/kg) and ketamine (9.0 mg/kg). Following the induction of anesthesia, blood samples (1.0 mL) were withdrawn from marginal ear vein of rabbits. Samples were withdrawn before dosing and 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 12.0, 18.0, 24.0 h post dosing. The light plane of anesthesia was maintained by an intramuscular (i.m) injection of one third of initial dose of xylazine and ketamine as needed. Blood samples were collected in 1.5 mL centrifuge tubes containing 100 μL of EDTA solution (1.0 mg/mL) and centrifuged at 4000 rpm for 4 min at 4 °C (Eppendorf centrifuge-5702R). The supernatant obtained was collected and stored at −20 °C till further processing for analysis.
2.4.11. In-vivo plasma sample processing and estimation of drug content
Stored plasma samples were thawed at ambient temperature (25 ± 2 °C) for at least 60 min. Before loading the sample in SPE cartridge, cartridge was conditioned (1 mL methanol, 2500 rpm) and equilibrated (25 mM potassium dihydrogen orthophosphate, 2500 rpm). Sample was loaded into cartridge and centrifuged at 2000 rpm to remove interfering components and matrix, washing step was performed using 3% v/v methanol (1 mL, 2500 rpm) to remove the remaining interfering components and finally elution was carried out using 0.025 M phosphate buffer pH 3.0 and acetonitrile (75:25, 1 mL, 3000 rpm) solvent system. The separation was performed using a centrifuge (Remi, India) and the elutions were collected in 5 mL disposable tubes.
The processed plasma samples were estimated for drug content using liquid chromatographic method mentioned earlier. The plasma concentration vs. time data of BS obtained during various sets of studies was subjected to non-compartmental analysis using WinNonlin Standard edition, Version 2.1 (WinNonlin Scientific Consultants, USA) to obtain various pharmacokinetic parameters. Absolute bioavailability of designed formulations was calculated using Eq. (2).
| (2) |
3. Results and discussion
3.1. Drug excipient compatibility
DSC thermogram of pure drug showed two endothermic (189.89 °C and 204.43 °C) and one exothermic (192.18 °C) peaks (Fig. 1). These values were close to values reported in the literature (Sheikhzadeh et al., 2007). The endothermic peak at 189.89 °C was due to melting of pure drug sample. The exothermic peak at 192.18 °C was due to conversion of polymorphic form-1 of the drug to polymorphic form-2. The last endothermic peak at 204.43 °C was due to melting of recrystallized form-2 of the drug. In the physical mixtures of BS with HPMC and BS with cellulose microcrystalline (1:1), all the three peaks were well retained (Fig. 1) indicating compatibility.
Figure 1.
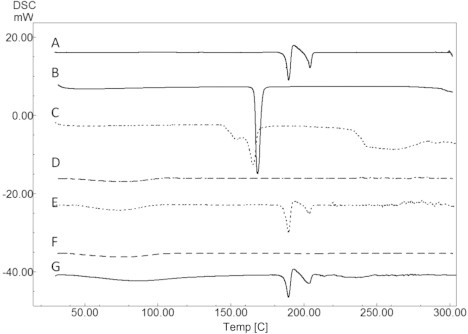
DSC thermogram obtained for pure drug BS (A), mannitol (B), BS and mannitol in 1:1 ratio (C), HPMC 15K (D), BS and HPMC 15K in 1:1 ratio (E), MCC (F) and BS with MCC in 1:1 ratio (G).
In DSC thermogram of pure mannitol, a sharp endothermic peak was observed at 168.4 °C very near to that of the drug. In physical mixture of drug and mannitol, a single, wide endothermic peak was observed which can be attributed to both BS and mannitol (Fig. 1). Thus, DSC being a thermal method of analysis should not be used singly to detect any inherent incompatibility. It has to be supported sufficiently by other non-thermal techniques such as FTIR or chromatographic techniques. To further understand the compatibility of BS with process excipients, FTIR studies were performed. FTIR spectroscopic analysis of pure drug revealed peaks specific to functional groups of BS. IR bands were obtained at 2949 cm−1 for aromatic C—H stretching of pyrimidine, 1724 cm−1 for C O stretching. Moreover, bands were also obtained at 1454 cm−1 for CH2 bending of piperazine, 1338 cm−1and 1165 cm−1 for C C bending and 980 cm−1 and 802 cm−1 for aromatic C—H bending. All the characteristic peaks of BS were retained in 1:1 physical mixtures of BS with mannitol (Fig. 2) and other excipients used, indicating compatibility. HPLC analysis of BS in the presence of mannitol did not alter peak characteristics and no degradation peaks were observed; peak area was also well retained with respect to analysis of pure BS. Thus, FTIR and HPLC studies together established the absence of interaction between BS and mannitol selected for this study. Overall, the results obtained from DSC and FTIR studies confirmed that BS is stable with the process excipients used for the study.
Figure 2.
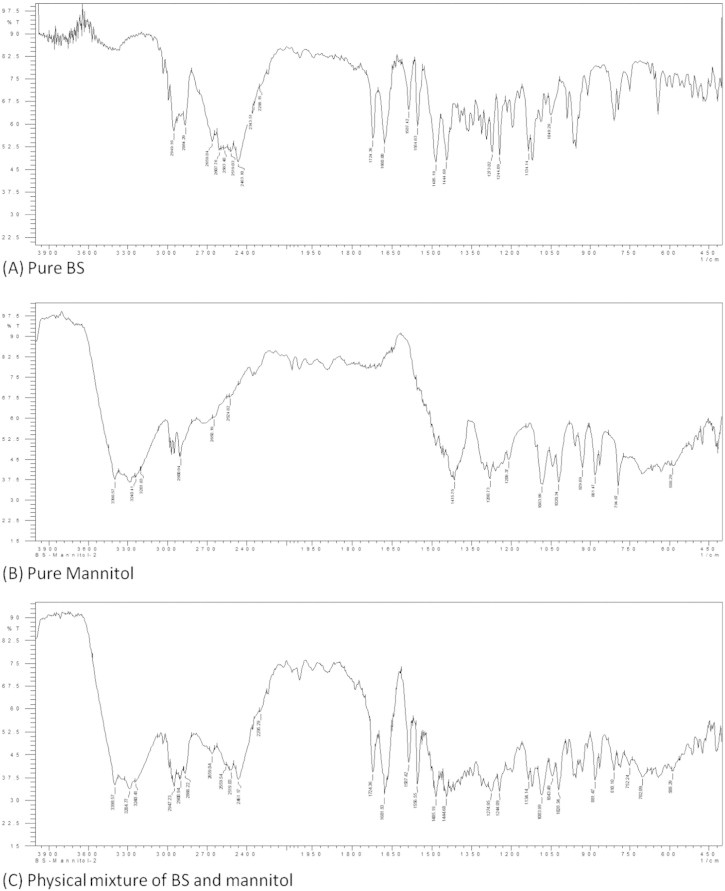
FTIR spectrum obtained for pure drug, pure mannitol and physical mixture of BS-mannitol.
3.2. Physical characteristics of designed buccal discs
Designed buccal discs demonstrated good physical characteristics indicating suitability of the method and excipients used for preparation. All the batches of buccal discs demonstrated permissible limit of percent error for weight variation (0.3–0.7%). Thickness measurements (ranging between 2.39 and 2.51 mm for all batches) indicated suitability of discs for buccal application (Table 1). Friability of all the batches was within limits (less than 1%) and surface pH was around 7.0 (Table 1). Assay values of designed formulations were found to be acceptable with a range of 98–102% (Table 1).
3.3. In-vitro water uptake studies
In in vitro water uptake studies, percent water uptake of designed buccal discs increased with increasing proportion of HPMC (Fig. 3). Increase in mannitol proportion also contributed to increased rate of water uptake (Fig. 3). This increased hydration behavior with increasing proportion of mannitol relative to HPMC can be attributed to faster permeability of aqueous medium into the matrix due to the formation of pores or channels caused due to higher solubility of mannitol.
Figure 3.
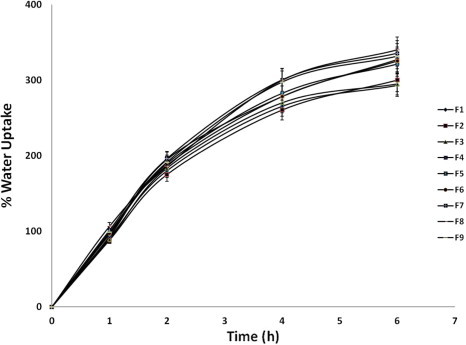
Water uptake behavior of buccal discs (n = 3).
3.3.1. In vitro bioadhesion studies
In-vitro bioadhesion studies for the designed buccal discs were investigated on porcine buccal mucosa using texture analyzer. Bioadhesion is the force of attachment of material or dosage form to the mucous secreting biological tissue or mucous membrane. The force required to detach designed discs form the mucosal membrane was determined using texture analyzer (Jaipal et al., 2013). HPMC is a hydrophilic polymer with many polar functional groups. Upon hydration the polymeric chains of HPMC get entangled with glycoprotein chains of mucin resulting in bioadhesion (Andrews et al., 2009, Jaipal et al., 2013). The degree of bioadhesion depends on type and amount of polymer, excipients used in the dosage form, degree of hydration, polymer chain length and molecular weight of polymer (Andrews et al., 2009, Charde et al., 2008, Salamat-Miller et al., 2005). Designed HPMC buccal discs (F1–F9) containing various proportions of HPMC and mannitol demonstrated good in vitro bioadhesion. Increase in polymer concentration resulted in increased force of adhesion (Table 3). When the concentration of polymer is low, the number of chains penetrating glycoprotein chains per unit volume of mucous is low resulting in weaker interaction (Leung and Robinson, 1990, Salamat-Miller et al., 2005, Smart, 2005). Various theories have been proposed for bioadhesive behavior of the polymers such as electronic interaction, hydrogen bonding, electronic theory, adsorption theory, wettability theory and the diffusion and interlocking theory (Smart, 2005, Sudhakar et al., 2006). Diffusion was the best suitable for bioadhesive behavior of HPMC buccal discs, due to its hydrophilic swellable characteristics (Heng et al., 2001). The equation obtained for bioadhesive force (FN) using full factorial design is as follows (Eq. (4))
| (4) |
Table 3.
Coded factors, percent drug released at end of 5 h (Q5), time required to release 50% of drug (T50) and bioadhesive strength in Newton (FN) of the designed buccal discs.
| B. no | Coded factors |
||||
|---|---|---|---|---|---|
| X1 | X2 | Q5 (%) | T50 (h) | FN (N) | |
| F1 | −1 | −1 | 82.69 | 1.58 | 9.57 |
| F2 | −1 | 0 | 86.31 | 1.53 | 9.47 |
| F3 | −1 | +1 | 99.59 | 1.39 | 9.61 |
| F4 | 0 | −1 | 79.02 | 1.76 | 10.39 |
| F5 | 0 | 0 | 82.30 | 1.59 | 10.13 |
| F6 | 0 | +1 | 93.40 | 1.25 | 9.89 |
| F7 | +1 | −1 | 69.95 | 2.62 | 12.00 |
| F8 | +1 | 0 | 73.20 | 2.29 | 12.14 |
| F9 | +1 | +1 | 79.15 | 2.18 | 11.97 |
The quadratic model was found to be significant with F value 55.24 (p < 0.0037). The relatively higher value (⩾0.9713) of regression coefficient for bioadhesive strength indicates a good fit. Fig. 4 represents the observed response values compared to that of predicted values. Bioadhesive force for the designed buccal discs increased significantly with increase in HPMC proportion (Fig. 4). High level of X1 gave increased bioadhesive force at all levels of X2 factor, this indicates factor X1 (HPMC) has significant effect on bioadhesive strength (Table 3). The results were according to previous theories suggested in the literature (Leung and Robinson, 1990, Peppas and Sahlin, 1996, Smart, 2005).
Figure 4.
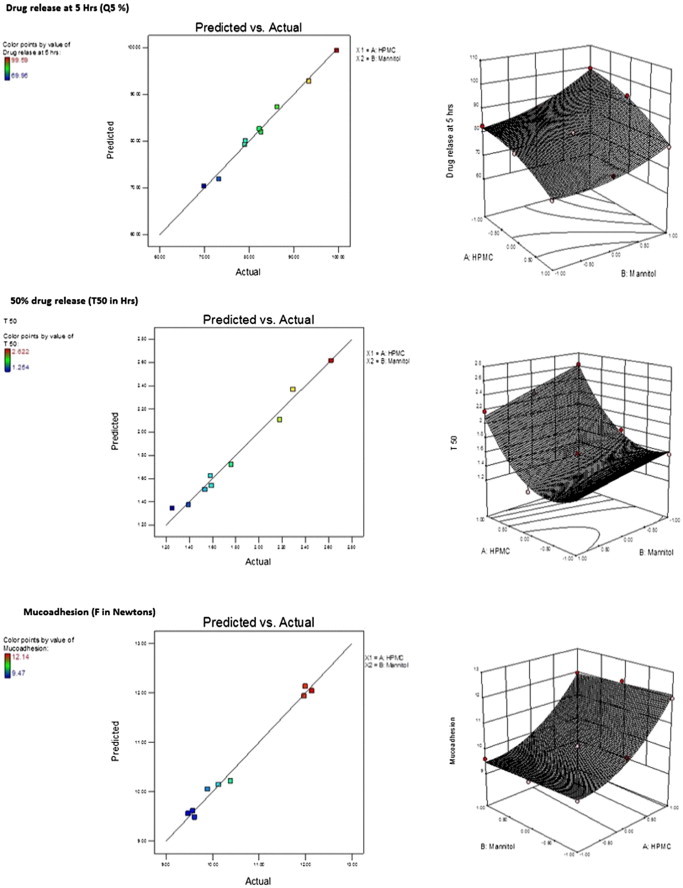
Predicted vs. actual and surface response plots of drug release at 5 H (Q5 in%), 50% drug release (T50 in h) and bioadhesion (FN in Newton) for the designed buccal discs.
3.3.2. In-vitro drug release studies: effect of formulation variables
The in vitro release profile of all the designed buccal discs is given in Fig. 5. The model terms of Q5 (drug release at 5 h) were found to be significant with the F value 83.67 (p < 0.0020), with actual values close to the predicted values (Eq. (5)). Regression coefficient value (0.9810) nearer to one indicated goodness of fit (all factors were found to be significant).
| (5) |
Figure 5.
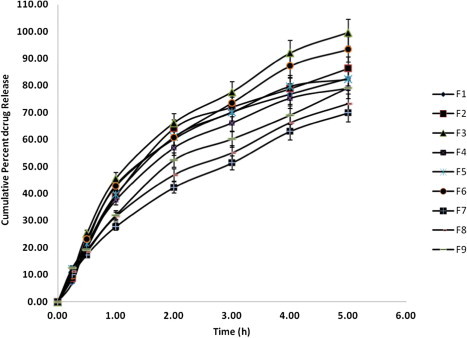
Drug release profile of designed buccal discs (n = 3).
The model terms for T50% were also found to be significant with F value 38.90 (p < 0.0063), with actual values close to predicted values (Fig. 4), with regression coefficient value of 0.9595 (Eq. (6)).
| (6) |
Changes in factor X1 (HPMC) and factor X2 (mannitol) influenced the drug release rate. The drug release rate decreased with increasing proportion of HPMC in disc matrix (Fig. 5). An increase in polymer proportion increases the viscosity of gel layer and also results in gel layer with longer diffusional path length resulting in greater retardation of drug release (Table 3; Fig 5). Increase in proportion of mannitol resulted in faster dissolution of drug and faster drug release rate. This can be ascribed to formation of pore in disc matrix due to higher and faster solubility of mannitol. This results in the formation of channel through which the media can enter the swollen polymer matrix and dissolve the drug. Information expressed by 3-dimensional surface plots matches closely with experimental results (Fig. 4). Low T50 values were observed for the batches having higher level of factor X2 (mannitol) due to pore formation. This effect was prominent at all levels of factor X1 indicating rapid drug release due to increased porosity of hydrogel matrix (Table 3).
Drug release observed from the designed HPMC matrices (F1–F9) followed first order release kinetics with n-values ranging from 0.463 to 0.544 (Table 4), indicating the drug release was non-Fickian anomalous type with polymer relaxation, diffusion and erosion being predominant mechanisms of drug release.
Table 4.
Model fitting of in vitro drug release study for determination of mechanism and kinetics of drug release.
| B. no | Zero order | First order | Higuchi | Korsmeyer–Peppas |
|
|---|---|---|---|---|---|
| (R2) | (R2) | (R2) | (R2) | n-value | |
| F∗ = ko × t | Mt = M0 e−k1t | ML,t = kH√t | F = kp × tn | ||
| F1 | 0.6846 | 0.9775 | 0.9535 | 0.9558 | 0.463 |
| F2 | 0.7154 | 0.9883 | 0.9577 | 0.9583 | 0.481 |
| F3 | 0.7235 | 0.9956 | 0.9713 | 0.9721 | 0.477 |
| F4 | 0.7587 | 0.9920 | 0.9814 | 0.9815 | 0.493 |
| F5 | 0.7455 | 0.9944 | 0.9780 | 0.9783 | 0.486 |
| F6 | 0.7326 | 0.9951 | 0.9745 | 0.9751 | 0.481 |
| F7 | 0.8381 | 0.9833 | 0.9907 | 0.9933 | 0.544 |
| F8 | 0.7782 | 0.9740 | 0.9872 | 0.9872 | 0.502 |
| F9 | 0.7610 | 0.9683 | 0.9858 | 0.9860 | 0.491 |
F∗, amount of drug dissolved at time t, ko, k1, kH, kp drug release rate constants of zero order, first order, Higuchi’s and Korsmeyer–Peppas model respectively.
“n” is dissolution exponent of Korsmeyer–Peppas model.
3.3.3. In-vivo pharmacokinetic study
In-vivo pharmacokinetic study was carried out for BS administered intravenously and for designed HPMC buccal discs (F3). The drug was detectable within 15 min of buccal administration indicating rapid absorption of released drug (Fig. 6). After buccal administration the maximum concentration was achieved within 2.5 h. In-vivo pharmacokinetic parameters obtained during the study are tabulated in Table 5. Absolute bioavailability of designed buccal discs was 0.41. This value is considerably higher than oral bioavailability of around 4% reported in the literature (Mahmood and Sahajwalla, 1999). Hence, this demonstrates usefulness of buccal delivery systems of BS for increase in bioavailability.
Figure 6.
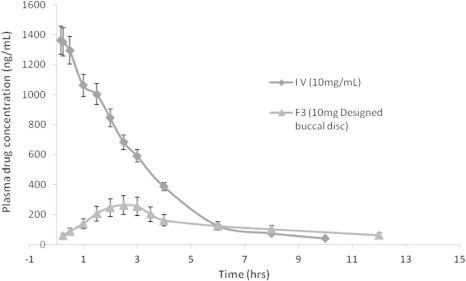
Mean plasma concentration time profile of BS following intravenous and buccal (F3) administration (n = 3).
Table 5.
Pharmacokinetic parameters of BS when administered intravenously and via buccal route.
| Pharmacokinetic parameters | 10 mg BS solution (i.v) | 10 mg buccal disc (F3) |
|---|---|---|
| AUC0−∞ (ng h/mL)a | 5153.90 ± 430.81 | 2129.57 ± 623.19 |
| F | – | 0.41 ± 0.09 |
| Cmax (ng/mL)a | 1384.68 ± 467.21 | 262.32 ± 43.29 |
| tmax (h) | – | 2.50 |
| T1/2a (h) | 2.53 ± 0.12 | 5.94 ± 0.37 |
Values are mean ± SD. n = 3.
4. Conclusion
Effect of mannitol and HPMC on drug release and bioadhesive behavior from the designed buccal discs was studied successfully using a 32 factorial design. It can be concluded that the drug release pattern can be changed by selection of appropriate levels of two factors viz HPMC and mannitol. Increase in mannitol proportion resulted in faster drug release rate and drug release rate decreased with increasing proportion of HPMC in buccal disc. Bioadhesive strength of designed discs increased with increasing proportion of HPMC in buccal discs. Designed HPMC buccal discs were successfully evaluated for pharmacokinetics parameters in rabbits and have demonstrated considerable absolute bioavailability. From the above study it can be concluded that HPMC bioadhesive buccal discs can serve as a promising option for delivery of BS and the drug release rate from the HPMC matrices can be adjusted by adding water soluble mannitol as release modifier.
Declaration of interest
The authors report no declarations of interest.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsps.2014.11.012.
Appendix A. Supplementary material
References
- Abruzzo A., Bigucci F., Cerchiara T., Cruciani F., Vitali B., Luppi B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr. Polym. 2012;87(1):581–588. doi: 10.1016/j.carbpol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Andrews G.P., Laverty T.P., Jones D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009;71(3):505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Badhan A., Mashru R., Shah P., Thakkar A., Dobaria N. Development and evaluation of sustained release gastroretentive minimatrices for effective treatment of H. pylori infection. AAPS PharmSciTech. 2009;10(2):459–467. doi: 10.1208/s12249-009-9231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baveja S.K., Ranga Rao K.V., Singh A., Gombar V.K. Release characteristics of some bronchodilators from compressed hydrophilic polymeric matrices and their correlation with molecular geometry. Int. J. Pharm. 1988;41(1–2):55–62. [Google Scholar]
- Blier P., Ward N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53(3):193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Boyapally H., Nukala R.K., Bhujbal P., Douroumis D. Controlled release from directly compressible theophylline buccal tablets. Colloids Surf. B. 2010;77(2):227–233. doi: 10.1016/j.colsurfb.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Charde S., Mudgal M., Kumar L., Saha R. Development and evaluation of buccoadhesive controlled release tablets of lercanidipine. AAPS PharmSciTech. 2008;9(1):182–190. doi: 10.1208/s12249-007-9031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.-G., Kim C.-K. Development of omeprazole buccal adhesive tablets with stability enhancement in human saliva. J. Control. Release. 2000;68(3):397–404. doi: 10.1016/s0168-3659(00)00276-5. [DOI] [PubMed] [Google Scholar]
- Debord B., Lefebvre C., Guyot-Hermann A.M., Hubert J., Bouché R., Cuyot J.C. Study of different crystalline forms of mannitol: comparative behaviour under compression. Drug Dev. Ind. Pharm. 1987;13(9–11):1533–1546. [Google Scholar]
- Giovino C., Ayensu I., Tetteh J., Boateng J.S. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): a potential approach for buccal delivery of macromolecules. Int. J. Pharm. 2012;428(1–2):143–151. doi: 10.1016/j.ijpharm.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Gohel M.C., Parikh R.K., Aghara P.Y., Nagori S.A., Delvadia R.R., Dabhi M.R. Application of simplex lattice design and desirability function for the formulation development of mouth dissolving film of salbutamol sulphate. Curr. Drug Deliv. 2009;6(5):486–494. doi: 10.2174/156720109789941696. [DOI] [PubMed] [Google Scholar]
- Gurny R., Doelker E., Peppas N.A. Modelling of sustained release of water-soluble drugs from porous, hydrophobic polymers. Biomaterials. 1982;3(1):27–32. doi: 10.1016/0142-9612(82)90057-6. [DOI] [PubMed] [Google Scholar]
- Harris D., Robinson J.R. Drug delivery via the mucous membranes of the oral cavity. J. Pharm. Sci. 1992;81(1):1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- Heng P.W.S., Chan L.W., Easterbrook M.G., Li X. Investigation of the influence of mean HPMC particle size and number of polymer particles on the release of aspirin from swellable hydrophilic matrix tablets. J. Control. Release. 2001;76(1–2):39–49. doi: 10.1016/s0168-3659(01)00410-2. [DOI] [PubMed] [Google Scholar]
- Holgado M.A., Caraballo I., Alvarez-Fuentes J., Fernández-Hervás M.J., Fernández-Arévalo M., Rabasco A.M. Influence of diluents and manufacturing method on the in vitro dissolution of carteolol hydrochloride matrix tablets. Int. J. Pharm. 1995;118(2):151–160. [Google Scholar]
- Jaipal A., Pandey M.M., Abhishek A., Vinay S., Charde S.Y. Interaction of calcium sulfate with xanthan gum: effect on in vitro bioadhesion and drug release behavior from xanthan gum based buccal discs of buspirone. Colloids Surf., B. 2013;111C:644–650. doi: 10.1016/j.colsurfb.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Jaipal A., Pandey M.M., Charde S., sadhu N., Ajjarapu S., Reddy P.G. Controlled release effervescent buccal discs of buspirone hydrochloride: in-vitro and in-vivo evaluation studies. Drug Delivery. 2014;1–7 doi: 10.3109/10717544.10712014.10917388. [DOI] [PubMed] [Google Scholar]
- Kianfar F., Antonijevic M., Chowdhry B., Boateng J.S. Lyophilized wafers comprising carrageenan and pluronic acid for buccal drug delivery using model soluble and insoluble drugs. Colloids Surf. B. 2013;103:99–106. doi: 10.1016/j.colsurfb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanisms of potassium chloride release from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J. Pharm. Sci. 1983;72(10):1189–1191. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15(1):25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Leung S.-H.S., Robinson J.R. Polymer structure features contributing to mucoadhesion. II. J. Control. Release. 1990;12(3):187–194. [Google Scholar]
- Lilja J.J., Kivisto K.T., Backman J.T., Lamberg T.S., Neuvonen P.J. Grapefruit juice substantially increases plasma concentrations of buspirone[ast] Clin. Pharmacol. Ther. 1998;64(6):655–660. doi: 10.1016/S0009-9236(98)90056-X. [DOI] [PubMed] [Google Scholar]
- Mahmood I., Sahajwalla C. Clinical pharmacokinetics and pharmacodynamics of buspirone, an anxiolytic drug. Clin. Pharmacokinet. 1999;36(4):277–287. doi: 10.2165/00003088-199936040-00003. [DOI] [PubMed] [Google Scholar]
- Martin L., Wilson C.G., Koosha F., Uchegbu I.F. Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur. J. Pharm. Biopharm. 2003;55(1):35–45. doi: 10.1016/s0939-6411(02)00118-2. [DOI] [PubMed] [Google Scholar]
- Morishita M., Barichello J.M., Takayama K., Chiba Y., Tokiwa S., Nagai T. Pluronic® F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin. Int. J. Pharm. 2001;212(2):289–293. doi: 10.1016/s0378-5173(00)00615-3. [DOI] [PubMed] [Google Scholar]
- Muchohi S.N., Kokwaro G.O., Ogutu B.R., Edwards G., Ward S.A., Newton C.R.J.C. Pharmacokinetics and clinical efficacy of midazolam in children with severe malaria and convulsions. Br. J. Clin. Pharmacol. 2008;66(4):529–538. doi: 10.1111/j.1365-2125.2008.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz A.M., Ch’ng H.-S. Evaluation of bioadhesive buccal tablets containing triamcinolone acetonide in healthy volunteers. Int. J. Pharm. 1995;121(2):249–254. [Google Scholar]
- Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev. Ind. Pharm. 2004;30(9):985–993. doi: 10.1081/ddc-200037245. [DOI] [PubMed] [Google Scholar]
- Narendra C., Srinath M.S., Prakash Rao B. Development of three layered buccal compact containing metoprolol tartrate by statistical optimization technique. Int. J. Pharm. 2005;304(1–2):102–114. doi: 10.1016/j.ijpharm.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Peppas N.A., Sahlin J.J. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17(16):1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- Pozzilli P., Manfrini S., Costanza F., Coppolino G., Cavallo M.G., Fioriti E., Modi P. Biokinetics of buccal spray insulin in patients with type 1 diabetes. Metabolism. 2005;54(7):930–934. doi: 10.1016/j.metabol.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Ratey J.J., Sovner R., Mikkelsen E., Chmielinski H.E. Buspirone therapy for maladaptive behavior and anxiety in developmentally disabled persons. J. Clin. Psychiatry. 1989;50:382–384. [PubMed] [Google Scholar]
- Realmuto G.M., August G.J., Garfinkel B.D. Clinical effect of buspirone in autistic children. J. Clin. Psychopharmacol. 1989;9:122–125. doi: 10.1097/00004714-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Riblet L.A., Eison A.S., Eison M.S., Newton R.E., Taylor D.P., Temple D.L., Jr. Buspirone: an anxioselective alternative for the management of anxiety disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1983;7(4–6):663–668. doi: 10.1016/0278-5846(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Rossi S., Sandri G., Caramella C.M. Buccal drug delivery: a challenge already won? Drug Discov. Today Technol. 2005;2(1):59–65. doi: 10.1016/j.ddtec.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Salamat-Miller N., Chittchang M., Johnston T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005;57(11):1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Sheikhzadeh M., Rohani S., Jutan A., Manifar T. Quantitative and molecular analysis of buspirone hydrochloride polymorphs. J. Pharm. Sci. 2007;96(3):569–583. doi: 10.1002/jps.20723. [DOI] [PubMed] [Google Scholar]
- Smart J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57(11):1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Sramek J.J., Zarotsky V., Cutler N.R. Generalised anxiety disorder: treatment options. Drugs. 2002;62(11):1635–1648. doi: 10.2165/00003495-200262110-00005. [DOI] [PubMed] [Google Scholar]
- Sudhakar Y., Kuotsu K., Bandyopadhyay A.K. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J. Control. Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Taylan B., Capan Y., Güven O., Kes S., Atilla Hincal A. Design and evaluation of sustained-release and buccal adhesive propranolol hydrochloride tablets. J. Control. Release. 1996;38(1):11–20. [Google Scholar]
- Taylor D.P., Eison M.S., Riblet L.A., Vandermaelen C.P. Pharmacological and clinical effects of buspirone. Pharmacol. Biochem. Behav. 1985;23(4):687–694. doi: 10.1016/0091-3057(85)90438-1. [DOI] [PubMed] [Google Scholar]
- Yehia S., El-Gazayerly O., Basalious E. Design and in vitro/in vivo evaluation of novel mucoadhesive buccal discs of an antifungal drug: relationship between swelling, erosion, and drug release. AAPS PharmSciTech. 2008;9(4):1207–1217. doi: 10.1208/s12249-008-9166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


