Abstract
Background
Recommendations regarding vancomycin dosing and monitoring in critically ill patients on continuous renal replacement therapy (CRRT) are limited. This is a retrospective study to assess the adequacy of current vancomycin dosing and monitoring practice for patients on CRRT in a tertiary hospital in Riyadh, Saudi Arabia.
Methods
A retrospective chart review of adult patients admitted between 1 April 2011 and 30 March 2013 to critical care and received intravenous vancomycin therapy whilst on CRRT was performed.
Results
A total of 68 patients received intravenous vancomycin therapy whilst on CRRT, of which 32 met the inclusion criteria. Fifty-one percent were males and median (range) age was 62.5 (19 – 90) years. Median APACHE II score was 33.5 (22–43) and median Charlson Comorbidity Score was 4 (0–8). The mean (± standard deviation) dose of vancomycin was 879.9 mg (± 281.2 mg) for an average duration of 5.9 days (± 3.7 days). All patients received continuous veno-venous haemofiltration (CVVH). A total of 55 vancomycin level readings were available from the study population, ranging from 6.6 to 41.3, with wide variations within the same sampling time frames. Vancomycin levels of > 15 mg/L or were achieved at least once in 24 patients (75.0%), but only 11 patients (34.3%) had 2 or more serum vancomycin level readings of 15 mg/L or more.
Conclusion
Therapeutic vancomycin levels are difficult to maintain in critically ill patients who are receiving IV vancomycin therapy whilst on CRRT. Aggressive dosing schedules and frequent monitoring are required to ensure adequate vancomycin therapy in this setting.
Keywords: Vancomycin, CRRT, Renal replacement therapy
1. Introduction
Vancomycin is an important antimicrobial agent for the treatment of Gram-positive bacterial infections (Rybak, 2006). It has been shown that for optimal clinical outcomes, the ratio of area under the curve (AUC) of serum vancomycin concentration to bacterial minimum inhibitory concentration (MIC) should be maintained at 400 or above (Kullar et al., 2011; Rybak et al., 2009). Such ratio is best predicted in clinical practice by serum vancomycin trough levels of 15 – 20 mg/L (Rybak et al., 2009). Vancomycin dosing recommendations have therefore been designed to achieve these pharmacokinetic targets for patients with different levels of renal function (Rybak et al., 2009; Liu et al., 2011). However, optimal vancomycin dosing regimens for critically ill patients on continuous renal replacement therapy (CRRT) remain unclear (Petejova et al., 2014; Chaijamorn et al., 2011).
Acute renal impairment is common in critically ill patients, many of who require renal replacement therapy pending the recovery of their kidney function (Uchino et al., 2005). CRRT has the advantage of closely simulating physiological renal clearance without the haemodynamic instability associated with intermittent haemodialysis (IHD) (Dirkes and Hodge, 2007). CRRT however, results in considerable alterations in the pharmacokinetics of various antimicrobial agents, including vancomycin (Chaijamorn et al., 2011; Bouman et al., 2006). Given the importance of early appropriate antimicrobial therapy for critically ill patients, effective vancomycin dosing is essential (Dellinger et al., 2013). This study was undertaken to assess the adequacy of current vancomycin dosing and monitoring practice in critically ill patients requiring CRRT in a large tertiary care centre in Riyadh, Saudi Arabia.
2. Materials and methods
We performed a retrospective chart review of all patients aged 18 years or more who were admitted to the intensive care unit (ICU) in our institution during the period from 1 April 2011 to 31 March 2013 and received two doses or more of intravenous vancomycin therapy whilst on CRRT. Interruptions totalling 6 h or less within any 24 h period of CRRT were permitted. We excluded pregnant women and patients with extensive burns. Details of vancomycin dosing regimens, renal replacement variables and serum vancomycin levels were recorded. Data were analysed using Microsoft Excel (Microsoft Inc., Redmond, USA).
3. Results
During the study period, a total of 68 patients received intravenous vancomycin therapy whilst on CRRT. Twenty-seven patients received less than 2 doses of intravenous vancomycin whilst on CRRT, 8 had incomplete CRRT data and 1 patient was aged less than 18 years. Thirty-two patients were included in the analysis, of which 18 (51.4%) were males. Median (range) patient age was 62.5 (19 – 91) years with median APACHE II score of 33.5 (22–43) and median Charlson Comorbidity Score of 4 (0–8). The mean (± standard deviation) length of stay in ICU was 28.4 (± 21.2) days. Mean body weight was 65.4 (± 12.8) kg. Patients received an average intravenous vancomycin dose of 879.9 mg (± 281.2 mg) for a mean duration of 5.9 days (± 3.7 days). The median number of doses of intravenous vancomycin received per patient was 4 (2–38). Mean serum creatinine at the time of starting CRRT was 271.8 μmole/L (± 179.4 μmole/L) with an average estimated creatinine clearance of 271.8 mL/min (± 179.4 mL/min). Mean serum albumin was 27.8 (± 13.9) g/L. CRRT mode in all patients was continuous veno-venous haemofiltration (CVVH).
A total of 55 vancomycin level readings were available from the study population. Overall, vancomycin serum levels ranged from 6.6 to 41.3 mg/L, with wide variations within the same sampling time frames (Fig. 1). Target vancomycin level of 15 mg/L or higher was achieved at least once in 24 patients (75.0%). However, only 11 patients (34.3%) had 2 or more serum vancomycin level readings of 15 mg/L or more.
Fig. 1.
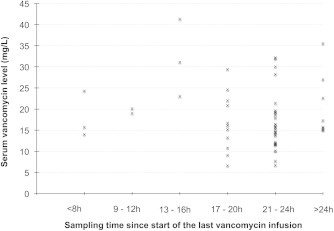
Serum vancomycin levels in relation to sampling time intervals.
4. Discussion
Our results demonstrate wide variation in serum vancomycin levels in critically ill patients on CRRT. Furthermore, vancomycin serum levels did not reach therapeutic levels in at least 25% of patients. Of more concern is the fact that only one third of patients (34.3%) can be shown to have maintained adequate vancomycin levels over at least two assessments.
Similar results were reported previously. For example, in a retrospective cohort study, Wilson et al. reported that 44% of medical ICU and 49% of surgical ICU vancomycin levels obtained from patients on CRRT were below the target of 15 mg/L (Wilson and Berns, 2012). Likewise, Petejova et al. found that in 10 out of 17 patients who received vancomycin whilst on CRRT, vancomycin serum levels dropped below 10 mg/L within the first 6 h of the infusion (Petejova et al., 2014). In a more recent study, only 1 out of 4 patients receiving intravenous vancomycin whilst on CRRT had any detectable serum vancomycin levels 24 h after the infusion (Paciullo et al., 2013).
It has become recently evident that vancomycin is removed very efficiently during CRRT. In a prospective pharmacokinetic study of 7 critically ill patients on CVVH, the mean sieving coefficient of vancomycin was 0.71 (± 0.13). Clearance of vancomycin by CVVH constituted 59.4 (± 20.8) of total vancomycin clearance (Chaijamorn et al., 2011). Continuous veno-venous haemodiafiltration (CVVHD) results in even more efficient vancomycin clearance, with a sieving coefficient of 0.7 (± 0.1) and total clearance reaching up to 76% (± 16.5%) of total vancomycin clearance (DelDot et al., 2004). In addition to the mode of CRRT, vancomycin clearance is significantly higher and thus therapeutic levels are more difficult to achieve in patients on higher rates of haemofiltration (Petejova et al., 2014; Frazee et al., 2012).
There have been a number of attempts to recommend dosing algorithms that ensure adequate vancomycin therapy during CRRT (Chaijamorn et al., 2011; Wilson and Berns, 2012; Heintz et al., 2009; Kuang et al., 2007). Those recommendations however, are based largely on limited data derived from small studies, simulation models or IHD studies. Given the apparent unpredictability of vancomycin serum levels in critically ill patients receiving CRRT, the variability of various factors that influence its clearance and the potentially rapidly changing clinical parameters in such patients, several authors recommend aggressive vancomycin dosing and monitoring regimens to ensure adequate and consistent therapy (Petejova et al., 2014; Chaijamorn et al., 2011; Wilson and Berns, 2012; Paciullo etal., 2013; Frazee et al., 2012).
5. Conclusion
Vancomycin serum levels are frequently insufficient in critically ill patients receiving CRRT. One possible strategy to optimize vancomycin therapy in this setting is to aim for 12 hourly dosing with serum level monitoring as early as 6–12 h after the first infusion followed by dynamic adjustment of dose and/or dosing intervals to maintain serum levels within therapeutic targets.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bouman C.S., van Kan H.J., Koopmans R.P., Korevaar J.C., Schultz M.J., Vroom M.B. Discrepancies between observed and predicted continuous venovenous hemofiltration removal of antimicrobial agents in critically ill patients and the effects on dosing. Intensive Care Med. 2006;32:2013–2019. doi: 10.1007/s00134-006-0397-x. [DOI] [PubMed] [Google Scholar]
- Chaijamorn W., Jitsurong A., Wiwattanawongsa K., Wanakamanee U., Dandecha P. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Int. J. Antimicrob. Agents. 2011;38:152–156. doi: 10.1016/j.ijantimicag.2011.04.010. [DOI] [PubMed] [Google Scholar]
- DelDot M.E., Lipman J., Tett S.E. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration. Br. J. Clin. Pharmacol. 2004;58:259–268. doi: 10.1111/j.1365-2125.2004.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger R.P., Levy M.M., Rhodes A. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkes S., Hodge K. Continuous renal replacement therapy in the Adult intensive care unit: History and current trends. Crit. Care Nurse. 2007;27:61–80. [PubMed] [Google Scholar]
- Frazee E.N., Kuper P.J., Schramm G.E. Effect of continuous venovenous hemofiltration dose on achievement of adequate vancomycin trough concentrations. Antimicrob. Agents Chemother. 2012;56:6181–6185. doi: 10.1128/AAC.00459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz B.H., Matzke G.R., Dager W.E. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacother. 2009;29:562–577. doi: 10.1592/phco.29.5.562. [DOI] [PubMed] [Google Scholar]
- Kuang D., Verbine A., Ronco C. Pharmacokinetics and antimicrobial dosing adjustment in critically ill patients during continuous renal replacement therapy. Clin. Nephrol. 2007;67:267–284. doi: 10.5414/cnp67267. [DOI] [PubMed] [Google Scholar]
- Kullar R., Davis S.L., Levine D.P., Rybak M.J. Impact of vancomycin exposure on outcomes in patients with Methicillin-Resistant Staphylococcus aureus Bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011;52:975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- Liu C., Bayer A., Cosgrove S.E. Clinical practice guidelines by the infectious diseases society of America for the treatment of Methicillin-Resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- Paciullo C.A., Harned K.C., Davis G.A., Connor M.J., Jr., Winstead P.S. Vancomycin Clearance in High-Volume Venovenous Hemofiltration. Ann. Pharmacother. 2013;47:e14. doi: 10.1345/aph.1Q488. [DOI] [PubMed] [Google Scholar]
- Petejova N., Martinek A., Zahalkova J. Vancomycin pharmacokinetics during high-volume continuous venovenous hemofiltration in critically ill septic patients. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2014;158:65–72. doi: 10.5507/bp.2012.092. [DOI] [PubMed] [Google Scholar]
- Rybak M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006;42:S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- Rybak M.J., Lomaestro B.M., Rotscahfer J.C. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009;49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- Uchino S., Kellum J.A., Bellomo R. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- Wilson F.P., Berns J.S. Vancomycin levels are frequently subtherapeutic during continuous venovenous hemodialysis (CVVHD) Clin. Nephrol. 2012;77:329–331. doi: 10.5414/CN106993. [DOI] [PMC free article] [PubMed] [Google Scholar]


