Abstract
5-Fluorouracil is used in the treatment of colorectal cancer along with oxaliplatin as first line treatment, but it is having lack of site specificity and poor therapeutic effect. Also toxic effects to healthy cells and unavailability of major proportion of drug at the colon region remain as limitations. Toxic effects prevention and drug localization at colon area was achieved by preparing enteric-coated chitosan polymeric nanoparticles as it can be delivered directly to large bowel. Enteric coating helps in preventing the drug degradation at gastric pH. So the main objective was to prepare chitosan polymeric nanoparticles by solvent evaporation emulsification method by using different ratios of polymer (1:1, 1:2, 1:3, 1:4). Optimized polymer ratio was characterized by differential scanning calorimetry (DSC), X-ray diffraction (XRD), entrapment efficiency and particle size and further subjected to enteric coating. In vitro drug release studies were done using dialysis bag technique using simulated fluids at various pH (1.2, 4.5, 7.5, 7.0) to mimic the GIT tract. 5-FU nanoparticles with drug: polymer ratio of 1:2 and 1:3 has shown better particle size (149 ± 1.28 nm and 138 ± 1.01 nm respectively), entrapment efficiency (48.12 ± 0.08% and 69.18 ± 1.89 respectively). 5-FU E1 has shown better drug release after 4 h and has shown 82% drug release till 24 h in a sustained manner comparable to the non-enteric coated tablets, which released more than 50% of the drug before entering the colon region. So we can conclude that nanoparticles prepared by this method using the same polymer with the optimized ratio can represent as potential drug delivery approach for effective delivery of the active pharmaceutical ingredient to the colorectal tumors.
Keywords: 5-Fluorouracil, Polymeric nanoparticles, Colorectal cancer, Enteric coated, Sustained
1. Introduction
Among the various types of cancers, colorectal cancer is a significant source of morbidity and mortality in the United States and other Western countries. The cancer is one of the most dreaded and threatening diseases in the world, causing more than 6 million deaths a year (Ramanathan et al., 2003). Colorectal cancer is the second leading cause of death followed by lung cancer (Dhawale et al., 2010).
Various cytotoxic drugs are used for the treatment of colorectal cancer like 5-Fluorouracil, Oxaliplatin, and Cisplatin. The first line treatment generally includes the combination of 5-fluorouracil and oxaliplatin by intravenous administration. The rationale behind using cytotoxic drug over others is their toxicity to the cells that are rapidly dividing which can be attributed to the fact that cancer cells undergo rapid growth proliferation. Most of these drugs are administered by intravenous route to attain maximum bioavailability, still treatment failure is observed for most of the cytotoxic drugs. The main problem for the treatment failure is the drug inability to act particularly at the target site, which leads to lack of site specificity leading to the side effects to both healthy cells and tumor cells by the drug. Some of the other limitations associated with the anti cancer drugs are their hydrophobic nature, improper biodistribution and their susceptibility to develop drug resistance (Wong et al., 2007; Duran et al., 2008).
Therapeutic strategies currently used in the treatment of colorectal cancer are chemotherapy, surgery, radiation and biological therapies (immuno therapy and hormonal therapy). The benefits of traditional chemotherapy are limited by the toxicity associated with anticancer drugs in healthy tissues. The common features of cancer and healthy cells make it difficult to achieve pharmacoselectivity of drugs at the target site (Cirstoiu-Hapcaa et al., 2009).
Lack of site specificity is one of the major reasons for the drug in reaching the target site in therapeutic concentrations in colorectal cancer (Michor et al., 2005; Krishnaiah and Satyanarayan, 2001). Generally in order to compensate the lack of site specificity, increase in the dose size is preferred which leads to various toxicities. Researchers have proved targeting the drug to the tumor tissues. Also localization of the drug at the colon area helps in getting drug released at colon region leading to the major amount of the drug entering the colon. Coating the drugs with various polymeric substances like cellulose derivatives (Levine et al., 1987) and acrylic polymers (Rasmussen et al., 1982) can deliver them directly to large bowel. The main rationale behind using these types of polymers is their ability to prevent drug degradation in the gastric environment in the stomach and their ability to release the drug after entering the distal ileum (Levine et al., 1987; Rasmussen et al., 1982).
5-Fluorouracil (5-FU or 5-fluoro-2,4-pyrimidinedione) has broad spectrum of activity with a broad spectrum of activity against solid tumors (of the gastrointestinal tract, pancreas, ovary, liver, brain, breast, etc.), alone or in combination with chemotherapy regimes. The mechanism behind the cytotoxicity and cell death activity of 5-FU is its interference with nucleoside metabolism in RNA and DNA (Arias, 2008).
To maintain therapeutic serum concentration, constantly dose administration of 5-Fluorouracil has to be given leading to severe toxic effects after certain limit (Zhang et al., 2008b; Arias et al., 2008). The overall response rate for advanced colorectal cancer of 5-FU alone is still only ≈ 10%, and the combination of 5-FU with other antitumor drugs has merely improved the response rates to just ≈ 45%. 5-FU injection is generally used with the specification of 0.25 g/10 ml (Grem, 2000; Malet-Martino and Martino, 2002).
So the main objective is to develop 5-fluorouracil chitosan nanoparticles with different polymer ratios using solvent emulsification evaporation method. Eudragit L-100 polymer was used which can provide enteric coating thereby protecting the drug at acidic environment in the stomach and releasing the drug at basic pH starting from the intestine to colon. The other novelty associated with this study is the administration of the nanoparticles orally to improve the patient compliance and also making available the dosage form directly to large bowel via the GIT tract so as to localize the maximum amount of drug in the colon area and also attaining the sustained release.
2. Materials and methods
2.1. Materials
5-Fluoro uracil, used as active pharmaceutical ingredient was obtained from Sigma Aldrich, Mumbai. Chitosan (purified), used as polymer was obtained from M/s Panacea Biotech, Chandigarh, India. Eudragit S100, used for enteric coating was obtained from Degussa, Germany. Dialysis bag and triethyl citrate were purchased from Himedia. Polyvinyl alcohol was obtained from BDH Laboratories. All other chemicals were of analytical grade. Doubly distilled water was used throughout the study.
2.2. Preparation of chitosan polymeric nanoparticles
The method used for the preparation of chitosan nanoparticles containing 5-FU is solvent emulsification evaporation technique. Different ratios of drug: polymer (1:1, 1:2,1:3, 1:4) was selected in order to optimize the best one and also to observe the effect of polymer on the formulation.
Acetic acid was used as organic solvent and PVA as surfactant in a fixed concentration of (0.5% w/v). Drug was dissolved in 40 ml water with varying polymer ratios (1:1, 1:2, 1:3, 1:4). Then it was followed by addition of aqueous surfactant polyvinyl alcohol using high speed emulsifier and was stirred continuously for 3 h. Then the emulsion was subjected to centrifugation (SIGMA, Germany) for 30 min at 12,000 rpm. Supernatant was removed and washed repeatedly three times and subjected to lyophilization using 1% mannitol as cryoprotectant (Christ Alpha 2-4 LD, Freeze Drying Solutions, UK).
2.3. Preparation of pellets and Enteric coating of nanoparticles (Jain et al., 2010)
The nanoparticles were made into pellets by extruder-spheronization technique(UFEE 60; Umang Pharmatech, Ahmedabad, India). Wet mass was formed by mixing the nanoparticles with deionized water and avicel which was extruded with an extruder having a sieve opening of 1 mm, screen thickness of 2.5 mm and extrude cut-off length of approximately 2–4 mm. then the extrudes were spheronized in a spheronizer at 2200 rpm for 10 min. Pellets formed were dried at 35–40 °C and subjected to enteric coating.
Enteric coating was done by conventional coating pan used in the laboratory. The conditions followed for the coating of the pellets were pan rotating speed at 20 rpm, atomizing air pressure of 2 bar; inlet air temperature of 60°–70 °C; outlet air temperature of 35°–40 °C. The coating solution was prepared by mixing Eudragit S100 with ammonia and triethyl citrate (which was an active ingredient generally used in coating process) and stirred properly was applied through 1.1 mm spray nozzle (Huyghebaert et al., 2005). Pellets were kept coated in the pan till they achieve complete weight gain. All the pellets were stored in a vacuum dessicator at room temperature until used.
2.4. Characterization of nanoparticles
2.4.1. Differential scanning calorimetry (DSC)
DSC analysis was performed using DSC Q200, TA instruments, Mumbai, India. The samples were heated in a sealed aluminum pans at a rate of 10 °C per/min in a 30–300 °C temperature under nitrogen flow of 40 mL/min. DSC analysis was performed for PLGA, Eudragit L100, Gliclazide, physical mixture of polymer and drug and finally for GL-PLGA, GL-EU polymeric nanoparticles.
2.4.2. X-ray diffraction studies (XRD) (Badry et al., 2009)
Molecular arrangements of drug gliclazide in nanoparticulate formulations were performed on an X-ray diffractometer (PANalytical X’pert PRO; Lelyweg, Almelo, The Netherlands) using Cu Kα radiation. The data were collected over an angular range from 3° to 50° 2θ in continuous mode using a step size of 0.02° 2θ and step time of 5 s.
2.4.3. Particle size and zeta potential (Naik et al., 2013)
The average particle size and zeta potential of the PLGA-Gliclazide and EU-gliclazide nanoparticles were determined by Particle Size Analyzer (Zetasizer Ver System; Malvern Instruments Ltd., Malvern, UK). To analyze particle size, nanosuspension was diluted with filtered (0.22 μm) ultra pure water.
2.4.4. Drug content and entrapment efficiency (Avinash et al., 2007)
Nanoparticles (20 mg) after freeze-drying were added to their specific solvent (10 ml) to facilitate the coat of the nanoparticles to get dissolved. The resultant suspension was subjected to evaporation for further removal of the solvent prior to filtration. Then the residue was washed and diluted appropriately with phosphate buffer of pH 6.8 to determine drug content and entrapment efficiency. Samples were measured at an absorbance of 269 nm in Double beam U.V Spectrophotometer. Drug content loading and entrapment efficiency of gliclazide in nanoparticles were determined by the following equations:
2.4.5. Statistical analysis
Statistical analysis was performed for different formulae by applying one-way analysis of variance ANOVA test. Further difference between two formulations was determined by paired t-test using statistical software package (Minitab express and Minitab 17). The differences were considered statistical significant based on the p-value whether it is equal or less than 0.05.
2.4.6. Scanning electron microscopy (SEM) (Mohideen et al., 2013)
Scanning electron microscopy (SEM) was used to verify uniformity of particle shape and size. Freeze-dried nanoparticles were resuspended in distilled water and were later dropped onto a silicon grid and dried under room temperature. The nanoparticle suspension was vacuum coated with gold for 3 min. The surface morphology of the samples was observed under a scanning electron microscope (JEOL–JAPAN) operated at 15-keV pulse at different resolutions.
2.4.7. In vitro drug release study (Jain et al., 2010)
In vitro drug release study was carried out using USP Dissolution apparatus (Type II). Enteric-coated chitosan nanoparticles were tested in various simulated fluids at different pH to evaluate the release of nanoparticles at particular pH and also to determine the drug release and localization. Dialysis bag method was preferred and nanoparticles were placed in a beaker containing 100 ml of simulated fluids at 37 ± 0.5 °C with a dialysis bag with molecular weight cut-off of 3500. The simulated fluids were maintained at proper sink conditions under continuous magnetic stirring. Various simulated fluids with pH 1.2 (indicative of gastric pH), 4.5 (indicative of gastric and intestinal fluid pH), 7.5 (indicative of intestinal fluid pH), 7 (colon pH). These fluids were changed according to the time intervals assuming that the drug keep on passing the GIT. So the fluids pH was changed accordingly to the area, which it was going to reach to calculate drug release effectively. First one hour was considered for gastric pH, 2–3 h was considered as mixture of gastric fluid and intestinal fluid with pH 4.5, 4–5 h was considered as intestinal fluid with pH 7.5 and 6–8 h were considered as colonic fluid with pH 7.0. Samples were withdrawn and the same amount was replaced using the same simulated fluid. The amount of the drug was quantified using double beam U.V spectrophotometer at 269 nm.
3. Results and discussion
3.1. Differential scanning calorimetry (DSC)
Thermogram of 5-Fluorouracil, chitosan, chitosan polymeric nanoparticles, Eudragit S-100 and Enteric coated polymeric nanoparticles was illustrated in Fig. 1. The DSC curve of 5-FU exhibits corresponding peak at peak temperature of 265.8 °C corresponding to its melting point. Polymers chitosan and Eudragit S-100 has shown peaks at a temperature of 82 and 172.5 °C respectively. 5-Fluoro uracil chitosan polymeric nanoparticles have shown a minor peak at 82 °C and peak position at 5-FU was found to be vanished, which can be attributed to the fact that drug can be molecularly dispersed into the polymer matrix. Enteric coated nanoparticles thermogram has shown peaks at 75.89 °C, 162.15 °C and a minor peak at the area where 5-FU present. These studies further strengthen the evidence that there is compatibility between the drug and the polymers and also the chance of entrapment of drug inside the polymer in the polymeric nanoparticles. Also the presence of polymer reduces the height and sharpness of the peaks, which can be observed in case of polymeric nanoparticles. The DSC studies support our rationale, as stability is the primary concern, which can effect the formulation in many ways. To achieve stability, compatibility between the drug and the polymer must be ensured which can be confirmed by the DSC and further by XRD studies.
Figure 1.
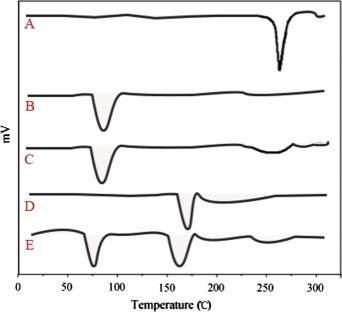
DSC thermograms of (A) 5-FU (B) chitosan (C) chitosan polymeric nanoparticles (D) Eudragit S-100 and (E) enteric coated chitosan polymeric nanoparticles.
3.2. XRD studies
An XRD peak mainly depends on the crystal size as they indicate the crystalline nature at particular value at 2θ range. In this study, pure drug 5-fluoro uracil had shown a sharp single peak and the highest one at 2θ equals 16.1° that indicates its crystalline nature. Polymers chitosan and Eudragit S-100 diffractograms had shown peaks at 18° and 14° respectively which can be seen in Fig. 2. The diffractograms of pure polymer were found to be different from the drug loaded polymeric nanoparticles, as we noticed a little decrease in the intensity of the peak which can be attributed to the lower level of detection of the encapsulated drug as it was dispersed in molecular level and moreover the slight disappearance of the 5-fluoro uracil peak indicates the entrapment of drug inside the polymers and also indicates the amorphous state of the encapsulated drug. Also the enteric-coated polymeric nanoparticles XRD peak was found to be of lower intensity and detection was found to be little difficult as they were enteric coated along with the chitosan polymeric nanoparticles.
Figure 2.
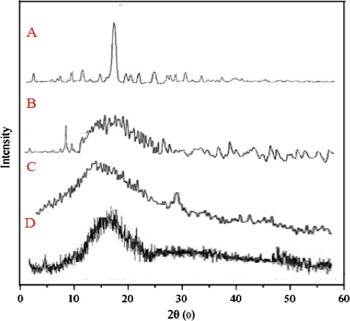
XRD patterns of (A) 5-FU (B) chitosan (C) Eudragit and (D) enteric coated polymeric nanoparticles.
3.3. Particle size and zeta potential analysis
The mean particle size of 5-FU nanoparticles was found to be smaller than 192 nm. So the nanoparticles size range was found to be satisfactory and was according to the specifications. This was performed in replicate of three times (n = 3) in order to ensure reproducibility to minimize the error. PDI values were found to be lesser than 0.2, which indicates that the system has a relatively narrow distribution. Zeta potential was found to be in the limit and it further proves the stability of the prepared polymeric nanoparticles, which justifies the rationale of preparing stable nanoparticles, as stable nanoparticles can be easily dispersed which enhances its solubility. The results evaluated were shown for different ratios of drug: polymer in Table 1. The particle size intensity for drug: polymer ratios of 1:3 and 1:2 were 138 ± 1.01 nm and 149 ± 1.28 nm respectively which can be seen in Fig. 3. Both these ratios were found to be satisfactory and can be optimized among the all by evaluating the entrapment efficiency, which was confirmed later.
Table 1.
Particle size, entrapment efficiencies and drug loading (%) of 5-FU polymeric nanoparticles.
| Drug: polymer ratio | Particle size (nm) | Entrapment efficiency (%) | Drug loading (%) |
|---|---|---|---|
| 1:1 | 192 ± 0.15 | 26.89 ± 0.09 | 17.51 ± 0.018 |
| 1:2 | 149 ± 1.28 | 48.12 ± 0.08 | 12.10 ± 0.027 |
| 1:3 | 138 ± 1.01 | 69.18 ± 1.89 | 28.14 ± 0.19 |
| 1:4 | 187 ± 0.09 | 32.18 ± 0.47 | 15.91 ± 1.25 |
Figure 3.
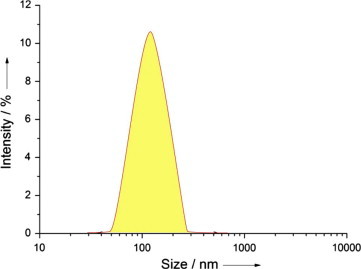
Average particle size of 5-FU (1:3) chitosan nanoparticle.
3.4. Drug loading and entrapment efficiency studies
The reason behind selecting polymeric nanoparticles over solid lipid nanoparticles is their ability to get drug incorporated and also exhibit more encapsulation efficiency. Entrapment efficiency is considered as an important parameter as improper entrapment leads to the initial burst release of the drug, which hinders its sustained release property. Also intended therapeutic dose has to be available for the formulation to achieve required therapeutic effect. Entrapment efficiency of all the polymer nanoparticles made of different polymer ratios was shown in Table 1.
Based on the evaluation parameters of entrapment efficiency and drug content, 5-FU polymeric nanoparticles of ratio 1:3 (138 ± 1.01 nm) and 1:2 (149 ± 1.28 nm) were optimized. Some past researches support the increase in the encapsulation efficiency with increase in the polymer, which was true to some extent in case of chitosan nanoparticles bearing 5-FU as entrapment efficiency increased up to 69.18% till 1:3 ratio of drug: polymer. The increase in the entrapment efficiency can be attributed to the hydrophilicity of the chitosan polymer. Also the use of chitosan correlates with the fact that it has very promising tumor inhibition rates (Zhang et al., 2008a).
But contrary to that chitosan polymeric nanoparticles had shown decrease in the entrapment efficiency (32.18 ± 0.47%) with increase in the polymer ratio after 1:3, which can be attributed to the more compact polymer coat, which limits its entrapment. GL-EU nanoparticles have shown an entrapment efficiency of 69.18 ± 1.89% and that decreased to 32.18 ± 0.47% for the drug: polymer ratio of 1:4. The optimized ratio of 1:2 and 1:3 ratio of 5-FU were given primary importance for further studies. Also the particle size of the optimized ratios was found satisfactory which enables us to select the both ratios for in vitro release studies.
3.5. Scanning electron microscopy
Scanning electron microscopy was performed for polymeric nanoparticles with drug: polymer ratio of 1:3 to obtain more information on the particle size and morphology. The photos of polymeric nanoparticles had shown that the formulated 5-FU chitosan nanoparticles of different polymers were of spherical shape with size range from 102 to 139 nm, which were shown in Fig. 4. Moreover the nanoparticles were observed with smooth surface, which may contribute to its release of the drug in a sustained manner when compared to the nanoparticles having rough surface. This property was evaluated thoroughly in the upcoming in vitro drug release studies.
Figure 4.
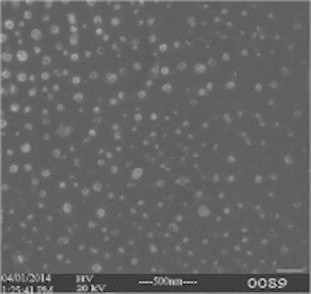
SEM image of GL-PLGA nanoparticles and GL-EU nanoparticles.
3.6. Enteric coated of optimized batches
Chitosan 5-FU nanoparticles with optimized drug: polymer ratios of 1:3 (5-FU E1) and 1:2 (5-FU E2) were subjected to enteric coating in order to protect the drug from getting released at gastric pH and thereby allowing the coat to get dissolved at basic pH which allows the major amount to get released at colon area.
3.7. In vitro drug release studies
The percentage drug release from 5-FU enteric coated nanoparticles with drug: polymer ratio of 1:3 (5-FU E1) and 1:2 (5-FU E2) was observed by using dialysis bag technique in various simulated fluids. These were compared with the plain chitosan nanoparticles, which were non-enteric coated (5-FU NE). This was shown in Fig. 5. Both the ratios did not release the drug till it reaches basic pH albeit minor amount of 5% which may be attributed to the slight improper coating.
Figure 5.
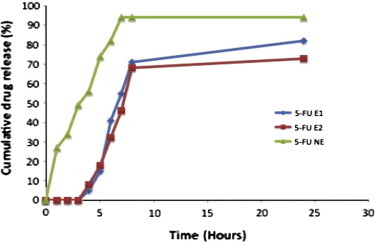
In vitro drug release profile of nanoparticulate pellets in various simulated fluids. Results were presented as mean ± SD, n = 3.
Drug started releasing only after 4 h once it enters simulated intestinal fluid with pH 7.4. At 2 and 3 h time interval, drug got released from the chitosan nanoparticles (52%) which were not enteric coated that can be attributed to the fact that chitosan polymer got degraded at gastric pH which is acidic and started releasing the drug prior to colonic fluid. 5-FU NE nanoparticles released the drug up to 70% before reaching the colonic fluid which decreases the drug intended to lower therapeutic effect.
The 5-FU E1 and 5-FU E2 nanoparticles showed considerable stability till it reaches basic pH and started releasing the drug once they reach basic pH which may be attributed to the carboxyl groups of Eudragit that ionize from neutral to alkaline media. Once the Eudragit nanoparticles enter the neutral and alkaline media, their integrity got disturbed due to the ionization leading to the release of the nanoparticles. As a result 5-FU E1 nanoparticles has shown enhanced drug release 82 ± 0.05% and 71 ± 1.02% at 24 h and 8 h respectively. The 5-FU E2 nanoparticles have shown a slight decrease in the drug release (73 ± 0.89% at 24 h and 68 ± 0.25% at 8 h), which may be attributed to the slightly improper drug entrapment and also improper coating with chitosan.
5-FU E1 nanoparticles have shown enhanced drug release when compared to the 5-FU E2 and 5-FU NE statistically by paired t-test to determine whether two sets of data were significantly different from each other. The statistical analysis using Minitab 17 and Minitab express has shown significant difference for 5-FU E1 nanoparticles. So these 5-FU E1 nanoparticles can be considered as a potential carrier, which can release and localize the drug at colonic pH. Also the use of chitosan leads to the sustained release and also benefitted by its tumor inhibiting property. The rationale of using enteric coating with Eudragit-S100 was supported by preventing drug degradation at gastric pH and allowing it to release once it enters the neutral and alkaline medium.
4. Conclusion
It can be evident from the study that 5-FU was successfully encapsulated in chitosan polymer by solvent evaporation emulsification method. 5-FU chitosan nanoparticles (drug: polymer ratio of 1:3) yielded more entrapment efficiency, drug content and cumulative drug release when compared to the other polymeric nanoparticles with different polymer ratios. In vitro release concluded that 5-FU E1 nanoparticles released drug after 4 h once it enters intestinal fluid therefore protecting the drug release in the gastric environment and enhancing its drug release at colonic region that fulfilled our objective. We can conclude that the formulated nanoparticles improved localization of the drug at the colon area and also achieved sustained release over a prolonged period of 24 h. This can leads to the decreased toxicity to healthy cells as more amount of drug is localized in the colon area. These changes benefit the patient in decreasing the dosing frequency and dose that can be administered. So we can conclude that nanoparticles prepared by this method using the same polymer with the optimized ratio can represent as potential drug delivery approach for effective delivery of the active pharmaceutical ingredient to the colorectal tumors.
Recommended future research
Further in vivo studies can be carried to estimate the pharmacokinetic parameters and the sustained release property of the 5-FU E1 nanoparticles.
Acknowledgments
Dr. M.N. Satish Kumar, professor guided my study in all areas to achieve the desired results and to ensure the study was carried out in a proper way. Ms. Ashwati Prakash supported the study with her kind help during the formulation stage and during manuscript preparation.
Footnotes
Peer review under responsibility of King Saud University.
References
- Arias Jose L. Novel strategies to improve the anticancer action of 5-Fluorouracil by using drug delivery systems. Molecules. 2008;13:2340–2369. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias J.L., Ruiz M.A., LopezViota M., Delgado A.V. Poly (alkylcyanoacrylate) colloidal particles as vehicles for antitumour drug delivery: a comparative study. Colloids Surf. B Biointerfaces. 2008;62:64–70. doi: 10.1016/j.colsurfb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Avinash B., Steven J.S., Karen I. Controlling the in-vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int. J. Pharm. 2007;51:87–92. doi: 10.1016/j.ijpharm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Badry M., Fetih G., Fathy M. Improvement of solubility and dissolution rate of indomethacin by solid dispersions in gelucire 50/13 and PEG 4000. Saudi Pharm. J. 2009;17(3):219–230. doi: 10.1016/j.jsps.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstoiu-Hapcaa F., Bucheggerb L., Bossya Nanomedicines for active targeting: physico-chemical characterization of paclitaxel-loaded anti-HER2 immuno nanoparticles and in vitro functional studies on target cells. Eur. J. Pharm. Sci. 2009;38:230–237. doi: 10.1016/j.ejps.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Dhawale S.C., Bankar A.S., Patro M.N. Formulation and evaluation porous microspheres of 5-fluorouracil for colon targeting. Int. J. Pharm. Tech. Res. 2010;2:1112–1118. [Google Scholar]
- Duran J.D.G., Arias J.L., Gallardo V., Delgado A.V. Magnetic colloids as drug vehicles. J. Pharm. Sci. 2008;97:2948–2983. doi: 10.1002/jps.21249. [DOI] [PubMed] [Google Scholar]
- Grem J.L. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest. New Drugs. 2000;18:299–313. doi: 10.1023/a:1006416410198. [DOI] [PubMed] [Google Scholar]
- Huyghebaert N., Vermiere N., Remon J. In vitro evaluation of coating polymers for enteric coating and human ileal targeting. Int. J. Pharm. 2005;298:26–37. doi: 10.1016/j.ijpharm.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Jain Anekant, Jain Sanjay K., Ganesh N., Barve Jaya, Aadil M. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomed. Nanotechnol. Biol. Med. 2010;6:179–190. doi: 10.1016/j.nano.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Krishnaiah Y.S.R., Satyanarayan S. CBS Publishers and Distributors; 2001. Colon-Specific Drug Delivery Systems: Advances in Controlled and Novel Drug Delivery; pp. 89–119. [Google Scholar]
- Levine D.S., Raisys V.A., Ainardi V. Coating of oral beclomethasone dipropionate capsules with cellulose acetate phthalate enhances delivery of topically active anti-inflammatory drug to the terminal ileum. Gastroenterology. 1987;92:1037–1044. doi: 10.1016/0016-5085(87)90980-2. [DOI] [PubMed] [Google Scholar]
- Malet-Martino M., Martino R.L. Clinical studies of three oral prodrugs of 5-fluorouracil Capecitabine, UFT, S-1): a review. Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- Michor F., Iwasa Y., Lengauer C., Nowak N.A. Dynamics of colorectal cancer. Semin Cancer Biol. 2005;15:484–493. doi: 10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Mohideen B., Ezhilmuthu R.P. Formulation and In Vitro characterization of gliclazide loaded polymeric nanoparticles. Int. J. Biol. Pharm. Res. 2013;4(7):533–540. [Google Scholar]
- Naik J.B., Mokale Vinod J., Shevalkar G.B., Patil K.V., Patil J.S., Yadava S., Verma U. Formulation and evaluation of poly (L-lactide-co-ε-caprolactone) loaded gliclazide biodegradable nanoparticles as a control release carrier. Int. J. Drug Deliv. 2013;5(3):300–308. [Google Scholar]
- Ramanathan Ramesh K., Clark Jeffery W. Safety and toxicity analysis of oxaliplatin combined with fluorouracil or as a single agent in patients with previously treated advanced colorectal cancer. Am. Soc. Clin. Oncol. 2003:2904–2911. doi: 10.1200/JCO.2003.11.045. [DOI] [PubMed] [Google Scholar]
- Rasmussen S.N., Rondesen S., Hvidberg E.F., Honore H.S., Binder V., Halskov S. 5-Aminosalicylic acid in a slow-release preparation: bioavailability, plasma level, and excretion in humans. Gastroenterology. 1982;83:1062–1067. [PubMed] [Google Scholar]
- Wong H.L., Bendayan R., Rauth A.M. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Zhang D.Y., Shen X.Z., Wang J.Y., Dong L., Zheng Y.L., Wu L.L. Preparation of chitosan-polyaspartic acid-5-fluorouracil nanoparticles and its anti-carcinoma effect on tumor growth in nude mice. World J. Gastroenterol. 2008;14:3554–3562. doi: 10.3748/wjg.14.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Yin Y., Xu S.J., Chen W.S. 5-fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–1569. doi: 10.3390/molecules13081551. [DOI] [PMC free article] [PubMed] [Google Scholar]


