Abstract
White pox disease (WPD) affects the threatened elkhorn coral, Acropora palmata. Owing in part to the lack of a rapid and simple diagnostic test, there have been few systematic assessments of the prevalence of acroporid serratiosis (caused specifically by Serratia marcescens) versus general WPD signs. Six reefs in the Florida Keys were surveyed between 2011 and 2013 to determine the disease status of A. palmata and the prevalence of S. marcescens. WPD was noted at four of the six reefs, with WPD lesions found on 8 to 40% of the colonies surveyed. S. marcescens was detected in 26.9% (7/26) of the WPD lesions and in mucus from apparently healthy colonies both during and outside of disease events (9%; 18/201). S. marcescens was detected with greater frequency in A. palmata than in the overlying water column, regardless of disease status (P = 0.0177). S. marcescens could not be cultured from A. palmata but was isolated from healthy colonies of other coral species and was identified as pathogenic pulsed-field gel electrophoresis type PDR60. WPD lesions were frequently observed on the reef, but unlike in prior outbreaks, no whole-colony death was observed. Pathogenic S. marcescens was circulating on the reef but did not appear to be the primary pathogen in these recent WPD episodes, suggesting that other pathogens or stressors may contribute to signs of WPD. Results highlight the critical importance of diagnostics in coral disease investigations, especially given that field manifestation of disease may be similar, regardless of the etiological agent.
INTRODUCTION
The combination of physical stress and disease has resulted in the decline of corals and coral reefs throughout the Caribbean (1, 2). In the Florida Keys and elsewhere, the iconic elkhorn coral (Acropora palmata) has experienced precipitous declines due in part to white pox disease (WPD) (3, 4). Following outbreaks of WPD in the late 1990s and early 2000s, the bacterium Serratia marcescens was isolated from diseased corals and subsequently identified as an etiological agent by fulfillment of Koch's postulates (4, 5). As originally proposed (4), to distinguish the disease caused by this bacterium from broader signs of WPD, it is referred to as acroporid serratiosis when, and only when, S. marcescens is confirmed from a lesion on an A. palmata colony.
Between 1999 and 2006, two strains of S. marcescens were associated with large outbreaks of WPD (acroporid serratiosis) in the Florida Keys. The strain found in association with outbreaks in 2002 and 2003 was identical to a strain concurrently found in human sewage from the nearby islands that compose the Florida Keys archipelago (5, 6). Until recently, septic systems and cesspits were the primary mechanism of wastewater disposal (7–9). In-ground disposal of waste led to sewage pollution in both nearshore and offshore waters of the Florida Keys (10–12).
In recent studies in the Florida Keys and elsewhere in the Caribbean, researchers have reported that S. marcescens could not always be isolated from colonies displaying signs of WPD (5, 13, 14). These observations suggest that other etiological agents may cause WPD signs similar to those of acroporid serratiosis. They also highlight the importance of differentiating acroporid serratiosis from, or identifying it as a specific type of, WPD. Given that there are only a limited number of outward manifestations that a coral may display in response to a stressor or pathogen, diagnosis and disease identification have been ongoing problems in coral disease ecology (15, 16).
A multiyear, multireef systematic analysis including tracking of the fates of individual coral colonies with diagnostics for a single disease agent has not previously been described. The primary objectives of this study were to describe WPD dynamics across reefs of the Florida Keys and to determine the relative importance of S. marcescens in the health of these reefs across space and time.
MATERIALS AND METHODS
Sample sites and collection strategy.
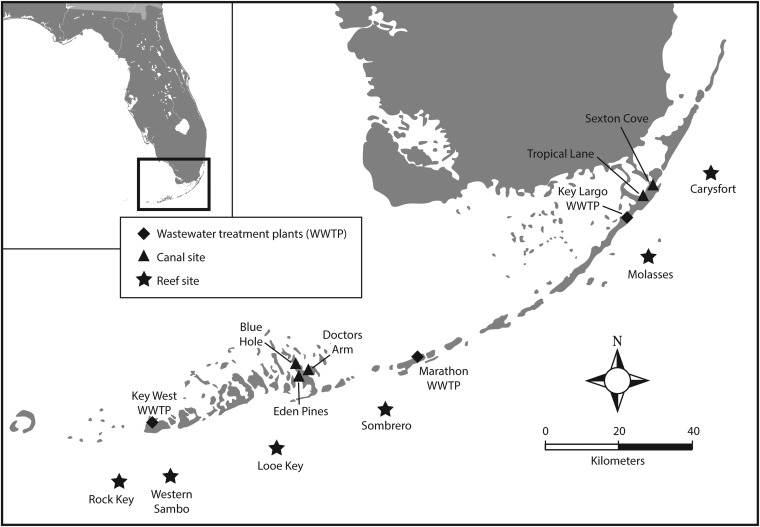
Surveys were conducted and samples were collected from 2011 to 2013 at six offshore shallow reefs spanning the length of the Florida Keys National Marine Sanctuary. Stations (listed from the Upper Keys, near Key Largo, to the Lower Keys, near Key West) included Carysfort Reef, Sombrero Reef, Molasses Reef, Looe Key Reef, Rock Key Reef, and Western Sambo Reef (Fig. 1). A. palmata colonies at each station were mapped and labeled with a unique identifier to allow the tracking of individual colonies between sampling events. At each sampling, colonies were examined for visual signs of WPD as described by Patterson and colleagues (4), as well as for overall health (e.g., discolored tissue, bleaching, or the presence of predation scars). At each station, scuba divers collected water and coral surface mucus. Water was collected in 1-liter sterile polypropylene bottles 1 m above the reef. Coral mucus was collected with 12- or 20-ml sterile needleless syringes from tissue on apparently healthy corals and from the margins of active disease lesions (when present). For corals with disease lesions or otherwise abnormal tissue, mucus was also collected from healthy tissue from a branch of the same colony where no lesions were present. On three occasions (February 2012, August 2012, and August 2013), mucus samples were collected from other nonhost corals. These included five other scleractinian coral species that do not exhibit WPD signs: Orbicella faveolata, O. annularis, Siderastrea siderea, Porites porites, and P. astreoides. In addition to coral mucus samples, sediment was collected at Molasses Reef. Sediment and Coralliophila abbreviata snails, which prey preferentially on A. palmata (1), were collected at Looe Key Reef. Sediment samples were collected from an open sandy area at each reef with sterile 50-ml polypropylene tubes. Unless otherwise noted, samples were collected three times per year between 2011 and 2013 (operationally defined as winter [December to February], spring [May to June], and summer [July to September]).
FIG 1.
Sample locations in the Florida Keys. Stars indicate reef sites, triangles indicate canal sites, and diamonds indicate wastewater treatment plants. Map created by Kip Carter (UGA Educational Resources) using Adobe Illustrator.
Water samples were also collected from residential canals in the Florida Keys that have a history of contamination from septic systems (5, 17). Tropical Lane and Sexton Cove are residential canals on Key Largo in the Upper Keys, an area undergoing conversion from septic to centralized wastewater treatment during the period of this study. Eden Pines and Doctors Arm are residential canals on Big Pine Key in the Lower Keys, an area that was not yet converted to centralized wastewater treatment at the time of this study. Blue Hole is a freshwater lens also located on Big Pine Key. Surface water from each site was collected as grab samples from about 0.5 m below the surface of the water with sterile 1-liter polypropylene bottles.
Finally, as a control for the detection of S. marcescens, sewage influent was collected from three wastewater treatment plants in Key Largo (25°6.041′N, 80°25.930′W), Marathon (24°43.855′N, 81°0.241′W), and Key West (24°34.115′N, 81°47.818′W) (Fig. 1). Wastewater samples were collected according to each plant operator's protocol and typically consisted of a 1-liter post-bar-screen grab sample.
All samples were immediately placed on ice, transported to the field laboratory, and processed within 2 h of collection.
WPD survey.
A. palmata colonies at each site were photographed with a standard scale bar; extra care was used to document disease lesions or any abnormal tissue. Except for mucus sampling, all colonies were left undisturbed. This allowed for natural loss or gain of corals (typically through asexual fragmentation of larger branches rather than through recombinant sexual recruitment) (Table 1). The number of A. palmata colonies surveyed varied by reef because of a wide range of abundance throughout the Florida Keys reef tract. The prevalence of disease at each reef was determined by calculating the percentage of the monitored colonies with white pox lesions. Lesions were defined as irregularly shaped white patches (at least 1 cm2) of tissue loss where the remaining exposed skeleton was bright white, indicating rapid tissue loss (4). Evaluations were made in the field and confirmed through analyses of high-definition photographs.
TABLE 1.
Prevalence of coral colonies exhibiting signs of WPD and total number of colonies surveyed by reef and sampling eventa
| Reef | Location | 2011 |
2012 |
2013 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Winter | Spring | Summer | Winter | Spring | Summer | ||
| Carysfort | 25°13.248′N, 80°12.594′W | 3/8 | 0/6 | 0/7 | 0/7 | 0/6 | 0/5 | 0/6 | 1/6 |
| Molasses | 25°00.528′N, 80°22.590′W | 1/12 | 0/10 | 0/10 | 0/10 | 4/10 | 0/7 | 0/6 | 1/6 |
| Sombrero | 24°37.518′N, 81°06.696′W | 2/8 | 0/8 | 0/9 | 0/8 | 2/8 | 0/8 | 0/8 | 2/8 |
| Looe Key | 24°32.700′N, 81°24.400′W | 0/32 | 0/31 | 0/28 | 0/31 | 10/31 | 0/31 | 0/27 | 6/27 |
| Rock Key | 24°27.270′N, 81°51.534′W | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| Western Sambo | 24°28.680′N, 81°43.026′W | 0/11 | 0/9 | 0/9 | 0/8 | 0/7 | 0/6 | 0/7 | 0/7 |
Boldface indicates that disease was observed. Any increase in colony number between seasons was generally due to a fragmented branch, resulting in an additional individual at the subsequent sampling time. Colony loss between sampling times was due to hurricane damage. No colonies were lost because of disease during the study period.
Molecular detection of S. marcescens.
Coral and water samples were split into 2-ml duplicate aliquots and centrifuged at ∼13,000 × g for 20 min. Sediment samples were vortexed with their overlying seawater and allowed to settle for up to 10 min. After settling, 10 ml of the seawater was transferred to a new sterile tube and saved. Sediment (up to 10 g) was transferred to a second sterile tube and resuspended in up to 10 ml of sterile deionized (DI) water. After vortexing and settling, 2 ml of the supernatant fluid was transferred into microcentrifuge tubes and centrifuged at ∼13,000 × g for 20 min. Snails were not used for molecular detection.
The supernatant fluid from all coral, water, and sediment aliquots was discarded, and the pellets containing bacteria were stored at −20°C pending DNA extraction. A modified ethanol precipitation protocol was used to extract environmental DNA from each of the replicate frozen pellets, maintaining a backup sample for future analyses (18). Briefly, lysis buffer (400 mM NaCl, 750 mM sucrose, 20 mM EDTA, 50 mM Tris-HCl [pH 9.0], lysozyme at 1 mg · ml−1) was added to the pelleted sample. Following incubation at 37°C for 30 min, proteinase K (final concentration, 100 μg · ml−1) and SDS (final concentration, 1% [wt/vol]) were added and tubes were incubated at 55°C for 16 to 18 h. To increase the precipitation of DNA, tRNA (a DNA carrier molecule; 50 μg), 0.1× volume of sodium acetate, and 2.5× volumes of ethanol (EtOH; 99%) were added and the mixture was kept for 1 h at −20°C. Samples were centrifuged (∼13,000 × g for 20 min), and the supernatant fluid was decanted to retain the pelleted DNA in the original tube. DNA pellets were then washed with 500 μl of EtOH (70%) and centrifuged (∼13,000 × g for 20 min), and the supernatant fluid was decanted. A SpeedVac (Eppendorf Concentrator 5301) was used to dry the DNA pellet, which was then resuspended in 100 μl of TE (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.0). The final DNA suspension was stored at −80°C.
Samples were screened by real-time quantitative PCR (qPCR) as technical replicates (19). Extraction controls and inhibition tests were completed according to the original assay design (19). The standard curve for the qPCR assay has a detection limit of 3 genome equivalents · μl−1 (a maximum quantification cycle [Cq] threshold value of 38.84). Samples were considered positive if at least one of the two technical replicates had Cq values below this threshold. Replicate positive controls for at least three dilutions and at least three negative (no-template) controls were included with every run. Any samples with both Cq values above 38.84 were considered negative.
Isolation of culturable S. marcescens.
To obtain isolates of S. marcescens for genetic fingerprinting, surface water (up to 400 ml) and coral mucus (up to10 ml) were filtered onto 47-mm-diameter 0.45-μm-pore-size mixed cellulose ester membranes (Millipore, Billerica, MA). Sediment samples were vortexed with their overlying seawater and allowed to settle for up to 10 min. After settling, 10 ml of the seawater was transferred to a new sterile tube and saved. Sediment (up to 10 g) was transferred to a second sterile tube and resuspended in up to 10 ml of sterile sea water. After vortexing and settling, 5 ml of the supernatant fluid was filtered through 0.45-μm-pore-size membranes in duplicate. Membrane filters were placed onto selective agar for S. marcescens (MacConkey sorbitol agar amended with colistin [MCSA] [5]). Snail tissue was macerated with a flame-sterilized razor blade, and tissue slurry was streaked onto MCSA with a sterile cotton swab. Sewage influent samples (10 to 100 μl) were spread directly onto MCSA plates. Sterile 1× phosphate-buffered saline was used for negative controls and rinse controls. MCSA plates were incubated overnight (18 ± 4 h) at 37°C, and presumptive Serratia colonies (pink colonies indicative of sorbitol fermentation) were transferred to duplicate DNase agar plates amended with toluidine blue and cephalothin (DTC) for phenotypic confirmation (indicated by red halos around colonies) (5, 20, 21). DTC plates were incubated at room temperature and at 41°C for 18 ± 4 h. Isolated colonies of presumptive S. marcescens from DTC were saved in deep-agar stabs of lysogeny broth agar. After two rounds of isolation for MCSA and DTC positive isolates, identification to the species level was completed by PCR (5). Pulsed-field gel electrophoresis (PFGE) was used for strain identification (5, 22).
Statistical analysis.
A generalized linear model with mixed effects using extensions for correlation analysis was applied to the data by using the PROC GLIMMIX procedure in SAS (version 9.3; SAS, Cary, NC). When detected, S. marcescens was generally found in the range of 3 to 10 genome equivalents, which was previously shown to be too low to quantify accurately (19); therefore, analyses focused on the prevalence rather than the concentration or abundance of S. marcescens. Preliminary analysis indicated that the rates of S. marcescens detection in samples from healthy coral, healthy areas on diseased coral, abnormal coral (generally discolored but not diseased), and coral WPD lesions were not significantly different; therefore, they were grouped together as coral samples for final analysis. The statistical model was used to determine if correlations existed between the presence or absence of S. marcescens (determined by qPCR) and the sample type (coral or water) or reef disease status. The collection year, season, and reef were linked together as the random effect in the model. Significance was determined at P < 0.05.
RESULTS
WPD survey.
Between 6 and 33 individual A. palmata colonies were tracked at each of the six reefs over the course of this study. WPD signs were noted at three reefs in 2011 (with a small number of colonies affected) but were observed at greater levels at three of the six reefs in 2012 and at four of the six in 2013 (Table 1). WPD signs were constrained to late summer months, with the exception of two colonies with WPD at Sombrero Reef, one at Molasses Reef, and three at Carysfort Reef in June 2011. Lesions generally appeared as small clusters that sometimes merged over time. Whole-colony death associated with disease was never observed, but temporary partial mortality in the immediate vicinity of active WPD lesions was common. No lesions were active by the subsequent winter sampling effort. Western Sambo and Rock Key Reefs never showed signs of WPD. Disease signs were observed at Sombrero Reef during three of the eight sampling events. At Molasses Reef, lesions were noted in all of the years, affecting six colonies. At Looe Key Reef, WPD signs were noted twice over the study period and the prevalence of disease was consistently high, with 32 and 22% of the colonies positive in 2012 and 2013, respectively (Table 1). Abnormal tissue, discolored but not showing WPD signs, was noted at least once at Carysfort, Sombrero, Looe Key, and Rock Key Reefs and was most common in the spring of 2011 (three reefs).
S. marcescens and acroporid serratiosis survey.
A total of 362 samples from five reefs and eight sampling periods were analyzed for the presence of S. marcescens by qPCR in conjunction with the surveys for WPD. Because of depletion of sample material, samples from Molasses Reef were not included in the survey for S. marcescens. Samples included 201 mucus samples from apparently healthy tissue, 26 samples from the margin of active WPD lesions, 13 mucus samples from areas of otherwise abnormal tissue, and 122 overlying water samples. When detected, S. marcescens was at levels between 3 and 10 genome equivalents, which have been previously shown to provide poor accuracy in quantitation (19). Therefore, analyses focused on overall prevalence. Of all of the samples collected during the 3-year study, 8.8% (32/362) were positive for S. marcescens. Detection rates ranged from 4.9% (6/122) in reef water to 26.9% (7/26) in WPD lesions (Table 2), but there was no significant association between diseased samples and the presence of S. marcescens. Additionally, the presence of disease on the reef was not significantly associated with the detection rate (P > 0.05). However, when healthy and diseased samples were combined, the prevalence of S. marcescens was significantly greater in A. palmata mucus than in the overlying water (P = 0.0177).
TABLE 2.
Prevalence of S. marcescens in A. palmata by disease status and sample type during the study period
| Sample type |
S. marcescens detection [no. of qPCR-positive samples/total(%)] |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2011 |
2012 |
2013 |
Total | ||||||
| Spring | Summer | Winter | Spring | Summer | Winter | Spring | Summer | ||
| Mucus from: | |||||||||
| Healthy tissue | 0/30 | 0/23 | 6/29 | 0/14 | 4/28 | 3/34 | 0/15 | 5/28 | 18/201 (9.0) |
| Abnormal tissue | 1/4 | 0/2 | 0/1 | 0/1 | 0/0 | 0/0 | 0/2 | 0/3 | 1/13 (7.7) |
| WPD lesion | 0/4 | 0/0 | 0/0 | 0/0 | 5/13 | 0/0 | 0/0 | 2/9 | 7/26 (26.9) |
| Overlying water | 0/12 | 0/17 | 1/19 | 0/7 | 4/23 | 0/19 | 0/10 | 1/15 | 6/122 (4.9) |
S. marcescens was detected by qPCR at three reefs: Rock Key, Sombrero, and Looe Key. One sample (water) was positive at Rock Key Reef (1.2%, 1/82), and three were positive at Sombrero Reef (one WPD lesion and two water samples; 3.9% [3/76] overall). S. marcescens was most often detected at Looe Key Reef. In total, 38.4% (28/73) of the samples from this reef were positive. Among the specific sample types, 18 (46.1%) of 39 apparently healthy coral samples were positive and 6 (42.9%) of 14 white pox lesions were positive (Table 3). S. marcescens was also detected in one or more sample types at Looe Key Reef during three of the four sampling events during nondisease periods (Table 3). However, all of the sample types collected during the two observed WPD events were positive for the bacterium (ranging from 12% of the water samples to 62.5% of the apparently healthy corals) (Table 3). No culturable isolates of S. marcescens were recovered from any A. palmata colonies or overlying water by the two-step medium approach.
TABLE 3.
Prevalence of S. marcescens at Looe Key Reefa
| Yr and seasona | WPD status |
S. marcescens detection (no. of qPCR-positive samples/total) |
||||
|---|---|---|---|---|---|---|
| Apparently healthy | Abnormal tissue | WPD lesion | Water | All | ||
| 2011, spring | No disease | 0/9 | 1/1 | —b | — | 1/10 |
| 2012 | ||||||
| Winter | No disease | 6/8 | 0/1 | — | 1/4 | 7/13 |
| Summer | Disease | 4/8 | — | 4/9 | 1/6 | 9/23 |
| 2013 | ||||||
| Winter | No disease | 3/9 | — | — | 0/3 | 3/12 |
| Summer | Disease | 5/5 | — | 2/5 | 1/5 | 8/15 |
Samples were not collected in the summer of 2011 or spring of 2012 and 2013.
—, not sampled or not applicable.
In the summers of 2012 and 2013, apparently healthy nonhost corals (i.e., P. porites, P. astreoides, and Orbicella species), which may be reservoirs of potential pathogens, were sampled during WPD events at Molasses and Looe Key Reefs. Nonhost corals from Rock Key Reef, where WPD was never observed during this study, were also sampled in the winter of 2012. By qPCR, 8 (80%) of 10 colonies sampled were positive for S. marcescens at Molasses Reef in 2012 and 1 (16.7%) of 6 was positive in 2013. At Looe Key Reef, 7 (70%) of 10 colonies sampled were positive in 2012 and 4 (66.7%) of 6 were positive in 2013. In winter of 2012, S. marcescens was detected in 9 (90%) of 10 colonies sampled at Rock Key Reef.
Nonhost corals, predatory snails (Coralliophila abbreviata), and sediment were also analyzed for culturable S. marcescens during summers at Molasses (sediment only) and Looe Key Reefs. No isolates were recovered from snails or sediment. Of 274 mucus samples collected from nonhost corals (P. porites, P. astreoides, O. annularis, O. faveolata, and S. siderea) over the study period, 4 were positive for S. marcescens by culture. All four isolates were obtained in 2012 at Looe Key Reef from P. porites and P. astreoides and matched the PDR60 PFGE pathogenic strain type (5, 6).
S. marcescens in nearshore and onshore environments.
S. marcescens was routinely detected in canals and sewage sources (Table 4). Detection rates in canals varied from 71.4% (5/7) at Doctors Arm in the Lower Keys to 0% (0/6) at Sexton Cove in the Upper Keys (Table 4). In general, S. marcescens was more commonly found in the canals and waters of the not-yet-sewered area of the Lower Keys (Blue Hole, Eden Pines, and Doctors Arm) than in canals in the areas of the Upper Keys, where septic systems have been decommissioned (Sexton Cove and Tropical Lane) (Table 4). Wastewater treatment plant influent (primary sewage) from the three plants was always positive for S. marcescens. Additionally, among cultured isolates, known disease strain PFGE type PDR60 was isolated three times, all from wastewater (twice at Key Largo and once at Marathon); no PDL100 PFGE types (another disease strain) were recovered (4).
TABLE 4.
Presence or absence of S. marcescens in Florida Keys canal samples determined by qPCR detection
| Site | Presence or absence of S. marcescensa |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2011 |
2012 |
2013 |
||||||
| Spring | Summer | Winter | Spring | Summer | Winter | Spring | Summer | |
| Upper Keys | ||||||||
| Sexton Cove | − | − | − | — | — | − | − | − |
| Tropical Lane | + | − | − | — | — | + | + | + |
| Lower Keys | ||||||||
| Blue Hole | − | − | − | — | + | + | − | + |
| Eden Pines | − | − | − | — | + | + | + | + |
| Doctors Arm | − | + | − | — | + | + | + | + |
+, present; −, absent; —, no sample collected.
DISCUSSION
WPD was present throughout the Florida Keys but was most commonly observed at Looe Key Reef; disease was observed in two of the three summers, affecting up to a third of the colonies surveyed (6 to 10 colonies at each event). Overall, WPD signs were observed less frequently during this study than in the 1990s and early 2000s after the disease was first described (4, 23). Between the late 1990s and mid-2000s, colony deaths were commonly noted following WPD signs in the Florida Keys (23); however, during the 3 years of this study, whole-colony death was never associated with WPD. Although partial death of WPD-affected tissue was common during our survey, lesions typically recovered. Any complete colony loss noted during this time was due to physical damage; two tropical storms and one tropical depression passed near the Florida Keys between 2011 and 2013 (NOAA National Hurricane Center). Results from the present study indicate a clear change in the severity of this disease and the resulting population dynamics of A. palmata in the Florida Keys.
In addition to documenting the fate of colonies and reefs with WPD, a major goal of this work was to examine disease etiology over time and geographical region, especially with regard to the presence of S. marcescens, which has been shown to elicit signs of WPD (4–6). Acroporid serratiosis may be just one manifestation of WPD (e.g., see references 4 and 5), and recent reports demonstrate that WPD signs are found in the absence of this agent (13, 14, 24). However, there has been no systematic assessment of the relative importance of S. marcescens in WPD in general. While coral diseases have long been defined only by their gross physical manifestations, it is clear that specific diagnostic assays are needed to identify the etiological agent(s) of disease (e.g., see references 16 and 25). For A. palmata, an endangered species, collection of sufficient materials for a large-scale systematic histological analysis is not always feasible. However, with the development of a rapid qPCR assay, we were able to collect and screen hundreds of samples concurrently to determine the presence of the suspected agent, providing a level of diagnostic evaluation not previously available (19). Using a molecular diagnostic approach, ∼9% of >370 samples collected from five reefs over the 3-year period tested positive for S. marcescens. While the detection rate was highest in WPD lesions (∼27%), this rate was not significantly different from that of other sample types, which may have been due, in part, to the relatively small number of lesions present (26 lesions compared to 201 healthy samples). During the 3-year period and across all of the reefs, collectively, the prevalence of WPD and the detection of S. marcescens were relatively low. Outside of Looe Key Reef, only one disease lesion was associated with S. marcescens, suggesting that during our survey period, acroporid serratiosis was rare.
It is worth noting that the patterns observed at Looe Key Reef were different from those found elsewhere. Looe Key Reef had the greatest number of colonies (∼30 compared to <10 at the other sites). When disease was present, it affected a larger absolute number (up to 10) and proportion of the colonies (up to 32%), and 40 to 44% of the lesions were positive for S. marcescens. In contrast, only 8% (1/12) of the lesions sampled at Carysfort and Sombrero Reefs were positive. Additionally, during WPD outbreaks, all of the sample types screened by qPCR at Looe Key Reef were found to be positive for S. marcescens, including 50 to 100% of the apparently healthy colonies. No S. marcescens isolates were cultured from A. palmata, indicating that levels may have been below the limit of detection for culture, cells were no longer culturable, or some cells may not have been recovered by the selective two-step medium approach. However, S. marcescens isolates were cultured from nonhost corals at Looe Key Reef both during and between WPD events. These isolates were all identified as PFGE type PDR60, which has been confirmed as a pathogenic strain causing acroporid serratiosis and, with the exception of type PDL100 isolated in 1999, is the only strain that has been detected in reef environments in the Florida Keys (5, 6). PDR60 was also reisolated from sewage sources in this study. Therefore, at least one pathogenic strain is still capable of reaching and circulating within the reef environment. It is possible that nonacroporid corals may be an important reservoir for this strain, but the overall impact may not be consistent across all reefs. One of the fundamental problems in the study of coral disease is that corals present illness through a limited number of signs. Therefore, diseases are described by their gross morphology (e.g., white band, Caribbean yellow band, black band, and white pox, among others) and specific pathogens have been determined for only a small number of these signs (reviewed in reference 25). Similar to human illness, where, for example, many different pathogens can result in a fever, it is likely that multiple agents (infectious or otherwise) are likely to be able to produce similar disease signs in corals. To address this, there has been an important push in the study of coral disease to better describe disease pathology (e.g., through histology) and improve disease diagnostics (e.g., see reference 26). The diagnostic evidence in this study indicates that other agents and conditions, in addition to S. marcescens, are likely to cause WPD signs and that these may co-occur with S. marcescens (26); however, an additional specific pathogen or causal agent has yet to be identified for WPD. Furthermore, unlike the case of Oculina patagonica, which has developed resistance to the Vibrio shiloi pathogen (27, 28), A. palmata appears to remain susceptible to S. marcescens, although disease severity has changed and there may be additional contributing factors.
Our understanding of infectious diseases is changing with discoveries of how both host- and microbiome-associated factors affect the disease process. These conceptual advances suggest that a potential pathogen does not necessarily act on its own to cause impairment but may require certain conditions to manifest impairment (29). WPD may provide an interesting example of these issues. While one infectious agent, S. marcescens, is known to cause WPD signs, S. marcescens is not always associated with the disease and can also be found in apparently healthy corals (as shown here and elsewhere [13, 14, 26]). In these cases, S. marcescens may be but one component of an infectious cascade, present but kept in check by the host or its microbial community. When disease signs do occur, they may suggest that the community has shifted to a dysbiotic state, which may promote secondary invaders such as ciliates (30).
The lethality of WPD in the Florida Keys (as noted here) and in the Virgin Islands (31) may be changing. In earlier studies, lesions with S. marcescens frequently resulted in whole-colony death (4), in the present study, similar death associated with WPD lesions was never observed. Further research is needed to determine if this change in WPD severity is due to the type of pathogen, an adaptive response of the coral or coral microbial community, or a combination of these factors. Some level of immunity to the disease may be conferred either by evolutionary modifications of the coral genome or by shifts in the associated microbial community living on the coral. In the Florida Keys, there are very few remaining A. palmata genotypes (32, 33), suggesting that changes in the microbial community may provide a more rapid response than evolution of resistance in the host. Such rapid responses could include shifts toward a microbial community capable of controlling the abundance of pathogenic bacteria or otherwise improving coral resistance. Colonies with either (or both) of these resistance strategies may have a strong selective advantage (27, 28). Continued investigations of WPD including spatial and temporal dynamics of microbial communities in the mucus of healthy and diseased corals, coral genomics, and finely scaled experiments to target the onset of infection are needed to address these outstanding issues. Such studies may provide insights into other diseases, or dysbiotic states, of corals and other animals that may be subject to complex interactions between a host and its microbial community.
ACKNOWLEDGMENTS
This work was supported by NSF grants EF1015342 (to E.K.L. and J.W.P.) and EF1015032 (to K.P.S.) as part of the joint NSF-NIH Ecology of Infectious Disease program. Additional support was provided by a NOAA Nancy Foster Scholarship (to J.L.J.), by the Rollins College Student-Faculty Collaborative Scholarship Program and the John Hauck Foundation (to H.N. and K.P.S.), and by a Rollins College Critchfield Research Grant (to K.P.S.).
Field work was conducted at the Mote Tropical Research Lab on Summerland Key and at the Keys Marine Lab on Key Largo. Mucus and other specimens were obtained under permit FKNMS-2010-131-A1.
REFERENCES
- 1.Miller M, Bourque A, Bohnsack J. 2002. An analysis of the loss of acroporid corals at Looe Key, Florida, USA: 1983–2000. Coral Reefs 21:179–182. doi: 10.1007/s00338-002-0228-7. [DOI] [Google Scholar]
- 2.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. 2003. Long-term region-wide declines in Caribbean corals. Science 301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 3.Miller S, Chiappone M, Rutten L, Swanson D. 2008. Population status of Acropora corals in the Florida Keys, p 781–778. Proc 11th Int Coral Reef Symp, Ft Lauderdale, FL. [Google Scholar]
- 4.Patterson KL, Porter JW, Ritchie KE, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci U S A 99:8725–8730. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland K, Porter J, Turner J, Thomas B, Looney E, Luna T, Meyers M, Futch J, Lipp E. 2010. Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata. Environ Microbiol 12:1122–1131. doi: 10.1111/j.1462-2920.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland KP, Shaban S, Joyner JL, Porter JW, Lipp EK. 2011. Human pathogen shown to cause disease in the threatened elkhorn coral Acropora palmata. PLoS One 6:e23468. doi: 10.1371/journal.pone.0023468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul JH, Rose JB, Jiang SC, Zhou XT, Cochran P, Kellogg C, Kang JB, Griffin D, Farrah S, Lukasik J. 1997. Evidence for groundwater and surface marine water contamination by waste disposal wells in the Florida Keys. Water Res 31:1448–1454. doi: 10.1016/S0043-1354(96)00374-0. [DOI] [Google Scholar]
- 8.Paul JH, Rose JB, Brown J, Shinn EA, Miller S, Farrah SR. 1995. Viral tracer studies indicate contamination of marine waters by sewage disposal practices in Key Largo, Florida. Appl Environ Microbiol 61:2230–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller BD, Causey BD. 2005. Linkages between the Florida Keys National Marine Sanctuary and the South Florida Ecosystem Restoration Initiative. Ocean Coast Manag 48:869–900. doi: 10.1016/j.ocecoaman.2005.03.008. [DOI] [Google Scholar]
- 10.Futch JC, Griffin DW, Lipp EK. 2010. Human enteric viruses in groundwater indicate offshore transport of human sewage to coral reefs of the Upper Florida Keys. Environ Microbiol 12:964–974. doi: 10.1111/j.1462-2920.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 11.Lipp EK, Futch JC, Griffin DW. 2007. Analysis of multiple enteric viral targets as sewage markers in coral reefs. Mar Pollut Bull 54:1897–1902. doi: 10.1016/j.marpolbul.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Lipp EK, Jarrell JL, Griffin DW, Lukasik J, Jacukiewicz J, Rose JB. 2002. Preliminary evidence for human fecal contamination in corals of the Florida Keys, USA. Mar Pollut Bull 44:666–670. doi: 10.1016/S0025-326X(01)00332-0. [DOI] [PubMed] [Google Scholar]
- 13.Polson SW, Higgins JL, Woodley CM. 2008. PCR-based assay for detection of four coral pathogens, p 251–255. Proc 11th Int Coral Reef Symp, Ft Lauderdale, FL http://www.nova.edu/ncri/11icrs/proceedings/files/m08-02.pdf. [Google Scholar]
- 14.Lesser MP, Jarett JK. 2014. Culture dependent and independent analyses reveal no prokaryotic community shifts or recovery of Serratia marcescens in Acropora palmata with white pox disease. FEMS Microbiol Ecol 88:457–467. doi: 10.1111/1574-6941.12311. [DOI] [PubMed] [Google Scholar]
- 15.Pollock FJ, Morris PJ, Willis BL, Bourne DG. 2011. The urgent need for robust coral disease diagnostics. PLoS Pathog 7:e1002183. doi: 10.1371/journal.ppat.1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Work TM, Aeby GS. 2006. Systematically describing gross lesions in corals. Dis Aquat Organ 70:155–160. doi: 10.3354/dao070155. [DOI] [PubMed] [Google Scholar]
- 17.Griffin DW, Gibson CJ III, Lipp EK, Riley K, Paul JH III, Rose JB. 1999. Detection of viral pathogens by reverse transcriptase PCR and of microbial indicators by standard methods in the canals of the Florida Keys. Appl Environ Microbiol 65:4118–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boström KH, Simu K, Hagström Å, Riemann L. 2004. Optimization of DNA extraction for quantitative marine bacterioplankton community analysis. Limnol Oceanogr Methods 2:365–373. doi: 10.4319/lom.2004.2.365. [DOI] [Google Scholar]
- 19.Joyner J, Wanless D, Sinigalliano CD, Lipp EK. 2014. Direct Detection of Serratia marcescens in marine and other aquatic environments using quantitative real time PCR. Appl Environ Microbiol 80:1679–1683. doi: 10.1128/AEM.02755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer JJ III, Silva JF, Williams D. 1973. Isolation of Serratia marcescens on deoxyribonuclease-toluidine blue-cephalothin agar. Appl Environ Microbiol 25:151–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasso G, d'Errico M, Schioppa F, Romano F, Montanaro D. 1988. Use of colistin and sorbitol for better isolation of Serratia marcescens in clinical samples. Epidemiol Infect 101:315–320. doi: 10.1017/S0950268800054248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter J, Dustan P, Jaap W, Patterson K, Kosmynin V, Meier O, Patterson M, Parsons M. 2001. Patterns of spread of coral disease in the Florida Keys. Hydrobiologia 460:1–24. doi: 10.1023/A:1013177617800. [DOI] [Google Scholar]
- 24.May LA, Avandanei AR, Rogers CS, Miller J, Woodley CM. 2010. Microbial community analysis of Acropora palmata mucus swabs, water, and sediment samples from Hawksnest Bay, St. John, U.S. Virgin Islands. NOAA Technical Memorandum NOS NCCOS 123 and CRCP 14. http://www.coris.noaa.gov/activities/aplm_usvi/. [Google Scholar]
- 25.Work T, Meteyer C. 2014. To understand coral disease, look at coral cells. Ecohealth 11:610–618. doi: 10.1007/s10393-014-0931-1. [DOI] [PubMed] [Google Scholar]
- 26.Muller EM, van Woesik R. 2014. Genetic susceptibility, colony size, and water temperature drive white-pox disease on the coral Acropora palmata. PLoS One 9:e110759. doi: 10.1371/journal.pone.0110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed KC, Muller EM, van Woesik R. 2010. Coral immunology and resistance to disease. Dis Aquat Organ 90:85–92. doi: 10.3354/dao02213. [DOI] [PubMed] [Google Scholar]
- 28.Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E. 2006. The coral probiotic hypothesis. Environ Microbiol 8:2068–2073. doi: 10.1111/j.1462-2920.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 29.Casadevall A, Pirofski L. 2014. Microbiology: ditch the term pathogen. Nature 516:165–166. doi: 10.1038/516165a. [DOI] [PubMed] [Google Scholar]
- 30.Sweet M, Bythell J. 2015. White syndrome in Acropora muricata: nonspecific bacterial infection and ciliate histopathology. Mol Ecol 24:1150–1159. doi: 10.1111/mec.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers C, Muller E. 2012. Bleaching, disease and recovery in the threatened scleractinian coral Acropora palmata in St. John, US Virgin Islands: 2003–2010. Coral Reefs 31:807–819. doi: 10.1007/s00338-012-0898-8. [DOI] [Google Scholar]
- 32.Baums IB, Miller MW, Hellberg ME. 2005. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol Ecol 14:1377–1390. doi: 10.1111/j.1365-294X.2005.02489.x. [DOI] [PubMed] [Google Scholar]
- 33.Baums IB, Miller MW, Hellberg ME. 2006. Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecol Monogr 76:503–519. doi: 10.1890/0012-9615(2006)076[0503:GVICSI]2.0.CO;2. [DOI] [Google Scholar]